Rosemary: A Promising Therapeutic Agent in Alleviating Nephrotoxicity
Abstract
Background: Nephrotoxicity, kidney damage caused by a variety of chemicals, presents a significant challenge in the medical field. To address nephrotoxicity, innovative therapeutic techniques must be investigated. Rosemary (Rosmarinus officinalis L.) has been studied for its antioxidant, anti-inflammatory, and cytoprotective effects; however, existing literature lacks a comprehensive analysis of its mechanistic role in nephrotoxicity. This narrative review evaluates current findings, highlighting gaps in previous studies and presenting an updated perspective on the molecular pathways of rosemary in renal protection.
Methods: This narrative review analyzed the nephroprotective effects of rosemary and its main components (rosmarinic acid, carnosic acid, carnosol, and ursolic acid) based on studies retrieved from Google Scholar, PubMed, Web of Science, and Scopus (2010–2025). Selection criteria focused on peer-reviewed research investigating the impact of rosemary on nephrotoxicity, including experimental and mechanistic studies. Studies unrelated to renal toxicity or lacking mechanistic insights were excluded. While no formal systematic review methodology was applied, a qualitative assessment of study relevance and methodological rigor was considered. Preclinical and mechanistic studies were prioritized to provide a comprehensive understanding of the nephroprotective properties of rosemary.
Results: This review consolidates evidence demonstrating the nephroprotective potential of rosemary and its main components. Unlike prior reviews, our analysis provides a comprehensive mechanistic overview of the effects of rosemary on nephrotoxicity, emphasizing its ability to modulate pyroptosis and pivotal molecular pathways, including NF-κB/NLRP3 inflammasome and TGF-β1/Smad/collagen IV signaling. These findings are primarily based on preclinical studies with limited direct evidence from clinical trials.
Conclusion: By addressing key mechanistic gaps, this review positions rosemary as a potential therapeutic agent for nephrotoxicity, based on promising findings from preclinical models. Our findings underscore its underexplored role in pyroptosis modulation and its ability to regulate key nephrotoxicity-related molecular pathways. Future research should focus on further elucidating these mechanisms to optimize rosemary’s potential as a nephroprotective agent.
Summary
- •
Nephrotoxicity remains a significant medical challenge, necessitating the search for effective therapeutic agents.
- •
Rosemary shows promise as a therapeutic agent for alleviating nephrotoxicity.
- •
Its bioactive compounds, such as rosmarinic acid and carnosic acid, help mitigate oxidative stress, inflammation, and apoptosis.
- •
Rosemary may also modulate key molecular pathways involved in nephrotoxicity.
- •
Further research is needed to understand its mechanisms and potential clinical applications fully.
1. Introduction
The kidney is the primary organ needed by the body to accomplish a number of vital tasks, such as excretion of toxic metabolites, homeostasis, detoxification, and extracellular fluid regulation [1]. The kidney is extremely susceptible to the impacts of dietary factors [2], environmental pollutants because of its excretory and concentrating functions [3].
Nephrotoxicity is a serious challenge in healthcare, characterized by kidney impairment caused by a variety of chemicals [1], including ethanol [4], as well as medications [5, 6], and environmental toxins [3]. Its prevalence is remarkable, with a high rate among individuals undergoing drug therapy [7], particularly those with previous renal problems [1]. Blood urea and serum creatinine, typically used indicators of nephrotoxicity and renal dysfunction, are thought to be low sensitive for identifying early renal injury. Serum levels of neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (Kim-1), and cystatin C are more sensitive than serum creatinine and blood urea in identifying acute kidney injury during nephrotoxicity [1]. Nephrotoxicity can occur through a variety of processes, including glomerular damage, inflammation, renal tubular toxicity, thrombotic microangiopathy, and crystal nephropathy [1], and can be induced by mechanisms such as oxidative stress [8, 9], inflammation [10, 11], apoptosis [12, 13], autophagy dysregulation [14], and mitochondrial dysfunction [15].
Moreover, renal fibrosis plays a central role in nephrotoxicity [16, 17], driven by transforming growth factor (TGF)-β1/mothers against decapentaplegic homolog (Smad) signaling, where Smad3 promotes fibrosis, while Smad7 suppresses it. Beyond TGF-β1, angiotensin II and advanced glycation end products also activate Smads, which interact with nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) pathways, linking inflammation and fibrosis. The NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome, conventionally associated with interleukin (IL)-1β and IL-18 activation, has also been implicated in fibrosis independent of its canonical role. It amplifies TGF-β/Smad signaling, enhancing Smad3 activity while downregulating Smad7, thereby contributing to renal scarring. Furthermore, NF-κB activation upregulates NLRP3 expression, prolonging inflammation and fibrosis [18, 19].
While existing therapeutic methods like hydration, dose adjustments, and nephroprotective agents are used, they frequently cause a variety of adverse effects, necessitating the exploration of novel, safer interventions.
In the field of medical research, traditional medicine has developed as a captivating source for researchers exploring alternative methods to treat complicated medical conditions [20–22] including diabetes [23], digestive system disorders [24], nervous system disorders [25, 26], and kidney injuries [27].
Rosemary (Rosmarinus officinalis L.; syn. Salvia rosmarinus Spenn.), a perennial herb of the Lamiaceae family [28], is appreciated for its aromatic fragrance, culinary use, and medicinal benefits. Although it originated in the Mediterranean region, it has been successfully grown in many other parts of the world [29]. The plant has thin, needle-like leaves and produces bright blue flowers. Rosemary contains a range of bioactive constituents, such as tannins, saponins, phenolic acids, flavonoids, diterpenes like rosmarinic acid and carnosic acid, and alkaloids, along with essential oils rich in monoterpene hydrocarbons, including camphor and alpha-pinene. These compounds are responsible for its distinctive aroma and exhibit a variety of pharmacological properties [30].
The investigations in the last decades revealed the antioxidant [31, 32], anti-inflammatory [33, 34], antiapoptotic [35], antidote [36], antiasthmatic [37], neuroprotective [38, 39], anticancer [40], antiobesity [41], antinociceptive [28], cardioprotective [42], antirheumatic [43], antidepressant [44], hypnotic [45], and renoprotective [46] properties of rosemary.
It is also essential to note that although rosemary is thought to be safe for food preservation, excessive and prolonged dosages should be used with caution because of possible teratology and negative effects on the reproductive system, liver, and kidneys. It is also important to consider drug interactions [30].
This narrative review aims to provide an in-depth overview of how rosemary and its components may provide protective effects against nephrotoxicity by collecting and analyzing a wide range of relevant data from various sources over the last decades. This review also attempts to bridge the gap between folk medicine and evidence-based medicine by elucidating the mechanisms through which rosemary exerts its renoprotective effects, providing insights that could potentially inform the development of novel therapeutic strategies for nephrotoxicity. Finally, the main objective of this study is to encourage further study into the use of rosemary as a complementary or alternative therapy in the management of nephrotoxic kidney injuries, with the hope of paving the way for the development of novel therapeutic formulations that prioritize efficacy and safety in renal care.
2. Methods
Articles published between 2010 and 2025 were systematically collected from prominent databases including Scopus, PubMed, Google Scholar, and Web of Science. The search was limited to peer-reviewed English-language publications. The inclusion criteria encompassed studies focusing on the therapeutic potential of rosemary and its main components in alleviating nephrotoxicity. Both in vitro and animal studies were considered. Despite the extensive search, no specific clinical trial focusing on the therapeutic effects of rosemary in nephrotoxicity was found.
The search strategy involved the utilization of Boolean operators (AND, OR) with a set of comprehensive keywords: “rosemary,” “Rosmarinus officinalis L.,” “Salvia rosmarinus Spenn.”, “rosmarinic acid,” “carnosic acid,” “carnosol,” “ursolic acid,” and “nephrotoxicity.” These keywords were applied across the titles, abstracts, and medical subject headings to ensure a thorough search process.
Articles were further screened based on their relevance to the topic, with a focus on studies that explored the mechanisms of action and efficacy of rosemary and its main components in mitigating nephrotoxicity.
The exclusion criteria involved the removal of non-English articles, non–peer-reviewed publications, and studies not directly related to the therapeutic effects of rosemary in nephrotoxicity.
3. The Effect of Rosemary and Its Main Components on Nephrotoxicity
3.1. Anticancer Drugs
3.1.1. Cisplatin
A very efficient and clinically advanced anticancer medication, cisplatin, is used to treat a variety of solid malignancies, including ovarian, stomach, and lung cancer. However, the main adverse consequence of administering cisplatin is nephrotoxicity. Clinically, patients receiving cisplatin have a 20%–35% chance of developing nephrotoxicity, which can result in acute kidney damage and mortality [47].
In light of the significant nephrotoxic effects associated with cisplatin administration, research has increasingly focused on exploring natural compounds such as rosemary and its bioactive constituents as potential protective agents against cisplatin-induced renal damage.
3.1.1.1. In Vitro
3.1.1.1.1. Carnosol
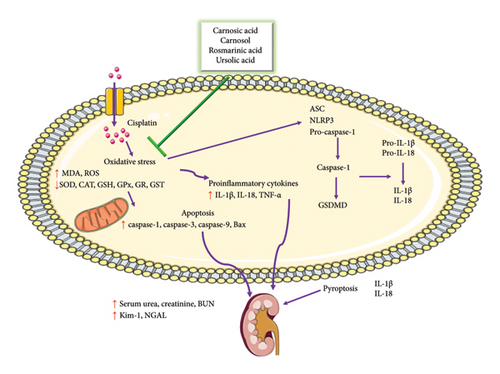
The researchers investigated the possible renoprotective effects of carnosol on acute kidney injury in HK2 cells exposed to cisplatin. The number of apoptotic cells and the expression of apoptotic proteins in HK2 cells were significantly decreased after carnosol treatment. By reducing tumor necrosis factor-alpha (TNF-α) and IL-1β levels, carnosol successfully reduced cisplatin-induced inflammation. Mechanistically, carnosol inhibited the NF-κB/NLRP3 signaling pathway activation stimulated by cisplatin, evidenced by decreased p-p65/p65, NLRP3, and apoptosis-associated speck-like protein containing a CARD (ASC) expression levels. By lowering the amounts of mature IL-1β and IL-18, gasdermin D (GSDMD), and cleaved caspase-1, carnosol also lessened pyroptosis. The NLRP3 activator nigericin, which increased GSDMD and NLRP3 protein expression, and the NLRP3 inhibitor MCC950, which decreased cleaved caspase-1 levels, further supported these results [48] (Figure 1).
3.1.1.2. In Vivo
3.1.1.2.1. Carnosic Acid
The effect of carnosic acid on protecting rats against cisplatin-induced nephrotoxicity was examined. Significant renal damage was caused by cisplatin, as evidenced by elevated serum creatinine, blood urea nitrogen (BUN), and kidney weight relative to normal control, as well as decreased tissue nitrite, superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR), and glutathione S-transferase (GST) levels and elevated kidney malondialdehyde (MDA), total reactive oxygen species (ROS), caspase-3, and glutathione (GSH) levels. When compared to cisplatin control, carnosic acid considerably reduced the rise in lipid peroxidation, caspase-3, and ROS formation while increasing GSH levels, tissue nitrite levels, and the activity of SOD, CAT, GPx, GR, and GST [49] (Table 1).
| Compound | Study design | Doses/duration | Results | Ref. |
|---|---|---|---|---|
| Cisplatin | ||||
| In vitro | ||||
| Carnosol | HK2 cells | — | ↓ Apoptotic proteins expression, TNF-α, IL-1β levels, NF-κB/NLRP3 signaling pathway activation, p-p65/p65, NLRP3, ASC expression, mature IL-1β, IL-18, GSDMD, cleaved caspase-1, pyroptosis | [48] |
| In vivo | ||||
| Carnosic acid | Female Wistar rats | 100 mg/kg, 5 and 10 days, p.o. |
|
[49] |
| Carnosol | Male C57BL/6 mice | — | ↓ NGAL, Kim-1, HMGB1, TNF-α, IL-1β levels, macrophage infiltration in kidney, NF-κB/NLRP3 signaling pathway activation, p-p65/p65, NLRP3, and ASC, pyroptosis, cleaved caspase-1, GSDMD, mature IL-1β, IL-18 | [48] |
| Rosmarinic acid | Male BALB/cN mic | 1, 2, and 5 mg/kg, 2 days, p.o. | ↓ Histopathological changes, blood creatinine, BUN, oxidative stress, renal HO-1, CYP2E1, 4-HNE, NF-κB, TNF-α, p53, phosphorylated p53, active caspase-3 | [50] |
| Rosmarinic acid | Male Swiss albino mice | 200 mg/kg, 7 days, p.o. |
|
[51] |
| Rosmarinic acid | Male BALB/c mice | 5, 10, 20 mg/kg, 5 days |
|
[52] |
| Rosmarinic acid | Male Swiss albino mice | 100 mg/kg, 2 days, p.o. |
|
[53] |
| Ursolic acid | Rats | 0.5, 1.0, and 1.5 mg/kg, twice a day, 5 days, i.v. | ↓Oxidative stress indicators of the kidneys, serum, and urine | [54] |
| Ursolic acid | Male Wistar rats | 5 and 10 mg/kg, 14 days, i.g. |
|
[55] |
| Cyclophosphamide | ||||
| In vivo | ||||
| Rosmarinic acid | Male Sprague–Dawley rats | 100 mg/kg, 8 days, gavage | ↓ Intertubular fibrosis, tubular epithelial vacuolization, brush border, basal membrane disruption, BUN levels, SOD levels | [56] |
| Doxorubicin | ||||
| In vivo | ||||
| R. officinalis leaves aqueous extract | Male Swiss albino mice | 30 mg/kg, 2 weeks, p.o. | ↓ Blood urea, serum creatinine levels, kidney damage | [57] |
| Rosmarinic acid | Male Sprague–Dawley rats | 75 mg/kg, 14 days, p.o. |
|
[58] |
| Etoposide | ||||
| In vivo | ||||
| R. officinalis extract | Male rats | 220 mg/kg, twice a week, 6 weeks, gavage |
|
[59] |
| R. officinalis aqueous extract | Male rats | 220 mg/kg, twice a week, 6 weeks, gavage |
|
[60] |
| R. officinalis aqueous extract | Male albino rats | 220 mg/kg, 3 times a week, 4 weeks, p.o. |
|
[61] |
| Methotrexate | ||||
| In vivo | ||||
| Rosmarinic acid | Wistar rats | 100 and 200 mg/kg, 12 days, gavage |
|
[62] |
- Abbreviations: 4-HNE, 4-hydroxynonenal; ASC, apoptosis-associated speck-like protein containing a CARD; Bax, Bcl2-associated X protein; BUN, blood urea nitrogen; CAT, catalase; Ctr1, copper transporter 1; CYP2E1, cytochrome P450 2E1; DNA, deoxyribonucleic acid; GPx, glutathione peroxidase; GR, glutathione reductase; GSDMD, gasdermin D; GSH, glutathione; GST, glutathione S-transferase; HMGB1, high mobility group box 1; HO-1, heme oxygenase-1; IL, interleukin; Kim-1, kidney injury molecule-1; MDA, malondialdehyde; NF-κB, nuclear factor Kappa B; NGAL, neutrophil gelatinase-associated lipocalin; NLRP3, NOD-, LRR- and pyrin domain-containing protein 3; NO, nitric oxide; Nrf2, nuclear factor erythroid 2-related factor 2; ROS, reactive oxygen species; SOD, superoxide dismutase; TNF-α, tumor necrosis factor-alpha.
