Biochemical Profiling and Bioactivity of Five Selected Microalgae Species as Potential Sources of Bioactive Compounds for Nutritional and Biotechnological Applications
Abstract
Microalgae have emerged as promising sustainable sources of bioactive compounds, essential for addressing global challenges. This study investigated the biochemical profiles of five microalgae species: Nannochloropsis sp., Tetraselmis chuii, Chaetoceros muelleri, Thalassiosira weissflogii, and Tisochrysis lutea. By using NMR spectroscopy, organic acids, amino acids, and other compounds were revealed. T. chuii was particularly rich in acetate, whereas the main organic acid in T. lutea was lactate. ICP-MS analysis indicated substantial variations in elemental concentrations across the species, with T. chuii showing the highest calcium content and C. muelleri having the highest iron content. SPME-GC/MS revealed that alcohols, acids, and aldehydes were the principal volatiles. Fatty acid profile was described by GC-MS technique. Carotenoid profiling highlighted T. lutea as having the highest total carotenoid content. Antioxidant activities were evaluated with C. muelleri demonstrating superior efficacy. Furthermore, the microalgae demonstrated moderate to significant activity against both bacterial and fungal pathogens. In addition, T. lutea and Nannochloropsis sp. demonstrate significant antibiofilm activity against various bacterial strains. This study highlights the potential of these microalgae as valuable sources of diverse metabolites contributing to nutritional and biotechnological advancements and addressing global food and health challenges.
1. Introduction
The search for new ways to treat diseases that are resistant to antibiotics is driving the search for effective natural compounds. While terrestrial sources have long been explored, the ocean began to offer new drug prospects in the 1940s, particularly advancements in diving techniques and marine exploration [1, 2].
Algae, a diverse group of predominantly photosynthetic organisms, represents one of the most genetically varied groups on Earth [3]. With estimates suggesting up to 50,000 different species, microalgae thrive in almost every type of environment [4]. Their vast diversity and distribution contribute to a rich array of biochemical compounds that enable them to adapt to extreme conditions and exhibit various important biological properties. Consequently, microalgae are considered valuable sources for producing secondary metabolites with significant commercial potential [5, 6]. These compounds endow microalgae with unique features, including phototactic responses, pharmaceutical potential, and rich nutritional profiles. Unlike other microorganisms, microalgae generally do not present toxicity issues and possess photosynthetic capabilities, making them highly valuable for applications in food supplements and biomedical fields [7].
Microalgae increase the nutritional value of standard food products due to their unique chemical composition, benefit human and animal health, and are also used as a biostimulant. They hold significant promise as dietary supplements for preventing, managing, and treating various physiological issues and offer a sustainable alternative to synthetic supplements [3, 8]. Microalgae produce valuable bioproducts such as β-carotene, astaxanthin, fucoxanthin, docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), bioactive pigments, natural dyes, polysaccharides, amino acids, vitamins, and antioxidants [9]. Their rich mineral content, including Ca, Mg, P, K, and trace elements like Fe, Zn, and Se, further positions them as a promising alternative to traditional mineral sources [10].
Beyond their nutritional value, microalgae are recognized for their unique compounds with greater bioactivity compared to terrestrial plants [2]; these include potential antimicrobial, antioxidant, anticancer, and anti-inflammatory ones [11]. Natural antioxidants from microalgae are increasingly sought as alternatives to synthetic ones. Additionally, microalgae offer significant advantages for biotechnological applications due to their high biodiversity, efficient photosynthetic yield, rapid growth, and metabolic flexibility, which can be tailored through specific culture conditions [12]. Carotenoids in microalgae, known for their antioxidant properties, protect against oxidative damage by neutralizing singlet oxygen and other free radicals through their conjugated double-bond system. They also play a crucial role in the xanthophyll cycle, which helps dissipate excess light energy and prevents damage to the photosynthetic machinery, providing benefits for health and industry [12]. It is noteworthy that carotenoids from microalgae exhibit antioxidant activity that surpasses both plant-based sources and synthetic alternatives. For example, fucoxanthin, a brown xanthophyll present in golden algae, has been shown to have antioxidant activity more than 13 times greater than vitamin E. Likewise, astaxanthin, a red keto-carotenoid, demonstrates antioxidant power that is 65 times greater than vitamin C and 100 times more effective than α-tocopherol [13]. In addition, microalgae are being investigated as alternative sources of antibiotics, preservatives, and plant disease control agent [14]. Their antimicrobial properties are linked to a range of chemical compounds, including indoles, terpenes, acetogenins, phenols, fatty acids, and volatile halogenated hydrocarbons. For instance, the antimicrobial effects of supercritical extracts from Chaetoceros muelleri have been associated with their lipid composition. Similarly, antimicrobial activity in pressurized extracts of microalgae can be attributed to fatty acids and compounds like α- and β-ionone, β-cyclocitral, neophytadiene, and phytol. The challenge of infections caused by pathogenic microorganisms within biofilms underscores the importance of finding effective solutions. Microalgae, with their range of biogenic substances, show promise in disrupting biofilm matrices and eliminating biofilms without harming other ecosystem organisms. Current research in phycology focuses on assessing the antifouling properties of various algal species [15]. Despite these benefits, only a few microalgae species are commercially utilized [11].
