Microvegetables and Their Potential Health Relevance: A Systematic Review of In Vitro and In Vivo Evidence
Abstract
A rise in noncommunicable disease (NCD) prevalence has been partly attributed to the low intake of food groups rich in phytochemicals, particularly fruits and vegetables. For this reason, microvegetables (MV) are an emerging functional food group. Nutrient-dense with bioactive compounds, MV exhibit bioactive properties that may be relevant for NCD and malnutrition risk modulation. No chronic MV supplementation human clinical trials have been conducted. However, experimental in vitro and in vivo studies have shown that MV can exert a range of beneficial effects. In this systematic review, we analysed the effects of MV in such investigations and discussed the potential mechanisms involved. A bibliographical search was performed in PubMed and Scopus, and based on the inclusion criteria, 28 articles were summarised and critically discussed. Twelve studies demonstrated direct antioxidant capabilities of MVs via in vitro assays. Few studies investigated the more physiologically relevant impact of MV on endogenous cellular antioxidant systems. MV demonstrated proapoptotic activity in immortalised cell lines, with limited in vivo evidence indicating potential anti-inflammatory, lipid and gut microbiota-modifying effects. These properties warrant further investigation in human trials to evaluate their translational potential, and our summary of the literature offers a rationale and basis for such clinical trials.
1. Introduction
Noncommunicable diseases (NCD), including but not limited to cardiovascular disease, type 2 diabetes (T2D) and cancer, account for 71% of all deaths and are of public health concern [1]. People are living longer with disabilities from NCD, with treatment and management across Europe costing at least 25% of total health expenditure [2]. National strategies for preventing and managing NCD across the globe, including the United Kingdom, have highlighted the importance of healthy eating [3]. Diet is a key modifiable risk factor for NCD. Extensive research has highlighted the importance of consuming fruits and vegetables (FV) for overall optimal health [4, 5]. A recently published meta-analysis found that for each 100 g/day increase in FV intake, the likelihood of mortality from cancer and cardiovascular disease is reduced by 4% and 7%, respectively [6]. In a separate meta-analysis, a nonlinear inverse association was observed between FV intake and NCD mortality; however, a benefit threshold was reached at approximately 5 servings daily [7].
Global recommendations for adult FV intake are at least 5 portions/day [8]. In the United Kingdom, however, adults aged 18–75 years consume on average 3.9 portions and only 30% of adults meet intake recommendations [9]. The content of phytochemicals, vitamins and other bioactives in FV is highly variable depending upon plant species, growing conditions and postharvest processing including storage conditions [10, 11]. Climate change is increasingly altering the phytochemical constituents of FV crops [5]. For example, increased ambient temperatures and carbon dioxide reduce the yield of radish and cabbage [12]. Moreover, increased ozone lowers the yield and nutritional quality of broccoli [13]. FV consumption also accounts for most of the pesticide exposure in the human diet [5], albeit current epidemiological evidence does not consistently link pesticide exposure from FV consumption with adverse health outcomes, such as all-cause mortality [14]. Nonetheless, if FV intake recommendations were to be increased and/or FV consumption increased in line with recommendations, the associations between increased pesticide exposure via FV consumption and health outcomes would need to be re-examined.
Microvegetables (MV) may represent a solution to these challenges. MV have lower barriers to production and consumption than other vegetables, and their growing conditions can be tightly controlled to greater standardise bioactive contents. For example, cabbage, beet [15] and basil [16] can be grown hydroponically, i.e., in water-based solutions and without the use of pesticides and chemical fertilisers. MV are vegetables grown for a short period of time, defined by the full expansion of the cotyledon leaves and the appearance of the first true leaves that generally occur within 7–21 days [17]. MV are rich in various phytochemicals and vitamins, and in greater amounts per unit weight compared to their mature counterparts [18]. Mustard MV have over double the amount of Vitamin C, compared to the mature counterpart. Furthermore, mustard MV had approximately 133% of the polyphenol content of the mature counterpart [19]. Polyphenols are the most plentiful and diverse group of phytochemicals and can be further classified into flavonoids and nonflavonoids (phenolic acids, stilbenes and lignans) [20]. High intake of polyphenols, the richest source of antioxidants in the human diet, is well-established in predicting better health outcomes, including cardiovascular disease [21] and metabolic disease [22]. While FV intake has been linked to improved health outcomes, the specific contribution of MV remains largely unexplored.
Recent reviews have summarised the chemical composition, growth, storage techniques and sensory evaluation of MV [17, 23–25]. These articles have highlighted the nutrient composition including phenolic compounds, vitamins (A, C, K and E) and minerals of various MV species and discussed the impact of stress or fortification of soil on the nutrient composition. However, the health benefits of MV have only been briefly reviewed and this has not been comprehensive or systematic. Therefore, the aim of this review was to systematically gather relevant literature on MVs and assess their therapeutic potential for human health.
2. Methods
2.1. PICOS
The protocol for this review was prospectively registered on the Open Science Framework [26] and adhered to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) reporting guidelines [27]. No chronic supplementation human trials have been performed. Nonetheless, many experimental in vitro and in vivo studies using MV have been conducted. The research question was formulated ‘what are the potential health properties of MV?’ This question was refined using the ‘PICOS’ acronym: population (P), animal (nonhuman) or in vitro experiments; intervention (I), MV; comparator (C), control group or a positive/negative control (in vitro) experiments; outcome (O), health property, including but not limited to antioxidant, anti-inflammatory, antiproliferative, or metabolic effects; study type (S), in vivo or in vitro.
2.2. Search Strategy
Searches were initially performed on 6 September 2024 (and rerun on 9 January 2025) using two online databases: PubMed and Scopus using a combination of the following terms: (‘micro-vegetables’ or ‘microgreens’) AND (‘antioxidant’ or ‘anti-inflammatory’ or ‘metabolic’ and associated keywords) AND (‘in-vitro’ or ‘in-vivo’ or ‘animal’ or ‘human’) to identify relevant articles.
2.3. Inclusion/Exclusion Criteria
All studies that clearly stated or described a MV intervention and that evaluated a ‘health’ property (including but not limited to antioxidant, anti-inflammatory or metabolic responses) were included. Articles that were published in English language were included. There was no restriction for the year of publication. Conference abstracts, editorials and review articles were excluded. Articles without clear description confirming that a plant or plant extract was an MV were excluded.