3.1.1.2.2. Carnosol
The possible renoprotective properties of carnosol on cisplatin-induced acute kidney injury in mice were investigated. Carnosol showed protective effects against renal dysfunction, histological changes, and tubular damage, as revealed by lower levels of NGAL, Kim-1, and high-mobility group box 1 (HMGB1). Carnosol reduced cisplatin-induced inflammation in mice by lowering TNF-α and IL-1β levels besides reducing macrophage infiltration in the kidney. Carnosol inhibited the activation of the NF-κB/NLRP3 signaling pathway, resulting in decreased expression levels of p-p65/p65, NLRP3, and ASC. Carnosol reduced pyroptosis by lowering levels of cleaved caspase-1, GSDMD, mature IL-1β, and IL-18. These findings were supported by the NLRP3 inhibitor MCC950, which reduced cleaved caspase-1 levels, and the NLRP3 activator nigericin, which increased GSDMD and NLRP3 protein expression [48].
3.1.1.2.3. Rosmarinic Acid
Treatment with rosmarinic acid effectively corrected histopathological changes and reduced the increase in blood creatinine and BUN caused by cisplatin in mice. Rosmarinic acid treatment significantly reduced the oxidative stress induced by cisplatin, as demonstrated by lower kidney levels of heme oxygenase-1 (HO-1), cytochrome P450 2E1 (CYP2E1), and 4-hydroxynonenal (4-HNE). Additionally, rosmarinic acid inhibited the production of TNF-α and NF-κB, showing anti-inflammatory properties. Furthermore, rosmarinic acid inhibited apoptosis by decreasing the levels of active caspase-3, p53, and phosphorylated p53 in the kidneys [50].
A study was conducted in order to understand the probable mechanisms underlying the nephroprotective properties of rosmarinic acid against cisplatin-induced nephrotoxicity in mice. Serum creatinine and BUN levels that were elevated by cisplatin were considerably reduced by rosmarinic acid pretreatment. Significant reversal was observed in the increased levels of MDA, TNF-α, decreased Bcl2-associated X (Bax), and caspase-9 in kidney tissues. Furthermore, rosmarinic acid markedly reversed the cisplatin-induced decrease in GSH levels. The obtained results were highlighted by the histological investigation [51].
The researchers studied the effects of rosmarinic acid pretreatment on cisplatin-induced kidney injury in mice, to better understand the underlying mechanisms. The study found that rosmarinic acid pretreatment decreased levels of kidney enzymes (BUN and creatinine), inflammatory cytokines (IL-1β, IL-6, TNF-α), and increased antioxidant capacity in kidney tissues. Rosmarinic acid also reduced oxidative stress indicators (MDA, myeloperoxidase, and nitric oxide (NO)), inhibited inflammatory gene expression, activated the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway, and increased downstream target genes [52]. Moreover, the preventive potential of rosmarinic acid against cisplatin-induced acute renal injury illustrated that rosmarinic acid treatment significantly recovered the decreased levels of the renal transmembrane transporter, copper transporter 1 (Ctr1), in cisplatin-exposed mice, as well as reduced serum albumin and globulin levels. Furthermore, rosmarinic acid therapy increased serum electrolyte levels (Ca2+, K+, and Na+) and vividly reduced the expression of the nephrotoxicity biomarker Kim-1 [53].
3.1.1.2.4. Ursolic Acid
Low-dose ursolic acid combined with intravenous amifostine was tested for its ability to protect rats from cisplatin-induced nephrotoxicity. Serum indicators of nephrotoxicity significantly increased and serum alkaline phosphatase (ALP) levels were decreased in rats treated with cisplatin. Rat kidney homogenates treated with cisplatin showed elevated oxidative stress. By preventing the oxidative stress indicators of the kidneys from being reduced, intravenous ursolic acid injection decreased oxidative stress in rats receiving cisplatin treatment. Changes in the urine and serum indicators of oxidative stress and nephrotoxicity were decreased by ursolic acid therapy in a dose-dependent manner. Rats treated with ursolic acid showed protection against cisplatin-induced damage in their kidney histology. Notably, ursolic acid, even given at a low dosage, protects against cisplatin-induced nephrotoxicity comparable with intravenous amifostine [54].
In a rat model, the contribution of ursolic acid to lowering cisplatin-induced nephrotoxicity and reducing proinflammatory cytokines and apoptosis was evaluated. Cisplatin caused histological damage and markedly raised serum creatinine, BUN, and uric acid levels. Cisplatin raised the amount of MDA while lowering GSH, SOD, and CAT levels. Furthermore, cisplatin markedly raised TNF-α, IL-1β, IL-6, caspase-3, and caspase-9 levels. When ursolic acid was administered, there was a notable recovery from cisplatin-induced nephrotoxicity; ursolic acid reduced uric acid, BUN, and creatinine levels and improved histological damage. Additionally, ursolic acid decreased the expression of caspase-3 and caspase-9 as well as the activity of IL-1β, IL-6, and TNF-α. Additionally, there was a considerable increase in GSH, SOD, and CAT levels and a decrease in MDA content [55].
Rosemary and its bioactive compounds, including carnosol, carnosic acid, rosmarinic acid, and ursolic acid, have gathered attention for their potential renoprotective properties against cisplatin-induced nephrotoxicity. These natural compounds have demonstrated significant protective effects in both in vitro and in vivo models, proposing promising opportunities for the development of adjunctive therapies in mitigating cisplatin-induced kidney injury.
Physiologically, rosemary and its active constituents exert renoprotective effects through multiple pathways. One key mechanism involves the modulation of pyroptosis, an inflammatory form of programmed cell death implicated in cisplatin-induced renal injury. By regulating the NLRP3 inflammasome, these compounds help suppress pyroptotic activation. Specifically, carnosol and rosmarinic acid have been shown to inhibit caspase-1 activation and GSDMD cleavage, thereby reducing the release of mature IL-1β and IL-18—hallmarks of pyroptotic cell death. In experimental models, rosemary-derived compounds effectively prevent NLRP3-driven inflammation, thereby preserving renal tubular integrity and function.
Beyond their role in pyroptosis suppression, rosemary compounds modulate inflammatory responses, attenuating proinflammatory cytokine levels such as TNF-α and IL-1β, which are typically upregulated during cisplatin-induced nephrotoxicity. Additionally, these bioactive compounds interfere with NF-κB/NLRP3 signaling, further reducing inflammatory and oxidative damage.
Besides, rosemary exhibits robust antioxidant properties, protecting renal tissues from cisplatin-induced oxidative stress. By enhancing the activity of SOD, CAT, and GPx and lowering ROS generation, lipid peroxidation, and oxidative damage markers, rosemary and its main components maintain cellular redox balance, mitigating oxidative stress-related renal injury.
By integrating antiapoptotic, anti-inflammatory, and antipyroptotic actions, rosemary and its bioactive compounds offer a comprehensive approach to mitigating cisplatin-induced nephrotoxicity.
Despite these promising findings, certain limitations remain. Human clinical trials are currently lacking, making it essential to conduct translational studies to validate the nephroprotective effects observed in preclinical models. In addition, future investigations should optimize therapeutic dosing strategies to ensure effective clinical applications of rosemary-based interventions.
3.1.2. Cyclophosphamide
Cyclophosphamide, a strong oxazaphosphorine alkylating agent, was originally synthesized in 1958 and is widely used to treat malignant tumors and autoimmune disorders such as lupus and vasculitides. This prodrug requires cytochrome P450 activation to generate active compounds, phosphoramide mustard and acrolein, which cause cell death upon interaction with deoxyribonucleic acid (DNA). Metabolites such as chloroacetaldehyde, a result of cyclophosphamide detoxification, add to its toxicity. Cumulative dosage is a major risk factor for the numerous toxicities related to cyclophosphamide. Notably, cyclophosphamide can cause nephrotoxicity, which limits its therapeutic value due to multiple organ toxicity [63].
Considering the nephrotoxic effects associated with cyclophosphamide, researchers have explored natural compounds like rosmarinic acid for their potential protective effects against the renal damage induced by this potent alkylating agent.
3.1.2.1. In Vivo
3.1.2.1.1. Rosmarinic Acid
The effect of rosmarinic acid on cyclophosphamide-induced nephrotoxicity revealed that the cyclophosphamide group had interstitial inflammation, vascular congestion, tubular atrophy, glomerular damage, and vacuolization. However, in the cyclophosphamide plus rosmarinic acid group, these histological changes were significantly less noticeable. Rosmarinic acid administration reduced intertubular fibrosis. Samples from the cyclophosphamide group showed tubular epithelial vacuolization, brush border, and basal membrane disruption; these effects were reduced in the cyclophosphamide plus rosmarinic acid group. Additionally, the cyclophosphamide plus rosmarinic acid group experienced less of the cyclophosphamide-induced rise in BUN levels, whereas rosmarinic acid treatment reduced the fall in SOD levels [56].
While providing valuable insights, additional research is needed to fully explore the underlying mechanisms and assess long-term effects. The strengths of the study are evident in its detailed assessments; however, additional mechanistic studies and clinical trials are necessary to validate these findings and effectively translate them into clinical applications. Moreover, further investigations into the effects of rosemary extract, rosemary essential oil, and other main components are warranted to comprehensively evaluate their protective properties.
3.1.3. Doxorubicin
The fungus Streptomyces peucetius is the source of doxorubicin [6], commonly known as adriamycin, a powerful chemotherapy drug used to treat a wide range of cancers, including acute leukemia, sarcomas, breast cancer, Hodgkin’s disease, and non-Hodgkin lymphomas [64]. The therapeutic effect of doxorubicin is limited due to a variety of toxicities, notably nephrotoxicity [65]. Several studies have demonstrated that rosemary and its main components can help reduce doxorubicin-induced nephrotoxicity, as discussed in the next section.
3.1.3.1. In Vivo
3.1.3.1.1. Rosemary Extracts
An investigation assessed the acute nephrotoxic effects of doxorubicin on mice. The animals were divided into four groups: one that received distilled water as a negative control, one that received a single intraperitoneal injection of doxorubicin, one that received an oral extract of R. officinalis leaves for 2 weeks before receiving a doxorubicin injection, and one that received only R. officinalis leaves extract. The animals were examined 2 days after the injection. Increased blood urea and serum creatinine levels, as well as significant kidney histological alterations, were indicators of nephrotoxicity induced by doxorubicin. In contrast to the doxorubicin group, pretreatment with R. officinalis leaves extract considerably lessened these effects by lowering urea and creatinine levels and showing less severe kidney damage [57] (Table 1).
3.1.3.1.2. Rosmarinic Acid
It has been reported that doxorubicin augmented serum urea and creatinine levels, declined renal GSH and CAT levels, and elevated tissue lipid peroxidation, TNF-α, and caspase-3. Rosmarinic acid treatment markedly improved each of these metrics. The biochemical results were confirmed by histopathological studies [58].
The research on the protective effects of rosemary extracts and rosmarinic acid against doxorubicin-induced nephrotoxicity provides valuable insights into potential therapeutic mechanisms and pathways. The underlying protective mechanisms of rosemary extracts and rosmarinic acid against doxorubicin-induced kidney injury involve their abilities to counteract oxidative stress, inflammation, and apoptosis. The strengths of these studies lie in their comprehensive assessments of biochemical markers, histopathological changes, and treatment effects, which contribute to a better understanding of the protective mechanisms of rosemary and its components. However, limitations may include the need for further elucidation of specific molecular targets and pathways involved, as well as the translation of preclinical findings into clinical applications.
Novel avenues for further exploration could include investigating the modulation of specific signaling pathways involved in the protective effects of rosemary extracts/essential oil and its main components, such as Nrf2-mediated antioxidant pathways, NF-κB signaling, and mitochondrial function regulation. Additionally, exploring the epigenetic modifications induced by these compounds and their interactions with the gut microbiota could provide new insights into their mechanisms of action against doxorubicin-induced nephrotoxicity.
3.1.4. Etoposide
Etoposide (VePesid), derived from Podophyllum peltatum, is a topoisomerase II inhibitor and a well-known antineoplastic medication used to treat a variety of human cancers. It targets a wide range of tumors, including bladder, lung, ovarian, prostate, stomach, testicular, and uterine cancer. Etoposide, also known as 4′-demethylepipodophyllotoxin 9-[4,6-O-(R)-ethylidene-β-D-glucopyranoside], targets both cancer and normal cells, including rapidly dividing cells in the body [66]. Transitioning from toxin discussions, studies have explored the protective effects of rosemary on etoposide-induced kidney injury, shedding light on potential therapeutic strategies against chemotherapy-induced nephrotoxicity.
3.1.4.1. In Vivo
3.1.4.1.1. Rosemary Extracts
Research was conducted to examine the possible properties of rosemary extract against etoposide-induced kidney injury. According to the results, as compared to the control group, the administration of etoposide resulted in lower levels of Na+, and Ca2+ and higher levels of creatinine, urea, K+, Cl−, and renal DNA damage. Co-treatment and post-treatment with rosemary along with etoposide reduced kidney damage and raised blood marker levels; the co-treatment group exhibited the least amount of damage [59].
