Effects of Acid Electrolytic Water Treatment on the Storability, Quality Attributes, and Metabolism of Reactive Oxygen Species of Citrus × Tangelo Fruit During Storage
Abstract
The effects of acid electrolyzed water (AEW) treatment on the storage quality and metabolism of reactive oxygen species (ROS) in Citrus × tangelo (tangelo) were investigated. AEW-treated tangelos had a lower risk of decay, a higher proportion of consumer-acceptable fruit, higher total soluble solids (TSS) and titratable acidity (TA) than untreated control fruit. During storage, AEW treatment decreased the cellular respiration rate and ameliorated the increase in cell membrane permeability, decreased superoxide anion radical (O2–•) production, the malondialdehyde (MDA) and hydrogen peroxide (H2O2) concentrations, and increased the activity of superoxide dismutase (SOD), catalase (CAT) and ascorbic acid peroxidase (APX). AEW treatment increased the concentrations of the endogenous antioxidant ascorbic acid (AsA) and glutathione (GSH). It appeared that AEW inhibited membrane lipid peroxidation by increasing the ROS scavenging rate and decreasing ROS; overall, AEW treatment was effective in maintaining the quality and storability of postharvest tangelos.
1. Introduction
Citrus × tangelo (tangelo) is a hybrid citrus cultivar, bred in Japan from the Citrus unshiu mandarin as the female parent. The maturity period of tangelo in the Northern hemisphere is from the end of November to the beginning of December. Tangelo has an orange–yellow appearance, a rough surface, a flat round shape, with an orange and pomelo aroma and golden yellow, delicate flesh. The juice is sweet, refreshing, and rich in vitamins and essential micronutrients, with considerable antioxidant content and health-beneficial biological activities [1]. However, the postharvest cellular metabolism of tangelo fruit is very active, making it susceptible to pathogen infection, oxidative stress, and nutrient depletion during storage [2]. This has a major impact on the storage, transportation, and sale of fresh tangelo fruit, restricting its market value and industrial development. Therefore, it is very important to find a solution to the postharvest quality deterioration of tangelo fruit, using safe and efficient postharvest preservation technologies.
Reactive oxygen species (ROS) are generated by the normal cellular metabolism of plants and are strong oxidizing species [3]. Plants normally produce ROS constantly, but excess ROS are quickly scavenged to maintain a low dynamic equilibrium concentration [4]. Plants have defense system against oxidative stress that can control excessive ROS and repair oxidative injury, consisting of a series of enzymes, such as superoxide dismutase (SOD), catalase (CAT), and ascorbic acid peroxidase (APX), as well as nonenzymatic antioxidants, such as reduced glutathione (GSH) and ascorbic acid (AsA) [5]. However, when plants are subjected to adverse environmental conditions, like aging, mechanical damage, or pathogen infection, the oxidative stress response system can break down and become ineffective. The aging of plants and the occurrence of diseases are closely related to imbalances in ROS metabolism and the resulting oxidative stress [6, 7]. For examples, the quality of stored navel orange was improved by regulating ROS metabolism [8]. Therefore, maintaining ROS metabolism homeostasis is beneficial to fruit quality and control of postharvest diseases.
Electrolytic water is produced by electrolysis of sodium chloride solution; there are three types, namely, acid electrolyzed water (AEW), slightly acid electrolyzed water (SAEW), and alkaline electrolyzed water (ALEW). AEW has a pH < 2.8, an oxidation–reduction potential (ORP) > 1050 mV, and an available chlorine concentration (ACC) > 5 mg/L [9]. AEW is regarded as an environmentally friendly antimicrobial sanitizer [10], which is nontoxic, food safe, produces minimal waste and no residues, has a simple formulation, and is cost-effective [11, 12]. It has been applied to control fungal infections on the fruit surface during storage and handling [13, 14]. AEW reduced blue mold growth, while maintaining the quality of the fruit [15, 16]. SAEW treatment improved the quality of carambola fruit [17], and AEW inhibited postharvest fungus infection of strawberries [18]. In a preliminary study, we isolated pathogenic fungi from rotten tangelo fruit and identified them based on morphological observation, molecular biological methods and pathogenicity analysis [2]. Two identified strains were Penicillium citrinum and Aspergillus sydowii. AEW treatment resulted in wrinkling and shrinkage of the hyphal surface and significant increases in the extracellular pH, extracellular conductivity, and absorbance at 260 nm. AEW disrupted the cell membrane integrity of Penicillium citrinum and Aspergillus sydowii, causing leakage of cellular components and inhibiting the growth of these pathogens. In our previous studies, AEW treatment (ACC 10, 20, 30, and 40 mg/L, respectively) maintained a higher proportion of commercially acceptable tangelo fruit, especially when fruit was treated with AEW treatment (ACC 30 mg/L), and its good fruit rate was 1.75 times that of the control [1]. However, the functions of ROS metabolism in AEW treated tangelo fruit in relation to disease prevention are still unclear. This study is aimed at exploring the influence of AEW treatment on ROS metabolism in tangelo fruit during postharvest storage; the effects on AEW on the storability and quality attributes of AEW treated tangelo fruit were evaluated. The findings provide a practical basis and technical reference for postharvest commercial treatment of tangelo fruit.
