Physicochemical Characteristics and Functionality of Burdekin Plum During Fruit Maturation
Abstract
Burdekin plum (Pleiogynium timoriense) is a rich source of phytochemicals with many health benefits, but the effect of maturation on its physicochemical properties and functionality is unknown. To fill this knowledge gap, Burdekin plums harvested at three different maturity stages (mature green, turning and dark maroon) were analysed, including basic physicochemical parameters, bioactive compounds, antimicrobial activity and antioxidant capacity. Results showed that the dietary fibre content decreased from around 50% to 30% during maturation. Three different solvents (water, 80% ethanol and 80% methanol) were used for extraction and tested for bioactive compounds and functionality. Bioactive compounds were identified and quantified using Ultra-High Performance Liquid Chromatography Tandem Mass Spectrometry. Water extracts contained higher content of gallic acid and galloylquinic acid content. However, ethanol and methanol extracts contained higher content of ellagic acid, anthocyanins and catechins. During maturation, most bioactive compounds decreased, except for anthocyanins, quercetin 3-glucoside and epicatechin, which increased. Similarly, the antioxidant capacity reduced during maturation. Regarding the antimicrobial activity, extracts from turning and dark maroon stages demonstrated a stronger inhibition against Staphylococcus aureus (inhibition zone > 8 mm) compared to mature green stage (inhibition zone < 6 mm), while no inhibition against the Escherichia coli and Candida albicans was observed. In conclusion, the physicochemical characteristics and functionality of Burdekin plums changed significantly during maturation, which should be considered when utilising the fruits as a functional food source.
1. Introduction
The growth of Australian native food industry has been primarily driven by the inherent Australian identity, unique character and functionality of Australian native foods [1, 2]. Australian native foods possess many functional properties. For example, Kakadu plum is renowned for its high vitamin C and hydrolysable tannin contents, consequently exhibiting strong antioxidant [3] and antimicrobial activities [4]. These functionalities, particularly the antioxidant and the antimicrobial activities, are strongly related to plant phytochemicals, offering various health benefits [5]. The growing interest in functional foods presents an opportunity for value addition and market expansion for native foods beyond their traditional application [6]. Besides a handful of commercialised Australian native edible plants, the functionality of many other Australian native plants is waiting to be rediscovered.
Pleiogynium timoriense (DC.) Leenh is a tropical rainforest tree native to Australia. It is renowned for its plum-like fruits, commonly known as Burdekin plum (BP) in Australia [7]. BP shares its botanical lineage with several mainstream crops such as mangoes and cashews, all being members of the Anacardiaceae family [8]. Traditionally consumed by Aboriginal people and subsequent settlers [9], BP fruits have been found to possess high antioxidant capacity [3] as well as anti-inflammatory, analgesic, hepatic and renal protective effects [10].
It is well known that many factors including growth environment and maturity stages affect the composition of fruits, consequently impacting their functionality [11]. Especially, the beneficial effects arising from the phytochemicals in fruits are affected by fruit maturity stages [12]. Pigmented fruits, such as plums, change colour during maturation as the green colour diminishes due to the reduction of chlorophyll, while the intensity of other pigments, such as anthocyanins and carotenoids, increases [13]. The concentrations of various phenolic compounds in tomatoes exhibit different trends during fruit development and are greatly influenced by variety. Certain compounds, such as chlorogenic acid, peak in green fruits, while others, such as naringenin glucoside and apigenin acetylhexoside are highest in half ripe and fully ripe fruits, respectively [14]. It has been found that the antioxidant capacity and antimicrobial activity increased as blueberries mature [15, 16], whereas the total phenolic content (TPC) and antimicrobial activity in jujube fruits decreased during maturation [17].
To the best of our knowledge, there are no published data on the physicochemical parameters and functionality of BP during fruit development. Therefore, this study investigated the evolution of key physicochemical parameters including dimension, weight, colour, firmness, pH, TA, TSS, fibre and phytochemical composition as well as functionality including antioxidant capacity and antimicrobial activity during the maturation of BP. Since extraction solvents play an important role on the composition of plant bioactive compounds [18], the present study also investigated the effects of three commonly used solvents (water, 80% aqueous ethanol and 80% aqueous methanol) on the composition of bioactive compounds and functionality of BP at different maturity stages.