Similarly, in research examining the preventive effects of rosemary extract on etoposide-induced renal toxicity and damage in rats, it was discovered that etoposide caused substantial rises in urea, creatinine, K+, and Cl−, while decreasing levels of Na+ and Ca2+. This was accompanied by serious damage and cellular infiltration in the renal histological structure. Immunohistochemical examination revealed increased Ki67-ir immunoreactivity and p53 protein responses in the etoposide group. In contrast, treatment with rosemary extract in co-treated and post-treated groups restored normal kidney function parameters, improved histological features, and reduced immunohistochemistry abnormalities compared to etoposide-exposed rats [60].
It has been shown that etoposide administration to rats enhanced the serum levels of total protein, whereas it decreased albumin levels in comparison with the control group. Compared to etoposide treatment alone, co-treatment with rosemary extract led to an increase in albumin levels and a decrease in total protein and total bilirubin levels. Significant changes in urea, creatinine, potassium ions, and chloride ions were also stimulated by etoposide therapy that was ameliorated with rosemary treatment [61] (Table 1).
Rosemary extracts have demonstrated a significant ability to mitigate the detrimental effects of etoposide on renal function and structure in preclinical models. The underlying protective mechanisms of rosemary against etoposide-induced nephrotoxicity likely involve its antioxidant, anti-inflammatory, and cytoprotective properties.
The strengths of these studies lie in their comprehensive assessments of kidney function parameters, histological changes, and biochemical markers, contributing to the understanding of the renoprotective potential of rosemary extracts. However, limitations may include the need for further mechanistic studies to elucidate specific targets and pathways involved, as well as the translation of preclinical findings into clinical applications.
To advance this field, future research could delve into the specific molecular pathways targeted by rosemary and its main components in counteracting etoposide-induced renal toxicity. Moreover, exploring the potential synergistic effects of different bioactive compounds within rosemary could unveil novel aspects of the protective mechanisms of rosemary against chemotherapy-induced nephrotoxicity.
3.1.5. Methotrexate
Methotrexate is a commonly used medication for treating inflammatory conditions like various cancers, leukemia, psoriasis, and rheumatoid arthritis. While it effectively targets cancer cells, it also harms healthy tissues in the body. Elevated levels of methotrexate can lead to damage in important organs such as the kidneys. Since methotrexate is eliminated through the renal tubules, the accumulation of methotrexate and its byproducts can disrupt renal function. Methotrexate generates ROS, impairs the function of antioxidant enzymes, triggers apoptosis, and impacts both malignant and normal cells by generating ROS while simultaneously reducing the effectiveness of antioxidant enzymes [62]. Moving on to protective strategies, an investigation has delved into the effectiveness of rosmarinic acid in alleviating methotrexate-induced kidney damage.
3.1.5.1. In Vivo
3.1.5.1.1. Rosmarinic Acid
Evaluating the effect of rosmarinic acid against methotrexate-induced nephrotoxicity revealed that in addition to lowering renal CAT activity, methotrexate caused substantial increases in urea, creatinine, and renal MDA levels. Necrosis, leukocyte infiltration, eosinophilic casts, and glomerular injury were all visible in the kidney tissues. Rosmarinic acid at a low dose significantly reduced urea and renal MDA. A high dose of rosmarinic acid markedly raised renal CAT and reduced leukocyte infiltration and necrosis in renal tissue [62].
The study on the protective effects of rosmarinic acid against methotrexate-induced nephrotoxicity highlights its potential to mitigate kidney damage through antioxidant and anti-inflammatory mechanisms. While the study provides valuable insights into the protective properties of rosmarinic acid, further research is needed to elucidate the detailed mechanisms underlying its renoprotective effects and to assess its translational potential in the clinic. Moreover, strengthening the evidence base on rosemary extracts, essential oils, and their main components could offer novel therapeutic strategies for mitigating chemotherapy-induced nephrotoxicity and improving patient outcomes.
3.2. Other Medicines
3.2.1. Folic Acid
Folic acid, a B-group vitamin, is found naturally in animal-based meals such as eggs, citrus fruits, green leafy vegetables, and legumes. Despite its prevalence in a variety of dietary sources, excessive ingestion has been linked with undesirable effects, including kidney damage. Folic acid causes renal impairment predominantly by oxidative stress, mitochondrial abnormalities impacting bioenergetics and ferroptosis, mitophagy, and pyroptosis [67]. The investigation into the protective effects of rosmarinic acid against folic acid–induced nephrotoxicity shows potential strategies for preserving kidney function in the presence of dietary toxins.
3.2.1.1. In Vivo
3.2.1.1.1. Rosmarinic Acid
Evaluating the potential ameliorative effects of rosmarinic acid in folic acid–induced renal injury in mice showed that levels of BUN and creatinine were significantly elevated in the folic acid group but decreased after rosmarinic acid treatment. Histopathological analysis revealed reduced tubular epithelial cell damage in mice treated with folic acid and rosmarinic acid compared to those treated with folic acid alone. Rosmarinic acid treatment in folic acid–exposed mice led to increased sirtuin 1 (SIRT1) expression, higher levels of GSH and SOD, and decreased expression of NADPH oxidase 1 (NOX1) and MDA [68] (Table 2).
| Compound | Study design | Doses/duration | Results | Ref. |
|---|---|---|---|---|
| Folic acid | ||||
| In vivo | ||||
| Rosmarinic acid | Male C57/BL6 mice | 50 and 100 mg/kg, 10 days, gavage |
|
[68] |
| Rosmarinic acid | Male C57/BL6 mice | 50 and 100 mg/kg, 10 days, gavage |
|
[67] |
| Gentamicin | ||||
| In vivo | ||||
| R. officinalis leaves aqueous extract | Male guinea pigs | 220 mg/kg, 10 days, gavage | ↓Histopathological alterations, serum urea, creatinine, uric acid | [69] |
| R. officinalis aerial parts aqueous extract | Male Sprague–Dawley rats | 8% rosemary aqueous extract, 10 mL/kg, p.o. | ↓BUN, creatinine, urea, plasma MDA, histopathological alterations, DNA fragmentation in kidney tissue | [70] |
| R. officinalis | Male albino rats | 100,150, and 200 mg/kg, 7 days, p.o. | ↑ Volume, weight, mean thickness, length of the kidney, mean weight of the rat | [71] |
| R. officinalis | Male albino rats | 100, 150, and 200 mg/kg, 7 days, p.o. | ↓ Serum urea and creatinine levels | [72] |
| R. officinalis | Male albino rats | 100, 150, and 200 mg/kg, 7 days, p.o. |
|
[73] |
| Rosmarinic acid | Sprague–Dawley rats | 50 and 100 mg/kg, 12 days |
|
[74] |
| Rosmarinic acid | Male albino Wistar rats | 50 mg/kg, 12 days, gavage |
|
[75] |
| Rosmarinic acid | Male Wistar albino rat | 50 mg/kg, 12 days, i.p. | ↓ Serum levels of creatinine, urea, BUN, total oxidative stress, histopathological alterations | [76] |
| Ursolic acid | Wistar albino rats | 2, 5, and 10 mg/kg, 8 days, p.o. | ↓ Serum urea, uric acid, creatinine, BUN levels, kidney injury, epithelium loss, granular degeneration | [77] |
| Isoniazid® | ||||
| In vivo | ||||
| R. officinalis leaves aqueous extract | Male albino rats | 440 mg/kg, 4 and 8 weeks, p.o. | ↓Serum creatinine, urea, uric acid, GGT activity | [78] |
| R. officinalis leaves aqueous extract | Male Wistar albino rats | 440 mg/kg, 8 weeks, p.o. |
|
[79] |
| Lithium | ||||
| In vivo | ||||
| R. officinalis leaves aqueous extract | Male albino rats | 220 mg/kg, 4 weeks, p.o. |
|
[80] |
| Paracetamol | ||||
| In vivo | ||||
| R. officinalis leaves aqueous extract | Male albino rats | 125 mg/kg, every other day, 8 weeks, gavage |
|
[81] |
| R. officinalis leaves and stems ethanolic extract | Male albino rats | 220 mg/kg, 6 weeks, gavage | ↓ Pathological alteration | [82] |
| R. officinalis oil | Male albino rats | 250 mg/kg |
|
[87] |
- Abbreviations: Bax, Bcl2-associated X protein; Bcl2: B-cell lymphoma 2; BUN, blood urea nitrogen; CAT, catalase; DNA, deoxyribonucleic acid; FoxO3:, forkhead box O3; GGT, gamma-glutamyl transferase; GPx, glutathione peroxidase; GSH, glutathione; IL, interleukin; iNOS, inducible nitric oxide synthase; LC3/B, microtubule-associated proteins 1A/1B light chain 3B; MDA, malondialdehyde; NF-B, nuclear factor Kappa B; NO, nitric oxide; NOX1, NADPH oxidase 1; SIRT1, sirtuin 1; SOD, superoxide dismutase; TNF-, tumor necrosis factor-alpha.
The nephroprotective effects of rosmarinic acid were determined against folic acid–induced kidney damage. The results demonstrated that rosmarinic acid therapy suppressed inflammation by increasing renal forkhead box O3 (FoxO3) expression and decreasing NF-κB, TNF-α, and IL-6 levels. Moreover, rosmarinic acid reduced renal caspase-3 levels, the Bax/B-cell lymphoma 2 (Bcl2) ratio, and p53 expression. The reduction of tissue damage was validated by histological and biochemical examinations [67] (Figure 2).
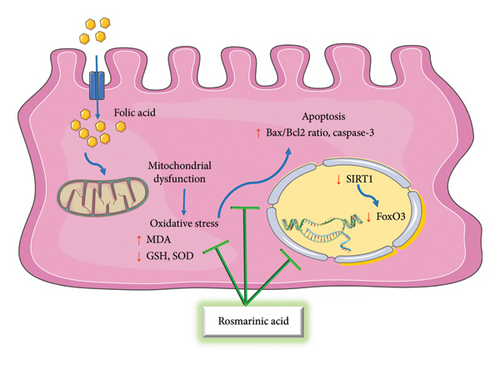
The protective mechanisms of rosmarinic acid against folic acid–induced nephrotoxicity involve a multidimensional approach, integrating antioxidant, anti-inflammatory, antiapoptotic, and pyroptosis-inhibitory effects. While the current studies have extensively explored the involvement of FoxO3, NF-κB, TNF-α, IL-6, caspase-3, Bax, Bcl2, and p53 pathways in rosmarinic acid–mediated nephron protection, future research could focus on elucidating potential crosstalk between these pathways and uncovering additional molecular targets that might contribute to the overall renoprotective effects of rosmarinic acid. Investigating the synergistic effects of rosmarinic acid in combination with other main components present in rosemary extracts or essential oils may unveil novel therapeutic strategies for opposing nephrotoxicity.
3.2.2. Gentamicin
The application of gentamicin, an aminoglycoside antibiotic known for its efficacy against gram-negative bacterial infections, is limited because of the possibility of ototoxic and nephrotoxic side effects. Gentamicin causes nephrotoxicity when it accumulates in the Golgi complex, endosomal and lysosomal vacuoles, and proximal renal tubules. This accumulation eventually results in acute tubular necrosis after causing oxidative stress, inflammatory responses, and vascular responses [83]. Some studies have demonstrated the preventive properties of rosemary and its main components against gentamicin-induced nephrotoxicity, which will be discussed below.
3.2.2.1. In Vivo
3.2.2.1.1. Rosemary Extracts
It has been illustrated that the administration of gentamicin to guinea pigs resulted in renal structural alterations, such as glomerular damage, vascular abnormalities, and tubular cell degeneration. These animals had higher amounts of urea, creatinine, and uric acid. On the other hand, co-administration of rosemary significantly decreased the levels of these blood markers and ameliorated kidney damage [69].
An in vivo study was designed to evaluate the nephroprotective properties of rosemary against gentamicin nephrotoxicity. Rats were divided into distinct groups: one that received gentamicin, one that received saline intraperitoneally as a normal control, and one that received rosemary aqueous extract in addition to gentamicin. In comparison with the gentamicin group, the rosemary group showed notable improvements in plasma kidney function biomarkers, decreased plasma levels of MDA, and rosemary extract significantly controlled electrolyte concentrations. The results were confirmed by histological examination and DNA fragmentation analysis, which showed that rosemary co-administration successfully reduced the detrimental histopathological alterations and enhanced DNA fragmentation caused by gentamicin on kidney tissue [70].
A study was conducted to evaluate the preventive properties of rosemary against acute renal damage caused by gentamicin in male albino rats. Volume, weight, mean thickness, and length of the kidney, as well as the mean weight of the rat, were significantly increased when a high dose of rosemary was co-administered in comparison to the positive control group that received gentamicin alone [71] (Table 2).
Examining the probable effects of rosemary against acute renal damage triggered by gentamicin revealed that gentamicin raised urea and creatinine levels in rats. However, administering high doses of rosemary at the same time helped keep urea and creatinine levels within normal ranges [72]. Likewise, in another investigation, adult male albino rats were used to test the preventive histological effects of rosemary against acute kidney damage caused by gentamicin. Five groups of rats were developed: gentamicin, low-dose rosemary plus gentamicin, medium-dose rosemary plus gentamicin, high-dose rosemary plus gentamicin, and control. Histological observations showed that Bowman space was damaged, proximal convoluted tubules were dilated, and glomeruli were reduced in the gentamicin and low- and medium-dose rosemary groups. On the other hand, the kidney histology of the control group and the high-dose rosemary group was similar and normal [73].
3.2.2.1.2. Rosmarinic Acid
The effect of rosmarinic acid on gentamicin sulfate-induced kidney oxidative damage in rats was examined. When compared to the gentamicin sulfate group, the co-treatment of gentamicin sulfate and rosmarinic acid (high dose) considerably boosted renal GSH, GPx, CAT, SOD, volume density of proximal convoluted tubule, and creatinine clearance while vividly lowering serum creatinine, MDA, urea, and tubular necrosis. Serum creatinine, proximal convoluted tubule volume density, renal GSH, GPx, SOD, and MDA were all substantially maintained at the same levels as the control group after receiving a high dose of rosmarinic acid [74].
The findings of a study indicated that the administration of rosmarinic acid to rats decreased the expression of proapoptotic (Bax), autophagic (microtubule-associated proteins 1A/1B light chain 3B (LC3/B)) markers, and inducible nitric oxide synthase (iNOS) triggered by gentamicin, as well as raised serum creatinine, BUN, and MDA in kidney tissue. Along with adjusting histopathological alterations, it also increased the expression of antiapoptotic proteins (Bcl2) and antioxidant enzyme levels (GSH, GPx, and SOD) [75].
