Research Progress on the Extraction, Structure Elucidation, Biological Activities, and Drug Carrier Applications of Polygonati Rhizom Polysaccharides
Abstract
As widely used in traditional Chinese medicine and nutritional food, Polygonati Rhizom is known for its diverse pharmacological properties, including hematopoiesis, immune enhancement, antitumor, anti-inflammatory, antioxidant, antiaging, antiviral, and hepatoprotection. Polygonati Rhizom polysaccharides (PRPs) are primary bioactive compounds that are water-soluble and mainly comprise fructose (Fru), glucose (Glc), galactose (Gal), arabinose (Ara), rhamnose (Rha), fucose (Fuc), xylose (Xyl), glucuronic acid (GlcA), and galacturonic acid (GalA). Meanwhile, different extraction methods, such as hot water and ultrasonic extractions, can significantly affect the yield of PRPs, Additionally, PRPs were also reported to hold promising potential as a drug carrier. Herein, the extraction, structure elucidation, biological activities, and drug carrier applications of PRPs are systematically summarized, providing reference for the further research and development of Polygonati Rhizom.
1. Introduction
Polygonati Rhizom, the dried rhizomes of Polygonatum sibiricum Delar. ex Redoute, Polygonatum kingianum Coll. et Hemsl, and Polygonatum cyrtonema Hua, is recorded in Chinese Pharmacopoeia (2020 edition) and is commonly known as “huangjing” [1]. These rhizomes are from perennial herbaceous plants belonging to the Liliaceae family, which are widely distributed in the temperate regions of Asia, Europe, and North America. These plants have edible tender stems, and the nutritious leaves are used as a vegetable [2]. Polygonati Rhizom is an important traditional medicine and shows great potential in modern drug research and health-product development [3]. In traditional Chinese medicine, Polygonati Rhizom is used to nourish the kidneys, moisten the lungs, and relieve coughs. Additionally, processed Polygonati Rhizom can be used to make healthy teas or medicinal dietary products [4].
It has been reported that Polygonati Rhizom contains abundant polysaccharides, saponins, flavonoids, and other bioactive components with various pharmacological effects, including immune modulation, antifatigue, and antiaging effects [5]. Among them, polysaccharides are considered to be the primary functional components, thereby supporting its traditional application [5]. To date, 53 Polygonati Rhizom polysaccharides (PRPs) have been reported, with important in vivo and in vitro pharmacological activities, such as immunomodulatory, antidepressant, antioxidant, osteogenic, antitumor, and so on [5]. In this review, the recent research progress on the extraction, structure elucidation, biological activities, and drug carrier applications of PRPs are summarized, which provide a theoretical basis for further studies on this traditional Chinese medicinal material.
2. Extraction, Separation, and Purification Methods of PRPs
The biological activity of polysaccharides primarily depends on their specific chemical structure, which is closely related to the extraction process employed [6]. Thus, this review first summarizes the extraction processes of PRPs from Polygonati Rhizom, which are presented in Figure 1.
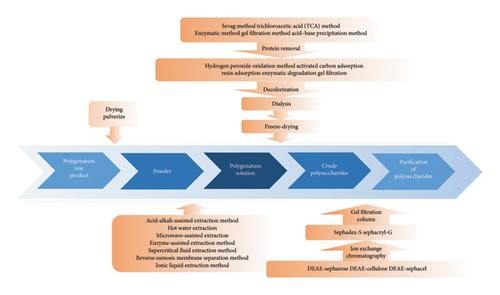
2.1. Extraction Methods
Polysaccharides often exhibit various characteristics depending on the extraction method employed. The commonly used extraction methods include acid–alkali enzymatic-assisted extraction, hot-water extraction, ultrasound-assisted extraction, microwave-assisted extraction, enzyme-assisted extraction, and supercritical-fluid extraction [7]. Hot-water extraction is the most common for PRPs. The extraction rate, chemical composition, and biological activities of the resulting polysaccharides are affected by the extraction method [8]. The advantages and disadvantages of these common extraction methods are summarized in Table 1.
| Extraction method | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Acid–alkali-assisted extraction | It can effectively degrade the cell wall and improve the extraction rate | It may lead to the structural damage of polysaccharides, rendering it necessary to control acidity, alkalinity concentration, and temperature | [9, 10] |
| Hot-water extraction | Simple operation, nontoxic, and pollution-free | Long extraction time and relatively low extraction efficiency | [11] |
| Microwave-assisted extraction | High extraction efficiency and short extraction time | It may cause the structural damage of polysaccharides | [12–14] |
| Enzyme-assisted extraction | Mild operating conditions and high extraction efficiency | Expensive and sensitive to temperature and pH | [15] |
| Supercritical fluid extraction | Low temperature, pollution-free, and high extraction efficiency | High equipment requirements and operating costs | [16] |
2.1.1. Acid–Alkali-Assisted Extraction
Acid-assisted extraction is used to obtain polysaccharides with high purity. However, acidic conditions may cleave glycosidic bonds and damage the original polysaccharide structure [9]. Alkaline conditions, on the other hand, facilitate acidic polysaccharide extraction and reduce extraction time but may increase viscosity and lead to structural damage [10]. Thus, the acidity, alkalinity, and temperature in acid–alkali-assisted extraction must be carefully controlled. Additionally, neutralization or dialysis is often required following this process [17]. DPSP is a polysaccharide extracted from Polygonati Rhizom using a deep eutectic solvent (DES; choline chloride and oxalic acid in a 1:1 mass ratio). This extraction method achieved a DPSP yield of 15.62 ± 0.71%, which was 1.53 times higher than that obtained using the pharmacopeia method (PSP) [18]. Compared to polysaccharides extracted by traditional methods, DPSP exhibited a lower molecular weight (3.2 × 106 Da) and higher contents of galactose (65.75%) and mannose (19.76%). In another study, a DES composed of choline chloride and 1,4-butanediol at a molar ratio of 1:4 was used to extract PRPs. This method significantly increased the polysaccharide yield, reaching 33.81 ± 0.36%, which was approximately four times higher than that achieved by hot-water extraction [19].
2.1.2. Hot-Water Extraction
Characterized by its simple equipment, convenient operation, low cost, and safety, the hot-water extraction method is currently the most commonly used approach for extracting polysaccharides. The principle is based on the fact that polysaccharides are soluble in hot water but insoluble in organic solvents, such as ethanol, while heat can be used to accelerate intermolecular movement [11]. Hot-water extraction expedites the separation of cell wall components, thereby facilitating the dissolution of cellular contents to obtain an aqueous extract containing polysaccharides [11]. Then, the polysaccharides are subsequently precipitated from the resulting aqueous extract using organic solvents. However, the hot-water extraction method has certain limitations, such as a low extraction efficiency and prolonged extraction time. Therefore, to improve polysaccharide extraction processes, various statistical design methods, including orthogonal and single-factor experiments and response surface analyses, have been widely applied for optimization. For example, Guo et al. used response surface methodology to determine the optimal extraction conditions for P. cyrtonema Hua, which were a solid-to-liquid ratio of 1:25, an extraction temperature of 85°C, and an extraction time of 2.0 h. Under these conditions, the polysaccharide extraction rate was 13.36% [20]. Furthermore, Wang et al. study indicated that the extraction conditions for P. cyrtonema Hua polysaccharides were a solid-to-liquid ratio of 1:25, an extraction temperature of 80°C, and an extraction time of 2 h, resulting in an extraction rate of 49.21% [21]. Xiao et al. optimized the extraction process of P. kingianum polysaccharides, and the results showed that the optimal extraction parameters for P. kingianum polysaccharides were a solid-to-liquid ratio of 1:30 (g/mL), an extraction time of 1.5 h, an extraction temperature of 80°C, with an extraction rate of 20.70% [22].
