Anti-Inflammatory Effects of Immature Pomelo Fruit Polysaccharides on Experimental Ulcerative Colitis Via Modulation of Gut Microbiota and the ERK1/2 Signaling Pathway
Abstract
The immature pomelo fruit (IPF) of Citrus maxima (Burm.) Merr. is an agricultural by-product rich in bioactive compounds, particularly the lipophilic flavonoid naringin. However, research on water-soluble polysaccharides, another critical component of IPF, including their fundamental structural characteristics and biological activities, remains limited. In this study, a crude polysaccharide named immature pomelo fruit polysaccharides (IPFP), with a polysaccharide content of 81.10%, was extracted from the IPF using the water extraction-alcohol precipitation method. High-performance gel permeation chromatography with refractive index detection (HPGPC-RID) revealed that IPFP consists of two components: IPFP-1 with a molecular weight of 9187 Da and IPFP-2 with a molecular weight exceeding 100 kDa. Analysis via 1-phenyl-3-methyl-5-pyrazolone-high performance liquid chromatography (PMP-HPLC) indicated that IPFP primarily comprises galactose, glucose, and xylose, with glucose being the predominant monosaccharide. To investigate the biological activity of IPFP, a mouse model of acute ulcerative colitis was established using 2.5% dextran sulfate sodium (DSS). Fifty BALB/c mice were randomly assigned to five groups: a control group, a DSS-induced model group, and three IPFP treatment groups with low, medium, and high doses. Molecular biological techniques, including quantitative polymerase chain reaction (qPCR), Western blotting, and 16S ribosomal RNA (16S rRNA) sequencing, were employed to evaluate the expression of inflammatory genes in colon tissues, activation of the extracellular signal-regulated kinase (ERK) signaling pathway, and composition of fecal microbiota. The animal experiments demonstrated that IPFP significantly alleviated DSS-induced symptoms, such as body weight loss and colon shortening. Additionally, it mitigated colonic histopathological damage, suppressed the expression of proinflammatory cytokines tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6), while promoting the expression of the anti-inflammatory cytokine interleukin-10 (IL-10). Notably, the high-dose IPFP group exhibited the most pronounced effects. Following IPFP treatment, there was a significant reduction in the phosphorylation levels of ERK1/2 protein and restoration of intestinal microbiota diversity and abundance. These findings suggest that IPFP may alleviate DSS-induced inflammatory damage by modulating gut microbiota, particularly bacteroidetes, and inhibiting the ERK1/2 pathway. Our study provides initial insights into the structure and pharmacological mechanisms of IPFP against ulcerative colitis, highlighting its potential as an anti-inflammatory agent for promoting intestinal health in inflammatory diseases.
1. Introduction
The Citrus maxima (Burm) Merr., a fruit belonging to the Rutaceae family, produces a considerable number of immature pomelo fruits during its growth cycle. This phenomenon is attributed to both plant physiological factors and artificial fruit thinning practices aimed at optimizing fruit yield and quality. According to statistical data, immature pomelo fruits constitute approximately 40%–50% of the total output [1]. In China, these immature pomelo fruits are utilized in traditional Chinese medicine for their health benefits, including regulating “Qi,” reducing phlegm, and relieving pain. However, their utilization rate remains low at only 5% [2]. Recent studies have focused on enhancing the economic value of immature pomelo fruits by investigating their chemical composition and pharmacological properties. Scientific evidence has shown that immature pomelo fruits contain various bioactive compounds, primarily naringin, which exhibit potent antioxidant and anti-inflammatory activities [3, 4]. Liu et al. employed an innovative continuous phase transformation technology to sequentially extract volatile oil, naringenin, and pectin from immature pomelo fruits [5]. Subsequent research has confirmed the significant immunomodulatory effects of the novel pectin obtained through this method [6]. Despite these advancements, limited research has been conducted on polysaccharide constituents other than pectin in immature pomelo fruits.
Ulcerative colitis (UC) is a chronic immune-mediated disease characterized by persistent and varying degrees of mucosal inflammation extending from the rectum to the proximal colon [7]. This condition primarily affects the mucosal layer, leading to superficial damage to the intestinal wall. Common symptoms include mucus-tinged bloody diarrhea, rectal urgency, tenesmus, and varying degrees of abdominal pain, all of which significantly impair patients’ quality of life [8]. Current pharmacological treatments for UC, such as anti-inflammatory agents, immunosuppressive drugs, antibiotics, and biologics, are associated with certain adverse effects [9]. In contrast, Chinese medicines, which possess both medicinal and dietary properties, tend to be safer and are favored by pharmaceutical researchers as a valuable source of natural compounds for developing novel therapeutics targeting various diseases [10, 11]. Many polysaccharides, including those derived from Chinese yam, Lycium barbarum, bergamot, and Ganoderma lucidum, have demonstrated anti-UC effects [12–15]. However, the pharmacological activity of polysaccharides from immature pomelo fruit remains unclear due to a lack of relevant studies.
