Innovative Applications of Hill Lemon (Citrus pseudolimon Tanaka) in Sustainable Bioprocessing: Bioactive Extraction, Nanoparticle Synthesis, and Functional Food Products
Abstract
Hill lemon (Citrus pseudolimon Tanaka) is an underutilized citrus fruit native to India, with significant potential in the food, pharmaceutical, and cosmetic industries due to its rich nutritional and bioactive profile. This review consolidates existing research on Hill lemon, highlighting its various components and their associated health benefits. Studies have documented its diverse health benefits, including antioxidant, antibacterial, antiviral, anti-inflammatory, and anticancer properties. The leaves, peel, and seeds of Hill lemon are rich sources of bioactive compounds like limonene, citronellol, pectin, essential oils, and vitamin C, contributing to its beneficial properties. Despite its promising potential, the short shelf life of the processed Hill lemon juice presents a challenge for commercialization. This review underscores the need for further research to fully explore the potential of Hill lemon and its bioactive compounds, particularly in developing value-added products with enhanced stability and extended shelf life.
1. Introduction
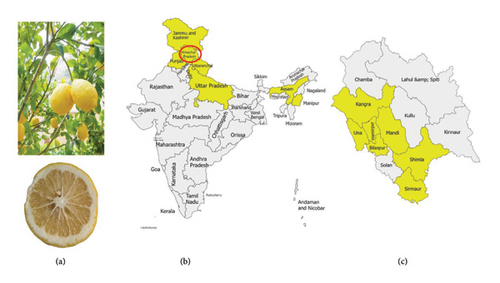
Hill lemon (Citrus pseudolimon Tanaka.) is a subtropical fruit (Figure 1(a)) that originated in India [1]. It belongs to the subfamily Aurantioideae (Citroideae) of the Rutaceae family, with a chromosome number of 2n = 18 [2, 3]. In various regions of India and Nepal, Hill lemon is known by different local names, including Khatta, Nibuwa, Galgal, Kumaon, Chukh, Chinara, Pahadi nibbu, Bada nimbu, and Jamir [3–9]. Although morphologically distinct from common lemon, Hill lemon shares some characteristics with another indigenous lemon variety, Alemow [10, 11]. It is grown organically and cultivated in the plains and sub-mountainous regions of the North-Western Himalayas (Shivalik mountain ranges) up to an average altitude of 1100 m above sea level [3, 7, 11, 12]. In India, it is primarily found in North-West hilly states such as Uttarakhand, Jammu Kashmir, Himachal Pradesh, and the north-eastern states of Assam and Manipur (Figure 1(b)). It also grows in other states like Punjab and Uttar Pradesh (Lucknow) [6, 12]. In Himachal Pradesh, it is cultivated organically and conventionally in different districts like Sirmour (Poanta valley), Shimla, Hamirpur, Una, Kangra, Bilaspur, and Mandi (Figure 1(c)) [3]. In Uttarakhand, its germplasm is widely distributed in areas like Mandal, Doon valley, and Gopeshwer in the Chamoli district [1, 13, 14]. Hill lemon grows in semiwild conditions and can thrive in rainfed areas [4]. It is resistant to citrus canker, tolerates high temperatures of up to 40°C, and withstands frost injuries by increasing its lipid content and decreasing its leaf water content [3, 11]. Hill lemon was previously used as rootstock for mandarin fruit, but this practice was discontinued due to its poorly grafted plant growth and susceptibility to Phytophthora diseases.
Hill lemon is a plant with various uses, containing diverse nutrients and bioactive compounds in its leaves, fruit peel, and juice. Some of these nutrients are pectin, protein, crude fibers, sugars, and organic acids [1, 15–17]. Major bioactive compounds include limonene, citronellol, d-limonene, nerolidol, bicyclo [2.2.1] heptane, 7,7-dimethyl-2-methylene, and Trans-β-ocimene. These bioactive compounds exhibit various health benefits, including anticancerous, anti-inflammation, antidiabetic, and anti-aging properties [1, 16, 18, 19]. There are different methods for extracting these bioactive compounds, ranging from traditional to advanced techniques. The traditional techniques include hydro-steam distillation, Clevenger apparatus, and steam distillation, while advance techniques include microwave-assisted extraction (MAE), ultrasonic-assisted extraction (UAE), and supercritical fluid extraction (SFE) [16, 18, 19].
Hill lemon fruit is primarily used by the local people to make pickles, chutney, and chukkh on a small scale [20]. Some studies have explored the production of juice powder, ready-to-serve (RTS) drink, squash, peel extract, and leaf extract, as well as the extraction of pectin and essential oil from this fruit [16, 21–23]. However, these products have not yet reached large-scale commercialization. Apart from these uses, Hill lemon fruit serves as a folk remedy for various ailments in some tribal areas. For example, the Gujjar tribe of Uttarakhand applies the juice to the skin for treating skin conditions [24]. Some people also mix its juice with raisins and dates to cure cough and cold [25, 26]. Hill lemon fruit has a high content of citric acid, which gives it a distinctive sour flavor [23]. Furthermore, it contains bitter compounds such as limonene and naringenin, which can impart bitterness after juice extraction. However, these compounds have antioxidant and anti-inflammatory properties [27].
Hill lemon is a seasonal crop harvested from October to December and faces postharvest losses of 20%–25% due to inadequate fruit management [20]. Additionally, it has notably a thick peel that limits its usage mainly to pickle and chutney preparation, reducing its overall utility [28]. Furthermore, handling of Hill lemon juice can be challenging due to its high acidity, as it tends to turn bitter upon exposure to oxygen [10, 29]. Hill lemon is also vulnerable to major pests and diseases, such as citrus leaf miner (Phyllocnistis citrella), citrus psylla (Diaphorina citri), citrus canker (Xanthomonas axenopodis p.v. citri), foot rot or gumosis (Phytophthora parasitica and palmivora), anthracnose or wither tip (Collectotrichum gloeosporioides), and citrus decline [13]. These factors have hindered the cultivation and commercial exploitation of Hill lemon by farmers in many parts of India. Processing Hill lemon fruit into various value-added products, such as jam, jelly, beverages, juice powder, spreads, etc., can help to utilize this underutilized fruit [30].
This compressive review stands as the first of its kind, offering a complete overview of Hill lemon, addressing the scattered and limited literature on this topic. It extensively covers its morphology, nutritional value, phytochemistry, and quality characteristics, as well as its medicinal and therapeutic value, along with the functional properties and value addition of Hill lemon fruit. Despite being an underutilized commodity, it holds significant potential for proper exploitation. Therefore, this detailed and all-encompassing review offers valuable insights into food manufacturers and scientists, ultimately enhancing its overall utility.
2. Scientific Description of Hill Lemon
2.1. Agricultural Practices
Hill lemon plays a significant role in agricultural practices, particularly in Punjab, where it is utilized for boundary plantations to alleviate agrarian crises [31]. Additionally, in the Kumaon Himalaya region, it is integrated into various fruit tree-based land use systems, such as Hill lemon with wheat [32].