2. Materials and Methods
2.1. Samples
BP fruits were harvested from Brisbane, Australia. Three fruit maturity stages in April (mature green stage), July (turning stage) and September (dark maroon stage) of 2022 were sampled. At each stage, fruits were harvested from three trees with around 50 fruits harvested from each tree. Samples for measurement were randomly selected from each tree and were free from defects or damage.
2.2. Physicochemical Parameters Measurement of BP
Fruit dimension, weight, firmness, TSS, pH, titratable acidity, moisture content and dietary fibre were measured based on our previous work [19].
The colour of the surface of peel and inner flesh was measured using a Minolta CR-400 Chroma Meter (Konica Minolta, Osaka, Japan). Colour space including a∗, b∗, C∗, h and L∗ were recorded [20].
2.3. Extract Preparation
400 mg of BP powder was extracted with 18 mL of water, 80% ethanol, 80% methanol in triplicate following the method reported [19], to maintain consistency with our previous study and enable easier comparison. The extracts were stored in −30°C freezer and filtered via a 0.22 μm GHP membrane filter (Pall, Melbourne, VIC, Australia) before further analysis.
2.4. Sugar Analysis
Sugar analysis followed the method reported by Rego et al. [21] with slight modifications. Briefly, the 80% ethanol extracts were analysed for sucrose, glucose, and fructose content with a Thermo Vanquish UHPLC system (Thermo Fisher Scientific, Waltham, MA, USA) equipped with a Thermo Orbitrap Exploris 120 mass spectrometer in negative ion mode and a Waters BEH Amide analytical column (100 × 2.1 mm, 1.7 μm particle size; Waters, Rydalmere, NSW, Australia) maintained at 50°C. The mobile phase was a mixture of A (30% acetonitrile with 0.1% ammonium hydroxide in water) and B (80% acetonitrile with 0.1% ammonium hydroxide in water) applied at a flow rate of 0.25 mL/min as follows: 0–1 min, 100% B; 1–8 min, 100%–60% B; 8–10 min, 60% B; 10–15 min, 60%–100% B. The injection volume was 1 μL, the column was set at 50°C, and the sample manager temperature was set at 15°C. The mass spectrometer detector conditions were set as follows: This procedure was operated in negative mode with a maximum mass tolerance of 5 ppm, a resolution of 60,000 FWHM, a normalised AGC Target at 200% and a maximum injection time of 100 ms. Commercial standards of sucrose, fructose and glucose were purchased from Sigma-Aldrich (Castle Hill, NSW, Australia).
2.5. Analysis of Bioactive Compounds
Major bioactive compounds were quantified as described in our previous work [22], using a Thermo Vanquish™ UHPLC system (Thermo Fisher Scientific, Waltham, MA, USA) with a Thermo Orbitrap Exploris™ 120 mass spectrometer and a Waters BEH C18 analytical column (150 9 2.1 mm, 1.8 μm particle size; Waters, Rydalmere, NSW, Australia). A product ion scan of targeted compounds was performed at a resolving power of 30,000 FWHM, with a mass tolerance of 5 ppm and respective collision energies as shown in Table S1. Quantification of the targeted and identified compounds was performed using external calibration curves. Commercial standards of cyanidin 3-galactoside, delphinidin 3-galactoside, gallic acid, ellagic acid, catechin, epicatechin, epicatechin gallate, epigallocatechin gallate, quercetin, quercetin 3-glucoside, and 1,3,6-tri-o-galloyl-beta-D-glucose were purchased from Merck/Sigma-Aldrich (Castle Hill, NSW, Australia) to make the individual calibration curves. Thermo Xcalibur 4.0 and TraceFinder 5.1 software (Thermo Fischer Scientific) were used for data acquisition and processing, respectively. Results were expressed as mg per kg of dry sample weight (mg/kg DW).