2.4. Data Extraction
Two researchers performed the data extraction of included articles sequentially, with the second confirming the data extraction by the first researcher. Any disagreement was resolved by discussion between researchers and reassessment of the data. Extracted data are shown in Tables 1, 2, 3, 4. Table 1 includes the in vitro studies and contains the following: MV species, total polyphenolic content (TPC; amount), antioxidant capacity assay and author. Table 2 includes in vitro studies and contains the following: MV, cell type, concentration (of MV), key results and author. Tables 3 and 4 include in vivo animal studies and contain the following: MV, dose (of MV), control, animal model, duration, key results and author.
| Microvegetable | Total polyphenol content | Antioxidant capacity | Author | |||
|---|---|---|---|---|---|---|
| Amount | ABTS | DPPH | FRAP | ORAC | ||
| Broccoli | ∼5.8 mg GAE/g | ∼7.5 mg TE/g | ∼22.5 mg FE/g | 30 | ||
| 15.41 mg GAE/g | 90.14% | 90.47% | 94.98% | 31 | ||
| 17.53 mg GAE/g | 57.18% | 88.23% | 97.74% | 32 | ||
| 142.79 mg GAE/g | 7.84 μM TE/g | 364.5 μM TE/g | 33 | |||
| Cress | 94.10 mg GAE/g | 105.95 mg TEAC/g | 9.43 mg TEAC/g | 34 | ||
| Kale | 144.77 mg GAE/g | 9.87 μM TE/g | 739.15 μM TE/g | 33 | ||
| Licorice | ∼400 mg GAE/g | ∼15 μmol TE/g | 35 | |||
| Mustard | ||||||
| Field | 2.96 mg GAE/g | 9.46 μmol TE/g | 36 | |||
| Green | ∼4.0 mg GAE/g | ∼2.2 mg TE/g | ∼15 mg FE/g | 37, 38 | ||
| Red | 82.06 mg GAE/g | 11.08 μM TE/g | 745.25 μM TE/g | 33 | ||
| Pea | 1050 μg/g | 300 μg/g | 39 | |||
| Radisho | ∼700 μg/g | ∼500 μg/g | 39 | |||
| 143.48 mg GAE/g | 13.77 μM TE/g | 525.89 μM TE/g | 33 | |||
| Thai rat-tailed | ∼7.8 mg GAE/g | ∼7.8 mg TE/g |
|
30, 40 | ||
| Red Ramb | ∼1490 μg/g | ∼1100 μg/g | 39 | |||
| Red cabbage | 3201.5 mg GAE/g | 700.1 μmol TE/g | 186.7 μmol TE/g | 324.9 μmol TE/g | 41 | |
| Rocket | ∼600 μg/g | 250 μg/g | 39 | |||
| Soy | ∼1200 μg/g | 450 μg/g | 39 | |||
- Note: Total polyphenolic content determined by Folin–Ciocalteu colorimetry. %, percentage, μg, microgram, μmol, micromolar, ABTS, 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid), DPPH, 2,2-diphenyl-1-picrylhydrazyl.
- Abbreviations: Fe, ferrous cation; FRAP, ferric reducing antioxidant power; g, grams; GAE, gallic acid equivalent; mg, milligrams; ORAC, oxygen radical absorbance capacity; TE, Trolox equivalent.
| Microvegetable | Cell type | Concentration | Results | Author |
|---|---|---|---|---|
| Beetroot | MCF-7 | Digested extract | Antiproliferative action: | 43 |
| - 27.38% cell growth inhibition | ||||
| Protein activity: | ||||
| - 97.5% Bcl-2 inhibition | ||||
|
||||
| - ↑ Cytochrome C, Smack/DIABLO, HtrA2/Omi, Caspase-7, Caspase-8, p38 | ||||
| - ↓ RIP | ||||
| MDA-MB-231 | Digested extract | Antiproliferative action: | 43 | |
| - 4.76% cell growth inhibition | ||||
| Protein activity: | ||||
| - 83.5% Bcl-2 inhibition | ||||
|
||||
| - ↑ Cytochrome c, Smack/DIABLO, p53, Caspase-7, Caspase-8, p38 | ||||
| - ↓ RIP | ||||
| Broccoli | Caco-2 | Digested extract (1:10 v/v) | Antiproliferative action: | 33 |
| - 10% reduction in overall cell growth. | ||||
| - ↓ G0G1 phase, GSH activity | ||||
| - ↑ Sub-G1 phase, mitochondrial depolarisation, intracellular ROS | ||||
| Digested extract (100 μL) | Antiproliferative action: | 44 | ||
| - N.S. on cell growth | ||||
| Iron bioavailability: | ||||
| N.S. in ferritin | ||||
| HaCaT | Crude extract (0.05–0.5 mg/mL) | - ↑ ROS inhibition (% not stated) | 31 | |
| Crude extract (0.05–0.8 mg/mL) | - Cytotoxicity ∼625 μg/mL | 32 | ||
| - ↓ Cell survival (dose–response) | ||||
| HepG-2 | Crude extract (0–250 μg/mL) | Antiproliferative action: | 30 | |
| - 87%–91% cell growth inhibition | ||||
| Crude extract (0.05–0.5 mg/mL) | - ↑ ROS inhibition (% not stated) | 31 | ||
| Crude extract (0.05–0.8 mg/mL) | - Cytotoxicity 243.23 μg/mL | 32 | ||
| - ↓ Cell survival (dose–response) | ||||
| HCT116 | Crude extract (0.05–0.5 mg/mL) | - ↑ ROS inhibition (% not stated) | 31 | |
| HCT116 | Crude extract (0.05–0.8 mg/mL) | - Cytotoxicity 544.67 μg/mL | 32 | |
| - ↑ Cell survival (dose–response) | ||||
| H460 | Crude extract (0.05–0.8 mg/mL) | - Cytotoxicity 435.24 μg/mL | 32 | |
| - ↓ Cell survival (dose–response) | ||||
| MEF | Crude extract (0.05–0.8 mg/mL) | - Cytotoxicity > 800 μg/mL | 32 | |
| - ↓ Cell survival (dose–response) | ||||
| Cress | TM3 | Crude extract (250–2000 μg/mL) | 1000–2000 μg/mL: | 34 |
| - ↑ Cytotoxicity, metabolic activity | ||||
| - ↓ Membrane integrity, lysosomal activity, progesterone, testosterone, GJIC inhibition | ||||
| 125–250 μg/mL: | ||||
| - ↑ Metabolic activity, progesterone, testosterone, GIJC inhibition | ||||
| - N.S. cell membrane integrity | ||||
| - N.S. lysosomal activity | ||||
| - ↓ ROS production | ||||
| Fenugreek | Caco-2 | Digested extract (100 μL) | Antiproliferative action: | 44 |
| - N.S on cell growth | ||||
| Iron bioavailability: | ||||
| - ↑ Ferritin | ||||
| Kale | Caco-2 | Digested extract (1:10 v/v) | Antiproliferative action: | 33 |
| - 11.7% reduction in overall cell growth | ||||
| - ↓ G0G1 phase, G2/M phase, GSH activity | ||||
| - ↑ Mitochondrial depolarisation, intracellular ROS | ||||
| ITC extract (0–250 μg/mL) | - IC50 151.33 μg/mL cell viability | 49 | ||
| - ↑ DNA fragmentation | ||||
| HepG2 | ITC extract (0–250 μg/mL) | - IC50 82.83 μg/mL cell viability | 49 | |
| - ↑ DNA fragmentation | ||||