The pathogenesis of UC is multifactorial, encompassing genetic predisposition, dietary factors, immune dysregulation, and alterations in the intestinal microbiota. Compared to healthy individuals, patients with UC exhibit reduced diversity in their intestinal microbiota, characterized by decreased levels of firmicutes and actinobacteria, as well as significantly increased levels of bacteroidetes and proteobacteria [16]. Modulating the balance of the intestinal microbiota has emerged as a promising therapeutic strategy for managing UC [17]. Recent studies have shown that various polysaccharide components derived from traditional Chinese medicines or natural plants can effectively modulate the composition of the gut microbiota, thereby alleviating inflammation and improving disease symptoms [18, 19]. Given the close relationship between intestinal microbiota and polysaccharides [20, 21], as well as the critical role of microorganisms in maintaining intestinal health, this study aims to prepare and characterize immature pomelo fruit polysaccharide (IPFP). Additionally, we investigate the pharmacological effects of IPFP on UC and explore its therapeutic mechanisms involving the gut microbiota in a DSS-induced mouse model, thereby addressing the research gap concerning the potential benefits of IPFP.
2. Materials and Methods
2.1. Materials
Citrus maxima (Burm.) Merr. cv. Shatian Yu’s immature pomelo fruits with a diameter of approximately 2.0 cm were harvested from Meizhou City, Guangdong Province, China (Figure 1). Antibodies against phospho-Erk1/2 (GB113492), Erk1/2 (GB11004), and GAPDH (GB15004) were obtained from Wuhan Sevier Biotechnology Co., Ltd. Dextran standards with varying molecular weights were sourced from Guangzhou Lantian Biotechnology Co., Ltd. Glucose (B33747), galactose (B21893), and xylose (D820550, Macklin) were supplied by Shanghai Yuanye Biotechnology Co., Ltd. A protein quantification detection kit (WK342256) was purchased from Thermo Fisher Scientific Co., Ltd. PCR-related reagents and consumables were acquired from Guangzhou Ruizhen Biotechnology Co., Ltd. DSS (Mw: 36–50 kDa) was obtained from MP Biomedicals, USA. Phenol, concentrated sulfuric acid, chloroform, n-butanol, 1-phenyl-3-methyl-5-pyrazolinone (PMP), KH2PO4, K2HPO4, and other reagents were of analytical grade.

2.2. Polysaccharide Sample Preparation
The immature pomelo fruit is sliced and dried (Figure 1) and then extracted three times using a heating reflux apparatus with a solid-to-liquid ratio of 1:20. The combined filtrate is filtered and concentrated under reduced pressure to a volume equivalent to 2.5 times the mass of the original immature pomelo fruit. Anhydrous ethanol is added sequentially to adjust the ethanol concentration to 40%, 60%, and 80% for fractional precipitation of polysaccharides. The precipitates obtained at 80% ethanol concentration are collected, deproteinized using the Sevag method [22], and subsequently dialyzed. After freeze-drying, crude IPFP is obtained for further experimentation.
2.3. Chemical Composition Analysis
The polysaccharide content of IPFP was quantified using the phenol-sulfuric acid method, as described by Chen and Huang [23], while the protein content was determined using the Thermo Scientific Pierce BCA Protein Assay Kit. Detailed protocols for both methods can be found in their respective manuals.
2.4. Molecular Weight Determination
The method for determining the molecular weight of polysaccharides was adapted from Tian et al. [24] with necessary modifications. The procedure is summarized as follows: High-performance liquid chromatography (HPLC) equipped with a refractive index detector (RID-20, Shimadzu, Japan) was employed to analyze IPFP and standard solutions, both prepared at a concentration of 1 mg/mL. The HPLC system utilized was a Nexera XR (Shimadzu, Japan). Chromatographic conditions were as follows: an UltrahydrogelTM 1000 gel column (7.8 mm × 300 mm, 5 μm, NanoCrom, Technology Co., Ltd., China) was used at a column temperature of 30°C and an RID temperature of 40°C. Ultra-pure water served as the mobile phase at a flow rate of 0.5 mL/min. Sample injection volume was set to 20 μL, and the total run time was 40 min.