2.6. Antioxidant Capacity
The antioxidant capacity was determined by using the TPC and the ferric reducing antioxidant power (FRAP) assays as reported in our previous work [19].
2.7. Antimicrobial Test
80% ethanol and 80% methanol extract were evaporated using nitrogen to remove ethanol and methanol, then the remaining were freeze dried with water extract (50°C, 0.04 mbar). The freeze-dried extract was reconstituted with 1% aqueous dimethyl sulfoxide to reach a concentration of 100 mg/mL for antimicrobial analysis. Agar well diffusion method was employed to test the antimicrobial activity of three BP extracts against Staphylococcus aureus (NCTC6571), Escherichia coli (NCTC9001) and Candida albicans (ATCC10231) following the method reported by Phan et al. [23]. Briefly, inoculums were prepared using overnight grown microbes adjusted to 106 CFU/mL using saline solution to a reading between 0.08 and 0.1 at 600 nm using a GENESYS 50 UV-Vis Spectrophotometer (Thermo Scientific, Australia). Mueller Hinton agar plates (Oxoid CM0337, Thermo Fisher Scientific, Melbourne, VIC, Australia) were impregnated with 100 μL bacteria inoculum in 8 mm wells aseptically. Potato dextrose agar plates were impregnated with 100 μL yeast inoculum in 8 mm wells aseptically. The plates were incubated at 37°C (for bacteria) and 30°C (for fungi) for 18 h. After incubation, the diameter of inhibition measured using a calliper minus the well diameter was expressed as inhibition zone (mm). Triplicate analysis was carried out with streptomycin (20 g/mL) as a positive control and 1% aqueous dimethyl sulfoxide as a negative control.
2.8. Data Analysis
Tests were performed in triplicate, and data analysis was carried out using XLSTAT (Addinsoft 2022, Paris, France). ANOVA was used to assess differences between samples. Tukey–Kramer HSD test was applied post-ANOVA for pairwise comparisons (p < 0.05). Principal Component Analysis (PCA) was performed on the quantified phenolic compounds in the samples and the contribution of each parameter to the PCA scores was interpreted based on their loading values for each PC (Table S3).
3. Results and Discussion
3.1. Physicochemical Parameters During BP Maturation
The changes in BP fruit dimension, weight and colour are shown in Table 1 and Figure 1. The fruit equatorial and vertical dimeters as well as whole fruit weight increased to around 4 cm, 3 cm and 30 g, respectively, while stone weight increased from around 10 g at mature green stage, then slightly decreased during maturation. The flesh stone ratio increased during development, reaching around 2 at the final stage. Endocarp usually reaches final size earlier than mesocarp, then lignify, whereas the mesocarp continues to grow during stone fruit development [24]. Peel colour changed significantly during development with significant increase in redness a∗ and hue angle h and decrease in lightness L∗, blueness b∗ and chroma C∗. Redness in flesh also increased from mature green stage to dark maroon stage with decreasing blueness, chroma and hue angle. The changes in colour can be predominately explained by the changes in pigments, including the degradation of chlorophylls and accumulation of anthocyanins. Colour change may also occur from the changes in cell wall structure leading to changes in reflectance [25, 26].