2. Materials and Methods
2.1. Preparation of Tangelos
Tangelo fruit at commercially acceptable maturity (defined as surface color CIELAB chromaticity values of L∗ = 65.2 ± 0.5, a∗ = 28.5 ± 0.05, and b∗ = 36.3 ± 0.5) was collected from Yanping District, Nanping City, Fujian Province, China, in the December 2022 harvesting season. Fruit with uniform color and size, disease-free, and with no mechanical damage was selected and transferred to the laboratory by refrigerated truck (4 ± 1°C) within 2 h.
2.2. Preparation of AEW
NaCl solution (0.03 g/100 mL) was electrolyzed using a hypochlorite water generator (Weyhill Environmental Technology Co. Ltd, Shanghai, China). The pH was determined with a pH meter (PHS-3E, Shanghai, China). Iodometry was applied to determine the initial ACC to be 300 mg/L. The AEW was diluted as required for testing, and the pH was adjusted to 2.5 ± 0.1 [19].
2.3. AEW Treatment Procedures for Tangelos
Two groups of 200 tangelos were treated. The treatment group was soaked in AEW (ACC 30 mg/L, pH 2.5) for 15 min [1], whereas the control group was soaked in distilled water. After air drying, the tangelos were packed in polythene bags (0.01 mm thickness, each with five fruits) and stored in a controlled-atmosphere storage box (10 ± 1°C and RH 70%). During storage, four bags (20 tangelos) from each group were removed at random at 30-day intervals and were used to analyze physiological and biochemical indices, fruit disease development, and ROS metabolism in the fruit pulp. Each experiment was conducted with a mixed sample, and all the analyses were performed in triplicate.
2.4. Determination of Fruit Disease Index
Forty tangelos were used to determine the fruit disease index, as described previously [20], using the following visual appearance scale to assess the average area of skin surface lesions: 0, no lesion; 1, lesion area < 1/4 of the fruit surface; 2, 1/4 ≤ lesion area < 1/2; 3, 1/2 ≤ lesion area < 3/4; 4, lesion area ≥ 3/4. The fruit disease index was calculated as Σ (disease score/the highest score × ratio of corresponding tangelo fruit with each score).
2.5. Determination of Total Soluble Solids (TSSs) and Titratable Acidity (TA) in Tangelo Pulp
After combining the pulp and juice, the TSS and TA were determined as described previously [21]. TSS was assessed with a pocket refractometer (WZ-214/ATC, Beijing, China). TA was determined by titration and calculated as citric acid equivalents. Both TSS and TA were expressed as ratios.
2.6. Measurement of Fruit Respiration Rate
As described previously, one tangelo was placed in a 2-L jar, tightly sealed for 10 min; then, the respiration rate was determined based on the CO2 production rate, using an infrared CO2 fruit respiration device (3051H, Zhejiang, China) and expressed as mg CO2 kg–1·h–1. The mean value of 20 tangelo fruits was calculated.
2.7. Measurement of Cell Membrane Permeability
Membrane permeability was determined as described previously [22] and expressed as relative electrical conductivity (percent). The electrical conductivity of samples was determined with a DDS-11A conductivity meter (Ningbo Hinotek Enterprise., Zhejiang, China).
2.8. Determination of Superoxide Anion (O2–•)Production Rate, Hydrogen Peroxide (H2O2), and Malondialdehyde (MDA) Concentrations
The superoxide production rate (expressed as mmol O2–•·kg–1·min–1) and H2O2 and MDA concentrations (expressed as moL·kg–1) were determined in 2.0 g samples taken from 20 tangelo fruit, as described previously [23].
2.9. Measurement of SOD, CAT, and APX Activities
These activities were determined in 2.0 g samples from 20 tangelo fruit, as described previously [24], and expressed as U·kg–1 protein.
2.10. Determination of GSH and AsA Content
These indices were determined in 2.0 g samples from 20 tangelo fruit, as described previously [12], and expressed as mg·kg–1.