The probable nephroprotective effects of rosmarinic acid were tested on gentamicin-induced nephrotoxicity. It was observed that the treatment of rosmarinic acid decreased blood serum levels of creatinine, urea, BUN, and total oxidative stress. The gentamicin plus rosmarinic acid group slightly reduced the significant histopathological alterations that gentamicin caused in the kidneys. The gentamicin and gentamicin plus rosmarinic acid groups both showed increased expression of the antiproliferative gene Ifi44. Although the administration of rosmarinic acid resulted in a reduction of oxygen radicals and an increase in antioxidant levels, the combination of rosmarinic acid and gentamicin only partially demonstrated a protective effect, not providing full protection against nephrotoxicity [76] (Table 2).
3.2.2.1.3. Ursolic Acid
Investigating the nephroprotective effects of ursolic acid in rats with gentamicin-induced kidney injury disclosed that in comparison with the saline-treated groups, gentamicin administration caused nephrotoxicity, demonstrated by a marked rise in serum urea, serum uric acid, serum creatinine, and BUN levels. These levels decreased in a dose-dependent manner when ursolic acid and gentamicin were administered together. Ursolic acid lessened the degree of gentamicin-induced kidney injury in rats, as evidenced by the histopathological investigation that revealed epithelium loss with severe granular degeneration [77].
Rosemary extracts, rosmarinic acid, and ursolic acid have shown promising effects in mitigating renal structural alterations induced by gentamicin. Exploring the underlying mechanisms, it has been observed that they exert nephroprotective properties by improving plasma kidney function biomarkers, reducing oxidative stress, modulating apoptotic and inflammatory pathways, and maintaining electrolyte concentrations.
While these studies demonstrate the potential of rosemary and its components in mitigating gentamicin-induced nephrotoxicity, further research could delve into elucidating the precise molecular mechanisms involved in their protective effects. Investigating potential synergistic interactions between different components of rosemary extracts and exploring novel targets may offer new insights for developing therapeutic interventions. Strengths of these studies include comprehensive assessments of biochemical and histological parameters, providing a complete understanding of the renoprotective effects of these compounds. However, limitations such as the need for more mechanistic studies and translation of findings to clinical applications should be considered for future research in this field.
3.2.3. Isoniazid®
Isoniazid is a semisynthetic macrocyclic antibiotic produced from Streptomyces mediterranei noted for its high lipid solubility. It is widely used in combination with ethambutol, pyrazinamide, and rifampicin to treat tuberculosis caused by organisms that are sensitive to it. Its sterilizing features make it very useful against fast-dividing organisms and semidormant bacterial populations. Although considered a safe medicine, isoniazid has been related to undesirable effects such as nephrotoxicity, which can occasionally result in acute renal failure. Isoniazid has been linked to structural abnormalities in the kidneys, such as glomerulonephritis, interstitial nephritis, and acute tubular necrosis [79]. As researchers investigate the potential side effects of isoniazid, some studies have emphasized the preventive properties of rosemary against its toxic effects, providing likely methods for minimizing its nephrotoxic results.
3.2.3.1. In Vivo
3.2.3.1.1. Rosemary Extracts
An in vivo study was carried out to evaluate the preventive properties of rosemary aqueous extract against isoniazid-induced nephrotoxicity in two short-term (4 weeks) and long-term (8 weeks) protocols. The rats were divided into different groups: control, rosemary extract, isoniazid alone, and isoniazid plus rosemary. The results showed that the combination of rosemary extract and isoniazid effectively reduced isoniazid-induced nephrotoxicity. This was demonstrated by considerable improvements in renal function, as seen by lower serum creatinine, urea, uric acid, and gamma-glutamyl transferase (GGT) activity. Moreover, in comparison with the short-term study, the long-term investigation showed a more noticeable improvement in the biochemical parameters [78] (Table 2).
Another study investigated the potential of rosemary aqueous extract to protect the kidneys against nephrotoxicity caused by Isoniazid®. Four groups of rats were formed: control, Isoniazid®, rosemary extract alone, and Isoniazid® plus rosemary extract. Results showed that the renal damage caused by Isoniazid® was effectively lowered when rosemary extract and Isoniazid® were administered together. Significant drops in serum urea, creatinine, uric acid, TNF-α, IL-1β, and Na+ levels, as well as lower kidney MDA, NO, and DNA fragmentation levels were also disclosed. Serum Na+ and K+ levels, kidney GSH levels, and Na+/K+ adenosine triphosphatase (ATP)ase activity all significantly increased. These results were supported by histological analyses, which indicated that rosemary extract provided protection against the tissue damage caused by Isoniazid® [79].
The protective effects of rosemary against isoniazid-induced nephrotoxicity involve its antioxidant and anti-inflammatory properties. The rich phytochemical composition of rosemary scavenges free radicals, reduces oxidative stress, and mitigates inflammatory responses triggered by isoniazid. The combined action of rosemary extract with isoniazid modulates key biochemical parameters, enhancing kidney function markers and restoring cellular homeostasis.
Strengths of these studies include the in vivo experimental design, which closely mimics the physiological conditions in living organisms, as well as the comprehensive assessment of biochemical, histological, and molecular markers to evaluate renal function. However, limitations such as the focus on animal models and the need for further clinical trials to validate these findings in human subjects underscore the importance of continued research in this area to translate these promising results into clinical applications effectively.
Moving forward, further research exploring the specific main components within rosemary and their individual contributions to nephroprotection against isoniazid toxicity could provide valuable insights for developing targeted therapeutic interventions. Investigating the synergistic effects of rosemary components and their interactions with isoniazid metabolites may unveil novel strategies for enhancing renal health in the context of antituberculosis treatment.
3.2.4. Lithium
Lithium, a commonly prescribed medicine for bipolar disease, effectively stabilizes mood swings, reduces suicide risk, and improves overall quality of life. However, prolonged lithium usage has been associated with chronic kidney disease, which can proceed to end-stage renal disease if not well treated. Chronic lithium usage may reduce glomerular filtration rate (GFR) while increasing blood creatinine and BUN, indicating kidney dysfunction. Antioxidants may have an important role in treating lithium-induced nephrotoxicity [84]. However, research has emphasized the potential protective function of rosemary against lithium-induced kidney damage, providing information for treating the renal problems associated with long-term lithium administration.
3.2.4.1. In Vivo
3.2.4.1.1. Rosemary Extracts
A study was conducted to examine the preventive effect of extract from rosemary leaves against lithium-induced kidney damage in rats. When compared to a healthy control group, lithium significantly increased plasma levels of MDA, creatinine, and urea while significantly decreasing those of SOD and GSH. Conversely, the amount of oxidative stress and regular blood tests for kidney function was considerably reduced after therapy with rosemary extraction [80].
While the use of animal models and extensive assessments in the reported study is beneficial, further investigation of specific bioactive components in rosemary extracts may uncover important mechanisms behind protection against lithium-induced nephrotoxicity, thereby providing new therapeutic targets for maintaining renal function during long-term lithium treatment. Moreover, the need for human validation, optimal dose studies, and treatment duration investigations highlight opportunities for future research to properly integrate these findings into clinical practice.
3.2.5. Paracetamol
One of the safest and most often used analgesics and antipyretics in the world is paracetamol [85]. At therapeutic dosages, it is a valuable and effective medication, but, at high doses, serious side effects have been documented. There have been reports linking paracetamol overdose to both isolated renal failure and renal failure along with liver failure. Paracetamol intoxication may result in severe renal failure and acute tubular necrosis. Elevated BUN and serum creatinine levels could be indicative of acute tubular necrosis triggered by paracetamol [86]. Nonetheless, several studies have shown that rosemary may protect against paracetamol-induced nephrotoxicity, providing novel ways to maintain renal health in cases of paracetamol toxicity.
3.2.5.1. In Vivo
3.2.5.1.1. Rosemary Extracts, Oil
The potential advantages of aqueous rosemary extract were investigated in preventing renal damage in rats that had overdosed on paracetamol. The findings showed that an overdose of paracetamol resulted in a considerable drop in Na+, total protein, and albumin levels and an increase in serum enzyme activity, urea, creatinine, and K+ levels. Additionally, the renal GSH levels and antioxidant enzyme activity decreased, whereas lipid peroxidation increased. These biochemical changes were confirmed by histological analysis. These biochemical and histological alterations induced by paracetamol were lessened by the administration of rosemary extract [81].
In addition, the potential protective advantages of an ethanolic extract of R. officinalis leaves and stems were assessed in reducing the renal damage caused by paracetamol in rats. Four groups were randomly selected: (1) control; (2) paracetamol; (3) concurrent administration of paracetamol and rosemary for 6 weeks; (4) two weeks of paracetamol, followed by 6 weeks of daily rosemary extract administration. Group 3 demonstrated a slight but statistically significant protective effect against renal toxicity. On the other hand, Group 4, which received only extract from R. officinalis, showed a remarkably high level of protection against kidney injury caused by paracetamol [82].
Furthermore, another study was designed to examine the renal protective effects of rosemary oil against paracetamol toxicity in rats by measuring renal oxidative stress indicators. The rats were divided into four groups: a negative control group, a paracetamol-intoxicated group, a rosemary oil-treated group, and a paracetamol plus rosemary oil group. Paracetamol significantly increased kidney function biomarkers (serum creatinine, urea, and uric acid levels) and renal MDA levels while decreasing renal antioxidant system components GSH, SOD, and CAT. Rosemary oil reduced the negative effects of paracetamol on kidney function indicators, as confirmed by histological examination [87].
Rosemary ameliorates biochemical imbalances from paracetamol overdose, restoring crucial markers, and reducing renal dysfunction. Its antioxidant properties prevent oxidative stress induced by paracetamol, improving renal health. Strengths of the studies include the comprehensive assessment of renal function biomarkers, histological analysis, and the demonstration of the protective effects of rosemary against paracetamol-induced kidney toxicity in animal models. However, limitations such as the need for human validation studies, optimal dosing investigations, and exploration of long-term effects emphasize fields for future research to effectively translate these findings into clinical applications for mitigating paracetamol-induced nephrotoxicity.
Exploring other main components of rosemary beyond aqueous extracts and oil could offer novel insights into the specific bioactive compounds responsible for its protective effects against paracetamol-induced nephrotoxicity. Further research focusing on these components and their interactions with paracetamol metabolites may uncover new therapeutic targets for the protection of renal function in cases of paracetamol toxicity.
3.3. Industrial Toxins
3.3.1. Acrylamide
Acrylamide, a water-soluble vinyl polymer used to make polyacrylamide products [88], is employed in a variety of industrial fields, including textiles, paper, and cosmetics. The production of acrylamide during high-temperature cooking procedures (over 120°C), such as roasting, grilling, baking, and frying foods high in carbohydrates, has been revealed by recent research from Stockholm University [89, 90]. Amino acids like asparagine and reducing carbohydrates like glucose interact to produce this molecule. It is estimated that eating foods cooked at high temperatures exposes people to about 0.5 mg/kg/day of acrylamide. The negative effects of acrylamide, such as hepatotoxicity, nephrotoxicity, genotoxicity, and carcinogenic effects in both humans and animals, have been reported in a number of study publications [89, 91]. Research has pointed to the potential protective role of rosemary oil against acrylamide-induced toxicity, which will be reported in the following section.
3.3.1.1. In Vivo
3.3.1.1.1. Rosemary Oil
The purpose of a study was to evaluate the harmful effects of acrylamide on the kidneys of rats and investigate if exposure to acrylamide might be improved by rosemary oil. The results of the investigation showed that rosemary provided some kidney protection. The normalized amounts of markers like creatinine and urea made this apparent. The potential of rosemary to prevent acrylamide-induced oxidative stress was proposed to be the cause of the nephroprotection. This was demonstrated by the increase of antioxidative enzymes, especially GSH and CAT, in the renal homogenate and the decrease in renal lipid peroxidation product MDA [92] (Table 3).
| Compound | Study design | Doses/Duration | Results | Ref. |
|---|---|---|---|---|
| Acrylamide | ||||
| In vivo | ||||
| R. officinalis oil | Male Wister rats | 250 mg/kg, 28 days, p.o. |
|
[92] |
| Cadmium | ||||
| In vitro | ||||
| Carnosic acid | Normal kidney epithelial | 1–10 μM, 24 h |
|
[93] |
| Rosmarinic acid | Mouse proximal tubular epithelial cells | 5–60 μM, 24 h |
|
[94] |
| In vivo | ||||
| R. officinalis leaves aqueous extract | Male albino rats | 220 mg/kg, 5 consecutive days per week, 8 weeks, p.o. |
|
[95] |
| Carnosic acid | Swiss albino mice | 10 mg/kg, 2 weeks, p.o. |
|
[93] |
| Rosmarinic acid | Swiss albino mice | 50 mg/kg, 14 days, p.o. |
|
[94] |
| Chromium | ||||
| In vivo | ||||
| R. officinalis essential oil | Male Wistar rats | 0.5 mL/kg, p.o. |
|
[96] |
| Rosmarinic acid | Male Wistar rats | 25 mg/kg, 60 days, p.o. |
|
[97] |
| Lead | ||||
| In vivo | ||||
| R. officinalis leaves ethanolic extract | Male albino rabbits | 30 mg/kg, 30 days, gavage |
|
[98] |
| Nickel chloride | ||||
| In vivo | ||||
| R. officinalis leaves methanolic extract | Male Wistar Albino rats | 100 mg/kg, 28 days, p.o. |
|
[99] |
- Abbreviations: α-SMA, alpha-smooth muscle actin; Apaf-1, apoptotic protease activating factor 1; Bad, Bcl2-associated death promoter; Bcl2, B-cell lymphoma 2; CAT, catalase; CRP, C-reactive protein; DNA, deoxyribonucleic acid; FAS, fatty acid synthase; GPx, glutathione peroxidase; GSH, glutathione; GSSG, oxidized glutathione; HO-1, heme oxygenase-1; IL, interleukin; Keap 1, Kelch-like ECH-associated protein 1; MDA, malondialdehyde; NADPH, nicotinamide adenine dinucleotide phosphate; NF-κB, nuclear factor Kappa B; NO, nitric oxide; Nrf2, nuclear factor erythroid 2-related factor 2; PKC-δ, protein kinase C-delta; ROS, reactive oxygen species; Smad, mothers against decapentaplegic homolog; SOD, superoxide dismutase; TBARS, thiobarbituric acid reactive substances; TGF-β1, transforming growth factor beta 1; TNF-α, tumor necrosis factor-alpha; TNFR2, tumor necrosis factor receptor 2.