| Test | Mature green | Turning | Dark maroon |
|---|---|---|---|
| Equatorial diameter (mm) | 35.8 ± 2.9c | 37.6 ± 2.3b | 39.7 ± 2.5a |
| Vertical diameter (mm) | 24.9 ± 2.8c | 27.4 ± 2.5b | 30.1 ± 3.0a |
| Whole fruit weight (g) | 18.5 ± 4.4c | 23.4 ± 4.5b | 28.5 ± 5.7a |
| Stone weight (g) | 10.4 ± 3.2a | 9.5 ± 3.1a | 9.3 ± 2.6a |
| Flesh stone ratio | 0.8 ± 0.2c | 1.6 ± 0.4b | 2.1 ± 0.4a |
| Peel L∗ | 48.5 ± 3.8a | 39.1 ± 3.9b | 27.1 ± 1.3c |
| Peel a∗ | −14.5 ± 1.0c | 4.1 ± 4.0b | 6.0 ± 2.6a |
| Peel b∗ | 30.8 ± 3.2a | 20.2 ± 4.9b | 3.7 ± 2.4c |
| Peel C∗ | 34.1 ± 3.0a | 21.0 ± 4.8b | 7.1 ± 3.4c |
| Peel h | 115.5 ± 2.8a | 78.3 ± 12.8b | 30.8 ± 6.2c |
| Flesh L∗ | 63.1 ± 3.4b | 71.3 ± 2.4a | 64.6 ± 4.5b |
| Flesh a∗ | −11.7 ± 1.7c | −4.7 ± 1.4b | 11.7 ± 6.2a |
| Flesh b∗ | 22.9 ± 2.4a | 19.0 ± 3.7b | 13.0 ± 4.8c |
| Flesh C∗ | 25.7 ± 2.2a | 19.6 ± 3.8b | 19.0 ± 2.7b |
| Flesh h | 117.3 ± 4.3a | 103.8 ± 2.7b | 48.2 ± 22.8c |
- Note: Data are mean ± SD (n = 3 × 10); L∗: lightness, a∗: redness, b∗: yellow, C∗: chroma, h: hue angle. Data without a common letter in each test indicate significant difference between samples for Tukey (HSD) at p < 0.05.

The firmness decreased during BP development (Table 2), which can be attributed to the relatively lower cell packing density and cell wall content due to the increase in cell size and air spaces, the reduction in cell wall thickness as well as changes in the cell wall composition [27].
| Test | Mature green | Turning | Dark maroon |
|---|---|---|---|
| Firmness (N) | 59.4 ± 9.2a | 46.5 ± 9.3b | 34.1 ± 6.8c |
| pH | 4.0 ± 0.3a | 3.5 ± 0.1b | 3.5 ± 0.2b |
| TA (% citric acid) | 2.2 ± 0.30.3c | 3.1 ± 0.2a | 2.8 ± 0.3b |
| TSS (°Brix) | 10.1 ± 2.7a | 8.6 ± 0.6b | 7.5 ± 0.7c |
- Note: Data are mean ± SD (n = 3 × 5). Data without a common letter in each test indicate significant difference between samples for Tukey (HSD) at p < 0.05.
At the same time, the dietary fibre content decreased to around 30 g/100 g DW during maturation (Table 3). The reduction in fibre content indicated its positive correlation with the loss of firmness in BP during maturation. Dietary fibre is the major constituent of plant cell walls [28] and the degradation of cell wall components, including pectin, hemicellulose, and cellulose, contributed to the reduction in dietary fibre [29, 30]. The reduction in firmness and fibre content has been commonly observed in the maturation of other fruits, such as apples [29].
| Test | Mature green | Turning | Dark maroon |
|---|---|---|---|
| Moisture content (g/100 g FW) | 81.9 ± 1.7a | 81.4 ± 2.0a | 81.4 ± 2.3a |
| Dietary fibre (g/100 g DW) | 56.2 ± 5.5a | 38.8 ± 4.5b | 33.2 ± 3.1c |
| Glucose (g/100 g DW) | 0.6 ± 0.2c | 2.2 ± 0.3b | 2.9 ± 0.7a |
| Fructose (g/100 g DW) | 0.4 ± 0.2c | 2.0 ± 0.3b | 2.9 ± 0.9a |
| Sucrose (g/100 g DW) | 0.1 ± 0.1a | 0.2 ± 0.2a | 0.1 ± 0.1a |
- Note: Data are mean ± SD (n = 3 × 3). Data without a common letter in each test indicate significant difference between samples for Tukey (HSD) at p < 0.05.
The moisture content of flesh remained stable during maturation, which has also been observed in other fruits during maturation such as tomato [31]. However, environmental conditions such as water supply can also affect fruit moisture content [32]. The pH decreased from mature green stage to turning stage then remained stable at dark maroon stage, with corresponding increase of TA from mature green to turning stage (Table 2). Total soluble solid (TSS) decreased during BP maturation which is in contrast to most fruits, with increasing TSS during maturation [33]. The reduction in TSS has also been observed during mango fruit maturation [34]. Since TSS is affected by many factors such as proteins and minerals, it may not reflect the sugar content well [35]. TSS and pH are more affected by fruit variety, growth location and season than fruit colour and firmness, as common maturity indices [36].