| MCF-7 | ITC extract (0–250 μg/mL) | - IC50 164.00 μg/mL | 49 | |
| - ↑ DNA fragmentation | ||||
| Licorice | Caco-2 | Crude extract (1.25–2.50 μg/mL) | - N.S. in cell proliferation (root extract) | 35 |
| - ↑ Cell proliferation (stem and leaf) | ||||
| - ↑ Viable cells (root, stem and leaf) | ||||
|
HCT116 | Crude extract (100 μg/mL) | - ↑ SOD, CAT activity | 42 |
| Protein activity: | ||||
| - ↑ Nrf2, HO-1 | ||||
| HaCaT | Crude extract (1–100 μg/mL) | - 17% reduction in cell viability | 36 | |
| - ↓ ROS production | ||||
|
||||
| - ↑ Nrf2 | ||||
| - ↑ p21WAF | ||||
| A549 | Crude extract (0–300 μg/mL) |
|
40 | |
| - ↑ Bax, Caspase-3, p21 | ||||
| - ↓ Bcl-2, MMP-9, cyclin D1 | ||||
| HepG2 | Crude extract (0–300 μg/mL) | - IC50 53.77 μg/mL cell viability | 37 | |
|
||||
| - ↑ Bax, Caspase-3 | ||||
| - ↓ Bcl-2, MMP-9 | ||||
| Crude extract (0–250 μg/mL) | - IC50 74.15 μg/mL cell viability | 38 | ||
|
||||
| - ↑ Bax | ||||
| - ↓ MMP-9 | ||||
| - N.S. in Bcl-2 and Caspase-3 | ||||
| MCF-7 | Crude extract (0–300 μg/mL) | - IC50 56.41 μg/mL cell viability | 37 | |
|
||||
| - ↑ Bax, Caspase-3 | ||||
| - ↓ MMP-9 | ||||
| Crude extract (0–250 μg/mL) | - IC50 79.56 μg/mL cell viability | 38 | ||
|
||||
| - ↑ Bax, Caspase-3 | ||||
| - ↓ MMP-9 | ||||
| - N.S. in Bcl-2 | ||||
| Caco-2 | Digested extract (1:10 v/v) | Antiproliferative action | 33 | |
| - 13.4% reduction in overall cell growth | ||||
| - ↓ G0G1 phase, G2/M phase, GSH activity | ||||
| - ↑ early apoptosis, mitochondrial depolarisation, intracellular ROS | ||||
| Pea | A673 | Crude extract (20 μL) | - ↓ cell proliferation | 39 |
| - ↑ necrotic area | ||||
| - N.S. in spheroid area | ||||
| L929 | Crude extract (20 μL) | - N.S. in cell proliferation | 39 | |
| RD-ES | Crude extract (20 μL) | - N.S. in cell proliferation | 39 | |
| - N.S. in spheroid/necrotic area | ||||
| Radish | A673 | Crude extract (20 μL) | - ↓ cell proliferation | 39 |
| - N.S. in spheroid/necrotic area | ||||
| Caco-2 | Digested extract (1:10 v/v) | Antiproliferative action | 33 | |
| - 13.1% reduction in overall cell growth | ||||
| - ↓ G0G1 phase | ||||
| - ↑ mitochondrial depolarisation | ||||
| - ↑ intracellular ROS production | ||||
| Thai rat-tailed | L929 | Crude extract (20 μL) | - ↓ GSH activity | 39 |
| - N.S. in cell proliferation | ||||
| RD-ES | Crude extract (20 μL) | - ↓ cell proliferation | 39 | |
| - N.S. in spheroid/necrotic area | ||||
| A549 | Crude extract (0–300 μg/mL) |
|
40 | |
| - ↑ Bax, Caspase-3, p21 | ||||
| ↓ Bcl-2, MMP-2, MMP-9, Cyclin D1 | ||||
| HCT116 | EVs (0–1000 μg/mL) | - IC50 451.02 μg/mL cell viability | 46 | |
| EVs (0–1000 μg/mL) | - IC50 675.4 μg/mL cell viability | 47 | ||
| - ↓ cellular lipid abundances | ||||
| - ↑ cellular nuclei acid and carbohydrate abundances | ||||
| Protein activity: | ||||
| - ↑ Caspase-8, Caspase-9 activity | ||||
| HepG-2 | Crude extract (0–250 μg/mL) | Antiproliferative action: | 30 | |
| - 75%–98% cell growth inhibition | ||||
| - ↓ colony formation | ||||
| - ↓ relative closure of wound | ||||
| Crude extract (0–250 μg/mL) |
|
30 | ||
| - ↑ Bax, Caspase-3 | ||||
| - ↓ Bcl-2, MMP-2, MMP-9 | ||||
| EVs (1xIC50; 2xIC50) | - 24 h IC50—685.50 | 48 | ||
| - 48 h IC50—139.70 | ||||
| - ↑ glycerophospholipid metabolism | ||||
| - ↓ lysophosphatidylethanolamine | ||||
| MCF-7 | Crude extract | Antiproliferative action: | 30 | |
| (0–250 μg/mL) | - 83%–87% cell growth inhibition | |||
| - ↓ colony formation, relative closure of wound | ||||
|
||||
| - ↑ Bax, Caspase-3 | ||||
| - ↓ MMP-2, MMP-9 | ||||
| Red Rambo | A673 | Crude extract (20 μL) | - ↓ cell proliferation | 39 |
| - N.S. in spheroid/necrotic area | ||||
| L929 | Crude extract (20 μL) | - N.S. in cell proliferation | 39 | |
| RD-ES | Crude extract (20 μL) | - ↓ cell proliferation | 39 | |
| - ↓ spheroid area | ||||
| - ↑ necrotic area | ||||
| — | ||||
| Red cabbage | DU145 | Crude extract (1%–5% cell media) | - ↓ cell proliferation (dose–time–response) | 41 |
| — | ||||
| Digested extract | - 32% reduction cell proliferation (24 h) | 45 | ||
|
||||
| - ↑ AIFM1, Apaf-1, FAS, FADD, Caspase-3, Caspase-7, Caspase-8 | ||||
| - ↓ NF-κB | ||||
| - N.S. in TRADD | ||||
| — | ||||
| LNCaP | Crude extract (1%–5% cell media) | - ↓ cell proliferation (dose–time–response) | 41 | |
| — | ||||
| Digested extract | - 44% reduction cell proliferation (24 h) | 45 | ||
|
||||
| - ↑ AIFM1, Apaf-1, TRADD, Caspase-3, Caspase-7, Caspase-8 | ||||
| - ↓ NF-κB | ||||
| PNT-2 | Crude extract (1%–5% cell media) | - N.S. in cell proliferation | 41 | |
| Digested extract | - N.S. in cell proliferation | 45 | ||
| Rocket | A673 | Crude extract (20 μL) | - ↓ Cell proliferation | 39 |
| N.S. in spheroid/necrotic area | ||||
| Caco-2 | Digested extract (100 μL) | Antiproliferative action: | 44 | |
| - ↑ Cell growth | ||||
| Iron bioavailability: | ||||
| N.S. in ferritin | ||||
| L929 | Crude extract (20 μL) | - N.S. cell proliferation | 39 | |
| RD-ES | Crude extract (20 μL) | - ↓ Cell proliferation | 39 | |
| - N.S. in spheroid/necrotic area | ||||
| — | ||||
| Soy | A673 | Crude extract (20 μL) | - ↓ Cell proliferation | 39 |
| - N.S. in spheroid/necrotic area | ||||
| L929 | Crude extract (20 μL) | - N.S. cell proliferation | 39 | |
| RD-ES | Crude extract (20 μL) | - ↓ Cell proliferation | 39 | |
| - N.S. in spheroid/necrotic area | ||||
- Note: μg, micrograms; μL, microlitres; %, percentage; A549, human adenocarcinoma alveolar basal epithelial cells; A673, Ewing sarcoma cell line.