2.5. Monosaccharide Composition Analysis
The PMP precolumn derivatization method coupled with HPLC was employed to analyze the hydrolyzed sample. Sample preparation procedures were adapted from the existing literature with specific modifications as follows [25]: 5 mg of IPFP and 3 mL of a 2 M trifluoroacetic acid (TFA) solution were sequentially added to the reaction vessel and incubated in an oil bath at 120°C for 3 h. Postreaction, the solution was subjected to spin-drying, with methanol being repeatedly added to ensure complete removal of TFA during each cycle. Subsequently, 1 mL of a 0.5 M PMP methanol solution and 1 mL of sodium hydroxide solution were introduced, followed by incubation in a water bath at 70°C for 2 h. The reaction mixture was then neutralized with hydrochloric acid, and impurities were removed via chloroform extraction. The aqueous phase was filtered through a membrane filter prior to HPLC analysis using a Nexera XR system (Shimadzu, Japan). Chromatographic conditions were set as follows: Eclipse Plus C18 column (4.6 mm × 250 mm, 5 μm, Agilent), mobile phase consisting of 0.1 M phosphate buffer-acetonitrile (80:20, v/v), detection wavelength of 245 nm, column temperature of 30°C, flow rate of 1 mL/min, injection volume of 20 μL, and elution time of 40 min.
2.6. Analysis of IPFP in the Treatment of DSS-Induced UC
2.6.1. Animal Experimental Design
Fifty specific pathogen-free BALB/c mice (18–22 g) were provided by Guangzhou Zitangjin Biotechnology Co., Ltd. and approved by the Ethics Committee of Jiaying College. The animals were housed in standard animal facilities under a 12 h light/dark cycle. Based on body weight, the mice were randomly divided into five groups: normal group (NG), model group (MG), low-dose group (LDG), medium-dose group (MDG), and high-dose group (HDG). Mice in the MG were administered a 2.5% DSS solution for 7 days. In the treatment groups, mice received varying doses of polysaccharide solution (200, 400, and 800 mg/kg) daily for 7 days, while also consuming water containing 2.5% DSS. The NG did not receive any pharmacological intervention. The experimental design is illustrated in Figure 2(a). At the end of the feeding period, mice were anesthetized and euthanized. Stool samples were collected from the cecal tissue postremoval and immediately cryopreserved in liquid nitrogen before being transferred to a −80°C refrigerator for storage. Intestinal contents were rinsed with precooled normal saline, followed by measurement of colon length. Spleen weight was recorded, and the spleen index was calculated for each mouse. From the distal colon, one 0.5 cm segment and two 1 cm segments were excised for histopathological examination and protein/gene expression analysis.

2.6.2. Histopathological Changes of Colon
The colon tissues were fixed in 4% paraformaldehyde for 24 h, dehydrated through a graded ethanol series, embedded in paraffin, and sectioned into 5 μm thick slices. The sections were subsequently subjected to standard Hematoxylin and Eosin (H&E) staining protocols [26], followed by microscopic imaging and histopathological evaluation.
2.6.3. Gene Expression of Inflammatory Cytokines
RNA was extracted from colon tissue using Trizol reagent and subsequently purified with lithium chloride to eliminate any residual DSS, which has been shown to interfere with experimental outcomes [27]. The RNA concentration and purity were assessed using a NanoDrop One ultramicrospectrophotometer (Thermo Scientific, USA). Complementary DNA (cDNA) synthesis was performed using the Accurate Biology reverse transcription kit (AG11718-S), and qPCR was conducted according to the instructions provided by the PerfectStart Visual Green qPCR SuperMix (R20314, TransGen Biotech, Beijing, China). Primers for TNF-α, IL-1β, and GAPDH were designed, synthesized, and purified by Sangon Bio-Engineering (Shanghai, China). The primer sequences are as follows: TNF-α forward primer (TGACTCCAAAGTAGACCTGC) and reverse primer (CTACTCCCAGGTTCTCTTCA); IL-1β forward primer (TGCCACCTTTTGACAGTGATC) and reverse primer (AAGGTCCACGGGAAAGACAC); IL-6 forward primer (TTCTTGGGACTGATGCTGGTGAC) and reverse primer (GTGGTATCCTCTGTGAAGTCTCCTC); and GAPDH forward primer (GCAAATTCAACGGCACAG) and reverse primer (TCGCTCCTGGAAGATGGTGATG). mRNA expression levels of TNF-α, IL-6, and IL-1β were normalized and subjected to statistical analysis.