2.2. Soil Water Characteristics
Hill lemon trees thrive in deep, fertile, loose, and well-drained soils without any hardpan layers of calcium carbonate in the root zone. These soils should have an electrical conductivity of up to 0.5 mmhos/cm, calcium carbonate levels of up to 5%, and lime concentration of up to 10%. The pH range that best suits Hill lemon cultivation is between 5.5 and 7.5 [13]. It is important to note that Hill lemon plants are sensitive to salt and do not grow well in saline or alkaline soils. However, these plants can be successfully grown in both rainfed and dryland conditions [13, 33].
2.3. Morphological Characteristics
Morphological characteristics play a crucial role in the identification and characterization of germplasm [34]. These characteristics encompass the visual attributes of Hill lemon, including the features of the tree, leaves, and fruit. However, it is important to note that changes in the environmental factors, particularly climate and elevation, significantly influence yield and other morphological characteristics [35]. The effect of elevation on the physical and chemical characteristics of Hill lemon fruit is presented in Table 1. The shape of Hill lemon trees includes growth habits such as spreading, erect, and drooping tendencies with spiny, stout spines, up to 2–3-cm long [12, 34] with a plant height of 3.55–3.65 m [4, 37]. The canopy of Hill lemon trees typically covers an area ranging from 3.85 to 5.21 m2, extending in both the north–south spread of 3.85 m and east–west 3.76 m. Notably, the spine length of these trees measures 21.52 cm [4, 34]. The fruit yield per tree was recorded at 13 fruit/tree or fruit yield of 1.88 kg/plant [4]. Regarding Hill lemon leaves, they are ovate with crenate-type margins and lamina lengths varying from 46.92 to 122.59 cm, with leaf width ranging from 26.79 to 78.35 mm [3, 12, 34, 36]. The leaf area of Hill lemon ranges from 1.81 to 63.76 cm3 and has the highest leaf area among all citrus spp. [3, 12, 34]. These unique attributes collectively contribute to the distinctive identity of Hill lemon.
| Elevation (m) | 135–154 | 296–315 | 389–417 | 714–979 |
|---|---|---|---|---|
| Areas | Tinsukia (Assam); Lakhimpur (UP) Indian Agricultural Research Institute, New Delhi | (CCRI) Nagpur; Fruit Research Station, Gangian (Dasuya) Hoshiarpur | Hamirpur (HP); Nalagarh (HP) | Mandi (HP); Kangra (HP); ICAR Research Complex, Barapani, Meghalaya Dehradun (UK) |
| Yield (kg/tree) | — | 145.50 | 48.80 | 1.88 |
| No. of segments | 11–12 | 8–13 | 9.2–10.75 | 9 |
| Fruit shape | Ellipsoid | Pyriform | Oblong/Spherical | Ellipsoid |
| Leaf lamina length (mm) | 46.92–122.04 ∗ | |||
| Leaf lamina width (mm) | 26.79–78.35 ∗ | |||
| Leaf thickness (mm) | 0.05–0.36 ∗ | |||
| Peel thickness (mm) | 4.40–4.92 | 5.06–10 | 4.56 | 5.46–9.20 |
| Peel content (%) | — | 23.09 | 9.2–23.0 | — |
| Juice content (%) | — | 33.4–59.6 | 40–44 | 30.55 |
| Weight (g) | 180.66–291.49 | 143.5–623.4 | 113.33–450 | 113.33–360.5 |
| TSS (°Brix) | 6.80 | 7.05–7.85 | 7.42–7.85 | 4.00–7.79 |
| TA (%) | 3.84 | 6.6–6.75 | 4.99–5.04 | 3.58–4.10 |
| AA (mg/100 mL) | 40.97 | 23.18–26.77 | 36.12–39.334 | 30 |
| References | [8, 36] | [14, 34] | [11, 15] | [4, 8, 37] |
- Abbreviations: AA, ascorbic acid; TA, titratable acidity; TSSs, total soluble solids.
- ∗Mean range (not from specific elevation) [3].
Under sub-tropical conditions, Hill lemon bears flowers during March–April, with clusters of four to nine solitary, mildly fragrant flowers that can be terminal or axillary [8, 14]. The fruit of Hill lemon has an elliptical to round shape, exhibiting a length ranging from 70.07 to 121.17 mm and maximum width ranging from 41 to 109 mm [4, 14, 34]. Hill lemon fruit weigh from 145.5 to 750 g per fruit [4, 12, 14, 34]. The number of segments in each fruit ranges from 7.33 to 16. The yield of Hill lemon juice ranged from 33.4% to 59.6% [12, 14, 34, 36]. The pulp of Hill lemon is light yellow and coarse, with cylindrical, loosely packed vesicles. The seeds of Hill lemon are light yellow, smooth, and conical-ovate with prominent ridges. They have a mean number of 29.50 per fruit. The seeds are polyembryonic and moderately recalcitrant, and their cotyledon is creamish. The embryos are used as the explant [4, 8, 38].
2.4. Nutritional Content
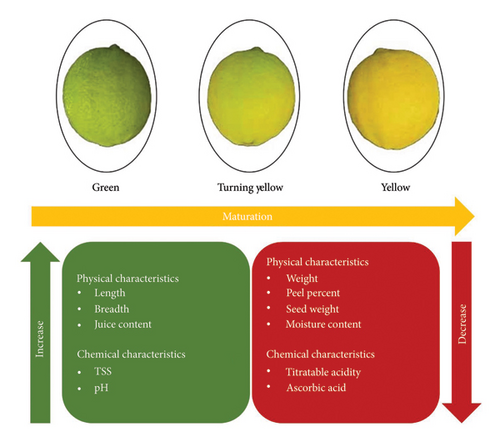
Hill lemon is a remarkable fruit as a whole, not only for its juice but also for its peel, which is rich in nutrients and bioactive compounds as shown in Table 2. The major nutrients include carbohydrates, protein, crude fiber, crude fat, and ash content. The moisture content of the peel ranges from 81.64% to 83.42% [16, 40]. The carbohydrates, which are the main source of energy, are present in both the juice (5.87 g/100 mL) and the peel (5.97 g/100 g), whereas the pectin, crude fiber, and crude fat are found in the peel at a concentration of 3.31%–20.80%, 1.55%–1.67%, and 0.25%–4.22%, respectively [16, 17]. Surprisingly, Hill lemon contains protein in juice (5.08%), peel (8.12%), and pomace (4.89%) [17, 39]. Furthermore, crude fibers, crude fat, total sugar, and total phenols are found in the pomace at concentrations of 60.12%, 2.17%, 4.81%, and 13 mg GAE/100 g, respectively [39, 41]. The Hill lemon sensory quality is determined by the balance between sugar and acid. Citric acid is the main organic acid in many citrus fruits, including Hill lemon, and it contributes to the sour flavor of juice. The acidity level of the Hill lemon juice ranges from 4.99% to 5.04%. The total sugar content is between 2.07% and 2.14%, and the total soluble solids (TSSs) vary from 5.4 to 8.7°Brix [15, 30]. Furthermore, the physical and chemical characteristics change during maturation as shown in Figure 2.