- Abbreviations: AIFM1, apoptosis-inducing factor mitochondria associated 1; Apaf-1, apoptotic peptidase activating factor 1; Bcl-2, B-cell leukaemia/lymphoma 2 protein; Caco-2, human colorectal adenocarcinoma cell; CAT, catalase; DU145, human prostate carcinoma (not detectably hormone sensitive); FADD, Fas-associated dealth domain; FAS, Fas cell surface death factor; GJIC, gap junctional intracellular communication; GSH, glutathione; H460, human non-small lung cancer cell; HaCaT, human keratinocyte cell; HCT116, human colorectal carcinoma cell; HepG-2, human hepatocellular carcinoma cell; HO-1, haeme oxygenase-1; L929, mouse fibroblasts; LNaCP, human prostate carcinoma (androgen receptor positive; oestrogen receptor positive); MCF-7, human breast cancer cell; MDA-MB-231, human metastasis breast cancer cell; mg, milligrams; mL, millilitres; MMP, matrix metalloproteinase; NF-κB, nuclear factor kappa B; Nrf2, nuclear factor erythroid 2-related factor 2; PNT-2, human normal prostate; RD-ES, rhabdomyosarcoma; RIP, receptor-interacting protein; ROS, reactive oxygen species; Smack/DIABLO, second mitochondria-derived activator of caspases; SOD, superoxide dismutase; TM3, mouse Leydig cell.
| Microvegetable | Dose | Control | Animal | Duration | Results | Author | ||
|---|---|---|---|---|---|---|---|---|
| Inflammation | Lipids | Gut microbiota | ||||||
| Broccoli | BMJ: 20 g/kg (BW) | HFD without MV | C57BL/6J mice (N = 32) | 10 weeks |
|
|
|
50 |
| Red cabbage |
|
HFD without MV | C57BL/6NCr mice (N = 60) | 8 weeks |
|
|
— | 51 |
| LFMG: 10.9 g/kg | HFD without MV | C57BL/6NCr mice (N = 40) | 8 weeks | — | — |
|
52 | |
- Abbreviations: CRP, C-reactive protein; F/B, firmicutes/bacteroidetes; HDL, high-density lipoprotein; HFD, high-fat diet; HFMG, high-fat diet with microgreen; IL, interleukin; LDL, low-density lipoprotein; LFMG, low-fat diet with microgreen; LPS, lipopolysaccharide; TNF, tumour necrosis factor.
| Microvegetable | Dose | Control | Animal | Duration | Results | Author | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucose/insulin | Liver function | Kidney function | Oxidative stress | Lipids | Inflammation | ||||||
| Barley | 10% of total diet | Corn starch | Albino rats (N = 48) | 6 weeks |
|
|
|
|
— | — | 53 |
| 10% of total diet | Corn starch | Albino rats (N = 48) | 6 weeks | — | — | — |
|
|
— | 57 | |
| Broccoli |
|
HFD without MV | C57BL/6J mice (N = 40) | 8 weeks |
|
— | — |
|
|
|
56 |
| Radish | 10% of total diet | Corn starch | Albino rats (N = 48) | 6 weeks |
|
|
|
|
— | — | 54 |
| Red clover | 10% of total diet | Corn starch | Albino rats (N = 48) | 6 weeks |
|
|
|
|
— | — | 55 |
- Abbreviations: γGT, gamma-glutamyl transferase; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CAT, catalase; GSH, glutathione; GST, glutathione transferases; HD, high concentration of broccoli with high-fat diet; HDL, high-density lipoprotein; HFD, high-fat diet; IL, interleukin; LD, low concentration of broccoli with high-fat diet; LDH, lactate dehydrogenase; LDL, low-density lipoprotein; MDA, malondialdehyde; SOD, superoxide dismutase; TNF, tumour necrosis factor.
2.5. Assessment of Quality and Bias
Included in vivo animal articles quality was assessed using the Collaborative Approach for Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES) [28], modified to contain additional nonoverlapping items from the SYRCLE risk of bias tool [29] by two researchers. Any disagreement was resolved by discussion between researchers and reassessment of quality/bias. The adapted CAMARADES criteria included the following: (1) regulatory/ethical compliance statement; (2) sample size calculation; (3) statement of temperature control; (4) appropriate animal model; (5) randomisation mentioned; (6) blinded application of treatment; (7) blinded assessment of outcomes; (8) statement of conflict of interest; (9) peer-reviewed journal; (10) random housing; (11) sequence generation; (12) baseline characteristics described; (13) allocation concealment; and (14) reporting bias.
3. Results
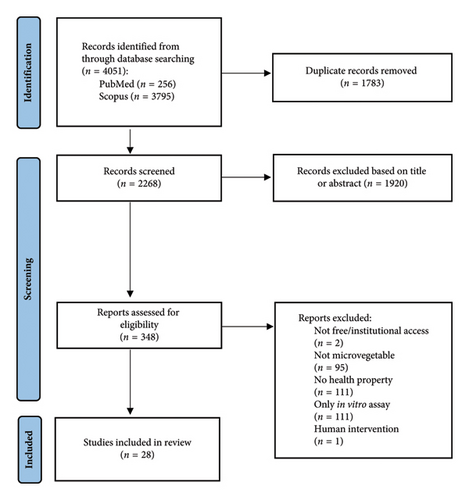
From initial searches in electronic databases and removal of duplicates, 2268 articles were screened by reviewing title or abstracts (Figure 1). Of these, 348 articles were fully screened for eligibility and were included if they were written in English, included clear description of ‘MV’ (either self-described or evident from growing conditions) and investigated a previously stated health property via in vitro cell culture experiments or in vivo animal (nonhuman) experiments. The 28 articles that fulfilled the inclusion criteria were divided into three subcategories, those assessing (a) in vitro antioxidant capacity (solely from in vitro cell culture articles); (b) in vitro cell culture investigations; and (c) in vivo animal investigations.