2.6.4. Expression of Proteins in the ERK Pathway
We employed the Western blot technique, as described in the literature with minor modifications, to quantify protein levels in mouse colon tissues [28]. The detailed procedural steps are outlined below. Tissue samples were homogenized using an automated homogenizer, and the resulting supernatant was collected. After measuring protein concentrations, an appropriate volume of loading buffer was added, followed by denaturation through boiling. The resultant mixture served as the test sample. Equal amounts (30 μg) of denatured proteins were separated by electrophoresis on a 10% SDS-PAGE gel and then transferred onto a 0.45 μm pore size PVDF membrane. The membrane was blocked with 5% skim milk powder for 1 h at room temperature, followed by incubation overnight at 4°C with primary antibodies specific to p-Erk1/2 and Erk1/2. Subsequently, HRP-conjugated rabbit secondary antibodies were applied and incubated for 1 h at room temperature. Protein bands were visualized using an enhanced chemiluminescence kit in conjunction with an automated imaging system. The intensity of each band was quantified using ImageJ software, normalized to GAPDH, and expressed as relative gray values for statistical analysis.
2.6.5. Fecal Bacterial Composition Analysis
Genomic DNA was extracted from fecal samples using the cetyltrimethyl ammonium bromide (CTAB) method, and its purity and concentration were evaluated by 1% agarose gel electrophoresis (111,860, Biowest, Spain). The samples were then diluted to a final concentration of 1 ng/μL and amplified via PCR targeting the V3 + V4 hypervariable regions [29, 30]. For efficient amplification, Phusion High-Fidelity PCR Master Mix (M0530S, New England Biolabs, USA) was utilized. Postamplification, the product concentration was quantified, and equimolar amounts of samples were pooled and purified. Library preparation was conducted using the NEBNext Ultra DNA Library Prep Kit for Illumina (E7370L, New England Biolabs, USA). Amplicon size and quantity were assessed using an Agilent 5400 Bioanalyzer (Agilent Technologies Co Ltd., USA). Qualified libraries were sequenced on the NovaSeq 6000 platform (Illumina, San Diego, CA, USA). The resulting sequencing data were analyzed using bioinformatics tools integrated into the Wekemo Bioincloud platform (https://www.bioincloud.tech/).
2.6.6. Statistical Analysis
The statistical analysis of the data was conducted using IBM SPSS Statistics 20.0 software. Data are presented as mean ± standard deviation (SD). For normally distributed data with homogeneity of variance, one-way analysis of variance (ANOVA) was performed, followed by the Bonferroni test for multiple comparisons. In cases where the assumption of homogeneity of variance was violated, Dunnett’s T3 test was used for statistical analysis. All tests were two-tailed, and a significance level of p < 0.05 was set to indicate statistical significance.
3. Results
3.1. Content of Polysaccharide and Protein in IPFP
The phenol-sulfuric acid method revealed that the sugar content of IPFP was 81.10%. According to the analysis performed using the protein quantitative kit, the protein content in IPFP was found to be 0.05%, which is considered negligible.
3.2. Molecular Weight of IPFP
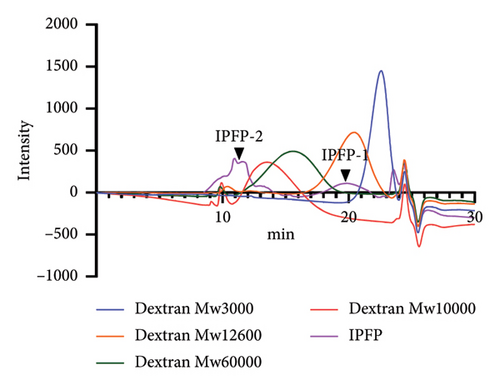
The analysis using high-performance gel permeation chromatography equipped with a refractive index detector revealed that IPFP can be characterized by two distinct chromatographic peaks, designated as IPFP-1 and IPFP-2. The results are illustrated in Figure 3. By comparing the retention time to that of the dextran standard, IPFP-1 was determined to have a molecular weight of 9187 Da. In contrast, IPFP-2 displays a complex chromatographic profile comprising at least three distinct polysaccharide fractions, each with a molecular weight exceeding 100 kDa.
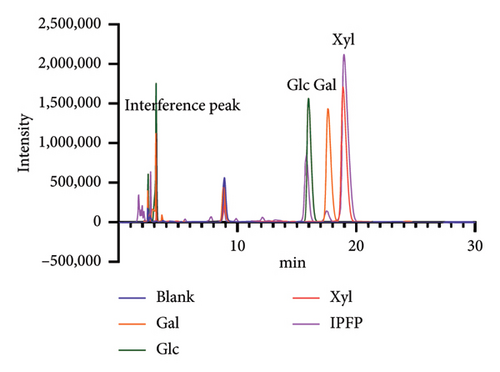
3.3. Monosaccharide Composition
The acetylated derivatives of standard monosaccharides were analyzed via precolumn derivatization coupled with liquid chromatography. By comparing the relative retention times, the monosaccharide composition of IPFP was identified as consisting of glucose, galactose, and xylose (Figure 4).