2.1.3. Ultrasound- and Microwave-Assisted Extraction
Ultrasound-assisted extraction utilizes the mechanical oscillations generated by ultrasound to accelerate polysaccharide molecular motion, effectively breaking down cell walls and tissues and promoting the large-scale release of polysaccharides [12]. In addition, microwave-assisted extraction uses high-frequency microwave heating to facilitate the release of intracellular polysaccharides and other active substances [13]. Both methods effectively increase the extraction rate and reduce extraction time but may significantly damage the structure of thermally unstable polysaccharides [14]. In a recent study, pretreated Polygonati Rhizom powder was added to an ultrasonic device with water at a 1:30 (w/v) ratio and then subjected to ultrasound extraction at 60°C and 100 W for 40 min. The mixture was subsequently heated in a water bath at 80°C for 80 min, a process repeated twice. Polysaccharides, labeled as PRP, were obtained via alcohol precipitation, filtration, and lyophilization, yielding 5.83% ± 2.52%. Another study determined the optimal conditions for PRP extraction as follows: a liquid-to-solid ratio of 26:1 (mL:g), an ultrasonic power of 82 W, an ultrasonic duration of 51 min, and an extraction temperature of 80°C. Under these conditions, the extraction yield of PSP reached a maximum of 43.61% [23].
2.1.4. Enzyme-Assisted Extraction
During the PRP extraction process, a significant number of insoluble substances, including cellulose, hemicellulose, and pectin, in the cell wall typically impacts extraction efficiency. Therefore, appropriate enzyme preparations are commonly added to the extraction solution to effectively degrade cellulose cross-linking, disrupt the dense structure of the cell wall, and increase the extraction rate of polysaccharides. The enzyme-assisted extraction method offers the advantages of mild conditions and a high extraction rate. However, it has several limitations, such as high cost, strong specificity, and sensitivity to temperature and pH [15]. In a previous study, pretreated Polygonati Rhizom powder with water (1:30, w/v) was added to 2% cellulase and papain. The mixture was activated at 40°C, and the pH was adjusted to 5.5 for a 2-h enzymatic hydrolysis. Subsequently, the solution was boiled for 10 min to deactivate the enzymes, and the mixture was filtered, concentrated, precipitated with 95% ethanol (1:4), and dried to obtain crude polysaccharides with a yield of 12.00% ± 3.26% [23].
2.1.5. Supercritical Fluid Extraction
Supercritical fluid extraction utilizes supercritical fluids, which exist in a state between a liquid and gas, to penetrate the matrix of the material being extracted and effectively extract desired components [16]. Its advantages include low-temperature and pollution-free operation, high extraction efficiency, high product purity, and the minimal degradation of biological activity. However, it requires high equipment specifications and operating costs and has a limited range of substances that can be extracted. Relevant literature reports that a liquid–liquid aqueous two-phase system has been developed, using tetrabutylammonium bromide (TBABr) to separate polysaccharides and proteins extracted from Polygonatum rhizomes. At 25°C, with 1.5 g of TBABr and 2.0 g of magnesium sulfate (MgSO4), the extraction yields of proteins and polysaccharides reached 98.6% and 93.5%, respectively [24, 25].
2.2. Protein Removal
The extract from polysaccharide extraction typically contains proteins. Thus, to enhance the purity of the extracted polysaccharides, certain separation and purification measures are required. These approaches include the Sevag [6], trichloroacetic acid [26], freeze–thaw [27], gel filtration [28], and acid–base precipitation methods [29]. Their advantages and disadvantages are detailed in Table 2.
| Protein removal method | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Sevag method | Simple operation, effective protein removal, and minimal impact on its structure | It cannot remove all types of proteins, and residual proteins may still be present | [6] |
| Trichloroacetic acid method | Noticeable effects | It may lead to the degradation and loss of polysaccharides, potentially affecting their properties and activities | [26] |
| Freeze–thaw method | Lower cost compared to other methods and suitable for various sample types | It may lead to the potential loss or denaturation of proteins | [27] |
| Gel filtration method | Simple and effective | It may require longer processing times and lacks specificity for certain proteins with small molecular weights | [28] |
| Acid–alkali precipitation method | Simple operation and low cost | The removal of proteins may not be thorough, thus affecting the properties and activity of polysaccharides, and it may pollute the environment | [29] |
2.3. Decolorization
To improve the purity of PRPs and prevent interference from pigments in experimental results, a decolorization process is commonly employed. Common methods for decolorizing polysaccharide solutions include hydrogen peroxide oxidation [30], activated carbon adsorption [31], resin adsorption [32], and enzymatic digestion [33]. The advantages and disadvantages of various pigment removal methods are summarized in Table 3.
| Method | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Hydrogen peroxide oxidation | Significant decolorization effect that rapidly reduces the concentration of pigments in the solution | It may cause polysaccharide oxidation, resulting in partial degradation | [30] |
| Activated carbon adsorption | Efficient adsorption capacity and effectively removes pigments and other impurities | It may reduce the polysaccharide yield | [31] |
| Resin adsorption | Resin exhibits notable selectivity and efficiently removes pigments and other ionic impurities | The operation is complex, requiring periodic resin replacement and regeneration, resulting in high operational expenses | [32] |
| Enzymatic digestion | Minimal impact on polysaccharide structure and high specificity | Longer reaction time and suitable conditions are required, and the activity and stability of protease enzymes may be affected | [33] |
2.4. Dialysis
The purpose of dialysis is to separate and purify polysaccharides from other small molecules or impurities in the solution by allowing small molecules and ions to pass through a semi-permeable membrane while retaining large molecules such as polysaccharides and proteins. This process helps improve the purity of the polysaccharide solution by removing impurities and adjusting the concentration difference between polysaccharides and other solutes, making it more suitable for specific applications [34].
2.5. Column Chromatography
Column chromatography is often used to separate polysaccharides from other compounds or impurities. This method uses adsorbents or gels packed in the column for physical or chemical separation, thereby enhancing the purity of the polysaccharide product [35].