| Parameters | Juice (per 100 mL juice) | Peel (per 100 g peel) | Pomace (per 100 g pomace) | References |
|---|---|---|---|---|
| Moisture content (%) | — | 81.64–83.42 | 10.66 | [16, 39] |
| Carbohydrates (g) | 5.87 | 5.97 | [17] | |
| Pectin (% DWB) | — | 3.31–20.80 | [16, 40] | |
| Crude fibers (%) | — | 1.55–1.67 | 60.12 | [16, 39] |
| Protein (mg) | 5.00 | 9.12–12.00 | 4.89 | [16, 17, 39] |
| Crude fat (%) | — | 0.25–0.4.22 | 2.17 | [16, 39] |
| TSS (°Brix) | 13.00 | 6.52–6.84 | [16, 17] | |
| Total sugars (%) | 2.07–2.14 | 6.69–7.40 | 4.81 | [15, 39] |
| Reducing sugars (%) | — | 3.31–3.82 | [16, 17] | |
| Titratable acidity (%) | 4.99–5.04 | — | [15] | |
| Ash content (%) | — | 2.52–2.89 | 3.21 | [16, 39] |
| Tannin (mg) | 14.15 | 35.70 | [17] | |
| AA (mg) | 19.58–21.00 | 34.00–39.00 | [16, 17] | |
| Total carotenoids (mg) | 0.11 | 79.54–81.54 | [16] | |
| Total chlorophyll (mg) | — | 13.45–15.69 | [16] | |
| Total phenols (mg GAE) | 4.00 | 7.26–8.25 | 13 | [16, 17, 41] |
| Total flavonoids (mg) | 3.00 | 5.00–7.00 | [16, 17] |
- Abbreviations: AA, ascorbic acid; DWB, dry weight basis; GAE, gallic acid equivalent; TSSs, total soluble solids.
The ash content, which indicates the mineral presence, is observed in the peel (2.52%–2.89%) and pomace (3.21%) [16, 39]. Another main attraction point of Hill lemon is its high content of vitamin C, which ranges from 14.70 to 39.58 mg/100 mL [15, 30]. Hill lemon fruit is also noteworthy for its high content of various bioactive components, including tannins (around 50 mg/100 g), carotenoids (79.54–81.54 mg/100 g), chlorophyll (13.45–15.69 mg/100 g), flavonoids (8–10 mg/100 g), and ascorbic acid (38–39 mg/100 g), all of which exhibit various antioxidant properties [16, 18]. A detailed discussion of Hill lemon’s bioactive compounds and their properties is provided in a further subsection.
3. Extracts From Hill Lemon
3.1. Phyto-Chemistry of Hill Lemon
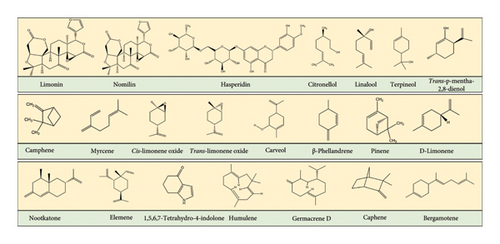
Hill lemon plant is a rich source of various nutrients, but it is particularly high in bioactive compounds that have multiple applications, such as nutraceuticals. Some of the bioactive compounds found in the peel of Hill lemon fruit are D-limonene, α-phellandrene, myrcene, α-pinene, α-elemene, camphene, caryophyllene, humulene, linalool, β-pinene, nootkatone, sabinene, germacrene, and β-myrcene (Table 3 and Figure 3). More than 300 volatile compounds have been identified in Hill lemon peel, primarily belonging to terpenoids. These compounds have different biological effects, such as antimicrobial, antioxidant, anticancer, antidiabetic, antiviral, anti-inflammatory, analgesic, antifungal, cholesterol-lowering, immunity-boosting, anti-depressant, and hepatoprotective [42, 43]. Several studies have confirmed the potential of these compounds for treating various diseases and disorders [16, 44, 45]. According to Sultana et al. [45], the leaves of Hill lemon plant contain the highest amount of bioactive compounds, followed by the peel and the lowest amount is found in the seeds. However, Manchanda et al. [22] reported that the in vitro extraction of bioactive compounds from the callus of leaves and peel showed higher phenol and flavonoid content in the peel than in the leaves. The total phenolic content of Hill lemon leaves, peels, and seeds ranged from 98.20 to 199.18 mg/g of dry matter, with the leaves having the highest values (199.18 mg/g of dry matter for phenolics and 39.60 mg/g for flavonoids). The juice of Hill lemon had lower phenol and flavonoid contents, ranging from 18.34 to 49.98 mg/100 mL and 13.67 to 37.09 mg/100 mL, respectively. A study by Revathy et al. [46] mentioned that citrus flavonoids (hesperetin) have potential in attenuating hyperglycemia in streptozotocin (STZ)-induced diabetes in rats by releasing insulin from β-cells of islets. The findings demonstrated that the supplementation of 40 mg/kg of hesperetin for 45 days resulted in a considerable increase in plasma insulin levels and a significant drop in plasma glucose levels. It improved the antioxidant status by boosting the activity of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). Additionally, hesperetin reduced the levels of cholesterol, free fatty acid (FFA), tau protein (TG), and phospholipid (PL) in diabetic rats likely through an insulin-mediated decrease in the synthesis of fatty acids and cholesterol. Moreover, it was suggested that the cholesterol-lowering effect of hesperetin is possibly due to the capability of hesperetin and other flavonoids to bind to bile acids within the intestine, resulting in enhanced bile acid secretion and a reduction in cholesterol absorption.