To fully realize the potential of microalgae, it is important to understand their chemical composition, especially when they are grown in controlled environments [16]. Consequently, the main aim of this study is to comprehensively evaluate the bioactive potential of five species of microalgae (Nannochloropsis sp., Tetraselmis chuii, Chaetoceros muelleri, Thalassiosira weissflogii, and Tisochrysis lutea) through the analysis of chemical composition, including carotenoid and chlorophyll content, volatiles, minerals and heavy metals concentrations, antioxidant activities, antimicrobial, antifungal, and antibiofilm properties. Besides, the evaluation of the efficacy of these microalgae extracts against various plant pathogens was also evaluated, to explore their potential use in agriculture for the management of plant diseases and the improvement of crop health. The selection of these five species was based on their ecological diversity, distinctive biochemical profiles, and broad biotechnological potential. Chosen for their widespread presence in marine environments, these microalgae are known for their varied metabolic pathways and the production of bioactive compounds, making them ideal candidates for biotechnological applications. They represent a range of characteristics essential for addressing global challenges, from enhancing nutrition to combating pathogens and advancing biotechnological innovations.
2. Material and Methods
2.1. Microalgae Samples
Powdered Nannochloropsis sp., Tetraselmis chuii, Chaetoceros muelleri, Thalassiosira weissflogii, and Tisochrysis lutea were acquired from Proviron (Hemiksem, Belgium). According to Proviron, these microalgae are cultivated under stringent conditions and undergo continuous monitoring for pathogen presence (certified under Hazard Analysis and Critical Control Points and Food Contact Materials standards by Société Générale de Surveillance, FCA certificate BE 01/1522.GF). All samples were purchased in 2022 and have been stored in a light-protected, dry environment at room temperature (∼20°C).
2.2. Nuclear Magnetic Resonance (NMR) Spectroscopy Analysis of Microalgae Extracts
All the chemicals and reagents employed in this study were of analytical grade. Potassium dihydrogen phosphate (KH2PO4, 99%), deuterium oxide (D2O, 99.9%), methanol-d4 (MeOD, > 99.8%), and methanol were sourced from VWR (Radnor, PA, USA). The sodium salt of 3-(trimethylsilyl) propionic-2,2,3,3-d4 acid (TMSP, 99%) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Ultrapure water was generated using a Millipore Direct-Q® 3 UV Water Purification System (Millipore Corp., Bedford, MA, USA) or from Merck KGaA (Darmstadt, Germany). Microalgae samples (50 mg each) were finely ground and extracted using a 1:1 v/v mixture of MeOD-D2O, following the procedure outlined by Mascellani et al. [17]. Proton nuclear magnetic resonance (1H NMR) WAS recorded on a Bruker Avance III spectrometer, equipped with a BBFO SmartProbe™ featuring z-axis gradients (Bruker BioSpin GmbH, Rheinstetten, Germany) and operating at a proton NMR frequency of 500.18 MHz. Spectra were acquired at 298 K using the pulse sequence “noesygppr1d” to suppress residual water signals. The acquisition parameters included a 4-s acquisition time over 64 K data points, a 16-ppm spectral width, a 1-s recycle delay, a 0.1-s mixing time, and 128 scans. Automated routines were used for tuning and matching, with consistent receiver gain settings applied throughout the measurements. All free induction decays were referenced to the internal standard TMSP at 0.0 ppm and processed using exponential apodization (0.3 Hz), zero filling, and phase and baseline corrections in Mnova software, version 14.1.0 (Mestrelab Research, S.L., Santiago de Compostela, Spain). Preprocessed 1r files were then imported into Chenomx NMR Suite version 9.02 (Chenomx, Edmonton, Canada) for quantification, utilizing the Chenomx library along with custom-developed compound signatures [17].