3. Chemical Composition and Structure of PRPs
Depending on the extraction techniques and procedures used, the monosaccharide composition of PRPs can significantly vary [36]. As shown in Table 4, 53 polysaccharides have been extracted and identified from Polygonati Rhizom. The relative molecular masses of homogeneous polysaccharides isolated from Polygonatum spp. primarily ranges from 3.8 to 630 kDa. They include various monosaccharides such as fructose (Fru), glucose (Glc), galactose (Gal), arabinose (Ara), rhamnose (Rha), fucose (Fuc), xylose (Xyl), glucuronic acid (GlcA), and galacturonic acid (GalA).
| Component | Relative molecular mass | Monosaccharide composition | Structure | Ref. |
|---|---|---|---|---|
| P. cyrtonema Hua | ||||
| PCP | 8.50 × 103 | Fru-Glc (28:1) | β-D-Fruf-(2⟶, ⟶6)-β-D-Fruf-(2⟶, ⟶1,6)-β-D-Fruf-(2⟶, ⟶1)-β-DFruf-(2⟶, ⟶6)-α-D-Glcp-(1⟶ | [37] |
| PCP | 8.84 × 103 | Fru-Glc-Gal (92.73:6.37:0.90) | — | [38] |
| HPCP | 5.52 × 103 | Fru-Glc-Gal-Xyl-Ara (60.16:22.35:13.03:1.35:3.12) | — | [38] |
| PCP | 8.91 × 103 | Fru-Glc (8.7:1) | β-Gal | [39] |
| PCP-1 | 4.80 × 103 | Fru-Glc (28:1) | ⟶1)-β-D-Fruf-(2⟶, ⟶6)-β D-Fruf-(2⟶, ⟶1, 6)-β-D-Fruf-(2⟶, β-D-Fruf-(2⟶, ⟶6)-3-acetyl-α-D-Glcp-(1⟶ | [40] |
| PCP1 | 2.09 × 103 | Ara-Gal-Glc-Man-GlcA-GalA (2.1:24:20.7:33.5: 0.5:19.3) | — | [41] |
| PCP2 | 3.86 × 104 | Ara-Gal-Glc-Man-Xyl-GlcA-GalA (18.5:59.8:9:2.3:0.4:5.3:4.7) | — | [41] |
| PCP3 | 4.26 × 104 | Ara-Gal-Glc-Man-Xyl-GlcA-GalA (22.2:58.7:3.9:4.9:0.5:8.5:1.5) | — | [41] |
| PCP4 | 3.43 × 104 | Ara-Gal-Glc-Man-Xyl-GlcA-GalA (21:61.3:2.7:6.7:0.4:7.9:0.1) | — | [41] |
| PCP5 | 2.41 × 104 | [41] | ||
| PPC1 | 7.02 × 103 | Gal | β-D-Galp-(1⟶, ⟶4)-β-D-Galp-(1⟶, ⟶4,6)-β-D-Galp-(1⟶ | [42] |
| DPC1 | 3.80 × 103 | Glc-Fru (1:26) | ⟶6)-β-D-Fruf-(2⟶, ⟶1, 6)-β-D-Fruf-(2⟶, β-D-Fruf-(2⟶, ⟶1)-β-D-Fruf-(2⟶, ⟶6)-α-D-Glcp-(1⟶ | [42] |
| PFOS-1 | 1.029 × 103 | Glc-Fru (1:5) | α-D-Glcp-1⟶, ⟶2-β-D-Fruf-1⟶, ⟶2-β-D-Fruf-1⟶ | [43] |
| PFOS-2 | 1.663 × 103 | Glc-Fru (1:9) | α-D-Glcp-1⟶, ⟶2-β-D-Fruf-1⟶, ⟶2-β-D-Fruf-1⟶ | [44] |
| PCP-F1 | — | Glc-Man-Gal-Rha-GalA (3.5: 2.5: 1.3: 1.8: 0.8) | ⟶3)-Glcp, ⟶1)-Manp (3⟶, ⟶3)-Rhap (4⟶, ⟶2)-Glcp (3⟶, ⟶1)--Glcp (2⟶, ⟶2) -Glcp (6⟶, ⟶2)-Galp (4⟶, ⟶2, 4)-Manp (6⟶, ⟶2)-GalAp (3, 4⟶, ⟶2)-Galp (3, 4⟶ | [22] |
| PKP | 3.60 × 104 | Man-Gal-Glc-Ara-Xyl-Fuc-Rha-GlcA-GalA (43.32:20.04:19.87:8.67:2.21:1.11:0.91:0.52:0.29) | — | [44] |
| P. kingianum Coll | ||||
| SPSP | 1.80 × 103 | Fru-Glc (10:1) | α-D-Glcp-(1⟶, ⟶1, 6)-β-D-Fruf-(2⟶, ⟶1)-β-D-Fruf-(2⟶, β-D-Fruf-(2⟶ | [45] |
| PKP | 1.41 × 105 | Gal-Gala-Ara-Glc (57.67:26.82:4.59:4.54) | — | [44] |
| PKPS-1 | 1.40 × 104 | Glc-Man-GalA-Gal-GlcA-Ara (7.22:1.0:0.16:0.11:0.05:0.02) | — | [20] |
| PSF | 1.79 × 105 | Man-GalA-Gal-Fuc | — | [46] |
| PS | 1.35 × 105 | Man-GalA-Gal-Fuc | — | [46] |
| P. sibiricum Delar | ||||
| PS | 6.3 × 105 | Fru-Rha-Ara-Gal-Glc-GalA (33.3: 6.6: 1.6: 22.9: 3.5: 32.1) | — | [47] |
| PsPs | — | Man-Rha-Gala- Glc -Xyl-Ara (6.6:15.4:4.5:8.8:40.7:24) | — | [48] |
| PSP | — | Gal-Rha-Man-Glc-Xyl (63.50:25.14:8.04:1.75:1.57) | — | [49] |
| PSP | 9.51 × 104 | Gal-Rha-Ara-Man-Glc (11.72:1.78:4.15:1.00:2.48) | α-D-Gal | [50] |
| PSP | — | Ara-Glc-GlcA-Gal-GalA-Man-Rha-Rib (13.7:82.9:3.7:36.2:4.3:52.5:3.3:1.0) | — | [51] |
| PSP | 1.0 × 104 | Fru-Gal-Glc-GalA (68.9: 11.0: 9.4: 10.7) | ⟶1)-β-D-Fruf-(2⟶, β-D-Fruf-(2⟶, ⟶1, 6)-β-D-Fruf-(2⟶, ⟶6)-β-D-Fruf-(2⟶ | [52] |
| PSP1 | 4.42 × 103 | Man-Glc-Gal (14.96:2.13:82.91) | β-Gal | [53] |
| PSP2 | 2.24 × 103 | Rha-Glc-Gal-Xyl (20.51:2.06:74.37:3.03) | β-Gal | [53] |
| PSP3 | 7.74 × 103 | Man-Rha-Glc-Gal-Xyl (1.38: 57.69: 2.02: 37.17: 1.74) | β-Gal | [53] |
| PSP4 | 6.47 × 103 | Man-Rha-Gal-Xyl (2.00: 72.63: 20.74: 4.63) | β-Gal | [53] |
| PS-WNP | 7.60 × 104 | Gal-Man (12.1: 5.4) | Manp-(1⟶, 6⟶)-Galp-(1⟶, 2, 6⟶)-Galp-(1⟶ | [53] |
| PSPJWA | 1.41 × 105 | Gal-Ara-Rha (14: 4: 1) | ⟶2, 4)-α-L-Rhap-(1⟶, α-L-Araf- (1⟶, ⟶3, 5)-α-L-Araf-(1⟶, ⟶5)-α-L-Araf-(1⟶, ⟶4)-β-D-Galp-(1⟶, ⟶4, 6)-β-D-Galp-(1⟶, β-D-Galp-(1⟶ | [54] |
| PSP-1 | 3.87 × 104 | Glc | β-D-Glcp-(1⟶, ⟶4)-α-D-Glcp-(1⟶, ⟶4, 6)-α-D-Glcp-(1⟶ | [55] |