Sucrose, glucose, and fructose are the most common sugars found in plants [37]. The results from the sugar analysis showed an increase in fructose and glucose content (Table 3). Sucrose was lower than fructose and glucose, which is also found in mangos [34]. Soluble sugars are produced mainly from photosynthesis and starch degradation during fruit development, and sucrose can be hydrolysed to fructose and glucose [38]. The increase in glucose and fructose during fruit development are common in fruits such as plums and peaches [39, 40].
3.2. Quantification of Bioactive Compounds
The composition of identified bioactive compounds by three commonly used solvents (water, 80% aqueous ethanol and 80% aqueous methanol) at different maturity stages is shown in Table S2. To illustrate the differences in bioactive compounds according to maturity stage and solvent effect, the quantified data were subjected to a PCA. As shown in Figure 2, the first two PCs (PC1 and PC2) accounted for 75% of the total variance. PC1 clearly distinguished extracts from different maturity stages, with mature green stage samples on the positive side, turning stage samples in the middle and dark maroon stage samples on the negative side. Compounds including quinic acid, galloylqunic acid, epigallocatechin gallate, epicatechin gallate and ellagic acid were heavily loaded on the positive side of PC1, and delphinidin 3-galactoside, cyanidin 3-galactoside, quercetin 3-glucoside and epicatechin were heavily loaded on the negative side of PC1. This differentiation responded to the decreasing concentrations of most phenolic compounds except the increasing content of delphinidin 3-galactoside, cyanidin 3-galactoside, quercetin 3-glucoside and epicatechin in BP from mature green stage to dark maroon stage. PC2 discriminated water extracts from ethanol or methanol extracts, where the positive side was water extract and the negative side was ethanol and methanol extracts. Water extracts were more correlated with PC2 due to the higher content of gallic acid and galloyl glucose and lower content of compounds in the negative side of PC2 than ethanol or methanol extracts. Therefore, the composition of extracts was influenced both by maturation stage and solvent.
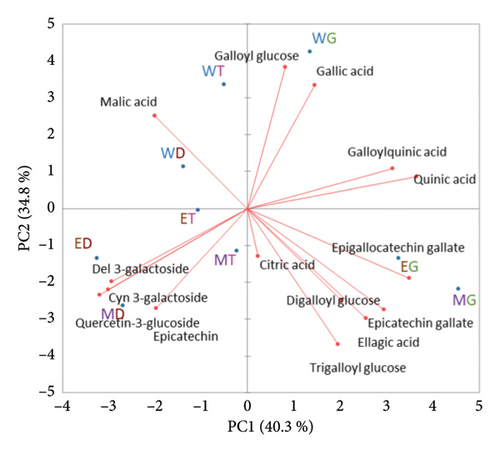
The decrease in most phenolic compounds during maturation are in agreement with observations in other fruits [41] including mangos [42], where green stage fruits are richer in phenolic compounds than ripe fruits. Some phenolic compounds increased during maturation including anthocyanins and quercetin glycosides. Anthocyanins were not detected at mature green stage when fruits were green, but the concentration increased during maturation. The increase in anthocyanins during fruit maturation is common in fruits that accumulate anthocyanins such as blueberries [43]. The accumulation of quercetin glycosides has also been reported in apple maturation [44]. The biosynthesis, accumulation and catabolism of phenolic compounds are regulated by specific genes, which are differentially expressed during fruit development [45]. Generally, an upregulation of anthocyanin biosynthesis-related genes can be observed in anthocyanin-accumulating fruits during maturation [46]. Furthermore, the degradation products from certain phenolic compounds can be used in the biosynthesis of other compounds [47], which may also explain the difference in phenolic compounds during maturation. Moreover, environmental factors such as light and water can also affect the phenolic content [48].