Rosemary oil has potential nephroprotective properties against acrylamide-induced kidney injury by regulating key indicators and increasing antioxidative enzymes while decreasing lipid peroxidation. Exploring rosemary extracts and their key bioactive components may reveal novel medicinal techniques. More study is needed to validate these findings in various models and apply them to human studies for the successful treatment of acrylamide-induced kidney damage.
3.3.2. Cadmium
Cadmium is one of the most prevalent metals in the environment, despite its very high level of toxicity. It can be found in food, beverages, and even the air. For the typical person, the main sources of cadmium are food, smoking, and exhaust pollution. Employees exposed to cadmium at work include those who make alloys, nickel-cadmium batteries, solar panels, anticorrosive coatings, plastics, poisons, and cadmium pigments. Workers in nonferrous metal smelters and welders are also vulnerable [100]. Chronic cadmium exposure causes significant kidney damage due to poor excretion and accumulation in the body, particularly in the kidneys. Cadmium accumulation in the kidneys, which can last for years to decades, leads to nephrotoxicity characterized by symptoms like polyuria and increased excretion of various substances in the urine, eventually resulting in irreversible kidney damage [101]. Several studies have suggested that rosemary and its main components may protect against cadmium-induced nephrotoxicity, as discussed in the next section.
3.3.2.1. In Vitro
3.3.2.1.1. Carnosic Acid
An experiment focused on the preventive effects of carnosic acid against cadmium chloride-induced nephrotoxicity. The findings showed that co-treating with carnosic acid and cadmium chloride reduced apoptosis in normal kidney epithelial cells by increasing the amounts of Bcl2, as well as reducing the levels of Bcl2-associated death promoter (Bad) and caspase-3, caspase-8, and caspase-9. Cadmium chloride exposure increased oxidative stress by boosting free radical production, inhibiting the redox defense system, and preventing Nrf2 activation. Furthermore, cadmium chloride–induced fibrosis. Nonetheless, carnosic acid efficiently defeated cadmium chloride–induced nephrotoxicity by decreasing free radicals, strengthening redox defense systems, and reducing fibrosis [93].
3.3.2.1.2. Rosmarinic Acid
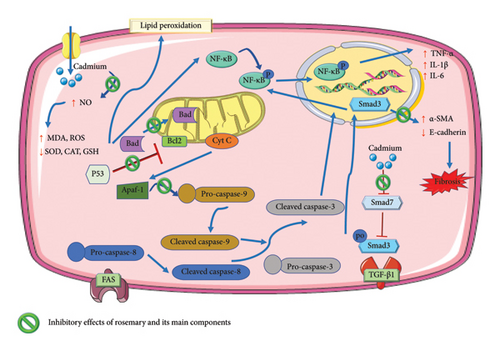
Rosmarinic acid demonstrated a dose-dependent ability to prevent cell death in isolated mouse kidney cells when exposed to cadmium chloride. The presence of cadmium chloride significantly induced oxidative stress in the kidney cells by promoting the excessive production of ROS, elevating NO levels, activating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, and impairing the defense mechanisms of the cell against oxidative stress. This oxidative stress caused by cadmium chloride notably promoted cell death in the mouse kidney cells by activating the NF-κB/protein kinase C-delta (PKC-δ)/tumor necrosis factor receptor 2 (TNFR2) pathways. Furthermore, cadmium chloride triggered the development of kidney fibrosis by activating TGF-β1/Smad3/alpha-smooth muscle actin (α-SMA)/collagen signaling pathways within the kidney cells. Conversely, rosmarinic acid effectively reduced cadmium chloride–induced oxidative stress and the related pathological signaling in the mouse kidney cells [94] (Figure 3).
3.3.2.2. In Vivo
3.3.2.2.1. Rosemary Extracts
It has been reported that the administration of cadmium chloride to rats rose the kidney MDA levels and attenuated GSH, CAT, and SOD activity in comparison with the control group. Serum creatinine and urea levels were also higher in the cadmium group. Leukocyte infiltrations, tubular dilatation, blood vessel congestion, and glomerular shrinkage were among the histological alterations induced by cadmium. Abnormalities in the brush border, renal tubules, endoplasmic reticulum, and nucleus were discovered through ultrastructural investigations. Treatment with rosemary aqueous extract, on the other hand, reversed these alterations [95].
3.3.2.2.2. Carnosic Acid
Examining the preventive effects of carnosic acid on nephrotoxicity caused by cadmium chloride in mice disclosed that cadmium chloride treatment increased oxidative stress by increasing free radical generation, decreasing the body’s redox defense mechanism, and inhibiting Nrf2 activation in kidney tissue. Cadmium chloride activated apoptosis and fibrosis by activating the apoptotic and TGF-β1/mothers against decapentaplegic homolog (Smad)/collagen IV signaling pathways. However, carnosic acid substantially mitigated cadmium chloride–induced nephrotoxicity by lowering free radicals, boosting redox defense systems (increasing GSH amounts, decreasing H2O2, NADPH oxidase, ROS, NO levels), apoptosis (augmenting Bcl2, attenuating Bad, and caspase cascade), and suppressing fibrosis. The restoration of blood and urine parameters in mice, together with supportive histological evidence, demonstrated the protective properties of carnosic acid [93].
3.3.2.2.3. Rosmarinic Acid
The goal of this study was to identify the mechanism by which rosmarinic acid protects against nephrotoxicity caused by cadmium chloride in mice. The obtained data illustrated that rosmarinic acid may considerably replicate cadmium chloride-mediated pathological alterations in blood and urine parameters. Moreover, rosmarinic acid significantly countered oxidative stress by enhancing renal antioxidant defenses and reducing ROS levels. Moreover, it exhibited anti-inflammatory properties by suppressing proinflammatory cytokines. Rosmarinic acid also mitigated apoptotic pathways and preserved mitochondrial integrity. Histopathological analysis further validated the protective effects, showing reduced tissue damage [94].
Rosemary and its components, such as carnosic acid and rosmarinic acid, show promise in protecting against cadmium-induced kidney damage by reducing apoptosis, boosting antioxidative enzymes, lessening fibrosis, and inhibiting proinflammatory pathways triggered by cadmium chloride.
Strengths of the studies include a complete assessment of oxidative stress indicators, apoptotic pathways, and histological changes to determine the protective benefits of rosemary components against cadmium-induced nephrotoxicity. However, limitations such as the need for additional research to validate these findings in various experimental models and the implementation of these results to clinical applications highlight areas for future research to effectively use rosemary and its components as protective agents against cadmium-induced kidney injury. Future research could focus on elucidating the complex molecular pathways involved in the nephroprotective effects of rosemary and its components against cadmium-induced nephrotoxicity.
3.3.3. Chromium
Chromium exists naturally in two stable forms, chromium (III) and highly toxic chromium (VI) compounds like potassium dichromate, known for their mutagenic and carcinogenic properties. Chromium (VI) exposure poses environmental risks due to its widespread use in various human activities, contributing to the pollution of soil, water, and air. Once inside cells, chromium (VI) converts to chromium (III), generating ROS through Fenton/Haber-Weiss reactions, causing oxidative stress and cellular damage. While chromium (VI) affects multiple tissues and organs, it predominantly accumulates in the kidneys, leading to elevated ROS levels, DNA damage, lipid peroxidation, enzyme inhibition, mitochondrial dysfunction, apoptosis, and nephropathy [102]. As the detrimental effects of chromium exposure on various biological systems, particularly the kidneys, continue to be a concern, studies have highlighted the potential protective effects of rosemary and rosmarinic acid against chromium-induced oxidative stress and cellular damage, offering new information about reducing the toxic effects of chromium exposure.
3.3.3.1. In Vivo
3.3.3.1.1. Rosemary Essential Oil
To evaluate the protective and therapeutic properties of rosemary essential oil against renal toxicity and oxidative stress induced by potassium dichromate in rats. The animals were divided into five groups: control, rosemary essential oil, hexavalent chromium for 14 days, protective group (rosemary essential oil administered 30 min before chromium hexavalent injection), and therapeutic group (chromium hexavalent 30 min followed by rosemary essential oil oral administration). The results indicated that rats intoxicated with chromium hexavalent had a significant decrease in renal GSH, total protein, and enzyme antioxidants (SOD, CAT, GPx, and GST) and a significant rise in the oxidative stress profile (thiobarbituric acid reactive substances (TBARS) and H2O2). Additionally, there was a significant increase in the markers of kidney function (urea, creatinine, and uric acid) in the serum. Additionally, rat kidney tissue changed histologically and immunohistochemically after receiving chromium hexavalent. Otherwise, the administration of rosemary essential oil either before or after treatment with chromium hexavalent improved the structure of kidney tissue and significantly recovered the majority of biochemical indicators. Additionally, oxidative stress markers showed an improvement with individual intake of rosemary essential oil [96].
3.3.3.1.2. Rosmarinic Acid
It has been reported that rats prolonged exposure to chromium resulted in an imbalance in oxidant/antioxidant levels, as evidenced by a large increase in MDA and a drop in GSH. Notable histopathological changes in kidney tissues were observed, which were supported by immunohistochemical staining for caspase-3, placental GST, and proliferating cell nuclear antigen, as well as a significant downregulation of the Nrf2 gene and an upregulation of the nibrin gene. However, the group that was co-treated with rosmarinic acid showed significant improvements in all toxicopathology markers [97] (Table 3).
Rosemary essential oil and rosmarinic acid exhibit significant protective effects in vivo against potassium dichromate–induced renal toxicity and oxidative stress in animal models by restoring antioxidant enzyme levels, reducing oxidative stress markers, and improving kidney function parameters in rats exposed to chromium hexavalent. The upregulation of protective genes and downregulation of toxicopathology markers further underscore the beneficial effects of these compounds against chromium-induced renal toxicity.
Strengths of the studies include the assessment of oxidative stress markers, kidney function parameters, histopathological changes, and gene expression profiles to elucidate the protective effects of rosemary and rosmarinic acid against chromium-induced renal toxicity. However, limitations such as the need for further investigations to validate these findings in different experimental models and clinical fields highlight areas for future research to effectively utilize rosemary and its components as protective agents against chromium-induced kidney injury.
3.3.4. Lead
One common heavy metal contaminant that poses a serious risk to human health and the environment is lead. Because lead is used so extensively in commercial products and human activities, its emission into different ecosystems, workplaces, and the food chain has been increasing. It is important to remember that lead is nonbiodegradable and can build up in different body tissues after being absorbed, which can have negative consequences on the liver, kidneys, and brain system. Among them, the kidneys are more vulnerable to lead toxicity because they serve as the principal excretory route, with the renal tubular epithelium being a critical site for possible renal impairment [103]. Below is a description of a study on the nephroprotective effect of rosemary extract against lead.
3.3.4.1. In Vivo
3.3.4.1.1. Rosemary Extracts
A study was designed to find out how rosemary ethanolic extract might protect rabbits from lead acetate–induced nephrotoxicity. The results demonstrated that lead-induced kidney damage markers and lipid peroxidation were substantially decreased by pretreatment with rosemary ethanolic extract. Additionally, the reduction of protein and antioxidant enzymes caused by lead was lessened by rosemary ethanolic extract pretreatment. According to histological and biochemical analyses, rosemary maintained the structural integrity of renal architecture [98] (Table 3).
The study investigating the protective effects of rosemary ethanolic extract against lead-induced nephrotoxicity in rabbits sheds light on the potential mechanisms underlying the renoprotective properties of rosemary. Future research could explore the specific main components of rosemary to elucidate their individual contributions to the protective effects against lead-induced nephrotoxicity. Investigating the molecular pathways and signaling cascades involved in the renoprotective effects of rosemary could also provide more information about developing targeted therapies for lead toxicity.
3.3.5. Nickel Chloride
Nickel is a harmful industrial and environmental contaminant having carcinogenic potential. The increase in mining activities and incorrect disposal of waste batteries lead to growing nickel levels in some areas. Excessive nickel exposure is harmful to people and animals, particularly the kidneys, which are a key target organ for nickel toxicity. Nickel has been shown to cause kidney injury, which can lead to systemic metabolic abnormalities and impaired renal function in adults. Excess nickel intake has been linked to renal dysfunction, tubular epithelial degeneration, necrosis, and apoptosis [104]. A study on the nephroprotective property of rosemary extract against nickel chloride is described below.
3.3.5.1. In Vivo
3.3.5.1.1. Rosemary Extracts
The evaluation of the potential renoprotective property of methanolic extract of rosemary against nickel chloride–induced nephrotoxicity in rats revealed that although receiving nickel chloride led to an augmentation of serum urea, creatinine, and uric acid, as well as renal oxidative stress, which was evidenced by enhanced GST activity and MDA levels, reduced amounts of CAT, GSH, and SOD, GPx activity, in addition to histopathological alterations in kidney tissue, the extract amended most of the parameters by its considerable metal-chelating and antioxidant power [99].
Future research might examine the molecular pathways and signaling cascades by which rosemary protects against nickel chloride–induced nephrotoxicity, potentially revealing new therapeutic targets for heavy metal poisoning. Investigating the synergistic effects of rosemary extracts and their primary components may provide novel findings to improve the efficiency of natural medications for heavy metal-induced nephrotoxicity.
3.4. Chemical Solvents
3.4.1. Carbon Tetrachloride
Carbon tetrachloride is a clear liquid known for its toxic effects on the liver and kidneys, causing conditions like cirrhosis and necrosis. Exposure to carbon tetrachloride generates free radicals in various tissues, including the kidneys. Historically used in dry cleaning and as an anesthetic, carbon tetrachloride is found in trace amounts in British foods. Its lipid solubility enables widespread distribution in the body. Carbon tetrachloride–induced nephrotoxicity begins with cytochrome P450-mediated electron transfer, leading to the formation of trichloromethyl radicals, initiating lipid peroxidation. This process generates reactive species like MDA, damaging cell membranes and contributing to acute and chronic renal issues [105]. Considering the renal toxicity of carbon tetrachloride caused by free radical formation, some research assessed the protective advantages of rosemary and ursolic acid, perhaps suggesting novel techniques for avoiding its adverse effects.