3.4. Oral IPFP Administration Attenuated Colitis in DSS-Treated Mice
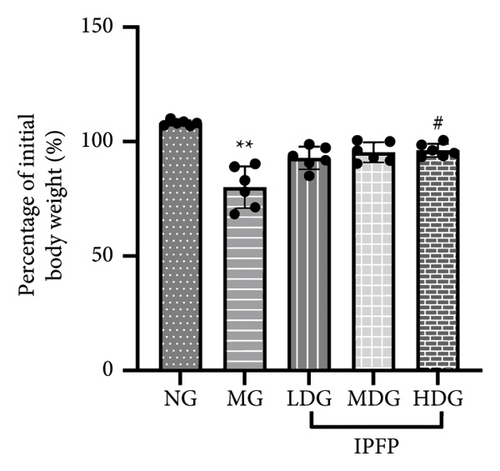
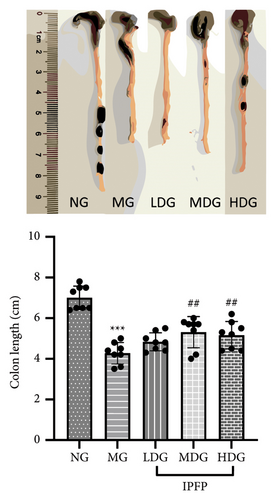
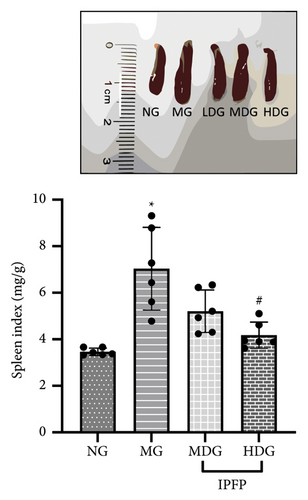
We evaluated the impact of IPFP on UC in mice from three perspectives: changes in body weight, colon length, and spleen index. We conducted a statistical analysis of the percentage change in body weight for each mouse group on day 7 relative to their initial body weight (Figure 2(b)). Mice treated with DSS exhibited significant weight loss ( ∗∗p < 0.01 vs. NG), while high doses of IPFP partially mitigated this weight loss (#p < 0.05 vs. MG). Additionally, mice in the LDG and MDG showed slight weight gain, although these differences were not statistically significant. DSS-induced colon shortening was significantly alleviated by medium and high doses of IPFP ( ∗∗∗p < 0.001 vs. MG), as shown in Figure 2(c). Following DSS treatment, mice experienced pathological hypertrophy and increased spleen weight ( ∗p < 0.05 vs. NG); however, oral administration of IPFP slightly attenuated these pathological features (#p < 0.05 vs. MG). These findings suggest that IPFP can protect the intestinal tract against DSS-induced damage in mice, with higher doses demonstrating superior protective effects.
3.5. IPFP Ameliorated Histological Damages in Colon
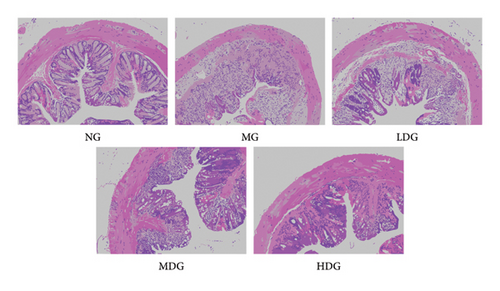
We conducted H&E staining on mouse colon tissue to further evaluate the therapeutic effects of IPFP. As shown in Figure 5, the colon epithelial cells of healthy, untreated mice exhibited a well-organized and intact structure. The crypt morphology was normal with minimal damage, and no inflammatory lesions were observed in the mucosa. In contrast, the MG displayed significant disruption in the crypt structure along with extensive infiltration of inflammatory cells. Conversely, the treatment group receiving IPFP showed limited inflammatory cell infiltration, preserved crypt architecture, reduced inflammation severity, and more pronounced improvement as the concentration of IPFP increased. Collectively, these findings indicate that IPFP can effectively mitigate DSS-induced pathological injury in the colon.