| Bioactive compounds | Peel (ppm) | References |
|---|---|---|
| Limonene | 10.6 ∗ | [18] |
| Citronellol | 0.50 (62.30 ∗) | [16, 18] |
| Linalool | 0.22 (1.70 ∗) | [16, 18] |
| Terpineol | 2.59 ∗ | [18] |
| β-Phellandrene | 0.13–0.58 | [16, 19] |
| α-Pinene | 2.65–4.69 | [16, 19] |
| β-Myrcene | 1.16 | [19] |
| D-limonene | 42.94–58.01 | [16, 19] |
| Linalool | 0.22 | [19] |
| trans-p-Mentha-2,8-dienol | 0.71 | [19] |
| cis-Limonene oxide | 2.04 | [19] |
| trans-limonene oxide | 1.09 | [19] |
| Cis-carveol | 2.11 | [19] |
| trans-Carveol | 1.32 | [19] |
| Caphene | 0.18 | [16] |
| (1S,4R)-p-Mentha-2,8-diene, 1-hydroperoxide | 0.82 | [19] |
| (2S,4R)-p-Mentha-6,8-diene, 2-hydroperoxide | 1.70 | [19] |
| α-Bergamotene | 0.52 | [19] |
| β-Bisabolene | 0.69 | [19] |
| Nerolidol | 0.43 | [19] |
| 3-Methylpentane | 4.46 | [19] |
| Trans-3-methyl-2-pentene | 7.95 | [19] |
| Toluene | 1.52 | [19] |
| 1,5,6,7-Tetrahydro-4-indolone | 0.78 | [19] |
| Limonene glycol | 0.65 | [19] |
| Bicyclo[2.2.1]heptane, 7,7-dimethyl-2-methylene | 5.24 | [16] |
| Trans-β-ocimene | 22.73 | [16] |
| β-Ocimene | 3.48 | [16] |
| Indolizine | 0.20 | [16] |
| Terpinen-4-ol | 0.66–0.87 | [16] |
| Elemene | 0.09–3.57 | [16] |
| Geranyl acetate | 0.15–0.36 | [16] |
| Cyclohexane, 1-ethenyl-1-methyl-2,4-bis(1-methylethenyl) | 0.59 | [16] |
| Caryophyllene | 0.12 | [16] |
| Caryophyllene oxide | 0.27 | [16] |
| N-(2,5Dimethylphenyl) piperazine | 10.42 | [16] |
| cis-β-bergamotene | 0.33–0.43 | [16] |
| Humulene | 1.18 | [16] |
| Germacrene D | 0.09–1.13 | [16] |
| (1S,2E,6E,10R)-3,7,11,11-Tetramethylbicyclo[8.1.0]undeca-2,6-diene | 2.28 | [16] |
| Neointermedeol | 0.23–0.37 | [16] |
| Nootkatone | 0.91 | [16] |
- Abbreviation: ppm, parts per million.
- ∗Content in leaves.
3.2. Essential Oil From Hill Lemon
Hill lemon waste, which encompasses peels, seeds, pomace, and leaves, is often overlooked despite it being rich in essential oils, aroma compounds, and polyphenols known for their anti-inflammatory and antimicrobial properties [47]. It is generally recognized as safe (GRAS), nontoxic, nonmutagenic, and noncarcinogenic. These properties have led to its wide application in the beverage, flavor, food packaging, pharmaceutical, and cosmetic industries [16]. Various extraction techniques have been used for the extraction of essential oil from Hill lemon peel, pomace, and leaves like Clevenger apparatus, steam distillation, MAE, UAE, and SFE [16, 18, 19]. The yield of essential oil from Hill lemon peel ranged from 6.25% to 9.40% using supercritical CO2 extraction. This technique is efficient but expensive due to high equipment and maintenance costs, whereas the Clevenger extraction technique had a lower yield ranging from 1.92% to 3.50% [16]. Furthermore, steam distillation was the least efficient method for the extraction of oil from Hill lemon peels, yielding only 0.79%–0.98% oil [16, 19]. The effect of different extraction methods on Hill lemon peel and pomace samples was also studied by Kaur et al., [44] using MAE, UAE, and shaking water bath extraction (SWE). The results showed that MAE had the highest extraction yield for peel samples, with the optimal conditions being 500 W, time 120 s, solid-liquid ratio—1:15, and pH 5.5. Similarly, Grover et al. [16] compared different methods for the extraction of essential oil from Hill lemon peels and showed that MAE (frequency of 2.45 GHz and time 20 min) provided a maximum yield of essential oil at 2.49%. UAE emerged as the second-highest yielding method for peel samples, employing power at 300 W for 30 min, at a solid–liquid ratio of 1:15, and pH = 5.5. Its extraction produced essential oil amounts ranging from 1.00% to 2.45%, with 30 min being the most effective duration [16, 44]. In contrast, SWE consistently delivered the lowest extraction yield for peel samples across various conditions [44]. However, for pomace samples, the trend diverged, showcasing UAE as the most efficient, followed by MAE and then SWE under similar conditions. This difference in yield between Hill lemon peel and pomace samples likely relates to their distinct characteristics. Notably, extraction temperature/power and the solid–liquid ratio emerged as pivotal factors significantly impacting the extraction yield [16, 22, 44]. The primary compounds in the essential oil were limonene, β-citronellal, β-citronellol, and linalool, therefore, contributing to beneficial effects, such as antioxidant, antimicrobial, anticancer, and anti-inflammatory properties [16, 18, 19]. In a study by Bi et al., [48], the therapeutic potential of citrus essential oils on primary dysmenorrhea was evaluated using both in vivo and in vitro models. The findings indicated a significant enhancement in the activity of antioxidant markers, including total antioxidant capacity (T-AOC), SOD, CAT, and glutathione (GSH). Concurrently, there was a notable reduction in malondialdehyde (MDA) levels, inducible nitric oxide synthase (iNOS) expression, and the PGF2α/PGE2 ratio in the rat uterus, which had been induced by estradiol benzoate and oxytocin. These biochemical alterations were associated with a substantial decrease in the writhing response, indicating a reduction in dysmenorrhea symptoms. Similarly, Castro et al. [49] investigated the anticancer properties of citrus peel oil, particularly rich in limonene, myrcene, and carotenoids. Their study demonstrated a dose-dependent inhibition of proliferation in A549 non-small-cell lung cancer (NSCLC) cells and a significant suppression of tumor growth in nude mice xenografted with A549 cells. Notably, a daily supplementation of 5.25 mg of peel oil per mouse over 7 days resulted in marked tumor growth inhibition. This effect was mediated through the downregulation of membrane-bound Ras protein, an increase in apoptotic cell death, and induction of cell cycle arrest at the G0/G1 phase.
3.3. Pectin Extraction From Hill Lemon
Pectin is a plant-based hydrocolloid that has unique structural and biochemical properties. It is widely used in various food products, pharmaceuticals, and other applications, such as edible films [50]. Hill lemon peel is a rich source of pectin, which is often discarded as waste (25%–35% of total fruit weight) by processing industries [40]. This causes environmental problems such as degradation and greenhouse gas emissions in landfills [50]. Therefore, it is important to recover pectin from Hill lemon peel and utilize it for various purposes. However, not many methods other than conventional methods have been exploited for pectin extraction from Hill lemon peel. Ethanol precipitation and aluminum salt precipitation are the two conventional methods explored. Ethanol precipitation produces higher quality and yield of pectin than aluminum salt precipitation. This might be due to the flocculation of pectin, which makes it easy to separate in ethanol precipitation [40]. The recovery of pectin from the conventional method ranges from 3.31% to 20.80% [16, 40]. In order to improve the quality and yield of pectin from Hill lemon peel, different novel techniques such as MAE, UAE, high-pressure-assisted extraction (HPAE), and enzyme-assisted extraction (EAE) should be explored [50]. These methods can enhance the efficiency of pectin extraction by reducing the time, temperature, and solvent consumption.