WG had the highest content of gallic acid (around 30 mg/100 g DW) and galloylglucose (around 20 mg/100 g). The galloylglucose content was similar to that in mango pulp [42] and the gallic acid content was comparable to that in mango kernel and peel which was higher than that in mango pulp [49, 50]. Gallic acid has a higher solubility in water than in organic solvents [51]. For galloylglucose, its hydrophobicity increases with increasing numbers of galloyl groups [52].
EG and MG had relatively higher concentrations of epigallocatechin gallate (around 20 mg/100 g), epicatechin gallate (around 20 mg/100 g) and ellagic acid (around 6 mg/100 g) compared to the remaining extracts. The ellagic acid concentration was also comparable to that from mango kernels which was higher than in mango flesh [49].
ED and MD were highest in anthocyanins (around 200 mg/100 g of cyanidin 3-galactoside) and quercetin 3-glucoside and its isomer (around 5 mg/100 g). Despite anthocyanins being highly soluble in water, their extraction is more efficient by using organic solvents such as methanol and ethanol [53]. This is due to the hydrophobic feature of the anthocyanin molecule [54].
3.3. Antioxidant Capacity
The antioxidant capacities had a declining trend during maturation as measured by TPC and FRAP (Table 4).
| Test | Extract | Mature green | Turning | Dark maroon |
|---|---|---|---|---|
| TPC (mg GAE/g DW) | 80% ethanol | 56.6 ± 40.6ab | 33.4 ± 19.0abcd | 29.3 ± 13.7bcd |
| 80% methanol | 70.1 ± 49.4a | 41.0 ± 14.9abcd | 29.5 ± 12.1bcd | |
| Water | 53.9 ± 19.2abc | 17.1 ± 9.5cd | 11.3 ± 6.9d | |
| FRAP (μmol Fe2+/g DW) | 80% ethanol | 688.8 ± 498.4abc | 412.1 ± 243.7bcd | 348.0 ± 164.3bcd |
| 80% methanol | 901.7 ± 637.3a | 520.7 ± 208.7abcd | 372.1 ± 159.9bcd | |
| Water | 782.4 ± 254.5ab | 268.4 ± 156.3cd | 173.2 ± 107.7d | |
- Note: Data are mean ± SD (n = 3 × 3). Data without a common letter in each test indicate significant difference between samples for Tukey (HSD) at p < 0.05.
The observed reduction in antioxidant capacity correlated with a decrease in most phenolic compounds. This has also been observed in other fruits during maturation, such as jujube and plum fruits [17, 41]. Furthermore, other bioactive compounds such as quinic acid [55], vitamin C [56] and metals [57] can also react with the TPC and FRAP reagents and therefore contribute to the total antioxidant capacity. In later stages of fruit development, most resources and energy are directed toward fruit growth rather than synthesising phenolics to combat frugivores [41] or environmental stress [58]. This shift can also explain the decrease in the antioxidant capacity in later maturity stages.
Methanol and ethanol extracts tended to have higher antioxidant capacity at turning and dark maroon stages (Table 4). This could be caused by antioxidant compounds which have a higher solubility in methanol and ethanol than in water. Methanol is a very efficient solvent for extracting antioxidant compounds from plant matrices [59].
3.4. Antimicrobial Activity
The antimicrobial activities of BP extracts against three microorganisms are shown in Table 5. Inhibition against the growth of the Gram-positive bacterium S. aureus was observed while there was no inhibition against Gram-negative bacterium E. coli and fungus C. albicans (Figure S1). The results are in line with previous findings that S. aureus is more sensitive than E. coli and C. albicans [60] to plant extracts. Gram-positive bacteria have a monolayer cell wall, which are generally less resistant than Gram-negative bacteria which have an outer membrane. The structural and compositional difference in the outer membrane has been identified as factors reducing the penetration of plant extracts into Gram-negative bacteria [61].