3.1. In Vitro Antioxidant Capacity
Table 1 lists 12 articles (from in vitro cell culture experiments) that investigated MV direct antioxidant capacity via in vitro assays: 2-2′-azinobis (3-ethylbenzothiazoline-6 sulfonic acid) (ABTS) assay, 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, ferric reducing/antioxidant power (FRAP) assay and oxygen radical absorbance capacity (ORAC) assay [30–41]. In total, 14 MV species were investigated, with broccoli (n = 4) and radish MV (n = 4) species being the most common. Red cabbage MV and red Rambo radish MV displayed the highest antioxidant capacity as determined by the FRAP assay, with results of 324.9 μmol TE/g [41] and ∼1100 μg/g [39], respectively. Red cabbage MV also displayed the highest antioxidant capacity assessed by the ABTS and DPPH assay and reported as 700.1 μmol TE/g and 186.7 μmol TE/g, respectively [41]. On ORAC assay, red mustard had the highest antioxidant capacity of the MV assessed at 745.25 μM TE/g [33]. The TPC of MV determined by Folin–Ciocalteu colorimetry are also summarised in Table 1. Red cabbage MV had the highest TPC, 3201.5 mg GAE/g [41]. Field mustard was observed to have the lowest TPC of the MV assessed at 2.96 mg GAE/g [36].
3.2. In Vitro Cell Culture Investigations
Table 2 lists 20 studies that investigated the health effects of MV using cell lines; in total, 17 MV were investigated. Crude extracts of MV were the most common formulation under investigation [30–32, 34–42], followed by digested extracts [33, 43–45] and extracellular vesicles [46–48]. One study used an isothiocyanate (ITC) extract from MV [49].
Radish MV were most commonly investigated (n = 7 articles), followed by broccoli and mustard MV (n = 6 articles). Cell viability assays indicated that MV, including beetroot, broccoli, cress, kale, mustard, radish and red cabbage inhibited cell growth in various immortalised cell lines (n = 16 articles). Beetroot [43], mustard [37, 38, 40], radish [30, 40, 47] and red cabbage MV [45] facilitated cell programmed death by increasing caspase activity/gene expression. Furthermore, these MV decreased Bcl-2 protein and gene expression while increasing the gene expression of Bax. A separate study reported that broccoli, kale, mustard and radish MV facilitated cell programmed death by arresting G0G1 and G2M cell cycle phases [33].
The antioxidant properties of MV were examined in five articles and were determined via the quantification of endogenous antioxidant protein activity or overall reactive oxygen species (ROS) inhibition. Mustard MV increased Nrf2 activity and decreased intracellular ROS production in HaCaT [36] and HCT116 cells [42]. In a separate study, mustard MV decreased intracellular glutathione (GSH) concentrations in Caco-2 cells [33]. Cress MV decreased ROS production in mice TM3 cells [34]. Broccoli MV increase ROS inhibition in various immortalised cell lines [31]. Digested extracts of broccoli, kale, mustard and radish MV had higher levels of intracellular ROS production in Caco-2 cells, compared to control [33]. Anti-inflammatory properties of MV were investigated in four articles, three of which quantified matrix metalloproteinase (MMP) gene expression as an inflammatory marker. Mustard and radish MV were observed to decrease MMP-2, MMP-9 expression in A549 [40], HepG2 and MCF-7 cells [30, 37]. Digested extracts of red cabbage MV significantly reduced NF-κB gene expression in prostate cancer cells, DU145 and LNCaP [45].
One article investigated the effect of MV on hormone production. Low concentrations of cress MV (125–250 μg/mL) increased progesterone and testosterone secretion from TM3 cells, while higher concentrations (1000–2000 μg/mL) reduced hormone secretion [34]. A separate article investigated the effect of MV on iron bioavailability. Broccoli, fenugreek and rocket MV had lower iron contents compared to mature counterparts. Fenugreek MV, however, had a significantly higher iron bioavailability compared to the mature counterpart. Moreover, ferritin uptake was significantly increased in Caco-2 cells treated with fenugreek MV [44].
3.3. In Vivo Animal Investigations
Table 3 describes three articles that investigated the effect of MV consumption in diet-induced obese mice models. Key outcomes included the characterisation of systemic and tissue inflammation, blood lipids and the gut microbiota composition. Concentrated broccoli MV juice (20 g/kg/d body weight [BW]) for 10 weeks significantly reduced serum inflammatory markers including interleukin (IL)-1β, IL-6, tumour necrosis factor (TNF)-α and lipopolysaccharide (LPS) in C57BL/6J obese mice [50]. Similarly, red cabbage MV (9.46 g/kg BW/d) for eight weeks significantly reduced inflammatory gene expression, such as C-reactive protein (CRP) and TNF-α in the liver of C5BL/6NCr mice [51]. Lipid profiles were quantified from sera of mice models; broccoli [50] and red cabbage MV [51] significantly reduced low-density lipoprotein (LDL). Additionally, broccoli MV reduced sera triacylglycerols (TG) and red cabbage MV reduced high-density lipoprotein (HDL). Broccoli MV significantly reduced the phylum Firmicutes and increased Bacteroides acidifaciens abundances [50]. Red cabbage MV (10.9 g/kg BW) consumption for eight weeks significantly increased alpha diversity indices: Chao1 and observed species in obese C57BL/6NCr mice [52]. Amongst high-fat-diet–fed mice, red cabbage MV supplementation did not induce any significant changes in phylum-level taxa; however, did significantly increase Clostridiales_other (family member of Firmicutes) when compared to high-fat diet alone.
Table 4 summarises five articles that investigated the effect of MV consumption in diabetic animal models, including C57BL/6J mice and albino rats. Barley [53], radish [54] and red clover MV [55] (10% of total diet) significantly reduced plasma glucose in diabetic albino rats, however, had no significant impact on serum insulin secretion. Broccoli MV (2 g/kg BW) significantly increased insulin, while having no influence on blood glucose in diabetic mice [56].
Barley, radish and red clover MV reduced serum aspartate aminotransferase (AST) and alanine transaminase (ALT) enzymes in diabetic albino rats [53–55]. Radish and red clover MV also reduced bilirubin and increased total protein. Barley, radish and red clover MV significantly decreased serum lactate dehydrogenase (LDH) activity in diabetic albino rats. Barley MV significantly increased serum urea and creatinine; radish and red clover had no significant impact on these markers. All of the MV summarised in Table 4 significantly reduced malondialdehyde (MDA) concentrations in the liver [53–56]. Barley, radish and red clover MV significantly increased liver GSH, glutathione transferase (GST) and superoxide dismutase (SOD), whereas broccoli MV significantly reduced liver GSH and SOD. Barley MV significantly reduced serum total cholesterol and LDL and increased TG [57]. Broccoli MV (2 g/kg/d BW) significantly reduced TG and LDL. Higher consumption of broccoli MV (2 g/kg/d BW) significantly increased serum IL-10 concentrations, while lower consumption of broccoli MV (0.2 mg/kg/d BW) significantly decreased serum IL-1β and TNF-α [56].