4. Biological Properties
4.1. Antioxidant Activity
To evaluate the total antioxidant activity and capacity of Hill lemon fruit peel, pomace, and seed, various antioxidant assays, such as DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging assay, FRAP (Ferric Reducing Antioxidant Power Assay), TEAC (Trolox Equivalent Antioxidant Capacity) assay, and ABTS (2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonate) assay, as well as metal chelating activity (MCA), have been used (Table 4). The results showed that the leaves had the highest DPPH radical scavenging activity, followed by the peel, while the seed had the lowest [44, 45]. The peel had a DPPH of 14.72 μM/mL, a FRAP of 7.82 mg/mL, an RPA of 13.88 mg/100 mL, an ABTS of 10.88 μM/mL, and an MCA of 5.67 mg/mL. The pomace had a DPPH of 16.34 μM/mL, a FRAP of 9.12 mg/mL, an RPA of 14.59 mg/100 mL, an ABTS of 11.89 μM/mL, and an MCA of 6.47 mg/mL [44]. The Hill lemon juice had an ABTS values of 2.99-5.25 mM/L and DPPH ranged from 5.11 to 19.01 μM/mL [45–52]. The seed had a DPPH scavenger activity of 42%, the peel had 50%, and the leaves had 49% [45]. The antioxidant activity also had a positive correlation with the percentage inhibition of linoleic acid peroxidation, ranging from 31% to 60%. The methanolic extract of Hill lemon leaves exhibited the highest peroxidation inhibition (60%), indicating superior antioxidant potential, while the lowest (31%) was noted in the case of Hill seed extract [45]. The effect of different extraction conditions, such as time, temperature, and solid solvent ratio, on the radical scavenging activity (%) of peel extract, leaf extract, and callus extract in in vitro conditions, was studied by [22]. The highest radical scavenging activity for peel (45.5%), leaf (40.2%), and callus extract (23.2%) was observed with an ethanol water ratio (E:W) of 50 at 50°C at 30 min, while the lowest for peel (20.8%), leaf (14.2%), and callus extract (10.5%) were observed with an E:W of 25 at 25°C at 10 min. The impact of extraction temperature emerged as a significant factor affecting the quantification of bioactive compounds. Elevated temperatures led to the softening of plant tissues, weakening the interactions of phenols with proteins and polysaccharides. Consequently, more polyphenols migrated into the solvent. However, prolonged extraction at high temperatures, such as 80°C, resulted in decreased yields due to oxidation, degradation of desired compounds, and solvent evaporation. Furthermore, according to [44], the phenolic content and antioxidant activity (DPPH) of Hill lemon peel and pomace increased with the increase in the microwave power from 250 to 500 W. The phenolic content increased by 14.11% and 17.53% and the antioxidant activity increased by 23.32% and 27.62% for peel and pomace, respectively. Similarly, the authors of [18] demonstrated the Hill lemon leaf antioxidant activity, as measured by the % DPPH inhibition assay, with an IC50 value of 18.43.
| Hill lemon plant part | DPPH | FRAP | RPA | MCA | ABTS | References |
|---|---|---|---|---|---|---|
| Leaves | 14.20%–77.70% | — | 06.48%–76.26% | — | — | [18, 22, 27, 45] |
| Peel | 20.0%–94.85% | 0.84–128.14 mg/mL | 0.84–47.73 mg/100 mL | 0.76–5.67 mg/mL | 27.7%–46.28% | [16, 22, 44, 45] |
| Juice | 4.46–9.44 mM/L | 0.95–2.03 mM/L | — | — | 2.99–5.25 mM/L | [1] |
| Callus | 10.5%–23.2% | — | — | — | — | [22] |
| Seeds | 10 mg·TE·g−1 DW | 50 mg·TE·g−1 DW | [53] |
- Note: DPPH—2,2-diphenyl-1-picrylhydrazyl radical scavenging assay, FRAP—Ferric reducing antioxidant power assay, ABTS—2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonate) assay.
- Abbreviations: DW, dry weight; MCA, metal chelating activity; RPA, reducing power assay; TE, trolox equivalent.
4.2. Antidiabetic Activity
Several studies have also investigated the antidiabetic activity of different parts of Hill lemon, such as the peel, seed, and leaves. The antidiabetic activity of Hill lemon leaves was investigated by Kumar et al. [27] using methanol extract (CPMLE) and ethyl acetate fraction (CPEALF). They performed different types of assays, including on key enzymes (D-glucosidase and D-amylase). They induced diabetes in rats by a single intraperitoneal injection of STZ (45 mg·kg−1) and monitored the fasting blood glucose level for 7 and 28 days. Only the animals with a level > 200 mg·dL−1 were included. They also evaluated the in vivo antidiabetic effect of CPMLE and CPEALF in STZ-induced diabetic rats. The results showed that IC50 (inhibitory concentration at 50.0%) values for α-amylase of CPMLE and CPEALF were 350.09 and 241.52, respectively, while the IC50 values of α-glucosidase for CPMLE and CPEALF were 291.17 and 166.46, respectively, whereas acarbose was taken as standard and its IC50 value was 125.88. They also demonstrated that the oral administration of CPMLE reduced blood glucose, oxidative stress, Hb1AC, and increased high-density lipoprotein (HDL) levels in STZ-induced diabetic rats. The blood glucose levels for CPMLE (200 mg·kg−1) were 304.08 on the seventh day and 245.66 on the 28th day, while at different concentrations of CPMLE (400 mg·kg−1), they were 306.92 on the seventh day and 206.50 on the 28th day. The blood glucose levels for CPEALF (100 mg·kg−1) were 303.62 on the seventh day and 216.33 on the 28th day. The standard drug gliclazide (10 mg·kg−1) showed blood glucose levels of 302.00 on the seventh day and 204.83 on the 28th day. The HDL levels for CPMLE (200 mg·kg−1) were 34.14 on the seventh day and 43.55 on the 28th day, while for CPMLE (400 mg·kg−1), they were 32.11 on the seventh day and 48.06 on the 28th day. The HDL levels for CPEALF (100 mg·kg−1) were 34.91 on the seventh day and 46.7 on the 28th day. The standard drug gliclazide (10 mg·kg−1) showed HDL levels of 33.12 on the seventh day and 46.27 on the 28th day. The oxidative stress enzyme activity of SOD and GSH enzymes was measured in STZ-induced diabetic rats treated with different doses of CPMLE and CPEALF. The standard drug was gliclazide at 10 mg·kg−1, and the SOD and GSH activities were 2.78 U·mg−1 and 88.16 μM, respectively. The results showed that SOD and GSH enzyme activities were higher in the CPMLE (SOD = 2.04 U·mg−1 and GSH = 71.92 μM at 200 mg·kg−1 and SOD = 2.86 U·mg−1 and GSH = 83.41 μM at 400 mg·kg−1) and the CPEALF (SOD = 2.84 U·mg−1 and GSH = 79.30 μM at 100 mg·kg−1) compared to the diabetic control rats (SOD = 1.07 U·mg−1 and GSH = 51.16 μM). The Hb1AC level was higher in the diabetic control rat compared to those administered CPMLE (200 mg·kg−1 and 400 mg·kg−1) and CPEALF (100 mg·kg−1). On the seventh day, the HbA1C level in the diabetic control group (NS, 2 mL) was 8.01, which was significantly higher than that in the normal control group (6.16). Treatment with CPMLE at 200 mg/kg reduced the HbA1C level to 7.26, and at 400 mg·kg−1, it was decreased to 6.92. CPEALF at 100 mg·kg−1 also decreased the HbA1C level to 7.47, while the standard drug treatment (10 mg·kg−1) brought it down to 6.89. On the 28th day, the HbA1C level in the diabetic control group (8.27) remained elevated compared to that of the normal control group (6.13). However, the CPMLE treatment at 200 mg·kg−1 decreased the HbA1C level to 6.81, and at 400 mg·kg−1, it was further decreased to 6.00. The CPEALF treatment at 100 mg·kg−1 also decreased the HbA1C level to 6.55, and the standard drug treatment brought it down to 6.14. These results show that while the HbA1C levels were elevated in the diabetic control group on both the 7th and 28th days, the CPMLE and CPEALF treatments effectively decreased the HbA1C levels compared to the diabetic control, and this reduction was more pronounced on the 28th day. In another study [44], the antidiabetic activity of bioactive compounds obtained by different extraction methods for peel and pomace was compared. The antidiabetic activity of the extracts was measured using α-glucosidase and α-amylase inhibition assays. Results showed that MAE extracts had the highest inhibition percentages (12.37%–27.95% for peel and 12.98%–28.79% for pomace). The antidiabetic activity increased by 18.44% and 17.00% for both peel and pomace extracts, respectively, as microwave increased from 250 to 500 W. Moreover, the essential oil of Hill lemon peel contained Myrcene compound (3.05%–27.09%), which had antidiabetic, antioxidant, antifungal, and anti-inflammatory properties [16]. In a study, Rufino et al. [54] studied the anti-inflammatory, anti-catabolic, and pro-anabolic effects of myrcene in a cell model of osteoarthritis. The results indicated that at non-cytotoxic concentrations, myrcene inhibited IL-1β-induced nitric oxide production (IC50 = 37.3 μg/mL). Myrcene, to a lesser extent, also decreased IL-1β-induced NF-κB, JNK and p38 activation and the expression of inflammatory (iNOS) and catabolic (MMP-1 and MMP-13) genes, while increasing the expression of anti-catabolic genes (TIMP-1 and -3 by myrcene).
4.3. Antimicrobial Activity
Hill lemon is a plant that has been traditionally used to treat viral infections and colds. It contains various bioactive compounds, such as polyphenols, flavonoids, antioxidants, terpenoids, alkaloids, and glycosides, that have antiviral, antifungal, and antibacterial properties. Viral and bacterial infections pose a significant global health threat, especially when bacterial respiratory tract infections develop as a consequence of viral infections. These include common colds, pneumonia, bronchitis, sinusitis, and tonsillitis [51]. Hill lemon peel essential oils exhibit antimicrobial activity against various bacteria, such as Pseudomonas aeruginosa, Stenotrophomonas maltophilia, Staphylococcus aureus, Haemophilus influenzae, Klebsiella pneumoniae, Streptococcus pneumoniae, Streptococcus agalactiae, and Streptococcus pyogenes [16, 17]. This is due to the presence of D-limonene and other bioactive compounds in the oils. The study conducted by Grover et al. [16] investigated the antibacterial activity of Hill lemon peel extracts and juice against two bacterial strains, namely, E. coli (Gram-negative) and Staphylococcus aureus (Gram-positive). The zone of inhibition of bacteria (E. coli and Staphylococcus aureus) measured by agar well diffusion assay ranged from 26 to 30 mm, which was somewhat similar to the positive control (Streptomycin), which was ranged from 28 to 31 mm. Therefore, Hill lemon can be considered as a potential source of natural antimicrobials for treating viral and bacterial infections. A similar study was conducted by [17] using the Kirby–Bauer well diffusion test method to evaluate the antibacterial activity of Hill lemon peel extracts and juice against two bacterial strains: E. coli (Gram-negative) and Staphylococcus aureus (Gram-positive). The results showed that juice exhibited the highest antibacterial activity against E. coli (24 mm), followed by Staphylococcus aureus (18 mm), while the peel extract showed no activity against either bacterial strain. The positive control showed different zones of inhibition against E. coli and Staphylococcus aureus, that is, 19 and 20 mm, respectively.
4.4. Anticancer and Anti-Inflammatory Activity
Cancer is one of the most frequently occurring diseases, affecting a large number of people worldwide. It can be generally described as an unstoppable growth and spread of abnormal cells in the body. Oxidative stress is the main factor involved in all the stages of cancer development, from initiation to progression [51]. One of the drawbacks of conventional chemotherapy is that it can harm healthy cells as well as cancer cells, resulting in poor disease management. Therefore, many people turn to natural products from plants as alternative treatments for various types of cancers. These products contain antioxidants that can protect the body from oxidative damage caused by free radicals [19, 51]. Polyphenols found in Hill lemon have shown anticancer effects on different cancerous tissues. Recently, Sajid et al. [19] reported that Hill lemon peel essential oil (CPEO) has remarkable anti-inflammatory and anticancer properties. CPEO was isolated through steam hydro-distillation and characterized by GC-MS. It contained a total of 22 chemical constituents (99.91%), mainly comprising 13 monoterpenes and 3 sesquiterpenes. The major constituents were d-limonene (58.01%), α-pinene (4.69%), β-myrcene (1.16%), limonene oxide (2.04%), and cis-carveol (2.11%). To evaluate the anticancer potential of CPEO, the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was performed against colon, leukemia, multiple myeloma, pancreatic, lung, and squamous carcinoma cells. Results revealed that CPEO inhibited cancer and inflammation through the downregulation of nuclear factor-kappa B (NF-kB) activation pathways and found that CPEO dose-dependently inhibits the cell proliferation up to 83%–98% for 100 μg/mL oil. Furthermore, it reduced the expression of Cyclin E1, ICAM-1, and XIAP, which are regulated by NF-kB, an inflammatory transcription factor. Moreover, flow cytometry analysis showed that CPEO decreased the DNA content in the S-phase of the cell cycle, suggesting that it arrests the cell cycle progression. These results demonstrate that CPEO exerts its anticancer activities by multiple mechanisms and routes. Therefore, CPEO might be a very effective herbal medicine against this deadly disease.
4.5. Insecticidal
The adverse effects of synthetic insecticides on the environment have raised significant concerns among the public. Hill lemon oil is a potential insecticide against dengue fever mosquitoes, especially Aedes albopictus. Akram et al. [55] suggested using it on the fourth instar larvae of these mosquitoes. The oil was extracted using a Soxhlet apparatus by steam distillation method. Then, 1 mL of oil was added to 100 mL of acetone to make a 1% stock solution. The fourth instar larvae were exposed to a mixture of 1 mL (1% stock) and 199 mL of distilled water. The observation was done after 24 and 48 h. The results showed that the LC50, that is, lethal concentration (ppm) to kill 50% population of the subjected organism, was 644.25 after 24 h and 177.44 after 48 h.