| Microorganism | Extract | Inhibition zone (mm) | ||
|---|---|---|---|---|
| Mature green | Turning | Dark maroon | ||
| Staphylococcus aureus (NCTC6571) | 80% ethanol | 6.3 ± 1.2b | 9.2 ± 0.7a | 9.5 ± 0.3a |
| 80% methanol | 4.4 ± 0.9c | 8.6 ± 1.4a | 9.4 ± 0.3a | |
| Water | 1.5 ± 2.5d | 8.4 ± 1.2a | 9.1 ± 1.3a | |
| Escherichia coli (NCTC9001) | 80% ethanol | 0 | 0 | 0 |
| 80% methanol | 0 | 0 | 0 | |
| Water | 0 | 0 | 0 | |
| Candida albicans (ATCC10231) | 80% ethanol | 0 | 0 | 0 |
| 80% methanol | 0 | 0 | 0 | |
| Water | 0 | 0 | 0 | |
- Note: Data are mean ± SD (n = 3 × 3). Data without a common letter in each test indicate significant difference between samples for Tukey (HSD) at p < 0.05.
The 8-9 mm inhibition zone of extracts from turning and dark maroon stages against the growth of S. aureus indicates moderate inhibition activity, while less than 7 mm inhibition zone from mature green stage result indicates low inhibition activity [62]. The larger inhibition zone at turning and dark maroon stages compared to mature green stage, indicates that antimicrobial activity of BP extracts against S. aureus was stronger for turning and dark maroon fruits than green fruits. In addition, there were no significant differences in the inhibition against S. aureus among the three extracts on turning and dark maroon stages. The increase in antimicrobial activity along with maturation has been observed in lemon [63], blueberry leaf [64] and fennel [65], while a decrease in antimicrobial activity has also been observed [17]. Usually, extracts with higher antimicrobial activity shows higher antioxidant capacity, while the opposite was observed in BP during maturation. Similar trend has also been described in extracts from wild blueberry fruit, where higher antimicrobial activity was accompanied with lower antioxidant capacity in mature fruits [15]. This may be explained by the increase in the content of anthocyanins. Anthocyanin extracts have been found to be active against various bacteria especially Gram-positive bacteria [66]. Anthocyanin rich fraction of extracts has been found to exert stronger antimicrobial activity than crude extracts [67]. This may also be explained by multiple mechanisms and synergies coming into play in antimicrobial activity due to the presence of a diverse array of compounds in the extracts such as organic acids, phenolics and amino acids [66].
4. Conclusion
This study demonstrated that the physicochemical characteristics, antimicrobial activity and antioxidant capacity of BP changed during maturation. As the fruit matured, size and weight increased significantly, accompanied by an increase in redness and hue angle, while firmness and fibre content declined. Most bioactive compounds, including gallic acid and epicatechin gallate, decreased, whereas anthocyanins, quercetin-3-glucoside and epicatechin increased during maturation. Compared to 80% ethanol and methanol extracts, water extracts exhibited comparable antimicrobial activity for the later maturity stages despite having relatively lower antioxidant capacity. This study further demonstrates that BP is a source of bioactive compounds at different maturity stages and has the potential to be used as a functional food to diversify our diets.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Gengning Chen: conceptualization; methodology; software; data curation; formal analysis; validation; investigation; visualization; project administration; writing–original draft. Michael E. Netzel: supervision; writing–review and editing. Daniel Cozzolino: supervision; writing–review and editing. Yasmina Sultanbawa: supervision; resources; writing–review and editing; funding acquisition; conceptualization.
Funding
This research was funded by the Australian Research Council (ARC) Industrial Transformation Training Centre for Uniquely Australian Foods (project number IC180100045).
Acknowledgements
The authors acknowledge the Traditional Owners of the lands on which the Burdekin plums are grown and harvested, and respect the knowledge and experience the Traditional Owners shared regarding this plant. The authors also acknowledge the Sherwood Arboretum and the curator for providing samples and sharing knowledge about this plant.
Supporting Information
Table S1: High resolution accurate mass data of identified bioactive compounds. Table S2: Quantification of major identified compounds. Table S3: PCA analysis factor loadings. Figure S1: representative photos showing the inhibitory activity of Burdekin plum extracts against S. aureus, E. coli and C. albicans.
Open Research
Data Availability Statement
The data that support the findings of this study are available within the article and/or its supporting information.