2.11. Statistical Analysis
Each result is presented as the mean ± standard error (n = 3). The statistics software of SPSS 20.0 was employed for processing and analyzing the data acquired from the present work based on the ANOVA (analysis of variance) and Duncan’s tests. Statistical significance was defined as p < 0.05 and high significance as p < 0.01. WPS software (https://www.wps.com) was used for graph plotting.
3. Results
3.1. Effects of AEW Treatment on Fruit Disease Index
The fruit disease index provides a quantitative assessment of fruit disease and spoilage during storage, in terms of the relative area of skin showing lesions. Lesions on control fruit first appeared on storage Day 30; then, the disease index increased rapidly (Figure 1a). Lesions first appeared on AEW-treated fruit on storage Day 60; then, the disease index increased significantly more slowly than for the control fruit (p < 0.01) until storage Day 120. The disease index of AEW-treated fruit was only 14.5% of that of control fruit on storage Day 120, that is, AEW treatment strongly inhibited fruit spoilage.
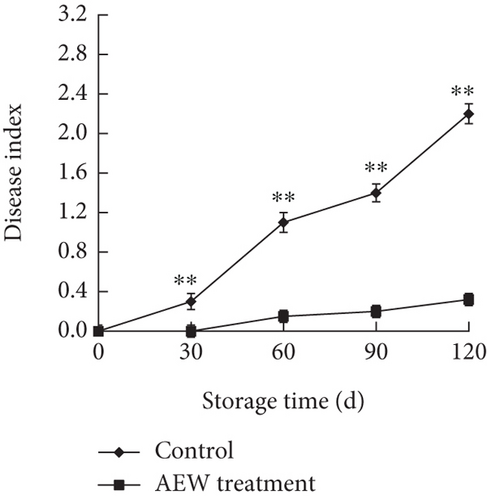
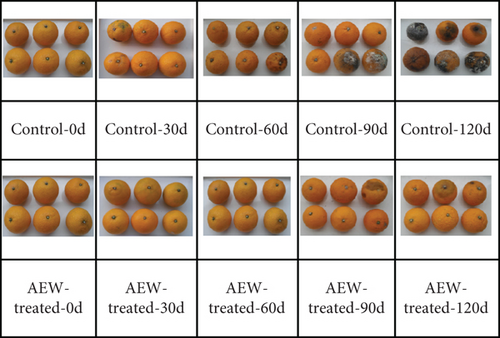
During the early stage of storage, the surface of AEW-treated fruit was intact and evenly colored, with no obvious wrinkles (Figure 1b). After 90 days, the surface of the tangelos began to show some browning, and skin wrinkling started; the flesh started to soften at Day 120 of storage. In contrast, the control fruit had obvious dehydration, wrinkling, and blackening by 30 days of storage, and after 90 days, serious fruit rot and other signs of decay were clearly visible.
3.2. Effects of AEW Intervention on TSS and TA of Tangelo Pulp
The TSS and TA are indicators of the soluble solid and acid contents, respectively, of fruit [17]. TSS includes the major component, sugars, and minor components, such as acids, vitamins, polyphenols, and minerals. Sugar is the main substrate for fruit respiration, so the TSS is a good indicator of sugar content and, therefore, fruit quality and maturity [25]. The TSS initially increased up to Day 60 of storage and then decreased (Figure 2a); the TSS of AEW-treated tangelos was higher (p < 0.01) than that of control tangelos on storage Days 60, 90, and 120. The TSS contents of control and AEW-treated tangelos were 12.27% and 12.94%, respectively, on Day 120, apparently because AEW inhibits cellular respiration, thereby reducing sugar consumption.
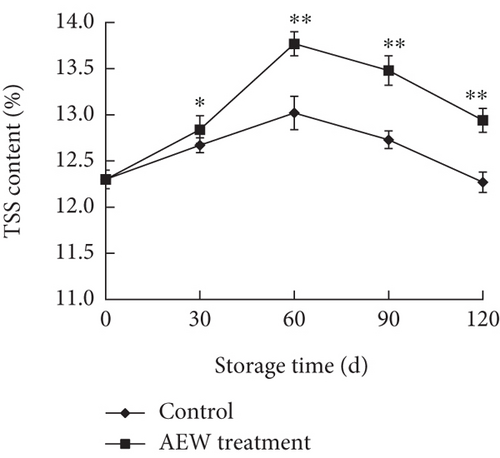
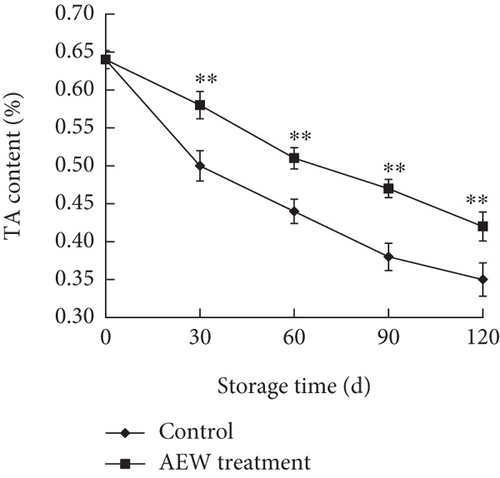
Citric acid is the main acidic component of tangelos; the acid content, measured by TA, has a strong influence on the taste of tangelos. The TA decreased steadily during storage (Figure 2b), but that of the AEW-treated tangelos was higher than control (p < 0.01). The TA of control tangelos decreased by 45.3%, whereas that of AEW-treated tangelos decreased by 34.4%, indicating that AEW treatment can improve tangelo taste.