3.6. IPFP Suppresses the Expression of Inflammation-Related Genes
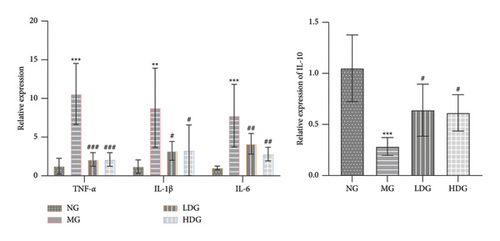
We performed PCR analysis to evaluate the expression of inflammation-associated genes in order to further substantiate the therapeutic efficacy of IPFP. As illustrated in Figure 6, the expression levels of TNF-α, IL-1β, and IL-6 in the colon tissue of DSS-induced mice were significantly elevated (p < 0.001 or p < 0.0001 vs. NG). After intervention with IPFP, there was a significant inhibition of the up-regulation of these inflammatory genes (#p < 0.05 or ###p < 0.001 vs. MG). These results indicate that IPFP has the potential to mitigate DSS-induced inflammatory damage. Additionally, for the anti-inflammatory gene IL-10, its expression level was markedly reduced in the MG ( ∗∗∗p < 0.001 vs. NG) but significantly increased in both the low-dose and high-dose IPFP groups (#p < 0.05 vs. MG). Collectively, these findings suggest that IPFP may alleviate DSS-induced colonic inflammation by suppressing proinflammatory genes and enhancing anti-inflammatory gene expression.
3.7. IPFP Inhibits DSS-Induced Inflammation Through the ERK Pathway
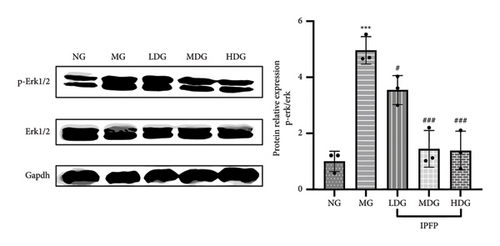
The ERK signaling pathway has been shown to play a pivotal role in the progression of UC, primarily by regulating inflammatory responses and modulating intestinal microbiota [31]. To investigate the anti-inflammatory mechanism of IPFP, we examined the expression of phosphorylated ERK protein in colon tissues. As illustrated in Figure 7, there was a significant increase in p-ERK1/2 expression in mice exposed to DSS ( ∗∗∗p < 0.001 vs. NG), indicating that DSS activates the ERK signaling pathway. However, this upregulation was effectively attenuated by IPFP treatment, particularly in the MDG and HDG. These findings elucidate the mechanism by which IPFP inhibits the ERK pathway and mitigates tissue inflammation in mice.
3.8. The Beneficial Effect of IPFP on Regulating Intestinal Flora
The role of intestinal flora in maintaining intestinal homeostasis has been well established, and dysbiosis of the intestinal microbiota is recognized as a critical factor in the pathogenesis of UC [32]. In this study, we examined whether the therapeutic effects of IPFP administration were associated with alterations in the composition of the intestinal microbiota. We utilized 16S rRNA sequencing to assess changes in microbial composition within cecal contents from mice across all experimental groups. The results are presented in Figure 8.
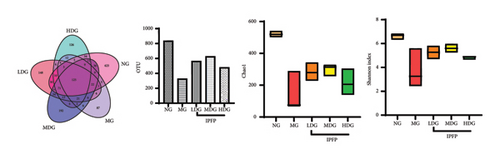
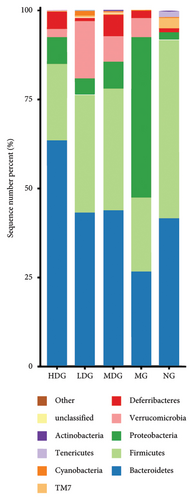
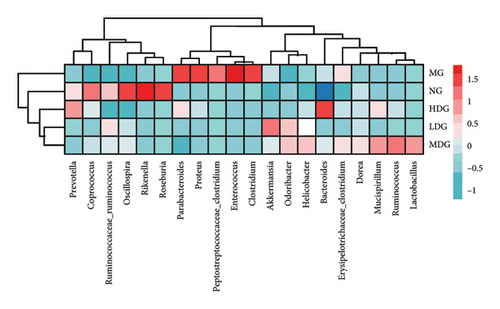
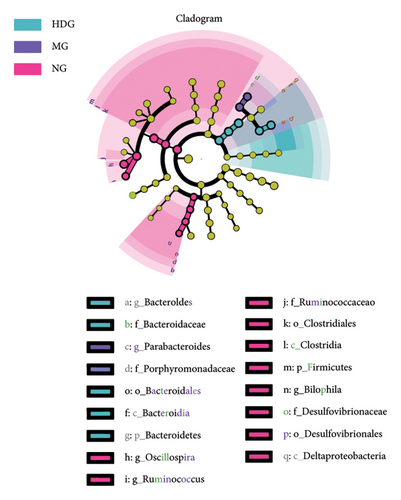
A Venn diagram analysis identified a total of 2831 operational taxonomic units (OTUs) across the five fecal sample groups, with 125 OTUs shared among all groups and 87 unique to the MG. The LDG and MDG exhibited significantly higher numbers of OTUs compared to the MG, approaching levels observed in the NG. Both the Chao1 index and Shannon index were reduced in DSS-induced colitis mice relative to controls; however, these indices increased following IPFP intervention, indicating that IPFP can enhance both richness and diversity within the intestinal microbiota. Notably, the Chao1 and Shannon indices were marginally higher in the LDG and MDG compared to the HDG group, consistent with the observed OTU values. Given the superior therapeutic effect of high-dose IPFP, we hypothesized potential differences in the leading OTUs among different dose treatment groups. Therefore, we further analyzed the relative abundance of intestinal microbiota at various taxonomic levels for each group of mice. At the phylum level, DSS treatment resulted in a decrease in bacteroidetes and firmicutes by 35.94% and 58.57%, respectively, while proteobacteria increased by 2000% compared