Furthermore, in addition to its insecticidal properties, some tribal areas of lower Uttarakhand use Hill lemon juice to cure body allergy while avoiding milk during this treatment [24].
5. Processing and Preservation of Hill Lemon Fruit
Processing of food not only adds value but also develops market opportunities and addresses issues related to shelf life, that is, seasonality and perishability. Hill lemon fruit is utilized in the development of value-added products such as beverages and juice powder, effectively extending its shelf life (Table 5). Hill lemon juice is widely used in various preparations, but it has limited shelf life due to its tendency to turn bitter. Different methods of juice extraction have been studied, such as screw-type extractor, hydraulic press, rosing machine, and pulper. The screw-type extractor was found to have the highest yield of 44.28%, followed by the rosing machine (41.79%) and pulper (40.00%) [15]. To preserve the juice quality, it can be subjected to heat pasteurization at 90°C for 10 s followed by rapid cooling to room temperature [15, 30]. Another approach involved boiling the juice until foam or leather formation and then adding an organic preservative such as salt with mustard, that is, 20 g of salt in 1 L of Hill lemon juice with 20 mL of mustard oil. This innovative value addition has resulted in the development of value-added products, such as beverages and juice powder, effectively extending the shelf life of Hill lemon [61]. Furthermore, inorganic preservatives, such as potassium metabisulfite at 0.7 gm/L of juice and sodium benzoate at 0.5 gm/L of juice, have been used, along with the addition of 500 ppm of sulfur dioxide [15, 61]. These preservatives increased the shelf life to 6 and 5 months, respectively. However, it appears that organic preservation methods may be a preferable alternative.
| Product | Best composition | TSS (°Brix) | Acidity (%) | Method of preservation | Shelf life | Reference |
|---|---|---|---|---|---|---|
| RTS | Hill lemon juice (5%) + basil extract (10%) | 14 | — | Heat pasteurization | 6 months | [21] |
| RTS | Hill lemon juice + pumpkin pulp (15%) | 13 | 0.3 | Heat pasteurization | 6 months | [56] |
| Squash | Hill lemon juice + pumpkin pulp (30%) + guava pulp (10%) | 45 | 1.2 | Chemical preservation | 6 months | [57] |
| Squash |
|
Chemical preservative | 6 months | [58] | ||
| Wine | Hill lemon juice | 11 | 1.47 | Chemical preservation | — | [59] |
| Juice powder | Hill lemon juice + stabilizer | 45 | 57.75 | Chemical preservative | — | [30] |
| Juice powder-RTS | Sugar (130 g) + lemon essence (4.9 mL) + cloud (2.6 mL) + coal tar dye (green l.5 mg) at a fixed level of 5-g Hill lemon juice powder | 12.5 | 0.3 | Heat pasteurization | — | [30] |
| Juice powder appetizer | Powdered spices (13.75 g ginger, 1.67 g each of mint and cumin, 3.25 g common salt, 5 g each of black salt, cardamom and black pepper) + 125 g sugar + 4.9 mL essence + 2.6 mL of cloud + 8.75 g of Hill lemon juice powder | 12.5 | 0.3 | Heat pasteurization | — | [30] |
| Juice powder—carbonated drink | Hill lemon juice (5%) + lemon residue extract (0.5%) | 12.5 | — | Heat pasteurization | — | [30] |
| Osmo-dried peel sticks | Hill lemon peel with 50% sugar | 50 | 0.52 | Sugar + blanching | 3 months | [60] |
- Abbreviations: RTS, ready to serve; TSSs, total soluble solids.
A series of studies involving the preparation of blended beverages utilized Hill lemon juice. Twelve functional drinks were prepared by using Hill lemon juice (5%–10%) and basil extract (5%–15%). The best combination contained 5% Hill lemon juice and 10% basil extract with 14°Brix TSSs without any exogenous addition. These drinks remained stable for 6 months [21]. In another study, pumpkin-based RTS beverages and squash were prepared. For the RTS beverages, 12 variations were developed, among which the recipe containing 15% pumpkin pulp, 13°Brix TSS, and 0.3% acidity received the highest sensory scores. The recipe’s acidity was maintained using citric acid, lime juice, and Hill lemon juice [56]. A blended squash of pumpkin and Hill lemon was prepared in which the pumpkin concentration was taken as 25%, 30%, 35%, and 40% and guava had 10% acidity with the help of citric acid, lime juice, and Hill lemon juice in which the highest amount of ascorbic acid and hedonic scores was observed in 30% pumpkin pulp + 10% guava pulp with Hill lemon-based squash. This squash can be stored in ambient conditions for 6 months [57]. Additionally, another study on blended squash, including 10 combinations with Rhododendron petal juice and Hill lemon, was prepared in which Rhododendron 15% + Hill lemon 5%, Rhododendron 10% + Ghengharu 5% + Hill lemon juice 5% + ginger 59%, and Rhododendron 15% + Ghengharu 5% + Hill lemon juice 5% outperformed other combinations in terms of overall quality. These products exhibited a shelf life of 6 months at ambient conditions. Remarkably, the cost of production for these products ranged from 38.33% to 50.00%, less than similar market-available products like mango squash. Also, it was rich in ascorbic acid with antioxidant activity and can be stored up to 6 months in ambient conditions, and in refrigerated conditions, it can be stored for up to 1 year [58]. In a separate study, wine was prepared from Hill lemon juice with a TSS of 11°Brix, while Hill lemon juice was diluted 10-fold due to its higher acidity. Various Hill lemon juices received SO2 at 100 ppm, 0.5% pectinase, 0.1% diammonium hydrogen orthophosphate, and additional sugar to reach a TSS of 24°Brix; 5% active yeast culture was introduced, initiating fermentation. After completion, wines were siphoned for clarity, undergoing this process 2–3 times. Sugar was added for palatability, and wines were pasteurized and bottle-matured for 2 years, resulting in a sweet Hill lemon wine [59].