3.3. Effects of AEW Treatment on Cellular Respiration Rate and Cell-Membrane Permeability
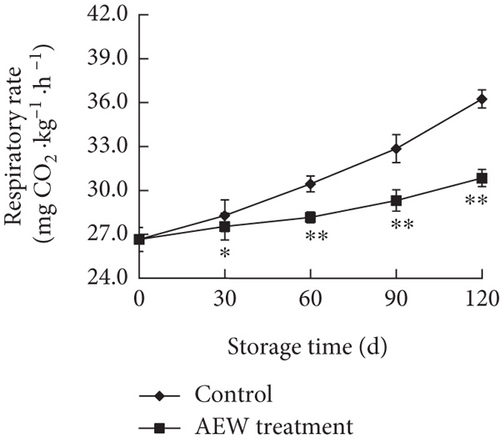
Respiration is a universal feature of living cells and is directly or indirectly related to various physiological and biochemical processes in fruit tissues and a major cause of fruit senescence. The respiration rate is often used as an indicator of the physiological state of fruit. The higher the respiration rate, the more vigorous the metabolism, the faster the nutrient consumption, and the shorter the practical storage period of the fruit [26]. The respiration rate of stored tangelo fruit increased during storage (Figure 3a), and that of control fruit increased faster than that of AEW-treated fruit. AEW-treated fruit had a lower respiration rate (p < 0.05) than control on Day 30, and the difference increased (p < 0.01) steadily with storage time up to Day 120. The respiration rate of AEW-treated fruit was 85.1% of that of control fruit on storage Day 120, that is, AEW treatment markedly decreased the respiration rate of tangelos during storage.
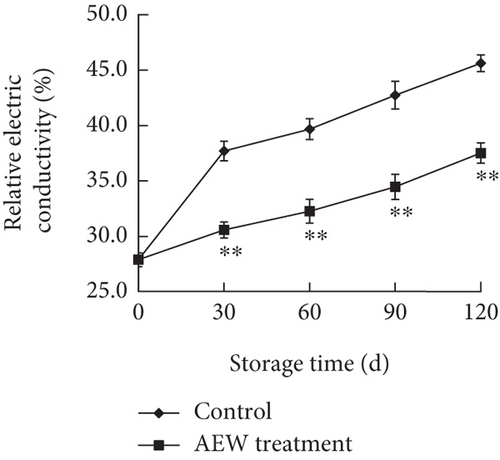
When fruit cells are stressed, cell-membrane permeability increases, and the membrane structure is disrupted, which changes membrane selectivity and results in the extravasation, or leakage, of intracellular substances [22]. The relative permeability is often used to assess membrane integrity and the degree of fruit senescence. That of the tangelos increased during storage (Figure 3b); at Day 30 of storage, that of AEW-treated fruit was markedly lower than control (p < 0.01). The relative electrical conductivity of the AEW-treated fruit increased by 34.5%, whereas that of the control fruit increased by 63.5%. Therefore, AEW treatment significantly reduced the deterioration of cell membrane integrity.