to the NG. IPFP administration significantly restored the abundance of bacteroidetes and firmicutes while reducing proteobacteria levels. Specifically, the HDG group demonstrated the highest increase in bacteroidetes abundance, reaching 137.51%, surpassing even the levels observed in the NG. The top 20 microbial communities at the genus level are presented here. Compared to the NG, a significant increase was observed in clostridium, enterococcus, proteus, and parabacteroides within the intestinal tracts of mice in the MG after IPFP supplementation. Conversely, rikenella and oscillospira exhibited notable decreases. However, following IPFP supplementation, these genera showed some degree of recovery as indicated by their variation trends. Finally, we utilized linear discriminant analysis effect size (LEfSe) to evaluate significantly altered bacterial groups in UC mice treated with IPFP. Based on the LDA score > 3.5 criteria, oscillospira, bilophila, and ruminococcus were identified as predominant genera in the NG, while parabacteroides emerged as a significant contributor in the intestinal tracts of mice with DSS-induced UC. Notably, there was a significant alteration in the abundance of bacteroidetes within the HDG group, which aligns with the aforementioned heatmap results and suggests a potential association between bacteroidetes and the therapeutic effect of IPFP in treating UC.
4. Discussion
In recent years, research on polysaccharides derived from natural sources has garnered significant attention. Studies have demonstrated that these polysaccharides exhibit a wide range of biological activities, including antioxidant, anti-inflammatory, immunomodulatory, and antitumor effects [33]. Notably, lentinan, a well-known polysaccharide, has been developed into various pharmaceutical formulations such as injections and capsules [34, 35]. In China, immature pomelo fruits, an important agricultural by-product and traditional Chinese medicine, have primarily focused on the investigation of flavonoid components such as naringin, while research on water-soluble polysaccharides has been largely overlooked [2, 5]. In this study, we successfully prepared a crude polysaccharide (IPFP) with a purity of 81.10% through water extraction, alcohol precipitation, and the Sevag method. We further explored the therapeutic potential of IPFP in UC and its underlying mechanisms through animal model studies. There were several observations conducted in this study.
Firstly, we performed an initial characterization of the structure of the prepared IPFP using HPGPC-RID and PMP-HPLC methods. The results revealed that it is a complex polysaccharide composed of three monosaccharides: galactose, glucose, and xylose, with an average molecular weight exceeding 9000 Da. This composition has not been previously documented in studies on immature pomelo fruits.
Secondly, the DSS-induced UC mouse model has emerged as one of the most widely utilized animal models for investigating the pathogenesis of UC and screening potential therapeutic agents or regimens in recent years [36]. This is due to its close resemblance to human UC patients. In this study, mice administered with 2.5% DSS in their drinking water developed characteristic colitis features such as colonic ulcers, shortened colon length, weight loss, loose stools, bloody stools, and lethargy, confirming the successful establishment of the UC mouse model. Based on this model, different doses of IPFP were administered via gavage. The results demonstrated that IPFP improved disease characteristics to varying degrees, as evidenced by increased body weight, longer colon length, and reduced pathological features in colon tissue observed through H & E staining. These findings confirm the efficacy of IPFP in treating UC, with higher doses of IPFP exhibiting superior therapeutic effects.