| PSP1 | 3.22 × 105 | Gal-Man-Glc-Fru | ⟶1)-β-D-Fruf-(2⟶, ⟶6)-α-D-Glcp-(1⟶, ⟶4)-β-D-Manp-(1⟶, ⟶1, 6)-β-DFruf-(2⟶, β-D-Fruf-(2⟶, ⟶6)-β-D-Fruf-(2⟶, ⟶4)-β-D-Glcp-(1⟶ | [56] |
| PSP50-2-1 | 7.70 × 103 | Gal-Glc-Fru (53.22: 15.59: 31.18) | β-D-Fruf-(2⟶, ⟶2)-β-D-Galp-(1⟶, ⟶2, 6)-β-D-Galp-(1⟶, α-D-Glcp-(1⟶ | [57] |
| PSP50-2-2 | 7.00 × 103 | Gal-Glc-Fru (64.85: 27.22: 7.92) | β-D-Galp-(1⟶, ⟶2)-β-D-Galp-(1⟶, ⟶6)-α-D-Galp-(1⟶, ⟶2, 6)-β-D-Galp-(1⟶, β-D-Glcp-(1⟶, β-D-Fruf-(2⟶ | [57] |
| F1 | 1.03 × 105 | Man-Glc-Ara-Gal (76.3:15.2: 4.00: 4.5) | ⟶ 4)-Man-(1⟶, ⟶ 4)-Glc-(1⟶, ⟶ 4, 6)-Man-(1⟶, ⟶ 5)-Ara-(1⟶, Glc-(1⟶, Gal-(1⟶ | [58] |
| F2 | 6.28 × 105 | Man-Glc-Ara-Gal (67.7: 20.3: 7.65: 4.35) | — | [58] |
| PSW-1b-2 | 4.20 × 104 | Gal | β-D-Galp-(1⟶, ⟶4)-β-D-Galp-(1⟶, ⟶4, 6)-β-D-Galp-(1⟶ | [59] |
| PSW-1a | — | Man-Gal (88.8: 11.2) | β-D-Galp-(1⟶, ⟶4)-β-D-Manp-(1⟶, ⟶4, 6)-β-D-Manp-(1⟶ | [59] |
| PSPC | 4.01 × 103 | Gal-Man-Glc-GalA (29.63: 36.1: 15.09: 10.20) | — | [60] |
| PSPW | 1.42 × 104 | Gal-Man-GalA-Rha (78.77: 5.50: 13.84: 1.85) | — | [60] |
| PSP | 6.1 × 103 | Man-Glc-Gal-Ara (51.2: 21.4: 26.9: 0.5) | — | [61] |
| PSP1 | 1.03 × 104 | Man-Glc-Gal-Ara (44.0: 40.2: 14.3: 1.5) | — | [61] |
| PSP2 | 4.6 × 103 | Man-Glc-Gal-Ara (38.6: 45.1: 13.9: 2.4) | — | [61] |
| PSP3 | 4.4 × 103 | Man-Glc-Gal-Ara (47.5: 39.1: 10.8: 2.7) | — | [61] |
| PSP4 | 5.1 × 103 | Man-Glc-Gal-Ara (22.2:64.0:9.0:4.9) | — | [61] |
| PSP5 | 4.43 × 104 | Man-Glc-Gal-Ara (18.2: 65.5: 13.4: 3.0) | — | [61] |
| PSP6 | 7.53 × 104 | Man-Glc-Gal--Ara (19.8: 57.1: 20.2: 2.9) | — | [61] |
| PSP7 | 6.44 × 104 | Man-Glc-Gal-Ara (18.0: 44.2: 32.5: 5.3) | — | [61] |
| PSP8 | 4.45 × 104 | Man-Glc-Gal-Ara (29.4: 33.4: 33.5: 3.8) | — | [61] |
| PSP9 | 5.75 × 104 | Man-Glc-Gal-Ara(17.5:46.5:30.1:5.9) | — | [61] |
3.1. Molecular Weight and Monosaccharide Composition of PRPs
Due to differences in extraction methods and chromatographic columns [62], the reported PRPs have different chemical structures, as shown in Figures 2 and 3. Polysaccharides that were extracted from P. cyrtonema Hua were primarily acidic, possessed molecular weights ranging from 1.029 to 42.6 kDa, and primarily comprised Fru, Glc, and Man. A homogeneous polysaccharide, PCP1, that primarily comprised Fru, Glu, and Man was extracted and purified from the rhizomes of P. cyrtonema Hua. A chemical structural analysis revealed that the main chain of PCP1 predominantly comprised ⟶1)-β-D-Fruf-(2⟶ and ⟶1,6)-β-D-Fruf-(2⟶, with minor amounts of ⟶6)-α-D-Glcp-(1⟶, ⟶4)-β-D-Manp-(1⟶, and β-D-Glcp-(1⟶. The side chain was β-D-Fruf-(2⟶ linked at the C-6 position of ⟶1,6)-β-D-Fruf-(2⟶ [36]. Meanwhile, the polysaccharides extracted from P. sibiricum were primarily neutral polysaccharides, possessed molecular weights ranging from 2.24 to 630 kDa, and comprised Glu, Man, and Gal. Two neutral polysaccharides, PSW-1b-2 and PSW-1a, were isolated and purified from P. sibiricum. PSW-1b-2 was characterized as a branched homogalactan, containing a 1,4-linked β-D-galactopyranosyl backbone with one β-D-galactopyranosyl stub substituted at the O-6 of every 7th backbone residue. Conversely, as a highly branched galactomannan possessing a 1,4-linked β-D-mannopyranosyl backbone, PSW-1a had a β-D-galactopyranosyl stub attached to the O-6 of every 9th mannosyl residue [20]. Among the polysaccharides from P. kingianum, the amounts of neutral and acidic polysaccharides were similar. They possessed molecular weights ranging from 1.8 to 179 kDa and primarily comprised Fru, Glu, Man, and Gal. Two acid polysaccharides (PSF and PS) were isolated from P. kingianum, and they exhibited uronic acid contents of 25.06% and 27.81%, respectively, and HPGPC revealed that the average molecular weights of PSF and PS were 178.6 and 134.7 kDa, respectively. Moreover, they both comprised Man, GalUA, Gal, and Xyl, with slight variations in their proportions [46]. A neutral polysaccharide with an average molecular weight of 14.05 kDa, PKPs-1, was isolated from P. kingianum and primarily composed of Glc and Man [20].