3.4. Effects of AEW Treatment on O2–• Production Rate and the Cellular H2O2 and MDA Concentrations
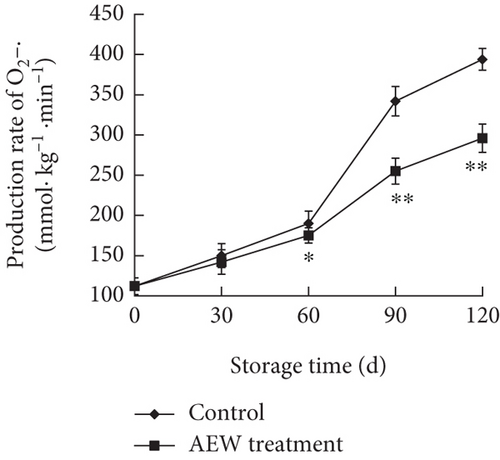
O2–• and H2O2 are intermediate products in the mitochondrial metabolic pathways for energy generation. They are strong oxidizing agents, and excessive accumulation generates oxidative stress, which results in cellular membrane lipid peroxidation, and increased membrane permeability [23]. Therefore, the O2–• production rate and H2O2 concentration are good indicators of the severity of oxidative stress in tissues. The O2–• production rate of stored tangelos increased slowly during the first 60 days, and there was little difference between AEW-treated and control fruit (Figure 4a). However, at Days 90 and 120, the O2–• production rate markedly increased in control fruit, but increased much less in treated fruit (p < 0.01).
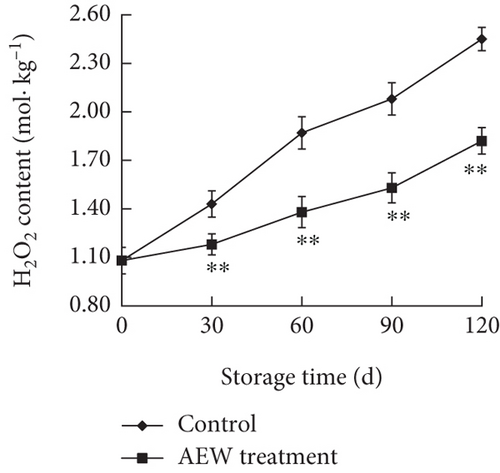
The H2O2 concentration in control and AEW-treated tangelos increased during storage (Figure 4b), but the increase was markedly greater in the control fruit (p < 0.01). The H2O2 content increases were essentially linear, except for a moderate increase in rate for treated fruit from 90 to 120 days.
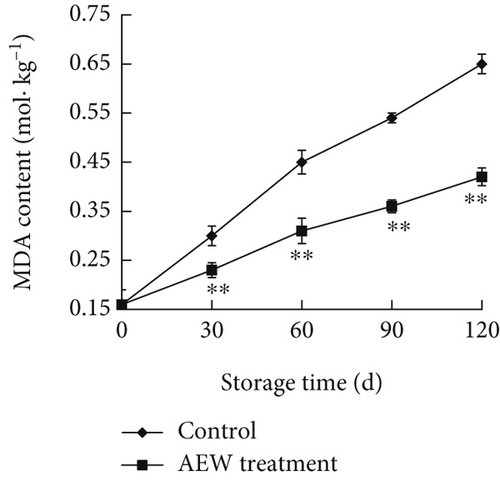
MDA is a major breakdown product of membrane lipid peroxidation and can be used as an index of cell membrane damage and cellular oxidative stress; the MDA concentration is a commonly used measure of membrane lipid peroxidation [23, 24]. The MDA content of stored tangelos increased, indicating increased membrane lipid peroxidation and more advanced senescence (Figure 4c). The MDA content of AEW-treated fruit (0.42 mol/kg at Day 120) was lower (p < 0.01) than control (0.65 mol/kg at Day 120) all through the storage period, indicating that AEW treatment reduces cellular membrane damage and delays senescence in stored tangelos.
3.5. Effects of AEW Treatment on Antioxidant Enzyme Activities
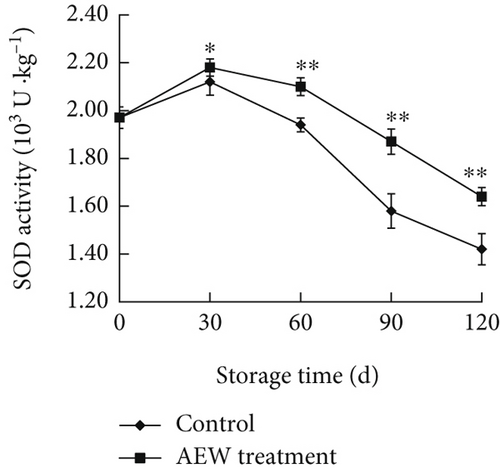
SOD, CAT, and APX are important antioxidant enzymes for scavenging ROS. They cooperate with each other to remove ROS and enhance the postharvest storage of fruit. The SOD activity increased slightly between storage Days 0 and 30 and then decreased rapidly until Day 120 (Figure 5a). SOD activity in AEW-treated fruit was slightly higher than that of control fruit at Day 30 and significantly higher from Day 60 to Day 120 (p < 0.01).