3.4.1.1. In Vivo
3.4.1.1.1. Rosemary Extracts
Investigating the preventive properties of aqueous rosemary extract against carbon tetrachloride–induced kidney injury revealed that rats exposed to carbon tetrachloride developed a variety of histological changes in their kidney cortex. The renal tubules lost their normal shape, including degraded epithelial cells. Glomeruli showed symptoms of shrinkage, whereas renal blood vessels were congested. Inflammatory leucocytic cells invaded intertubular spaces, leading to increased α-SMA expression in the interstitium compared to the control group. Carbon tetrachloride also caused large increases in serum creatinine and urea. Animals treated with carbon tetrachloride and an aqueous rosemary extract showed improvements in both biochemical and histopathological characteristics [106] (Table 4).
| Compound | Study design | Doses/Duration | Results | Ref. |
|---|---|---|---|---|
| Carbon tetrachloride | ||||
| In vivo | ||||
| R. officinalis aqueous extract | Male albino Wistar rats | 220 mg/kg, twice a week, 6 weeks, p.o. | ↓ Leukocytic cells invasion to intertubular spaces, α-SMA expression in the interstitium, serum creatinine, urea, histopathological alterations | [106] |
| R. officinalis aerial parts ethanolic extract | Male Wistar albino rats | 100 and 250 mg/kg, 30 days, p.o. |
|
[107] |
| R. officinalis leaves aqueous extract | Wistar female mice | 100 mg/kg, 15 days, p.o. |
|
[108] |
| Ursolic acid | Male ICR mice | 25 and 50 mg/kg, 6 weeks, i.g. |
|
[109] |
| Diethylnitrosamine | ||||
| In vivo | ||||
| R. officinalis leaves aqueous extract | Male Wistar albino rats | 200 mg/kg, 14 days, p.o. | ↓ Serum urea, creatinine, uric acid, renal and serum MDA, TNF-α, renal tubule epithelial lining vacuolation | [110] |
| R. officinalis powder and essential oil | Male albino rats |
|
|
[111] |
| Rosmarinic acid | Male Wistar albino rats | 50 mg/kg, 14 days, p.o. | ↓ Serum urea, creatinine, uric acid, renal and serum MDA, TNF-α, renal tubule epithelial lining vacuolation | [110] |
| Naphthalene | ||||
| In vivo | ||||
| R. officinalis leaves aqueous extract | Male Sprague–Dawley albino rats | 10 mL/kg, 30 days, gavage |
|
[112] |
- Abbreviations: α-SMA, alpha-smooth muscle actin; ALP, alkaline phosphatase; BUN, blood urea nitrogen; CAT, catalase; COX-2, cyclooxygenase-2; GPx, glutathione peroxidase; GSH, glutathione; IL, interleukin; MDA, malondialdehyde; NF-δB, nuclear factor Kappa B; ROS, reactive oxygen species; SOD, superoxide dismutase; STAT3, signal transducer and activator of transcription 3; TNF-α, tumor necrosis factor-alpha.
Additionally, the ethanolic extract of rosemary was tested for its ability to protect rats against carbon tetrachloride–induced nephrotoxicity. According to the findings, kidney biomarkers like BUN and creatinine, and kidney biochemicals like lipid peroxidation and xanthine oxidase were elevated, and antioxidant enzyme levels like SOD, CAT, GPx, and GSH were significantly decreased after carbon tetrachloride treatment. Administration of ethanolic extract of rosemary, however, vividly and dose-dependently reversed these changed biomarker levels. Furthermore, ethanolic extract of rosemary assisted in reducing the histological changes triggered by carbon tetrachloride [107].
Another study was designed to evaluate the preventive effects of R. officinalis aqueous extract against renal damage in mice caused by carbon tetrachloride. The findings showed that carbon tetrachloride–induced kidney damage was linked to elevated oxidative stress, as shown by substantial increases in TBARS and protein carbonyl levels, coupled with decreased GSH levels and lower activity of antioxidant enzymes SOD, CAT, and GPx. Histological analysis also revealed that nephropathology markers such as creatinine, BUN, and urea were significantly high. By preventing lipid peroxidation, increasing cellular antioxidant production, decreasing renal biomarkers, and restoring kidney structure, pretreatment with rosemary extract successfully reduced the harmful effects of carbon tetrachloride [108].
3.4.1.1.2. Ursolic Acid
It has been found that ursolic acid successfully protected carbon tetrachloride–induced nephrotoxicity in a dose-dependent manner, as evidenced by histological analysis and diagnostic markers for kidney damage. By lowering lipid peroxidation levels and restoring the total antioxidant capacity of the kidney, ursolic acid inhibited the substantial rise in ROS and oxidative stress caused by carbon tetrachloride. 8-hydroxy-2-deoxyguanosine, a hallmark of oxidative DNA damage, was decreased by ursolic acid. In mice treated with carbon tetrachloride, ursolic acid markedly reduced the production of proinflammatory markers such as TNF-α, IL-6, IL-17, and cyclooxygenase-2 (COX-2). It was shown that ursolic acid elevated signal transducer and activator of transcription 3 (STAT3), which in turn activated the NF-κB pathway and subsequently modified the inflammatory cytokines of the kidney [109].
The protective properties of rosemary and ursolic acid against carbon tetrachloride–induced kidney damage show promise in alleviating the adverse effects of this toxic compound by improving histological changes, reducing inflammation, and restoring kidney biomarkers. The antioxidant properties of rosemary and ursolic acid play a key role in reversing lipid peroxidation, restoring antioxidant enzyme levels, and mitigating histopathological changes induced by carbon tetrachloride.
Future research could explore the synergistic effects of rosemary extracts and ursolic acid or the other main components in amending carbon tetrachloride–induced nephrotoxicity, potentially uncovering novel therapeutic combinations for renal protection. Investigating the specific molecular pathways and signaling cascades through which rosemary and its main components exert their protective effects could offer new information for developing targeted therapies against the toxic effects on kidney function.
3.4.2. Diethylnitrosamine
Diethylnitrosamine, a highly carcinogenic compound, is found in cheese, processed meats, soybeans, tobacco smoke, and several other dietary products. During its metabolism, diethylnitrosamine generates increased levels of ROS, which play a significant role in promoting mutagenesis and cancer development [111]. It has also been shown that diethylnitrosamine caused nephrotoxicity through inducing oxidative stress [113]. Multiple studies have shown that rosemary and its main components have the ability to mitigate diethylnitrosamine-induced nephrotoxicity, as detailed in the subsequent section.
3.4.2.1. In Vivo
3.4.2.1.1. Rosemary Extracts, Powder, or Essential Oil
The possible chemoprotective effects of rosemary extract were assessed against ferric nitrilotriacetate-promoted and diethylnitrosamine-initiated nephrotoxicity in rats. Before receiving a diethylnitrosamine injection, the extracts were taken orally for 14 days. Ferric nitrilotriacetate was then used to cause nephrotoxicity. Following diethylnitrosamine, ferric nitrilotriacetate treatment resulted in acute nephrotoxicity, as evidenced by substantial increases in serum urea, creatinine, uric acid, MDA, TNF-α, and renal tubule epithelial lining vacuolation. The elevated levels of these indicators were considerably reduced by pretreatment with rosemary extract [110].
A study aimed to determine whether R. officinalis powder and essential oil could protect rats’ kidneys from diethylnitrosamine-induced damage. The effects on antioxidant biomarkers, kidney functions, and histopathological characteristics were assessed in rats who received the drug orally over a 2-month period. Serum GPx activity significantly increased, while serum creatinine and urea levels were decreased. Histopathological analyses of the kidneys confirmed the preventive effect of rosemary against renal abnormalities induced by diethylnitrosamine [111] (Table 4).
3.4.2.1.2. Rosmarinic Acid
In rats, the potential chemoprotective benefits of rosmarinic acid were evaluated against diethylnitrosamine-induced and ferric nitrilotriacetate–promoted nephrotoxicity. For 14 days, rosmarinic acid was taken orally prior to receiving an injection of diethylnitrosamine. Then, nephrotoxicity was induced using ferric nitrilotriacetate. Serum urea, creatinine, uric acid, MDA, TNF-α, and renal tubule epithelial lining vacuolation all significantly increased after diethylnitrosamine therapy with ferric nitrilotriacetate, indicating acute nephrotoxicity. Pretreating with rosmarinic acid significantly decreased the high levels of these markers [110].
Rosemary and rosmarinic acid protect against diethylnitrosamine-induced kidney nephrotoxicity, suggesting effective methods to counter the harmful effects of this carcinogenic compound. Research indicates that these compounds reduce nephrotoxicity markers and histopathological abnormalities caused by diethylnitrosamine, potentially alleviating acute kidney damage by targeting oxidative stress and inflammation. The antioxidant properties of rosemary and rosmarinic acid are key factors in their protective effects against diethylnitrosamine-induced renal injury.
While these studies provide important information about the protective effects of rosemary and rosmarinic acid against diethylnitrosamine-induced nephrotoxicity, future research might explore new pathways and molecular mechanisms by which these natural compounds exert their renoprotective effects. Investigation into the synergistic effects of rosemary components and their interactions with cellular signaling pathways may provide new methods for establishing targeted therapeutics against nephrotoxicity caused by carcinogenic chemicals such as diethylnitrosamine.
3.4.3. Naphthalene
Naphthalene, a bioactivated chemical, is a common environmental pollutant found in both the air and water. Human exposure to naphthalene comes from a variety of sources, including industrial processes such as the synthesis of phthalic anhydride for use in resins, plastics, medicines, and insect repellents. Nonoccupational exposure also occurs as a result of diesel fuel emissions. Notably, naphthalene is a major polycyclic aromatic hydrocarbon in cigarette smoke, which poses potential risks to nonsmokers. Originally, naphthalene exposure at home was related to the use of mothballs, which is becoming less common due to its dangerous properties and flammability. Exposure to naphthalene can cause kidney injury, which manifests as changes in kidney tissue structure as well as a significant increase in serum urea and creatinine [112]. Additionally, research on the properties of rosemary to prevent kidney damage from naphthalene has revealed possible treatment approaches for reducing the harmful effects of this dangerous substance, which will be discussed in the following section.
3.4.3.1. In Vivo
3.4.3.1.1. Rosemary Extracts
Rats exposed to naphthalene were shown to exhibit histological alterations such as glomerular congestion, mesangial expansion, tubular dilatation, and vascular congestion, as well as a marked rise in serum urea and creatinine levels. Additionally, changes in the expression of ALP and iNOS in kidney tissues were discovered by immunohistochemistry examination. However, in the group that received rosemary extract, these effects were reversed [112] (Table 4).
Rosemary has shown potential in preventing naphthalene-induced kidney injury by amending histological alterations and reducing urea and creatinine levels. Its antioxidant and anti-inflammatory properties help to inhibit cellular damage induced by naphthalene exposure, making it a favorable treatment option for kidney protection. Investigating the combined benefits of rosemary extracts, essential oils, and components such as rosmarinic acid in preventing naphthalene-induced kidney injury should provide useful findings for renal protection. More research is needed to establish the renoprotective effects of rosemary in other animals and clinical conditions, including dosage effects, long-term results, and potential combinations with other medications to improve its efficacy against nephrotoxicity.
3.5. Food Additives
3.5.1. Aspartame
Aspartame, a widely used sugar substitute, is found in antiulcer medications, soft drinks, and various dietary products to control blood sugar levels and combat obesity. Upon ingestion, it breaks down into aspartic acid, methanol, and phenylalanine. While approved by the Food and Drug Administration (FDA), aspartame remains a topic of controversy due to its potential health effects. Prolonged consumption can lead to liver toxicity by reducing antioxidant levels, particularly GSH. The kidney, known for its crucial role in metabolite excretion, has gained attention in studies investigating its composition and function to assess the toxic effects of aspartame [114]. Research on the nephroprotective effects of rosemary against aspartame will be explored in the following section.
3.5.1.1. In Vivo
3.5.1.1.1. Rosemary Extracts
Examining the nephroprotective properties of rosemary extract against aspartame-induced kidney damage in rats illustrated that pretreatment with rosemary extract significantly raised serum sodium levels while significantly lowering serum urea, creatinine, and K+ levels. In addition, compared to the aspartame group, rosemary extract substantially lowered the increase in lipid peroxidation, raised GSH levels, and enhanced antioxidant enzyme activity in the kidney. Furthermore, when comparing the treated groups to the aspartame group, the histological structure of the kidney was found to be almost normal [115] (Table 5).
| Compound | Study design | Doses/Duration | Results | Ref. |
|---|---|---|---|---|
| Food additives | ||||
| Aspartame | ||||
| In vivo | ||||
| R. officinalis leaves aqueous extract | Male albino rats | 125 mg/kg, every other days, 3 months, p.o. |
|
[115] |
| Potassium bromate | ||||
| In vivo | ||||
| Rosmarinic acid | Male albino Wistar rats | 50 mg/kg, 4 weeks, gavage |
|
[116] |
| Pesticides | ||||
| Chlorpyrifos | ||||
| In vivo | ||||
| R. officinalis leaves ethanolic extract | Male Wistar rats | 100 and 200 mg/kg, 7 days, gavage |
|
[117] |
| Rosmarinic acid | Rats | 25, 50, and 100 mg/kg, 28 days, p.o. |
|
[118] |
| Cypermethrin | ||||
| In vivo | ||||
| R. officinalis leaf ethanol extract | Male rabbits | 100 and 200 mg/kg, 6 weeks, p.o. |
|
[119] |
| Deltamethrin | ||||
| In vivo | ||||
| Rosmarinic acid | Male Wistar albino rats | 20 mg/kg, 7 days, gavage | ↓ BUN, creatinine levels, kidney damage, total antioxidant, total oxidant grades | [120] |
| Others | ||||
| Nicotine | ||||
| In vivo | ||||
| R. officinalis leaves aqueous extract | Adult male guinea pigs | 220 mg/kg, 30 days, gavage |
|
[121] |
- Abbreviations: Bax, Bcl2-associated X protein; Bcl2, B-cell lymphoma 2; BUN, blood urea nitrogen; DNA, deoxyribonucleic acid; GPx, glutathione peroxidase; GSH, glutathione; HO-1, heme oxygenase-1; IL-1_, interleukin-1 beta; iNOS, inducible nitric oxide synthase; Keap1, Kelch-like ECH-associated protein 1; MDA, malondialdehyde; NF-_B, nuclear factor Kappa B; NO, nitric oxide; Nrf2, nuclear factor erythroid 2–related factor 2; ROS, reactive oxygen species; SIRT1, sirtuin 1; SOD, superoxide dismutase; TNF-_, tumor necrosis factor-alpha.