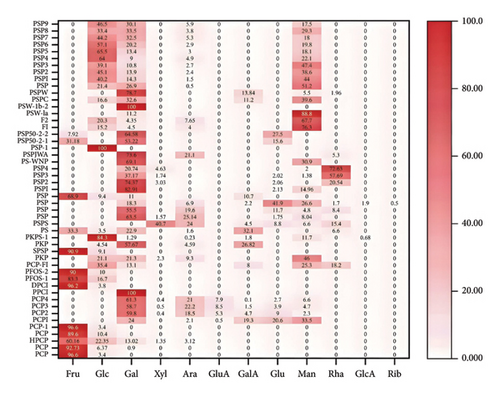
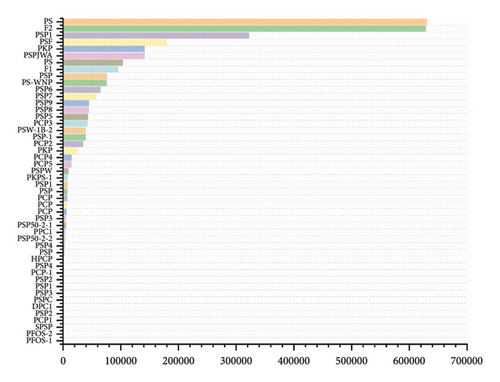
3.2. Modification of PRPs
The biological functionality of polysaccharides can typically be altered by modifying their structure [63]. These modifications can either add or degrade structural components of the polysaccharides. Various methods are employed for this purpose, including chemical, biological, and physical modifications.
Regarding chemical methods, response surface methodology can optimize the preparation conditions for cubes of PRPs modified with cetyltrimethylammonium bromide (CTAB). This specific modification can slow release behavior in vitro, reduce cytotoxicity to a certain extent, and more effectively promote cell proliferation [64]. Regarding biological methods, PRPs can be treated via fermentation, followed by ultrafiltration to fractionate the hydrolyzed polysaccharides. Fermentation can reduce the primary molecular weight distribution of PRPs from 50–650 to 2–100 kDa, altering their chemical composition and monosaccharide profile. Notably, PRPs modified using fermentation exhibited enhanced antiaging activities in vitro, including antioxidant, hypoglycemic, and hypolipidemic effects, and improved cellular antiaging properties [65]. Four polysaccharides have been isolated from crude Polygonati Rhizoma (P-1: 71.40%, P-2: 1.95%, P-3: 1.14%, and P-4: 1.64%) using physical methods. The molecular weight of P-1 decreased from 2.99 × 105 to 2.33 × 103 Da following microwave-assisted degradation. Notably, the degraded P-1 exhibited approximately eight times higher antioxidant activity than the natural P-1 [59].
3.3. Structure of PRPs
High-performance gel permeation chromatography (HPGPC), Fourier-transform infrared spectroscopy (FT-IR), ultraviolet (UV) spectrophotometry, gas chromatography–mass spectrometry (GC–MS), and nuclear magnetic resonance (NMR) are key techniques for studying the structure and composition of PRPs. Specifically, HPGPC is primarily used to determine the homogeneity and molecular weight of polysaccharides. FT-IR is employed to detect functional groups of polysaccharide molecules and identify the glycosidic bond configuration (α-type or β-type). UV spectrophotometry is used to detect the presence of nucleic acids or protein impurities in polysaccharide (the absence of absorption peaks at 260 and 280 nm indicates high purity). GC–MS analyzes the types and proportions of monosaccharides after hydrolysis to determine the monosaccharide composition of PRPs. NMR is a key tool for elucidating the chemical structure of polysaccharides. Combined with one- and two-dimensional NMR techniques, it can further reveal the complex structures of the polysaccharide main chains and side chains. Together, these techniques provide a comprehensive characterization of polysaccharide structures.
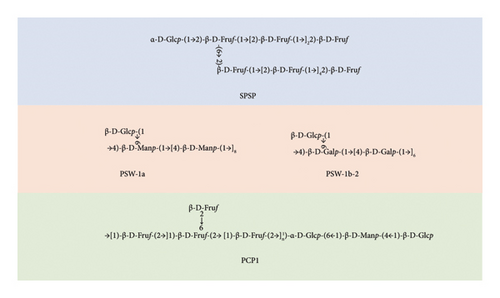
Two neutral polysaccharides were isolated from the rhizome of P. sibiricum, labeled as PSW-1b-2 and PSW-1a. Among them, PSW-1b-2 had a backbone comprising ⟶4)-β-D-Galp-(1⟶ linkages, with β-D-Galp-(1⟶ branches occurring every seven residues. In contrast, PSW-1a featured a backbone comprising ⟶4)-β-D-Manp-(1⟶ linkages, with β-D-Galp-(1⟶ branches appearing every nine residues [66]. A polysaccharide named PCP1 was isolated from P. cyrtonema Hua, and a chemical analysis indicated that the main chain of PCP1 primarily comprised ⟶1)-β-D-Fruf-(2⟶ and ⟶1,6)-β-D-Fruf-(2⟶, with minor components including ⟶6)-α-D-Glcp-(1⟶, ⟶4)-β-D-Manp-(1⟶, and β-D-Glcp-(1⟶. Its side chain comprised β-D-Fruf-(2⟶ linked at the C-6 position of ⟶1,6)-β-D-Fruf-(2⟶ [67]. A low-molecular-weight polysaccharide (SPSP) was isolated and purified from steamed P. sibiricum. Its main chain included α-D-Glcp-(1⟶, β-D-Fruf-(2⟶, ⟶1,6)-β-D-Fruf-(2⟶, and ⟶1)-β-D-Fruf-(2⟶ residues. Furthermore, its side chain comprised terminal β-D-Fruf-(2⟶ and ⟶1)-β-D-Fruf-(2⟶ residues, which were sequentially attached to the O-6 position of the ⟶1)-β-D-Fruf-(2⟶ residues [45]. Figure 4 and Table 4 list the well-defined PRPs and illustrate their structural formulas.
4. Pharmacological Activities of PRPs
4.1. Immunomodulatory Activities
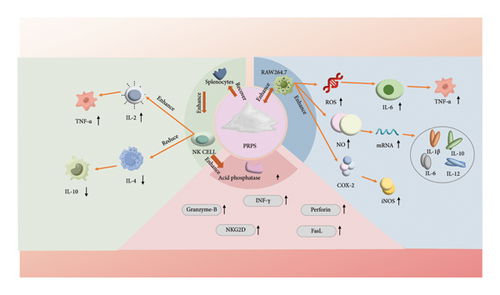
The immune system is a crucial defense mechanism in the human body that protects against external pathogens such as bacteria, viruses, and other infections. Immunomodulation refers to the process of regulating the activity of the immune system through various mechanisms and pathways [68]. It includes enhancing or suppressing immune responses to maintain health and prevent diseases [69]. PRPs can enhance the protein expressions of COX-2 and iNOS while increasing the release of NO, reactive oxygen species (ROS), and cytokines, such as TNF-α and IL-6, in RAW 264.7 cells [70]. Moreover, PRPs significantly restore organ indices, enhance splenocyte proliferation and NK cell activity, increase IL-2 and TNF-α levels, and decrease IL-4 and IL-10 levels [68]. In particular, polysaccharides extracted from the roots of P. sibiricum can significantly activate RAW264.7 cells, resulting in the production of NO and the upregulation of mRNA expression for cytokines such as IL-1β, IL-6, IL-10, and IL-12. When these polysaccharides undergo sulfation, the cytotoxicity of NK cells against HT-29 cells is enhanced, with corresponding increases in the expression of genes such as INF-γ, granzyme B, perforin, NKG2D, and FasL. Thus, the activation of RAW264.7 cells is likely mediated through MR and TLR4 signaling pathways, while CR3 and TLR2 may play a key role in the stimulation of NK cells [71]. Additionally, Wang et al. found that CTAB-modified PSP-Cubs/OVA enhanced the secretion of relevant cytokines and lymphocyte proliferation, thereby boosting cellular immune responses and increasing the levels of humoral immunity. These CTAB-modified PSP-Cubs exhibited effective adjuvant properties [72]. RNA-seq analysis revealed that PRP treatment resulted in 2160 differentially expressed genes, with 1142 and 1018 genes up- and downregulated, respectively. Further analysis indicated that PRP treatment upregulated key genes related to immunoregulation, including the expressions of iNOS, COX-2, and IL-2, suggesting its potential regulatory role in promoting macrophage activation and enhancing cellular immune responses. To explore potential regulatory mechanisms of PRPs, a bioinformatics analysis was employed to focus on the NF-κB, MAPK, and JAK-STAT signaling pathways. These findings were further confirmed using Western blot experiments, where the JAK-STAT pathway (indicated by reduced JAK1 in the cytoplasm and increased phosphorylated STAT3 in the nucleus), MAPK pathway (elevated phosphorylated p38, ERK1/2, and JNK), and NF-κB pathway (increased phosphorylated IκBα in the cytoplasm, decreased cytoplasmic p65, and enhanced nuclear presence of phosphorylated p65) in macrophage activation were induced by PRPs [73].