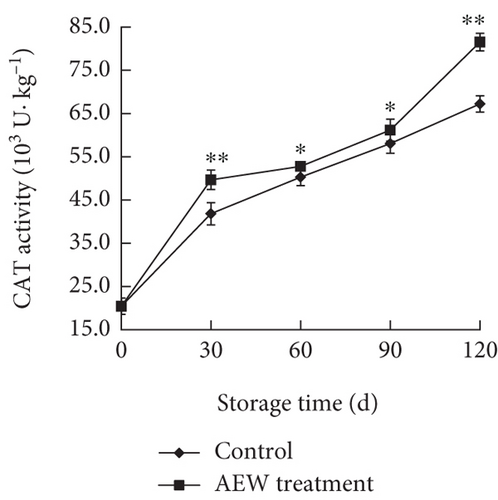
The CAT activity in control tangelos increased relatively steadily, whereas that in AEW treated fruit increased rapidly during the first and last 30 days, but relatively slowly from Day 30 to Day 90, reaching 81.54 × 103 U · kg–1 protein on Day 120 (Figure 5b). The control fruit CAT activity was lower than that of AEW treated fruit and the difference was greater at Days 30 and 120 (p < 0.01).
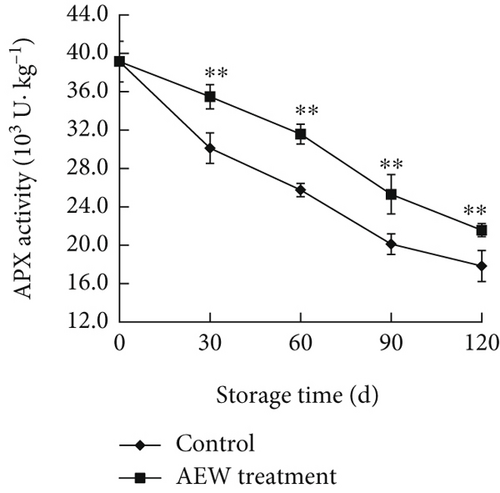
The APX activity of control stored tangelos decreased much faster (p < 0.01) until Day 30 than that of AEW-treated fruit; then, the rates of decrease were similar until Day 120 (Figure 5c). The APX activity of AEW-treated fruit was 21.57 × 103 U · kg–1 protein on Day 120, about 1.2 times that of control.
The enzyme activities were increased relative to control by AEW treatment, which would strengthen the oxidative stress response and delay senescence in stored tangelos.
3.6. Effects of AEW Treatment on Nonenzymic Antioxidants
AsA and GSH are abundant nonenzymic antioxidants in plants, with important functions in ROS scavenging. The AsA concentration in control stored tangelos decreased (Figure 6a) and was markedly lower than that in AEW-treated fruit (p < 0.01). The control AsA concentration decreased rapidly during the first 60 days and then decreased slowly, whereas that of the AEW-treated fruits initially decreased slowly and then decreased rapidly.
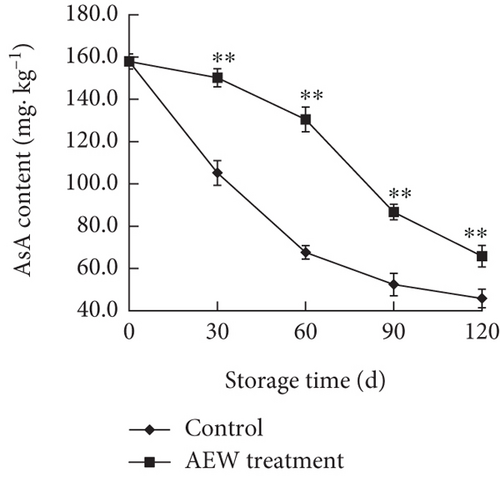
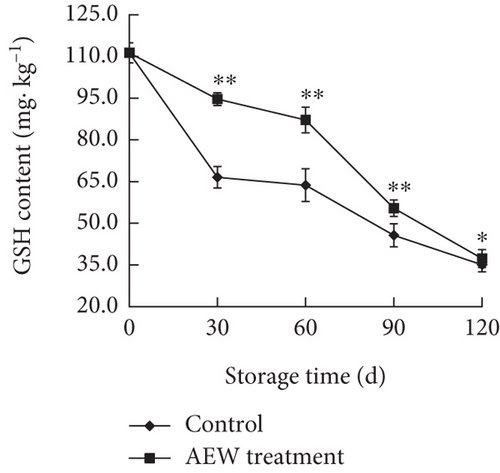
The GSH content of control fruit decreased rapidly for the first 30 days, slowly from Days 30 to 60, and then rapidly again after Day 60 (Figure 6b). The GSH content of AEW-treated tangelos decreased slowly until Day 60 and then rapidly thereafter (p < 0.05). The control and AEW GSH contents were similar on Day 120, whereas the AEW treated fruit was markedly higher (p < 0.01) at Days 30, 60, and 90. AEW treatment consistently maintained the AsA and GSH concentrations in tangelos above that of control fruit.