3.4. Study Quality and Bias
In this review, we used the CAMARADES criteria (with some adaptions from SYRCLE risk of bias tool) on eight included in vivo animal studies. The awarded scores ranged from 4 to 7 from a total of 14 points (Table 5). All included studies used an appropriate animal model, included a statement of regulatory/ethical compliance and conflicts of interest, and published in a peer-reviewed journal. No article described treatment/outcome blinding, randomised sequence generation, baseline characteristics, allocation concealment or reporting bias. The absence of key bias-reduction measures reduces the reliability of these findings and warrants cautious interpretation.
| Criteria ∗ | Author | |||||||
|---|---|---|---|---|---|---|---|---|
| Aly et al. 2023 | Huang et al. 2016 | Khattab et al. 2022 | Li et al. 2021 | Ma et al. 2022 | Mohamed et al. 2022 | Mohamed et al. 2023 | Wu et al. 2023 | |
| Regulatory/ethical compliance statement | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Sample size calculation | ||||||||
| Statement of temperature control | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Appropriate animal model | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Randomisation mentioned | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Blinded application of treatment | ||||||||
| Blinded assessment of outcomes | ||||||||
| Statement of conflict of interest | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Peer-reviewed journal | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Random housing | ✓ | ✓ | ||||||
| Sequence generation | ||||||||
| Baseline characteristics described | ||||||||
| Allocation concealment | ||||||||
| Reporting bias | ||||||||
| Score | 5 | 7 | 4 | 5 | 4 | 6 | 6 | 6 |
- ∗CAMARADES checklist (adapted with SYRCLE risk of bias tool).
4. Discussion
In this systematic review, 28 articles investigated the potential health effects of MV via in vitro and in vivo animal (nonhuman) experiments. These studies indicated MV consumption may have beneficial effects for human health as they exhibit antioxidant, proapoptotic, and anti-inflammatory properties and modify lipid profiles and gut microbiota composition. The principal known health-related effects of MV and their putative mechanisms of action will be critically discussed based on the key results from the studies identified by this review.
4.1. The Antioxidant Effects of MV
ROS and/or reactive nitrogen species (RNS) play important roles in cellular processes, including combatting infectious pathogens, inducing mitogenic responses and maturation of cellular structures, via reacting with lipids, proteins and nucleic acids (DNA and RNA). However, in excess, ROS and RNS can lead to oxidative stress, resulting in deleterious effects on cellular function [58].
More than half of the included in vitro immortalised cell line experiments assessed the direct antioxidant capacity of MV via in vitro assays, namely, ABTS, DPPH and FRAP. These methods are relatively simple, sensitive and cost-effective and act on the principle of either hydrogen atom transfer and/or single electron transfer [59]. Nevertheless, they have certain limitations. For example, DPPH assay is only suitable for assessing antioxidants with lipophilic properties. Crucially, the outcomes from these assays have no clear biological correlation or translation to physiology; it is not known how these antioxidants/oxidants will act in vivo. It is recommended, when assessing the antioxidant activity of compounds that lipid peroxidation should be monitored and assessed using analytical methods, such as gas chromatography [59]. The studies identified in this review did not assess lipid peroxidation. Most in vitro studies summarised in this review determined total MV polyphenol content by Folin–Ciocalteu colorimetry, a well-known method in determining plant polyphenols [60]. This method, however, has several drawbacks. For example, poor sensitivity due to the interference from organic acids and reducing sugars, and resulting in an overestimated polyphenol content [61]. Polyphenolic compounds have complex structures [20]; high-performance liquid chromatograph-mass spectroscopy (HPLC-MS) should be used to quantify the TPC where possible [62].
This review highlighted the antioxidant effects of certain MV in immortalised cell line experiments. Specifically, crude extracts of broccoli and mustard MV were shown to inhibit intracellular ROS production [31, 36]. In a separate study, broccoli and mustard subjected to in vitro digestion evoked higher levels of intracellular ROS compared to a control [33]. Digested extracts contain lower quantities of phytochemicals compared to crude extracts. Although this was not directly measured by de la Fuente et al. (2020), this may explain the discrepancies between findings. Nonetheless, in vitro digestion mimicking the action of the gut microbiota may provide a greater approximation of the in vivo phytochemical bioavailability from MV and their possible health-related effects for humans [63–65]. Intracellular ROS production was quantified in these experiments via the dichlorofluorescein diacetate (DCFDA) assay. The DCFDA has complex intracellular redox chemistry, and researchers have concluded that this assay is not reliable in determining intracellular ROS [66–69]. However, recent findings have identified the DCFDA assay to be most sensitive when partnered with tert-butyl hydrogen peroxide (TBHP), yielding an accurate estimate of ROS in an immortalised cell line [70]. This approach might be considered in future in vitro MV investigations.
Nrf2 is a transcription factor that regulates the expression of antioxidant proteins, including haeme oxygenase-1 (HO-1) [71]. Mustard MV grown with salinity (100 mM NaCl solution) and under fluorescent light caused the greatest expression of Nrf-2 in HCT116 cells, compared to red-blue light and no salinity [42]. It is notable that mustard MV grown under these conditions had a higher TPC (∼3300 mg GAE/g), compared to other growing conditions. Yet in contrast, mustard MV grown with blue light and salt yielded greater effects on HO-1 gene expression and SOD and catalase (CAT) activity. The effect of growing conditions on the MV phenolic content has been extensively reviewed [18, 25] with no optimal growing method having yet been defined. Indeed, such optimal conditions are likely to vary between plant species. Another study showed growing mustard MV in white light significantly increased Nrf2 levels of HaCaT cells, compared to those grown under red-blue light [36]. Interestingly, mustard MV grown under white light displayed a TPC of 2.96 mg GAE/g; this was lower compared to the red-blue light regime (4.45 mg GAE/g). Thus, the relationship between the TPC and physiological effects does not appear to be linear. Furthermore, the TPC was assessed via Folin–Ciocalteu colorimetry with its limitations discussed above. The white light increases the flavonoid quantities, such as quercetin in fruits [72] and mature broccoli florets [73] compared to other light regimes. To bring greater clarity, the effects of different light regimes on the content and profile of individual polyphenol compounds and classes should be identified using techniques such as LC-MS-MS and UHPLC-MS. This can then be further related to physiological outcomes.