The investigation into the nephroprotective potential of rosemary extract against aspartame-induced kidney damage in rats unveils the underlying mechanisms and pathways involved in this protective effect. However, it is necessary to further explore the specific molecular targets and signaling pathways affected by rosemary extract or its main components. Optimizing dosages and treatment procedures for maximal renoprotective effects is crucial.
3.5.2. Potassium Bromate
Potassium bromate is commonly used in various industries, including food production and processing, with applications in items such as beverages, cosmetics, and pharmaceuticals. Exposure to potassium bromate can occur through bakery products and treated tap water. Once inside the body, potassium bromate generates free radicals, disrupting cellular redox balance and causing tissue damage. Metabolized potassium bromate can produce aggressive byproducts that damage cellular structures and genetic material. Classified as a Class 2B carcinogen, strong oxidant properties of potassium bromate can lead to severe health issues like renal failure, cirrhosis, and cancer with prolonged exposure [122]. Rosmarinic acid has been found to have renoprotective properties against potassium bromate–induced nephrotoxicity, as will be discussed in the following section.
3.5.2.1. In Vivo
3.5.2.1.1. Rosmarinic Acid
The findings of a study that examined the potential of rosmarinic acid in mitigating potassium bromate–induced renal oxidative damage in rats revealed that rosmarinic acid treatment significantly reduced markers of renal toxicity induced by potassium bromate, ameliorated histopathological changes, and modulated the expression of Bax, Bcl2, and iNOS influenced by potassium bromate [116].
The investigation of the preventive advantages of rosmarinic acid against potassium bromate–induced kidney oxidative injury in rats revealed promising results. Rosmarinic acid significantly lowers kidney toxicity markers, improves histopathological alterations, and regulates crucial protein expressions influenced by potassium bromate. Further investigation into the molecular interactions of rosemary components may lead to novel treatment techniques for preventing kidney injury. Understanding these pathways is important for developing methods of therapy and investigating the clinical applications of rosemary extracts and essential oils in preventing potassium bromate–induced kidney oxidative damage.
3.6. Pesticides
3.6.1. Chlorpyrifos
Chlorpyrifos is a common pesticide used indoors as well as outdoors to fight a variety of pests that target crops, fruits, and vegetables. Because chlorpyrifos is commonly used in homes to control flies, cockroaches, and ants, humans may be exposed to it on a daily basis by direct touch, skin absorption, or inhalation. In addition to direct exposure, contaminated food can transmit chlorpyrifos to humans and animals, and residues have been found on the surface of agricultural products at concentrations higher than the acute reference limit. The kidney is one of the major organs that has been related to exposure to chlorpyrifos in various ways. Increased ROS seems to be associated with chlorpyrifos toxicity and that of its metabolites [123]. The preventive effects of rosemary and rosmarinic acid against chlorpyrifos-induced nephrotoxicity will be discussed in the following section.
3.6.1.1. In Vivo
3.6.1.1.1. Rosemary Extracts
The effect of rosemary ethanolic extract was assessed on chlorpyrifos-induced nephrotoxicity in rats. The obtained data illustrated that compared to the control group, chlorpyrifos significantly damaged the kidneys and changed the levels of MDA and GPx. The amounts of MDA and GPx were significantly altered by concurrent exposure to low-dose rosemary extract and chlorpyrifos. Changes in MDA and GPx levels were more in line with the control group when the rosemary extract dosage was increased [117] (Table 5).
3.6.1.1.2. Rosmarinic Acid
An in vivo investigation examined the antioxidant property of rosmarinic acid to prevent kidney damage caused by chlorpyrifos. It was found that chlorpyrifos induced a number of histopathological changes and raised blood urea, creatinine, and renal Kim-1. The kidneys of rats intoxicated with chlorpyrifos showed increased levels of ROS, MDA, NO, NF-κB p65, TNF-α, and IL-1β. In chlorpyrifos-intoxicated rats, rosmarinic acid reduced NF-κB p65, TNF-α, and IL-1β in a dose-dependent manner; avoided tissue damage, lowered ROS, MDA, and NO, and improved kidney function indicators. In rats given chlorpyrifos, rosmarinic acid increased Nrf2, HO-1, and SIRT1, decreased Bax, caspase-3, oxidative DNA damage, and Kelch-like ECH-associated protein 1 (Keap1), and increased antioxidant enzymes and Bcl2. Using molecular docking simulation, the binding affinity of rosmarinic acid for NF-κB, Keap1, HO-1, and SIRT1 was discovered [118].
The strengths of studies lie in its thorough assessment of biochemical markers and histopathological changes, contributing to a detailed understanding of the protective effects of rosemary and rosmarinic acid against chlorpyrifos-induced nephrotoxicity. The demonstrated modulation of crucial molecular targets highlights the therapeutic potential of these compounds in preserving kidney function.
Nevertheless, some limitations persist, necessitating further exploration of the specific molecular pathways influenced by rosemary and rosmarinic acid. Furthermore, the optimization of dosages and treatment programs is crucial to maximize the renoprotective effects of these compounds. Investigating the bioavailability and pharmacokinetics of rosemary and rosmarinic acid could provide valuable results in their mechanisms of action and potential clinical applications for ameliorating chlorpyrifos-induced renal damage.
3.6.2. Cypermethrin
Cypermethrin, a synthetic pyrethroid, has developed as a popular insecticide since the prohibition of dichloro-diphenyl-trichloroethane (DDT). Overuse and unregulated application of this chemical can cause unintended poisoning and have long-term consequences. Enzymes responsible for breaking down pyrethroids in the body include esterases and oxidases, which oxidize via the liver mixed-function oxidase system. As a result, the metabolites produced are removed by the liver and kidneys. The adverse effects of cypermethrin-induced toxic stress can cause kidney damage, potentially leading to renal failure [124]. Several studies have shown that rosemary has the ability to ameliorate nephrotoxicity caused by cypermethrin, which is covered in the next section.
3.6.2.1. In Vivo
3.6.2.1.1. Rosemary Extracts
A study examined the probable protective effects of R. officinalis leaf ethanol extract on rabbits’ kidneys with cypermethrin-induced toxicity. A control group, a group exposed to cypermethrin, a protective group treated with low-dose rosemary extract and cypermethrin, and a group treated with a higher dose of rosemary extract and cypermethrin comprised the four groups of rabbits. The results showed that the group exposed to cypermethrin alone had significantly higher levels of kidney function tests (BUN, creatinine, urea, and uric acid). These values, however, significantly decreased and returned to normal in rabbits treated with rosemary extract and cypermethrin [119].
Even though the study provides insightful information, future research might examine the pharmacological properties of rosemary components, such as bioavailability and metabolism, in order to fully explore their medicinal potential. Researchers may establish novel approaches in nephroprotection and pesticide toxicity control by clarifying the precise mechanisms by which rosemary provides protection against cypermethrin toxicity.
3.6.3. Deltamethrin
Deltamethrin, a very effective synthetic pyrethroid insecticide, is widely used in agriculture due to its low toxicity and quick action. Despite its benefits, its hydrophobic nature causes environmental accumulation, making it a potentially harmful pollutant. Residual deltamethrin threatens human and animal health via a variety of exposure mechanisms, particularly affecting the kidneys, which play an important role in deltamethrin excretion. Accumulation in the kidney can cause renal cell damage, highlighting the susceptibility of organs to deltamethrin exposure [125]. The next part will address how rosmarinic acid can protect against nephrotoxicity caused by deltamethrin.
3.6.3.1. In Vivo
3.6.3.1.1. Rosmarinic Acid
Evaluating the effects of rosmarinic acid against the harmful effects of deltamethrin on rats’ kidneys indicated that the BUN and creatinine levels were lower in the rosmarinic acid plus deltamethrin group than in the deltamethrin-only group. Furthermore, compared to the rosmarinic acid plus deltamethrin group, the damage ratings of the deltamethrin group were more noticeable, and their total antioxidant and total oxidant grades were greater [120] (Table 5).
While the study provides evidence for the protective properties of rosmarinic acid, more research is needed to determine the particular molecular pathways via which it provides renoprotection. The efficacy of rosmarinic acid in alleviating deltamethrin-induced nephrotoxicity may be improved by optimizing dosages and treatments, as well as investigating its pharmacokinetics. By addressing these issues, researchers can improve the understanding of the therapeutic potential of rosmarinic acid and help in finding innovative kidney-protective therapies.
3.7. Others
3.7.1. Nicotine
Smoking tobacco is widely recognized as a prevalent risk factor for multiple illnesses, including kidney disease. Nicotine, a primary constituent of cigarette smoke, is accountable for the addictive nature of smoking. Its influence extends to biological processes such as cell-mediated immunity, apoptosis, and angiogenesis, achieved through interactions with nicotinic acetylcholine receptors. Nicotine plays a crucial role in the renal dysfunction associated with smoking, triggering apoptosis by raising the generation of ROS, arresting the cell cycle, and initiating the MAPK and NF-κB signaling pathways within human renal tubular epithelial cells [126]. Moving on to the protective effects of rosemary against nicotine, research has revealed its ability to reduce the negative effects of this toxic substance.
3.7.1.1. In Vivo
3.7.1.1.1. Rosemary Extracts
The effectiveness of aqueous rosemary extract in reducing nicotine-induced kidney damage in guinea pigs was examined in one study. The animals were divided into four groups: one that received only nicotine, one that received both nicotine and rosemary, a control group, and a rosemary group. In comparison with the control group, rats receiving nicotine showed markedly higher levels of renal enzymes, urea, uric acid, creatinine, and K+, as well as lower levels of total proteins, albumin, and Na+. However, the albumin/globulin ratio and globulin concentrations were not affected. All biochemical markers were markedly ameliorated by co-administration of rosemary extract [121] (Table 5).
Although the study offers convincing proof of the ability of rosemary to prevent nicotine-induced nephrotoxicity, more investigation is required to determine the exact physiological mechanisms at play and improve dose recommendations. To improve the knowledge of the therapeutic potential of rosemary and make additional protection against the nephrotoxic effects of nicotine, it will be crucial to address these issues as well as investigate the pharmacokinetics of rosemary extracts and their main components.
4. Comparative Analysis of Key Studies
To enhance the quantitative comparison, dosage ranges, experimental models, and nephroprotective mechanisms were summarized across studies evaluating rosemary and its primary components against various compounds.
4.1. Cisplatin
Across cisplatin-induced nephrotoxicity, multiple studies demonstrate dose-dependent protective effects of carnosic acid, carnosol, rosmarinic acid, and ursolic acid, with doses ranging from 1 to 200 mg/kg in rodent models. The observed benefits include antioxidant activity, anti-inflammatory effects, and apoptosis inhibition. Notably, rosmarinic acid (200 mg/kg, 7 days) and carnosic acid (100 mg/kg, 10 days) were among the most effective in reducing oxidative stress and inflammatory cytokines.
4.2. Cyclophosphamide
Rosmarinic acid (100 mg/kg, 8 days) demonstrated notable reductions in fibrosis, tubular epithelial vacuolization, and oxidative markers, emphasizing its potential therapeutic application in nephrotoxic injury linked to chemotherapy.
4.3. Doxorubicin
Rosemary aqueous extract (30 mg/kg, 14 days) and rosmarinic acid (75 mg/kg, 14 days) showed significant reductions in blood urea, serum creatinine, lipid peroxidation, and TNF-α, alongside improvements in renal antioxidant enzyme activity.
4.4. Etoposide
Rosemary extract (220 mg/kg, 6 weeks) modulates electrolyte balance and inhibits DNA damage, providing insight into its role in preserving renal function.
4.5. Methotrexate
Methotrexate-induced nephrotoxicity is mitigated by rosmarinic acid (100–200 mg/kg, 12 days), which significantly reduces renal MDA, leukocyte infiltration, and necrosis while improving renal CAT activity.
4.6. Folic Acid
Studies using C57/BL6 mice demonstrate that rosmarinic acid (50–100 mg/kg, 10 days) exerts nephroprotective effects by upregulating SIRT1 and FoxO3 expression, increasing antioxidant defenses, and reducing inflammation, as well as apoptosis markers. These results suggest a dose-dependent improvement in renal function, highlighting rosmarinic acid as a promising candidate for mitigating folic acid–induced renal damage.
4.7. Gentamicin
Multiple studies in rats and guinea pigs confirm the protective role of rosemary extracts, administered at 100–220 mg/kg for 7–12 days. Observed benefits include reductions in serum creatinine, BUN, urea, oxidative stress markers, and histopathological improvements. Notably, rosmarinic acid (50–100 mg/kg, 12 days) enhances antioxidant enzyme activity and improves creatinine clearance, reinforcing its mechanistic role in renal protection.
4.8. Isoniazid®
Studies in albino and Wistar rats using rosemary leaves aqueous extract (440 mg/kg, 4–8 weeks) show modulation of electrolyte balance, restoration of kidney GSH levels, and inhibition of TNF-α and IL-1β-mediated inflammation. Notably, reductions in serum urea, creatinine, uric acid, and oxidative stress further confirm rosemary’s protective effects against isoniazid-induced renal toxicity.
4.9. Lithium
Rosemary extract (220 mg/kg, 4 weeks) significantly reduces plasma MDA, creatinine, and urea, while increasing GSH and SOD levels, confirming its antioxidant and nephroprotective potential in lithium-induced kidney dysfunction.
4.10. Paracetamol
Studies using rosemary extract (125–220 mg/kg, up to 8 weeks) highlight significant improvements in renal antioxidant defenses, electrolyte balance, and reductions in lipid peroxidation and histopathological alterations. These findings indicate rosemary’s therapeutic potential in mitigating oxidative damage caused by paracetamol toxicity.
4.11. Acrylamide
A study using Wistar rats (250 mg/kg, 28 days) demonstrated that rosemary oil enhances renal CAT and GSH levels, while reducing serum creatinine, urea, and lipid peroxidation. These findings suggest potential nephroprotection through antioxidant defense mechanisms.