4. Discussion
Fruit cellular metabolism and respiration continue during postharvest storage, and uncontrolled respiratory metabolism results in the stored fruit becoming senescent, reducing quality and consumer acceptance [20, 22]. Therefore, effective treatments are needed to inhibit respiratory metabolism and slow down the decline in quality, thereby delaying senescence and extending the practical storage period. In this study, AEW treatment significantly inhibited the respiration of tangelo fruit (Figure 3a) and delayed the decrease in TSS and TA (Figures 2a,b), in agreement with previous reports [21, 26]. These findings indicate that AEW inhibited respiration in stored tangelos, thereby reducing the consumption of sugars and other nutrients, and prolonging their shelf life.
Fruit produces ROS during postharvest storage and handling because respiration and other metabolic processes continue [27, 28]. The main ROS species in stored fruit are·OH, O2–• and H2O2, which are strong oxidizing agents that cause oxidative stress and damage to biomolecules [12]. Excessive accumulation of these substances can accelerate membrane lipid peroxidation, disrupting the structure and function of cell membranes and generating toxic by-products such as MDA [23]. Therefore, ROS must be degraded to maintain oxidative homeostasis. The rate of O2–• production increased in control tangelos during postharvest storage, indicating that the fruit tissues were subjected to oxidative stress, including accumulation of the highly oxidative H2O2 and increased lipid peroxidation, resulting in an increased cellular MDA concentration. Lipid peroxidation, in turn, increased cell membrane disruption, demonstrated by increased electrical conductivity, indicating increased membrane permeability. AEW treatment significantly inhibited the increased O2–• production rate (Figure 4a) and reduced the accumulation of H2O2 and MDA (Figure 4b,c), in agreement with a previous report on longan fruit [23–25].
Plants have a well-developed oxidative stress response based on a ROS scavenging system involving antioxidant enzymes, such as SOD and CAT, and nonenzymic antioxidants, such as AsA and GSH. These oxidative stress response components work together to control the cellular ROS concentration, thereby maintaining oxidative homeostasis [29]. SOD is a vital antioxidant enzyme, converting the extremely reactive O2–• into the less reactive H2O2. CAT and APX then convert H2O2 to harmless H2O and O2. In plants, APX catalyzes the reduction of H2O2 into water by oxidizing AsA to monodehydroascorbic acid (MDHA) [30]. Subsequently, MDHA can spontaneously convert into dehydroascorbic acid (DHA). Additionally, monodehydroascorbate reductase (MDHAR) regenerates AsA from MDHA, using reduced nicotinamide adenine dinucleotide phosphate (NADPH) as the electron acceptor. Dehydroascorbate reductase (DHAR) converts DHA and GSH into GSSG and AsA. Glutathione reductase (GR) converts GSSG to GSH using NADPH. A study on apples demonstrated that ferulic acid enhances both the activities and the gene expression of enzymes involved in ROS metabolism, specifically SOD, CAT, APX, MDHAR, and DHAR, which increases the accumulation of antioxidants, and in turn, reduces the levels of H2O2 and MDA in the fruit tissues. These findings suggest that ferulic acid may regulate ROS metabolism, thereby increasing disease resistance in apples. In a postharvest analysis, phenyllactic acid (PLA) application was found to induce disease resistance in apples by stimulating ROS metabolism through enhanced activities and gene expression of SOD, CAT, MDHAR, DHAR, and APX. Additionally, PLA treatment facilitated the accumulation of GSH and AsA, further supporting its role in enhancing the antioxidative defense mechanisms in apple tissues [31]. AEW treatment enhanced the enzymic activities of SOD, CAT, and APX (Figure 5); the findings of this study agree with a previous report on blueberries [9]. AsA and GSH, the most important nonenzymic antioxidants, also function as ROS scavengers [23]. This study found that AEW treatment markedly increased the concentrations of AsA and GSH (Figure 6), enhanced ROS scavenging capacity, reduced peroxidative damage to the cell membrane, and maintained membrane integrity, thereby enhancing the storability of tangelos. There are many similar previous reports: AEW treatment at pH 2.82 and an ACC of 103 mg/L reduced the pathogen counts in fruits and vegetables [32]; AEW treatment at an ACC of 50 mg/L inhibited pathogen invasion of strawberry [18]; AEW treatment at pH 2.8 and an ACC of 48 mg/L inhibited postharvest diseases in blueberry [9]. For practical application of AEW, it is necessary to optimize the effective chlorine concentration and pH depending on the microbial pathogens and food matrices involved, to effectively control the pathogens. In this study, AEW (30 mg/L ACC, pH 2.5) was used to treat tangelo fruit effectively. However, the application of AEW technology still faces some challenges, including the low stability of the active components in AEW and potential impacts on the sensory quality of fruits and vegetables. Therefore, it is necessary to optimize the electrolyzed water generation process, control the storage conditions, and develop synergistic combinations of AEW treatment, modified atmosphere storage, coating preservation, and other technologies to improve the effectiveness of preservation technology. Future research should include combined treatment of tangelos with AEW and other physical preservation technologies, such as ultrahigh pressure and mild heating treatment, which could improve the storage and preservation of fruits and vegetables and has broad development potential.