The mechanisms of immunomodulation currently involve multiple pathways. PRPs participate in immunomodulation through multiple mechanisms, including enhancing the expressions of specific enzymes and signaling molecules, such as COX-2, regulating the release of cytokines, and affecting mRNA expression. These actions synergistically promote the balance of the immune system and enhance the defense of the body against diseases [74]. Through these mechanisms (Figure 5), PRPs can enhance immune responses, combat infections, and oppose certain autoimmune and inflammatory diseases. Overall, PRPs possess immunomodulatory activity and have the potential to become an immune stimulant.
4.2. Antidepressant Activity
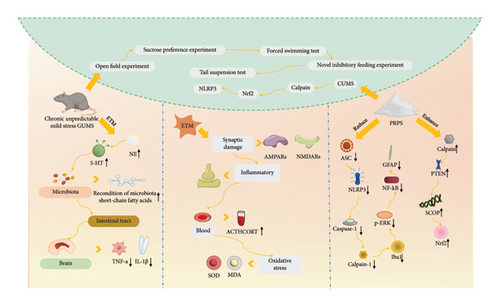
Multiple studies have reported the potential antidepressant effects of PRPs, which may interact with neurochemical pathways related to depression, thereby influencing mood and cognitive functions [75, 76]. Depression and chronic stress are often accompanied by overactivity of the hypothalamic–pituitary–adrenal (HPA) axis, which is characterized by elevated adrenocorticotropic hormone and cortisol levels. Polysaccharides from P. sibiricum can modulate the HPA axis, thus reducing cortisol levels under stress conditions and alleviating stress-related depressive symptoms. This effect may be achieved by regulating the synthesis and release of neurotransmitters, such as 5-hydroxytryptamine (5-HT) and norepinephrine (NE). On the other hand, it was demonstrated that fecal microbiota transplantation (FMT) from mice treated with P. sibiricum alleviated depressive-like behaviors in mice, who were experiencing chronic unpredictable mild stress (CUMS), via the microbiota–gut–brain axis (Figure 6) [77]. There are also related studies reporting that Polygonatum polysaccharides prevent the impairment of intestinal barrier function induced by CUMS, inhibit the levels of corticosterone and lipopolysaccharide (LPS), and increase the levels of 5-HT in the serum. Furthermore, Polygonatum polysaccharides prevent the abnormal neuronal activation in the paraventricular nucleus and the changes in local field potential (LFP) in CUMS mice, particularly the decrease in power spectral density in the delta and theta frequency bands [78]. Additionally, another study demonstrated that PRP treatment reduced the levels of calpain-1, NLRP3, ASC, caspase-1, cleaved caspase-1, Iba1, p-ERK, NF-κB, and GFAP while increasing the levels of calpain, PTEN, SCOP, and Nrf2. Administering PRPs mitigated the changes in the calpain system and Nrf2 and NLRP3 signaling pathways that were triggered by CUMS. This effectively reduced behaviors associated with depression [79]. Moreover, PRPs alleviated depressive-like behaviors in mice treated with LPS by inhibiting oxidative stress, hypercortisolemia, inflammatory responses, and synaptic damage [52]. In conclusion, PRPs could potentially alleviate depressive-like behaviors and synaptic and neuronal damage by diminishing oxidative stress, hyperactivity of the HPA axis, and inflammatory responses.
4.3. Antioxidant Activity
Antioxidant activity analysis typically includes chemical methods and biological studies involving cells or animals. Standard chemical tests include evaluating the scavenging activities for free radicals such as 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), and hydroxyl radicals [77]. In addition to effectively scavenging DPPH, hydroxyl, and superoxide anion radicals, PRPs chelate ferrous ions [56]. The scavenging ability of PRPs has been attributed to the high content of GalA residues, hemicellulose, and galacturonic acid ester in their structure [77, 80]. Research on the antioxidant effects of PRPs has shown that the oral intake of PRPs alleviates heart aging and damage caused by D-galactose in vivo [81]. Specifically, PRPs reduced ROS and malondialdehyde (MDA) levels while increasing superoxide dismutase levels in the cardiac tissue of D-galactose-treated mice. Furthermore, PRPs were found to reduce oxidative stress-induced DNA damage and lipid peroxidation [81]. In conclusion, PRPs mitigate D-galactose-induced heart aging by combating oxidative stress, indicating their potential as a herbal antiaging agent, particularly in reducing oxidative stress-related damage.
4.4. Osteogenic Activity
Osteoporosis is a skeletal disorder characterized by reduced bone density and mass and the deterioration of bone microarchitecture, which results in increased bone fragility and a higher risk of fractures [82, 83]. Researchers conducted studies on the osteogenic differentiation effects of PRPs on mouse bone marrow stromal cells (BMSCs) [84]. The PRPs promoted the osteogenic differentiation of mouse BMSCs without affecting the BMP signaling pathway. This result was due to an increased nuclear accumulation of β-catenin, which enhanced the expression of genes associated with osteoblasts. Their research highlighted that PRPs inhibited the activation of the NF-κB receptor activator of ligand-induced osteoclastogenesis and provided preventive protection against LPS-induced bone resorption in vivo. Because β-catenin accumulated in the nucleus, osteoclast-specific gene expression was downregulated [85]. The existing literature has reported that PRPs could inhibit glycogen synthase kinase 3β (GSK-3β) activity and prevent nuclear β-catenin accumulation by engaging Wnt signaling pathways that are not dependent on low-density lipoprotein receptor-related protein 5 [85]. Moreover, PRPs decreased GSK-3β levels, which destabilized nuclear β-catenin through phosphorylation via the extracellular signal-regulated kinase signaling pathway. However, Ref. [85] conducted cell-based experiments, reporting that PRPs significantly increased the expression levels of alkaline phosphatase (ALP), osteocalcin (OC), type I procollagen N-terminal propeptide, and bone morphogenetic protein-2. Thus, PRPs could enhance both the proliferation and activity of BMSCs during osteogenic differentiation, highlighting the significant potential of PRPs in osteoporosis prevention.