4.12. Cadmium
Both in vitro and in vivo models reveal significant protective effects of carnosic acid and rosmarinic acid against cadmium-induced renal toxicity.
In vitro: Carnosic acid (1–10 μM, 24 h) and rosmarinic acid (5–60 μM, 24 h) modulate key pathways, including Nrf2 signaling, TGF-β1/Smad3 inhibition, apoptosis suppression, and oxidative stress reduction.
In vivo: Rosmarinic acid (50 mg/kg, 14 days) and carnosic acid (10 mg/kg, 2 weeks) enhance redox defense systems, GSH/GSSG balance, and reduce histopathological damage. Additionally, rosemary aqueous extract (220 mg/kg, 8 weeks) significantly improves antioxidant enzyme activities and reduces glomerular shrinkage and leukocyte infiltration.
4.13. Chromium
Studies on Wistar rats using rosemary essential oil (0.5 mL/kg, p.o.) and rosmarinic acid (25 mg/kg, 60 days) highlight reductions in histopathological alterations, renal TBARS and H2O2 levels, alongside improved oxidative balance.
4.14. Lead
In albino rabbits (30 mg/kg, 30 days, gavage), rosemary ethanolic extract significantly increased renal SOD, CAT, and protein levels, while decreasing serum urea and creatinine, suggesting protective effects through oxidative stress mitigation.
4.15. Nickel Chloride
Studies in Wistar albino rats (100 mg/kg, 28 days, p.o.) report that rosemary methanolic extract enhances renal GPx, CAT, and SOD activity while reducing MDA levels and kidney tissue damage, supporting its antioxidant and nephroprotective role.
4.16. Carbon Tetrachloride
Studies using Wistar albino rats and female mice reveal that rosemary aqueous and ethanolic extracts administered between 100 and 250 mg/kg for 15–30 days enhance antioxidant enzyme activity and mitigate serum creatinine, BUN, lipid peroxidation, and histopathological damage. Notably, ursolic acid (25–50 mg/kg, 6 weeks, i.g.) further supports renal protection by modulating STAT3 and NF-κB signaling, reducing ROS levels and inflammatory cytokines.
4.17. Diethylnitrosamine
Studies in Wistar albino and male albino rats demonstrate that rosemary powder (2.5% diet, 2 months), essential oil (0.02% diet, 2 months), and aqueous extracts (200 mg/kg, 14 days) reduce serum creatinine, urea, uric acid, MDA, and renal TNF-α levels, while improving GPx enzyme activity. Rosmarinic acid (50 mg/kg, 14 days) exhibits similar benefits, further confirming its nephroprotective role.
4.18. Naphthalene
Using Sprague–Dawley rats (10 mL/kg, 30 days, gavage), rosemary aqueous extract increases ALP reaction in proximal tubules, suggesting a protective effect on tubular function. Moreover, reductions in histopathological alterations, serum urea, and creatinine indicate restoration of renal integrity.
4.19. Aspartame
In male albino rats (125 mg/kg, every other day, 3 months), rosemary aqueous extract enhances antioxidant enzyme levels while reducing serum creatinine, urea, lipid peroxidation, and electrolyte imbalances. The prolonged administration suggests potential efficacy in mitigating chronic toxicity from food additives.
4.20. Potassium Bromate
Studies on male albino Wistar rats (50 mg/kg, 4 weeks, gavage) reveal that rosmarinic acid increases antioxidant capacity while significantly reducing oxidative stress markers, serum creatinine, and BUN levels. These findings support its potential role in preventing oxidative damage caused by food additives.
4.21. Chlorpyrifos
Studies in male Wistar rats (100–200 mg/kg, 7 days, gavage) using rosemary ethanolic extract show significant improvements in renal GPx levels and reductions in MDA levels, confirming its role in combating oxidative stress induced by pesticide exposure.
In rats (25–100 mg/kg, 28 days, p.o.), rosmarinic acid enhances Nrf2/HO-1/SIRT1 antioxidant pathways, suppresses NF-κB-driven inflammation, and reduces oxidative stress and DNA damage, highlighting its multidimensional renal protection.
4.22. Cypermethrin
Male rabbits (100–200 mg/kg, 6 weeks, p.o.) treated with rosemary ethanol extract exhibit higher total protein levels and reduced urea, creatinine, and uric acid, indicating protective effects against pesticide-induced nephrotoxicity.
4.23. Deltamethrin
In male Wistar albino rats (20 mg/kg, 7 days, gavage), rosmarinic acid demonstrates significant reductions in BUN, creatinine levels, and total oxidative stress, reinforcing its nephroprotective properties.
4.24. Nicotine
Studies in adult male guinea pigs (220 mg/kg, 30 days, gavage) show that rosemary aqueous extract increases total protein, albumin, and Na+ levels, while reducing urea, uric acid, creatinine, and electrolyte imbalances, suggesting its role in mitigating nicotine-induced renal dysfunction.
Across diverse nephrotoxic models, rosemary and its main components exhibit dose-dependent protective effects through antioxidant, anti-inflammatory, and antiapoptotic mechanisms. Rosmarinic acid consistently demonstrates high efficacy, with optimal doses ranging from 50 to 200 mg/kg, significantly reducing oxidative stress markers, inflammatory cytokines, and histopathological damage in models of cisplatin, methotrexate, and folic acid toxicity. Similarly, carnosic acid (10–100 mg/kg) modulates key pathways like Nrf2 signaling, reinforcing its role in cadmium-induced renal injury. Comparatively, rosemary extracts (100–250 mg/kg) improve electrolyte balance and renal function across gentamicin, lithium, and pesticide-induced nephrotoxicity, suggesting broad applicability. Notably, mechanistic studies reveal that ursolic acid and rosmarinic acid exert superior protection against heavy metal toxicity via STAT3/NF-κB modulation. These findings collectively highlight a consistent pattern of nephroprotection, where efficacy is closely linked to dosage, treatment duration, and mechanistic engagement, supporting rosemary’s therapeutic potential against chemically induced renal damage.
5. Future Perspectives
- 1.
Further investigation into the underlying processes by which rosemary and its main components provide renoprotective advantages against various nephrotoxic substances may reveal important findings. Understanding these pathways at the cellular level can help develop targeted therapeutic strategies.
- 2.
Researching the synergies between rosemary extracts, essential oils, and main components such as rosmarinic acid could reveal the full potential of rosemary in preventing nephrotoxicity. Future research should focus on combinations that improve efficacy while reducing toxicity.
- 3.
Modifying the dosages and treatment procedures of rosemary-based therapies is critical for increasing therapeutic benefits while reducing side effects. Dose–response studies and pharmacokinetic evaluations can assist optimize the use of rosemary to treat nephrotoxicity.
- 4.
Translating preclinical discoveries into clinical trials is essential for proving the efficacy and safety of rosemary-based treatments in humans. Well-designed clinical studies may provide strong evidence of the efficacy of rosemary as a nephroprotective medication.
- 5.
Designing novel formulations of rosemary extracts, such as nanoparticles or liposomes, can improve bioavailability and target delivery to the kidneys. Novel delivery strategies can enhance therapeutic outcomes while minimizing systemic side effects.
- 6.
Longitudinal experiments are required to assess the long-term safety and efficacy of rosemary treatments in reducing nephrotoxicity. Assessing outcomes over time can provide useful information about the long-term advantages of rosemary.
6. Limitations and Risk of Bias
- 1.
Lack of clinical validation: All of the findings discussed in this review are derived from preclinical studies, including in vitro and animal models. No clinical trials have been conducted to establish rosemary’s nephroprotective effects in human populations, which limits its direct translational applicability.
- 2.
Variability in experimental designs: Differences in dosage, extraction methods, administration routes, and nephrotoxic models across studies create challenges in drawing definitive conclusions about rosemary’s effectiveness. Some studies report significant protective effects, while others indicate neutral or inconsistent outcomes, highlighting the need for standardized protocols.
- 3.
Potential risk of publication bias: There may be an inclination for studies reporting positive effects to be more frequently published than those with negative or inconclusive results, possibly influencing the perceived efficacy of rosemary. Addressing this bias requires further investigation into studies with contrasting findings.
- 4.
Limited understanding of long-term safety: While rosemary exhibits promising nephroprotective mechanisms, its long-term safety profile, potential toxicities, and interactions with conventional nephroprotective treatments remain insufficiently studied, necessitating further research.
- 5.
Absence of large-scale comparative analyses: Few studies directly compare rosemary’s efficacy with standard nephroprotective agents, limiting our ability to evaluate its therapeutic advantage or synergistic potential with existing treatments.
Future studies should prioritize clinical validation, standardization of experimental protocols, and comparative analyses to provide a clearer, more objective assessment of rosemary’s nephroprotective effects. Addressing these limitations will help advance its role in renal health while ensuring its safe and effective therapeutic application.
7. Conclusion
A growing body of preclinical evidence suggests that R. officinalis (rosemary) and its main components (carnosic acid, carnosol, rosmarinic acid, and ursolic acid) have the potential for mitigating nephrotoxicity caused by anticancer drugs, pharmaceuticals, industrial toxins, and environmental pollutants. The nephroprotective effects of rosemary are primarily attributed to its antioxidant, anti-inflammatory, and antiapoptotic properties, as well as its ability to regulate key molecular targets, including Nrf2, HO-1, SIRT1, NF-κB, and Keap1.
Additionally, recent findings highlight rosemary’s influence on pyroptosis, an inflammatory form of programmed cell death implicated in renal damage. Rosemary and its bioactive components suppress pyroptosis by modulating NF-κB/NLRP3 inflammasome activation, downregulating caspase-1/GSDMD-mediated cell death, and reducing inflammatory cytokine release in models of cisplatin- and folic acid–induced nephrotoxicity. However, further mechanistic studies are needed to confirm these effects across broader nephrotoxic models.
While significant progress has been made in understanding the mechanistic role of rosemary in renal protection, several translational gaps remain. Future research must prioritize pharmacokinetic evaluations to define the absorption, distribution, metabolism, and excretion (ADME) profiles of rosemary’s bioactive compounds. Optimizing therapeutic formulations and drug delivery systems is crucial for enhancing bioavailability, as some active components exhibit limited systemic absorption. Additionally, long-term safety assessments and investigations into potential interactions with conventional nephroprotective agents are essential to ensure clinical viability.
Further efforts should focus on rigorous evaluation of dosage relevance, comparing effective preclinical concentrations to practical human doses. Exploring synergistic effects with existing treatments and combination therapies may enhance efficacy while minimizing toxicity. By advancing research on pharmacokinetics, bioavailability, and safety profiles, rosemary-based interventions could evolve into viable nephroprotective strategies with applications in preventive care and adjunct treatment for kidney disorders. Bridging these research gaps will be key to translating experimental findings into clinical applications, paving the way for new therapeutic innovations in renal health.
Nomenclature
-
- 4-HNE
-
- 4-Hydroxynonenal
-
- α-SMA
-
- Alpha-smooth muscle actin
-
- ALP
-
- Alkaline phosphatase
-
- Apaf-1
-
- Apoptotic protease activating factor 1
-
- ASC
-
- Apoptosis-associated speck-like protein containing a CARD
-
- Bad
-
- Bcl2-associated death promoter
-
- Bax
-
- Bcl2-associated X protein
-
- Bcl2
-
- B-cell lymphoma 2
-
- BUN
-
- Blood urea nitrogen
-
- CAT
-
- Catalase
-
- COX-2
-
- Cyclooxygenase-2
-
- CRP
-
- C-reactive protein
-
- Ctr1
-
- Copper transporter 1
-
- CYP2E1
-
- Cytochrome P450 2E1
-
- DDT
-
- Dichloro-diphenyl-trichloroethane
-
- DNA
-
- Deoxyribonucleic acid
-
- FAS
-
- Fatty acid synthase
-
- FDA
-
- Food and Drug Administration
-
- FoxO3
-
- Forkhead box O3
-
- GFR
-
- Glomerular filtration rate
-
- GGT
-
- Gamma-glutamyl transferase
-
- GPx
-
- Glutathione peroxidase
-
- GR
-
- Glutathione reductase
-
- GSDMD
-
- Gasdermin D
-
- GSH
-
- Glutathione
-
- GSSG
-
- Oxidized glutathione
-
- GST
-
- Glutathione S-transferase
-
- HMGB1
-
- High-mobility group box 1
-
- HO-1
-
- Heme oxygenase-1
-
- IL
-
- Interleukin
-
- iNOS
-
- Inducible nitric oxide synthase
-
- Keap1
-
- Kelch-like ECH-associated protein 1
-
- Kim-1
-
- kidney injury molecule-1
-
- LC3/B
-
- Microtubule-associated proteins 1A/1B light chain 3B
-
- MAPK
-
- Mitogen-activated protein kinase
-
- MDA
-
- Malondialdehyde
-
- NADPH
-
- Nicotinamide adenine dinucleotide phosphate
-
- NF-κB
-
- Nuclear factor kappa B
-
- NGAL
-
- Neutrophil gelatinase-associated lipocalin
-
- NLRP3
-
- NOD-, LRR-, and pyrin domain-containing protein 3
-
- NO
-
- Nitric oxide
-
- NOX1
-
- NADPH oxidase 1
-
- Nrf2
-
- Nuclear factor erythroid 2–related factor 2
-
- PKC-δ
-
- Protein kinase C-delta
-
- ROS
-
- Reactive oxygen species
-
- SIRT1
-
- Sirtuin 1
-
- Smad
-
- Mothers against decapentaplegic homolog
-
- SOD
-
- Superoxide dismutase
-
- STAT3
-
- Signal transducer and activator of transcription 3
-
- TBARS
-
- Thiobarbituric acid reactive substances
-
- TGF-β1
-
- Transforming growth factor beta 1
-
- TNFR2
-
- Tumor necrosis factor receptor 2
-
- TNF-α
-
- Tumor necrosis factor alpha
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Jalileh Jalali rose to the notion and helped in gathering the data. Mahboobeh Ghasemzadeh Rahbardar wrote the manuscript together. Both authors approved the final version of the manuscript.
Funding
No funding was received for this manuscript.
Acknowledgments
Declaration of Generative AI and AI-Assisted Technologies in the Writing Process. During the preparation of this work, the author(s) used ChatGPT and QuillBot in order to rephrase to reduce plagiarism and improve the language and grammar. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Open Research
Data Availability Statement
No new data were created or analyzed during this study.