5. Limitations of the Study
This research omitted how AEW affects flavor and aroma. In the next stage, we will further study the influence of AEW treatment on the flavor, aroma, and sensory evaluation of tangelo fruit. The study was conducted under a single storage condition (10 ± 1°C, 70%). In subsequent research, we will evaluate the effects of AEW treatment under varying temperature and humidity conditions to better promote the research results.
6. Conclusion
In conclusion, the disease index of AEW-treated fruit was only 0.32 on storage Day 120. The TSS and TA contents of AEW-treated tangelos were 12.94% and 0.42%. The indicated that AEW treatment delayed the decay of tangelo fruit and retained higher concentrations of nutrients including sugars and acids in the later period of storage. Next, the respiration rate of AEW-treated fruit was 85.1% of that of control fruit on storage Day 120. AEW treatment on O2–• production rate and the cellular H2O2 and MDA concentrations were 296 mmoL·kg–1·min–1, 1.82 moL·kg–1, and 0.42 moL·kg–1. AEW treatment could prevent the cell membrane lipid peroxidation, hence maintaining the integrity of cellular membrane structure and inhibiting the disease development. In addition, AEW treatment could enhance the activities of SOD, CAT, and APX. AEW also delayed the decrease in concentration of the endogenous antioxidants AsA and GSH.
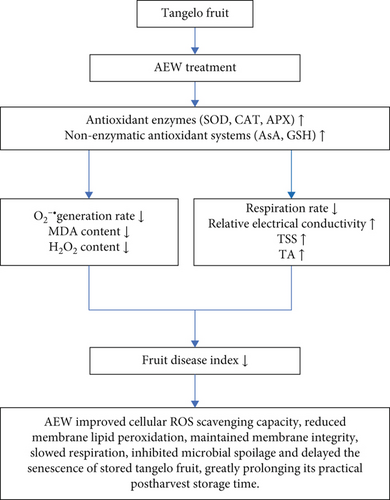
A possible mechanism for the delayed occurrence of disease development in harvested tangelo fruit, through enhancing the oxidative stress response, is shown in Figure 7. The findings of this study demonstrate that AEW treatment is a promising method to control postharvest disease in tangelo fruit. However, the mechanism of action by which AEW induces postharvest disease resistance in tangelo fruit requires further research, specifically, identification of the genes and proteins involved in enhancing postharvest disease resistance and elucidation of the molecular mechanisms of AEW-induced disease resistance.
Disclosure
A preprint has previously been published (Ji, Ying and Wang, Jieqiong and Huang, Hua-Ming and Huang, Jing-Yi and Ma, Yan-Hong, Effects of Acid Electrolytic Water Treatment on the Storability, Quality Attributes and Metabolism of Reactive Oxygen Species of Tangelo Fruit During Storage. Available at SSRN: https://ssrn.com/abstract=5039719).
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Conceptualization, Y.J.; methodology, Y.J. and J.-Q.W.; formal analysis, J.-Q.W.; resources, H.-M.H.; data curation, Y.J., J.-Y.H., J.-Q.W., and Y.-H.M.; writing—original draft preparation, Y.J.; writing—review and editing, J.-Q.W.; visualization, J.-Y.H.; supervision, Y.J. and H.-M.H.; project administration, Y.J.; funding acquisition, H.-M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by the Natural Science Foundation of Fujian Province (10.13039/501100003392) (2022J01388) and the Natural Science Foundation of Nanping (2019J09).
Acknowledgments
This work was supported in part by the Fujian Provincial Department of Science and Technology.
Open Research
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on a reasonable request.