MV supplementation appeared to alter endogenous oxidant/antioxidant balance in vivo. Obese mice and diabetic rats fed broccoli MV for 8 and 10 weeks, respectively, had significantly lower concentrations of MDA in the liver compared to those receiving high-fat diet alone [50, 56]. MDA is one of the final products of lipid peroxidation and contributes to oxidative stress [58]. The reduction in this biomarker by broccoli MV consumption may indicate reduced oxidative stress and cellular damage. Oxidative stress, however, is a balance between oxidants and antioxidants [58]. Diabetic mice fed the higher dose of broccoli MV (2 g/kg/d) displayed significantly lower endogenous antioxidant activity (GSH and SOD) compared to control [56]. However, other concentrations (0.2 g/kg/d or 20 g/kg/d) had no significant impact on the liver GSH and SOD activity in diabetic and obese mice [50, 56]. Broccoli MV has direct radical scavenging capabilities ex vivo [31, 33], and this may reduce the requirement for endogenous antioxidants. However, the relative importance of direct radical scavenging by MV metabolites in vivo has not been established. Broccoli MV antioxidant potential needs further examined particularly in human cohorts, particularly as other polyphenol-containing foodstuffs, such as tart cherry can significantly increase tissue-level expression of endogenous antioxidant enzymes in humans [74].
In three separate studies, liver concentrations of MDA were significantly lower and GSH, SOD and GST activity were significantly higher in diabetic rats fed barley MV [53], radish MV [54] or red clover MV [55]. These MV were fully characterised via gas chromatography-mass spectrometry; barley and red cover had high abundances flavonoids, including 3-benzyloxy-5,6,7,4′-tetramethoxyflavone and gardenin, respectively [53, 55]. Recently, flavonoids (from Citrus changshan-huyou) have been shown to attenuate oxidative stress in a mouse model of nonalcoholic fatty liver disease [75]. Radish MV, however, had higher abundances of the essential oils, phytane and norphytan and relatively low abundances of polyphenols [54]. This is surprising as radish MV has previously been suggested by the less accurate Folin–Ciocalteu method to contain relatively high quantities of polyphenols [33, 39]. Impairment of glucose regulation by the liver is a characteristic of diabetes mellitus pathology [76]. The aforementioned studies all reported a significant decrease in plasma glucose levels following MV consumption [53–55]; this may be related to the reduction in liver oxidative stress.
4.2. The Proapoptotic Effect of MV
Apoptosis, or cell programmed death, is a required homeostatic process of eliminating cells. It is critical for whole-organism homeostasis with dysregulated apoptosis being associated with not just malignancies but so too age-related, degenerative and inflammatory diseases [77]. At a cellular level, apoptosis can occur in mammalian cells by extrinsic or intrinsic pathways. The extrinsic pathway is activated by specific ligand binds, such as TNF-α and/or FasL [78]; MV manipulation of this pathway has yet to be examined. The intrinsic pathway can be activated by endogenous and/or exogenous oxidants, resulting in oxidative stress [78]. The mitochondria and mitochondrial proteins, such as the antiapoptotic proteins (Bcl-2) and proapoptotic pore formers (Bax and caspases) are highly associated with the intrinsic pathway [79, 80].
In this review, beetroot, mustard, radish and red cabbage MV exhibited proapoptotic effects on various immortalised cell lines by inhibiting Bcl-2 and/or increasing activity of Bax and caspases gene expression accompanied by cell growth inhibition [30, 37, 40, 43, 45, 47]. Of these MV, beetroot was reported to have high levels of sinapinic acid (nonflavonoid), hesperidin (flavonoid) and rutin (flavonoid) [43]. Mustard and radish were identified to have high abundances of myricetin (flavonoid) and ellagic acid (nonflavonoid) [40]. Previously, a polyphenol blend from Camellia sinensis (containing 40% polyphenols; 5%–8% epigallocatechin-3 gallate; 1.3% theaflavins) significantly increased Bcl-2 in muscle tissue from untrained males 1 h after a resistance exercise protocol [80]. However, other polyphenol-rich supplements have previously shown no effect on tissue Bcl-2/Bax levels in the prostate gland from prostate cancer patients [81] and adenoma polyps from colorectal cancer patients [82]. Mustard and radish MV significantly reduced the mRNA expression of Cyclin D1 in HepG2 cells, a key regulator of cell cycle progression [40]. However, the converse has been observed in adenoma polyps from colorectal cancer patients receiving a Brazilian propolis supplement rich in the p-coumaric acid derivative Artepillin C [82]. It appears that MV findings in vitro may not be replicated in vivo. Researchers used crude extracts or extracellular vesicles derived from beetroot, mustard and radish MV, which may explain the inconsistencies between in vitro MV findings and human studies of other polyphenol-rich supplements in human cancer patients; MV remain to be studied in this population. Digested extracts of broccoli, kale, mustard and radish MV exhibited proapoptotic effects on an immortalised cell line via arresting the cell cycle [33]. Although the comprehensive polyphenolic characterisation of their intervention was not performed by de la Fuente and others, the MV were estimated to have relatively high amounts of the TPC after in vitro digestion (82.06–144.77 mg GAE/g) [33]. Polyphenols, including flavonoids and nonflavonoids, have been shown to arrest the cell cycle by upregulating miR-195-5p and miR-497-5p in colon cancer cells [83], and downregulating phosphor-Cdc2 and cyclin B1 in breast cancer cells [84].
4.3. The Anti-Inflammatory Effects of MV
Inflammation is a common mechanism uniting all NCDs, including cardiometabolic diseases, e.g., T2D [85]. In vitro experiments with immortalised cell lines demonstrated that mustard, radish and red cabbage MV reduced the mRNA expression of MMP [30, 37, 40] and NF-κB [45], respectively. This is in keeping with the literature where in THP-1 macrophages, polyphenols have exhibited anti-MMP activity by reducing intracellular ROS and thereby inhibiting the NF-κB pathway [86]. Notably, polyphenols are zinc-chelating agents [87]; zinc is a known cofactor required for MMP activity [88].
This review also identified anti-inflammatory properties of MV described by in vivo investigations. Obese mice fed red cabbage MV had significantly lower liver CRP and TNF-α gene expression [51]. Polyphenols have previously been shown to increase peroxisome proliferator-activated receptor (PPAR)-α expression and subsequently inhibit NF-kB pathway and CRP in T2D animals [89]. The anti-inflammatory effects of broccoli MV varied across animal models and dosages. In obese mice, a 20 g/kg/d BW dose significantly reduced serum IL-1β, IL-6 and TNF-α [50]. In another study, diabetic mice receiving 2 g/kg/d BW MV showed no change in these inflammatory cytokines, while a lower dose of 0.2 mg/kg/d BW resulted in significant reductions in IL-1β and TNF-α, but not IL-6 [56]. In a human clinical trial with T2D patients, broccoli supplementation (not in MV form) alone did not cause a significant reduction in serum IL-6 concentrations [90]. To date, no study has examined the anti-inflammatory effects of broccoli MV supplementation in humans. Translating these murine findings to humans will require controlled investigations, including dose-dependent studies.