In addition, the pathogenesis of UC is characterized by intestinal inflammation. Proinflammatory factors such as TNF-α, IL-1β, and IL-6 are considered important pathogenic indicators for the progression of UC, as they can aggravate the aggregation and infiltration of inflammatory cells such as neutrophils and macrophages in the colon tissue, leading to intestinal edema and eventually ulcer formation [37]. Their secretion levels are positively correlated with the severity of the disease, while the expression of the anti-inflammatory cytokine IL-10 is closely related to the alleviation of the disease [38]. Therefore, inhibiting the expression of proinflammatory cytokines and promoting the expression of anti-inflammatory factors can effectively alleviate the symptoms of UC. This study shows that IPFP can effectively inhibit the expression of TNF-α, IL-1β, and IL-6 genes, promote the expression of IL-10 genes, and simultaneously reduce the tissue infiltration of inflammatory cells and the destruction of the crypt structure caused by DSS-induced colitis, thereby demonstrating a significant anti-UC effect. To clarify the anti-inflammatory mechanism of IPFP in the treatment of UC, we used Western blot to detect the expression of p-ERK1/2 protein in colon tissue. The close correlation between the elevated expression of p-ERK1/2 and the progression of UC has been reported in the literature [39]. Our current study shows that IPFP can significantly inhibit the activation of the ERK signaling pathway in DSS-induced UC mice. Other signaling pathways, such as the PI3K/Akt/mTOR signaling pathway, NF-κB, and HIF-1α, have been reported to be involved in the disease process of UC [36, 40]. However, in this study, we only focused on the ERK1/2 signaling pathway and did not detect and analyze other signaling pathways. In the future, we will further explore whether IPFP has a multipathway treatment approach.
Finally, the dysbiosis of the intestinal microbiota, characterized by a reduction in beneficial probiotics and an increase in pathogenic bacteria, is a critical factor in the pathogenesis of UC. Typically, the gut microbiota of healthy individuals is predominantly composed of firmicutes and bacteroidetes, with relatively low levels of proteobacteria [41]. In contrast, UC patients often exhibit decreased microbial diversity, overgrowth of proteobacteria, and depletion of firmicutes [42]. Previous studies have demonstrated that fecal microbiota transplantation can effectively modulate the relative abundance of specific gut microbiota, thereby ameliorating DSS-induced colitis in mice, underscoring the pivotal role of the gut microbiota in both the pathogenesis and treatment of UC [43]. Since most natural polysaccharides cannot be enzymatically degraded in the human gastrointestinal tract, they are transported directly to the colon where they serve as substrates for the gut microbiota [44]. To investigate the impact of IPFP treatment on the gut microbiota of UC mice, this study analyzed the composition and abundance of microbiota in the feces of normal, model, and IPFP-treated groups through 16S rRNA gene sequencing. Compared to the NG, DSS treatment resulted in significant alterations in gut microbiota composition, including a 58.57% reduction in firmicutes and a 20-fold increase in proteobacteria, consistent with previous reports. Moreover, our experimental results revealed that IPFP treatment could effectively reduce the abundance of pathogenic bacteria such as clostridium, enterococcus, and proteus, while increasing the levels of beneficial probiotics such as rikenella. Previous research has shown that Enterococcus faecalis strains can enhance reactive oxygen species secretion, thereby exacerbating UC symptoms [45]. The increase in rikenella abundance is associated with the preventive effect of exogenous probiotic supplementation on DSS-induced UC in mice, underscoring the beneficial role of rikenella [46]. Furthermore, bacteroidetes species have been shown to be closely linked to the disease activity of UC [47], which aligns with our observation of a significantly elevated abundance of bacteroidetes in the HDG.
Our study possesses certain limitations. Specifically, we did not examine the alterations in endogenous and polysaccharide metabolites within the colons of mice when polysaccharides interact with gut microbiota. Additionally, we have not investigated whether the roles and mechanisms of these metabolites in vivo are associated with our reported findings. Future research is warranted to address these gaps.
5. Conclusions
In summary, our findings demonstrate that IPFP is a complex polysaccharide with a molecular weight exceeding 9000 Da, composed primarily of glucose, galactose, and xylose. The amelioration of DSS-induced UC by IPFP can be attributed to its capacity to suppress the inflammatory response, decrease the abundance of pathogenic bacteria such as enterococcus and clostridium, and enhance the abundance of beneficial bacteria such as rikenella. Consequently, IPFP shows significant potential as an adjunctive therapeutic agent for the prevention and treatment of UC.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Keman Yin: data curation; Ming Xie: formal analysis; Zuoliang Zheng: methodology; Zuoliang Zheng and Yuping Zhong: writing – original draft; Zuoliang Zheng, Yuping Zhong, and Zhiwei Liu: project administration; Yuping Zhong and Zhiwei Liu: review and final approval of the version to be published.
Funding
This study was supported by the Guangdong science and technology innovation cultivation project (grant number: pdjh2023b0489), Jiaying University Research Projects (grant numbers: 2021KJM03 and 2022KJM02), and the Undergraduate Innovation and Entrepreneurship Training Program (grant number: 202310582010).
Acknowledgments
We extend our sincere gratitude to Lihui Mou for providing valuable language editing support for this research.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