4.5. Antitumor Activity
Polysaccharides can exhibit significant antitumor effects, primarily through inhibiting tumor growth, inducing apoptosis, and enhancing immune functions [86–88]. It was reported that PRPs can significantly inhibit tumor growth, improve the indices of the spleen and thymus, enhance cytokine secretion, and regulate the CD4/CD8 lymphocyte ratio [89]. PRP treatment resulted in a significant increase in mRNA and protein levels at the main nodes of the TLR4-MAPK/NF-κB signaling pathway and elevated levels of nitric oxide and cytokines.
Relevant reports indicate that triple-negative breast cancer (TNBC) tumors significantly increase myeloid cells in peripheral blood, bone marrow, and spleen while reducing hematopoietic stem and progenitor cells (HSPCs). Some researchers have studied the effects of PRPs on hematopoiesis in TNBC mice and found that PRPs inhibit myeloid hematopoiesis. Furthermore, PRPs suppressed the expansion of hematopoietic cells in the spleen induced by TNBC tumors and significantly increased the numbers of HSPC and common lymphoid progenitors in the bone marrow, which were suppressed by TNBC tumors [90]. Thus, PRPs could protect the hematopoietic function in the bone marrow suppressed by TNBC tumors. Xie et al. used different extraction solvents to isolate four types of Polygonatum polysaccharides (HBSS, CHSS, DASS, and CASS). Among them, CASS, extracted using a concentrated alkaline solution, exhibited the strongest anti-HeLa cell activity, effectively blocking the G2/M phase of the HeLa cell cycle. The study found that CASS triggered caspase-3 activity through both the death receptor and mitochondrial pathways [91].
4.6. Antidiabetes Activity
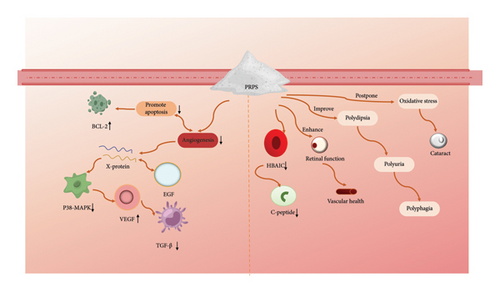
Diabetes mellitus is a severe global health issue, and Polygonati Rhizom plays a significant role in blood sugar control in traditional Chinese medicine. Refs. [92, 93] investigated the potential therapeutic benefits of PRPs on vascular abnormalities in the retinas of diabetic rats. They reported that PRPs reduced the levels of factors promoting apoptosis and angiogenesis, including Bcl2-associated X protein, epidermal growth factor, p38 mitogen-activated protein kinase, vascular endothelial growth factor, and transforming growth factor-β [94]. Moreover, PRPs enhanced the expression of antiapoptotic factor B-cell lymphoma-2. Thus, PRPs could protect against vascular damage in diabetic retinopathy by modulating critical pathways involved in cell apoptosis and new blood vessel formation. Moreover, PRPs effectively reduced fasting blood glucose and glycosylated hemoglobin levels in rats while dose-dependently increasing insulin and C-peptide levels in plasma. They also improved clinical symptoms such as polydipsia, polyphagia, polyuria, and weight loss in diabetic rats, alleviated oxidative stress, delayed the development of cataracts, and enhanced retinal function and vascular health [95]. Lei et al. demonstrated that the mice treated with PRPs showed decreases in both body weight and blood glucose levels. These polysaccharides also improved heart function and exerted minimal effects on MDA activity, indicating their potential for combating oxidative stress. Further analyses revealed that PRPs reduced endoplasmic reticulum (ER) and oxidative stresses in the hearts of mice. This process was linked to enhance cyclic guanosine monophosphate-dependent protein kinase G signaling and the inhibition of phosphodiesterase type 5, which mitigates heart dysfunction caused by a high-fat diet and reduces ER stress [96]. In conclusion, PRP supplementation could be an alternative method for preventing diabetic retinopathy (Figure 7).
4.7. Other Activities
PRPs exhibit diverse biological activities, including cardiovascular protection [97], anti-inflammatory [98], antibacterial [99], and antiaging [100] properties, as evidenced by various studies. For example, it was demonstrated that PRPs prevent doxorubicin-induced acute heart failure by reducing oxidative stress, suppressing inflammation, and inhibiting cardiomyocyte apoptosis [101]. Similarly, PRPs were shown to alleviate DSS-induced colitis in mice by downregulating pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6 [102]. Additionally, chemical modification was shown to significantly enhance the antiviral activity of these polysaccharides [103]. Notably, the antiaging effects of PRPs were shown to be mediated by regulating the IIS pathway, activating the DAF-16/FOXO transcription factor, and improving oxidative defense and stress resistance [100]. Table 5 summarizes additional biological activities of PRPs, further underscoring their therapeutic potential.
| Biological activity | Type | Experimental model | Mechanism | Ref. |
|---|---|---|---|---|
| Anti-inflammatory | In vitro | MH7A cells | IL-11β, IL-6↓ IL-10↑ | [21] |
| In vitro | RPE cells | ROS, MDA, Bcl-2↓ SOD, GPx, bax, caspase-3↑ | [104] | |
| In vivo | Gentamicin-induced acute renal injury rats | NGAL, KIM-1, IL-1β, IL-6, TNF-α, p38-MAPK mRNA, p-p38 MAPK, p-ATF2,CRE, urea, and kidney coefficient↓ | [102] | |
| In vivo | C57BL/6J mice | TNF-α, IL-1β, IL-6↓ SOD1, GPX2, Nrf2↑ | [105] | |
| In vivo | Colitis mice | iNOS, IL-6, COX-2↓ alleviated the pathological damage to the colon | [101] | |
| Cardiovascular protection | In vivo | Mice suffering from acute heart failure induced by adriamycin | HR, LVSP, ±dp/dtmax, sSOD, Na+-K+-ATPase, Ca2+-Mg2+-ATPase, SDH, Bcl-2, Caspase-3 ↑ LVEDP, cTnI,CK-MB,TNF-α, Bax, MDA, NO↓ | [106] |
| In vivo vitro | RAW264.7; SD rats | NO, TNF-α, IL-6, IL-1β, NF-κB activity, κB, TLR4, TGF-β1, MyD88 mRNA expression↓ | [107] | |
| In vivo | Isoproterenol-induced cardiac hypertrophy rats | MDA, LPO, TNF-α, IL-6, p-JAK2, p-STAT3↓ SOD, GSH-Px↑ | [108] | |
| Neuroprotective | In vitro | PC12 cells | Mitochondrial dysfunction, cytochrome c, caspase-3↓, Akt↑ | [53] |
| In vivo Vitro | Parkinson’s disease mouse; N2acell | MPP+↓ MPTP-↑Gclm, Gclc, HO-1, NQO1, Nrf2 activation | [109] | |
| Hypolipidemic | In vivo | High-fat diet-induced obesity in mice | Body weight, blood lipids, blood glucose, insulin, resistin, adiponectin, liver weight, abdominal fat pad weight, SREBP-1, FAS, IL-1β, TNF-α, iNOS, IL-6 mRNA ↓ PPARα, CPT-1 ↑ | [110] |
| In vivo and in vitro | High-fat diet-induced obesity in rabbits; ECs | TC, LDL-C, Lp(a), intimal foam cells↓ | [111] | |
| Hepatoprotective | In vivo vitro | HepG2 cells; CCl4-induced liver injury in mice | Antioxidant enzyme activity↑ lipid peroxidation, pro-inflammatory mediators↓ | [112] |
| In vivo | Septic mice | IL-18, IL-1β, AST, ALT, ALP, TBIL, MPO.↓; reduce mortality, alleviate liver damage | [113] | |
| In vivo | Ethanol-induced mouse liver injury | ALT activity, TBIL, TG levels, NOX1, p67phox, gp91phox, CYP2E1↓; alleviation of hepatic histopathological lesions; reduction in liver oxidative stress | [103] | |
| Antiviral | In vitro | In HSV2-infected vero cells | IC50 (P-PD, S-PD) of HSV-2 were 1.22, 2.20 mg/mL, respectively | [114] |
- Note: “↑” Indicates that the expression increases, “↓” indicates that the expression decreases.