4.4. The Lipid-Modifying Effects of MV
Dyslipidaemia is a hallmark of obesity and T2D, whereby TG and LDL are elevated, along with low levels of HDL. Previous human clinical trials have reported that polyphenol-rich foods significantly alter the lipid profile by increasing HDL and lowering total cholesterol and LDL in T2D patients [91–93]. To date, no research has examined the lipid-modifying effect of MV in humans. Broccoli MV significantly reduced sera TG and LDL in obese [50] and diabetic [56] animal models. Polyphenols, such as quercetin and genistein, can upregulate LDL receptor expression in the liver and subsequently increase LDL clearance in vitro and in vivo [94, 95]. Broccoli MV had no effect on modifying HDL levels in mice [50, 56]. The inhibition of cholesterol ester transfer protein (CETP) can increase HDL concentrations. Several berry polyphenols have been shown to reduce plasma CETP activity and increase HDL concentrations in overweight adults [96, 97]. The differences in polyphenol profiles between MV and berry sources may lead to different effects on HDL. Preliminary findings suggest MV may influence lipid metabolism, although human studies are needed to confirm translational relevance.
4.5. Gut Microbiota Modulation From MV Consumption
Obese mice fed broccoli MV had significantly lower abundances of Firmicutes and higher abundances of B. acidifaciens [50]. Diabetic mice fed broccoli MV additionally displayed higher abundances of Mucispirillum schaedleri [56]. Gut microbial species have vital functions, such as immune modulation [98], and MV anti-inflammatory effects described in the animal studies summarised above could be related to the changes in the gut microbiota composition. Although the results in animal studies show promise in MV acting as a ‘prebiotic’, recently published data reported that a single dose of 16-g broccoli MV had no significant impact on the human gut microbiota composition [99]. This is unsurprising given the limited duration of the intervention. To the author’s knowledge, this is the first human study investigating the gut microbiota composition following MV consumption. The planning of future human trials should account for the effect of long-term MV consumption on gut microbiota composition, as well as other health outcomes. Red cabbage MV was shown to alter the gut microbiota alpha diversity and composition in obese mice; this was accompanied with strong positive associations between Rikenellaceae abundances and hepatic TG, and Coprococcus abundances and body weight [52]. The link between these health benefits from MV consumption and gut microbiota composition is plausible but remains to be definitively established. The gut microbiota’s role in the bioavailability of dietary polyphenols [64, 65] is another important factor to be considered for future investigations.
4.6. Other Health Effects
Plant-derived polyphenols have previously shown promise in attenuating oxidative stress in male reproductive organs [100], and articles included in this review suggest that this expands to the field of MV. Mouse Leydig cells supplemented with cress MV (125–250 μg/mL) significantly increased progesterone and testosterone [34]. Authors identified cress MV had high concentrations of phenolic acids, including ferulic acid (333.66 mg/kg) and 4-OH benzoic acid (74.64 mg/kg). Conversely, higher doses of cress MV (1000–2000 μg/mL) reduce progesterone and testosterone. Similar results have been observed in human sperm cells, as concentrations of resveratrol and genistein above 100 μM caused adverse effects on spermatozoa [101]. This dose-dependent in vitro response further calls into question the translation of these observations to an in vivo scenario. Barley MV consumption, with high abundances of 3-benzyloxy-5,6,7,4′-tetramethoxyflavone [53], significantly increased sperm count and reduced sperm cell abnormalities in diabetic rats [57]. Mitochondria are key organelles supporting many sperm functions [102]; polyphenols (flavonoids and nonflavonoids) improve overall mitochondrial capabilities, which, in turn, can benefit the male reproductive system.
Iron deficiency is of public health concern, with approximately 40% of the worldwide population affected [103]. The current recommendations for iron deficiency are supplementation or consumption of iron-containing foodstuffs with high bioavailability [104]. Broccoli, fenugreek and rocket MV were reported to have relatively lower concentrations of iron compared to their mature counterpart. Nevertheless, fenugreek MV had higher iron bioavailability compared to the mature counterpart in an immortalised cell line [44]. Iron fortification in MV growing conditions has shown to increase the iron content of spinach and pea MV [105]. Plant-based diet popularity is steadily increasing due to being a sustainable food group, and these results show the potential of plant-based diets managing iron deficiency, although further exploration in human cohorts is required.
5. Limitations
This is the first systematic review examining the bioactive properties of MV, and evidence is limited to preclinical models (in vitro cell and in vivo animal models). The limitations of these models have been discussed throughout and are highlighted again here. Specifically, the in vitro cell culture experiments highlighted provide useful mechanistic insights and signposts toward appropriately designed trials in human cohorts. Nonetheless, cautious interpretation of in vitro results is warranted due to nonphysiological environmental conditions and their lack of consideration of whole-body effects. In vivo animal investigations account for whole-body effects and share some physiological similarities with humans. However, such animal experiments are also tightly controlled to limit a myriad of confounding factors and such findings do not always translate to human populations. Quality and risk of bias assessment of the in vivo animal experiments highlighted the absence of key bias-reduction measures, and further cautious interpretation of these findings is warranted.
6. Conclusion
This review identified that MV may hold relevance for dietary strategies targeting NCD risk due to their antioxidant, anti-inflammatory, lipid and gut microbiota-modifying effects. MV demonstrated potent direct antioxidant capacities when assessed via an in vitro assay; albeit their effects on endogenous antioxidant status in vivo require extensive further exploration, as does the question of the relative importance of their direct and indirect antioxidant actions in vivo. The proapoptotic and to a lesser extent anti-inflammatory actions of MV have been well described using immortalised cell line experiments; these actions should now be explored in vivo with efforts made to establish whether there exists a mechanistic link between such effects and health outcomes. Evidence from in vivo studies suggests the anti-inflammatory responses may be dose dependent. The issue of MV dose is one that should be addressed promptly in human trials. However, as the human MV literature expands, it is inevitable that diverse single MV and MV blends will be studied, further complicating this issue. To allow researchers to unpick these issues as the literature expands, comprehensive characterisation of the phytochemical constituents of MV supplements will be essential. In animal models, MV appear to positively regulate lipid profile; given their anti-inflammatory effects, an evaluation of the effect of MV supplementation on those at cardiometabolic risk should be a key priority. The gut microbiota is vital to overall host health, and when examining the health benefits of MV consumption, it will be important to consider the gut microbiota composition and their metabolism of parent MV compounds. Overall, the in vitro and in vivo evidence discussed in this review provides useful insight into the promising bioactive properties of MV. Their translational relevance now should be confirmed in robust human clinical trials with controlled interventions.
Disclosure
Registration of this review can be found at https://commons.datacite.org/doi.org/10.17605/osf.io/59fa4.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
There was no external funding for this study.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study (the article is a review article).