To further improve storage, the transformation of Hill lemon juice into powder form has been considered followed by heat pasteurization (90°C for 10 s), along with immediate cooling and preservation in 500 ppm SO2. The pasteurized juice after enzymatic clarification using 0.2% pectinase CCM for 2 h at 50 ± 2°C was then concentrated to different folds (°brix) in a rotary-type vacuum evaporator; then, the lemon juice powder was prepared by mixing juice concentrate (45°brix) with 2% carboxymethyl cellulose (CMC), wiping at 500–700 rpm for 1–2 min in a household mixer to prepare foam, followed by drying in a mechanical dehydrator (55 ± 2°C) and finishing in vacuum oven. The dried foam was then turned into a powder in a grinder along with the addition of an anticaking agent (2% dicalcium phosphate). This powder contained 57.75% acidity (citric acid), 185.88 mg/100 mL ascorbic acid, and other essential nutrients [23, 30]. For practical utilization of the juice powder, various recipes were developed, like beverages including RTS beverage, appetizer, carbonated beverage, apple jam, apple jelly, and Brahmi syrup. The recipe of RTS included sugar (130 g), lemon essence (4.9 mL), cloud (2.6 mL), and coal tar dye (green 1.5 mg), with a fixed amount of 5 g of lemon juice powder. Similarly, an appetizer powder was created by adding the appropriate number of powdered spices (13.75 g ginger, 1.67 g each of mint and cumin, 3.25 g common salt, 5 g each of black salt, cardamom, and black pepper), 125 g sugar, 4.9 mL essence, and 2.6 mL of cloud to 875 g of lemon juice powder. Furthermore, a Hill lemon beverage with a 5% lemon juice residue extract and 12.58 TSS was successfully carbonated at 5.28 kg/cm2 of CO2 gas pressure [23, 30]. Another study prepared ready-to-eat appetizers in two variants: ginger munch (ginger powder, raisins, and sweetener) and fruit munch (ginger paste, lemon juice, and Hill lemon juice). The final product, fruit munch with ginger paste, lemon juice, and Hill lemon juice, was optimized, and it was observed that the sensory points increased as the concentration of Hill lemon and ginger increased [62]. The juice of Hill lemon has various applications, but the peel of this fruit is often wasted. However, an innovative research endeavor converted the Hill lemon peel into an edible candy using osmotic drying techniques. The sticks were made by dipping them in different concentrations of sugar and jaggery solution (30, 40, and 50°Brix). The osmo-dried Hill lemon peel sticks were stored for 3 months, and the chemical and organoleptic changes were recorded every month. The maximum values of TSS and ascorbic acid were 54.02°Brix and 120.05 mg/100 g, respectively. However, the TSS increased, while the titratable acidity, moisture, ascorbic acid, and carotenoids decreased during the storage period. The treatment with 50°Brix sugar had the highest overall acceptability (8.19) after 3 months [60].
6. Nanoparticles From Hill Lemon
Nanoparticles can be prepared by physical and chemical methods, which allow precise control over their shapes and sizes, but these methods are costly and involve harmful chemicals [63]. Therefore, biological methods have gained attention in the last 2 decades for synthesizing metal and metal oxide nanoparticles, such as gold and silver, with antibacterial properties. Biological methods are simpler, cheaper, and eco-friendly, but they have some drawbacks in terms of size and shape diversity. For instance, they usually produce spherical nanoparticles, while anisotropic nanoparticles show much more efficiency than the spherical particles [2]. However, other problems are multidrug-resistant (MDR) pathogens, which pose a major crisis in the 21st century; some studies have explored the use of biological nanoparticles as an alternative to conventional antibiotics [64]. These nanoparticles have the advantage of having no side effects and can be derived from different biological sources. One example is endophytic fungi from Hill lemon leaves, which have been used to produce silver nanoparticles with antibacterial activity [2]. Hill lemon is an underutilized crop that can provide various parts of the plant, such as the peel and leaves of endophytic fungi for nanoparticle synthesis. Endophytic fungi are microorganisms that live inside plant tissues without harming them and form a symbiotic relationship with the host plant. Examples of endophytic fungi are Aspergillus versicolor, Cladosporium sp. Fusarium solani, Amylomyces roudi Aspergillus sp., Penicillium citrinum, Colletotrichum sp., Altemaria sp., and Cryptosporiopsis ericae [2]. These fungi have the potential to produce nanoparticles with different shapes and biomedical applications. They also secrete some unique and medicinally important compounds that could enhance the functionality of the nanoparticles [65, 66]. The preparation, properties and applications of Hill lemon nanoparticles are discussed below.
Aman et al. [64] used Hill lemon peel for the preparation of specialized silver nanoparticles (G-Ag NPs) that displayed specificity against various MDR bacterial strains. The spherical-shaped G-Ag NPs, approximately 40 nm in size with a surface charge of-31 mV, proved effective against MDR, serving as an eco-friendly tool. The study also demonstrated the biocompatibility of G-Ag NPs with human cells. The antibacterial activity of G-Ag NPs was evaluated against MDR strains using colony-forming units (CFU) count method. Compared to untreated cells, G-Ag NPs exhibited bactericidal activity against all tested MDR strains after 6 h of incubation. Mechanistically, the bactericidal property of G-Ag NPs might be attributed to their ability to generate reactive oxygen species and induce bacterial membrane disruption. Biocompatibility tests showed G-Ag NPs’ compatibility with human red blood cells (RBCs) and peripheral blood mononuclear cells (PBMCs). G-Ag NPs exhibited 4.8% hemolysis (acceptable for biomaterials) compared to chemically synthesized Ag nanoparticles (98% hemolysis) and showed no cytotoxic effects on PBMC.
Another study conducted by Kumar et al. [2] utilized Hill lemon leaf samples to isolate eight different types of endophytic fungi. Among them, Colletotrichum plurivorum showed optimum antimicrobial activity and interestingly induced the formation of cuboid-shaped Ag2O nanoparticles, which were further applied against pathogenic bacteria. Field emission scanning electron microscope (FESEM) and high-resolution transmission electron microscopy (HRTEM) images showed that silver nanocuboids with fungal extract had a size range of 200–250 nm in length and 80–150 nm in width, with a fringe spacing of 0.34 nm. The antibacterial property of Ag2O nanoparticles was tested against the pathogenic Gram-negative bacteria E. coli (MTCC 443) and Gram-positive bacteria B. subtilis (MTCC 441) by using the well-diffusion method. The inhibition zone size for silver nanocuboids with a fungal extract was 9 mm for E. coli and 11 mm for B. subtilis.
7. Conclusion and Future Scope
Hill lemon is a valuable yet underutilized subtropical fruit, abundant in sugars, organic acids, phenolics, flavonoids, and various bioactive compounds. Its leaves are particularly rich in bioactives like limonene and citronellol, exhibiting the highest antioxidant capacity among all plant parts. The peel, which includes both the flavedo and albedo layers, is packed with nutrients such as pectin, vitamin C, and protein, along with bioactives like limonene. The seeds also offer significant compounds, including limonene and nomilin. Collectively, these bioactive components contribute to health benefits, such as protection against skin disorders, diabetes, cancer, and inflammation. A range of in vitro and in vivo studies have confirmed Hill lemon’s antioxidant, antibacterial, antiviral, anti-inflammatory, antimetastatic, and anticancer activities. Nonetheless, certain bioactives remain unexplored, underscoring the need for further research and clinical studies to unlock their potential for various scientific applications.
A lack of awareness about Hill lemon’s benefits results in lower market prices for farmers and considerable postharvest losses due to inefficient handling. Hill lemon juice, in particular, poses challenges in terms of bitterness and shelf life; debittering techniques, whether chemical or physical, could help overcome this. Additionally, byproducts like seeds, peels, and leaves offer valuable resources for pectin extraction, essential oil production, and development into marketable products, such as osmotic-dried peel. These uses could significantly boost the market value of Hill lemon and promote effective waste management. Continued research is essential to advance processing methods that would fully realize the potential of Hill lemon.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received to conduct this study.
Open Research
Data Availability Statement
Data are available on request to the corresponding author.