5. Drug Carriers
PRPs exhibit unique biocompatibility, biodegradability, and excellent compatibility with biological systems, positioning them as ideal materials for drug delivery systems. These properties enable PRPs to significantly enhance drug efficacy and facilitate targeted release, showcasing tremendous potential in this field [103, 113–115]. For example, PRPs have been shown to improve the solubility of poorly soluble drugs. Xu et al. developed an ibuprofen-Polygonatum polysaccharide (IBU-PSP) drug delivery system, which increased the solubility of ibuprofen by 8.22 times and its bioavailability by 2.52 times [116]. Additionally, IBU-PSP enhanced anti-inflammatory effects and reduced ibuprofen-induced renal damage, providing a novel and safer approach for developing nonsteroidal anti-inflammatory drug (NADI) formulations. Moreover, PRPs can be chemically modified to achieve targeted drug delivery. Liu et al. demonstrated that CTAB-modified PSP-Cuboids (PSP-Cubs) stimulated cytokine secretion and lymphocyte proliferation, thereby enhancing both cellular and humoral immune responses. This finding highlights their potential for application in vaccine development [71]. In certain formulations, PRPs also function as stabilizers, protecting drugs from degradation caused by external factors. Liu et al. utilized PRPs to synthesize selenium nanoparticles (PSP-SeNPs) in a simple redox system. These nanoparticles exhibited excellent stability, with an average particle size of 105 nm and a zeta potential of −34.9 mV, and remained stable for 30 days at 4°C. Such properties position PSP-SeNPs as promising antioxidants for applications in food and nutraceutical industries [117]. Furthermore, PRPs have demonstrated significant potential as vaccine adjuvants, attributed to their immunomodulatory properties. Shen et al. investigated the adjuvant activity of calcium carbonate-modified microparticle PRPs (MP-PSP). Their study revealed that MP-PSP enhanced humoral immune responses by slowing antigen release, activating the Toll-like receptor 4 signaling pathway, and promoting dendritic cell activation and interleukin-6 secretion. These findings suggest that MP-PSP is a safe and effective novel vaccine adjuvant with promising applications [118]. Despite these advances, several challenges remain. The structural complexity of PRPs can complicate modifications for specific applications, and issues related to drug release control and bioavailability require further investigation. Addressing these challenges is essential to fully unlock the potential of PRPs in drug delivery systems.
6. Conclusions and Prospects
This review comprehensively discusses the significant progress in the understanding of the properties of PRPs and their medical applications. PRPs exhibit a wide range of structures, molecular weights, and monosaccharide compositions. Biologically, they demonstrate a broad spectrum of activities, including immunomodulatory, antitumor, anti-inflammatory, and antidiabetic effects. These functionalities are associated with their roles in regulating various biological pathways and their interactions with cellular receptors. Structural modifications, such as sulfation and carboxymethylation, have further enhanced the antioxidant, antitumor, and antiviral properties of PRPs. In their application as stabilizers, PRPs improved the stability and functionality of selenium nanoparticles, showing excellent antioxidant and antitumor properties. In the field of immunomodulation, modified PRPs activated Toll-like receptor 4 signaling pathways, enhancing both humoral and cellular immune responses, and exhibiting excellent safety, highlighting their potential as vaccine adjuvants. Moreover, the antiaging, antidiabetic, and gut microbiota-regulating properties of PRPs have garnered widespread attention. PRPs exhibit remarkable potential for delaying aging and improving glucose metabolism by regulating the IIS pathway, activating DAF-16/FOXO transcription factors, and reducing ROS levels. Advanced analytical techniques, such as NMR and HPLC, have further elucidated the complex structure–activity relationships of PRPs, driving their application in functional foods and pharmaceuticals. Polygonatum is also widely used in the food, cosmetics, and health industries. Due to its medicinal properties, various functional products derived from Polygonatum have been developed, such as tea, powder, beverages, cakes, porridge, paste, wine, and yogurt.
In conclusion, this review provides a comprehensive examination of key areas of PRP research. It highlights recent advancements in efficient extraction techniques, clarifying their molecular structure and elucidating a wide range of biological activities, including immunomodulation, antitumor, and anti-inflammatory effects. Additionally, the review discusses the potential of PRPs as drug carriers, particularly in nanoparticle-based drugs and vaccine adjuvants. Together, these studies lay the foundation for the further development and practical application of PRPs in functional foods and pharmaceuticals. Despite significant advances in PRP research, several challenges remain. Most studies have focused on in vitro experiments and animal models. However, these conditions differ greatly from the complex human physiological environment, which may introduce biases when applying the findings to human health. Additionally, there is a lack of consistency in the extraction methods, purity, and structure identification standards used across studies. This makes it difficult to compare results and hinders systematic data collection and analysis. Although PRPs have demonstrated various biological activities, the mechanisms underlying these effects are still not fully understood, particularly regarding their interactions with complex intracellular signaling pathways. This limits our ability to regulate and harness the full potential of PRPs in therapeutic applications. Finally, clinical research on PRPs is limited by the small sample sizes and short study durations, making it challenging to evaluate their long-term safety and efficacy and determine the optimal dosage and administration methods for clinical use.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Qiangbao Xu, Qiuyue Lv, and Zhu Yang made equal contributions to this work.
Funding
This work was supported by the Academic Support Project for Top-Notch Talents in Disciplines of universities in Anhui Province (gxbjZD2022043), the Project of Natural Science Foundation of the Department of Education of Anhui Province (2024AH040169, 2024AH051934, 2024AH051923), the Research Funds of Center for Xin’an Medicine and Modernization of Traditional Chinese Medicine of IHM (2023CXMMTCM011), and the Inheritance and Innovation research of TCM of Anhui Province (2024CCCX260, 2024CCCX016).
Open Research
Data Availability Statement
No data were used for the research described in the article.