2.3. Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) Analysis
A variety of elements (Ag, Al, As, Ba, Ca, Cd, Co, Cr, Cu, Fe, K, Li, Mg, Mn, Mo, Na, Ni, Pb, Sb, Se, Sr, and Zn) were examined in selected microalgae samples using ICP-OES [18]. For sample preparation, mineralization was carried out in a microwave digestion system (Ethos UP, Milestone Srl, Sorisole, BG, Italy) with a mixture of 5 mL HNO3 (≥ 69.0%, Trace SELECT®, Honeywell Fluka, Morris Plains, USA), 1 mL H2O2 (≥ 30%, Sigma-Aldrich, Saint-Louis, Missouri, USA), and 2 mL ultrapure water (18.2 MΩ-cm; 25°C, Synergy UV, Merck Millipore, France). The samples were heated to 200°C for 15 min and then cooled to 50°C over the next 15 min. Postmineralization, the samples were filtered (filter paper no. 390, Munktell & Filtrak GmbH, Bärenstein, Germany) and diluted to a final volume of 50 mL with ultrapure water. Elemental analysis was conducted using ICP-OES (700 Series, Agilent Technologies, USA) equipped with axial argon plasma and an automated sampler (SPS-3, Agilent Technologies, USA). Calibration was achieved using a multielement standard solution prepared from individual element standards for ICP (Sigma-Aldrich Production GmbH, Switzerland). Detection limits (μg/kg) for each element were as follows: Ag 0.3; Al 0.2; As 1.5; Ba 0.03; Ca 0.01; Cd 0.05; Co 0.2; Cr 0.15; Cu 0.3; Fe 0.1; K 0.3; Li 0.06; Mg 0.01; Mn 0.03; Mo 0.5; Na 0.15; Ni 0.3; Pb 0.8; Sb 2.0; Se 2.0; Sr 0.01; and Zn 0.2. The accuracy of the method was verified using a certified reference material (CRM-ERM CE278K, Sigma-Aldrich Production GmbH, Switzerland).
2.4. HPLC Analysis of Carotenoids and Chlorophylls
We measured the concentrations of specific carotenoids and chlorophylls in ethanol and hexane extracts of microalgae using HPLC-PDA analysis. The Shimadzu Prominence HPLC system was employed with defined settings. The mobile phase consisted of (A) tetrahydrofuran, (B) a water–acetic acid mixture (100:1 v/v), and (C) methanol. The flow rate was set at 1 mL/min, with a 20 μL injection volume and a column temperature of 35°C. The elution process involved a linear gradient from 10% to 8% B and from 87% to 89% C over 0–6 min, followed by a gradient from 8% to 0% B and from 89% to 90% C from 6 to 15 min, and then an isocratic phase at 90% C from 15 to 20 min. Quantification of fucoxanthin, violaxanthin, astaxanthin, lutein, zeaxanthin, lycopene, α-carotene, chlorophyll b, and chlorophyll a was performed at 420 nm, with UV/VIS spectra scanned from 200 nm to 800 nm. For HPLC analysis, 10 mg of dried microalgae extract (prepared as outlined in Section 2.8.1) was dissolved in 1 mL methanol and filtered through a 0.45-μm filter (Millipore, Billerica, MA, USA). Chromatographic optimization was achieved using standard compounds dissolved in methanol at 1000 μg/mL. A methanol stock solution with standard compounds at 1000 μg/mL was made for quantitative analysis, which was then diluted to prepare working solutions ranging from 100 to 0.00625 μg/mL to establish calibration curves. All standard solutions were kept at 4°C. The concentrations of standard compounds in the extracts were determined by analyzing peak areas and using linear regression equations from the calibration curves. Results are reported as mean values ± standard error (SE).
2.5. Total Carotenoids
2.6. Volatile Compound Analysis
The analysis of volatile compounds of microalgae extracts with water was performed following the approach outlined by Issa-Issa et al. [20, 21]. A Shimadzu GC2030 gas chromatograph paired with a TQ8040 NX triple quadrupole mass spectrometer was used, along with a GC-MS system (Shimadzu Scientific Instruments, Inc., Columbia, MD, USA) fitted with an AOC-6000Plus autosampler. For headspace analysis, approximately 0.5 g of microalgae was combined with 5 mL of water and 0.5 g of NaCl to facilitate the release of volatile compounds into the vial headspace. Volatile compounds were extracted using the HS-SPME technique with a DVB/CAR/PDMS fiber (Supelco, Bellefonte, PA, USA). The samples were incubated in the autosampler at 500 rpm and 40°C for 20 min to mimic conditions of food mastication. The gas chromatograph was programmed to hold at 40°C for 2 min and then increase at +3°C/min to 250°C. Helium was used as the carrier gas, set at 50.4 kPa with a linear flow rate of 36.3 cm/s. The injection, ion source, and interface temperatures were maintained at 260°C, 200°C, and 250°C, respectively, with a total helium flow rate of 1.01 mL/min. Volatile compounds were identified through three methods: (i) retention indices determined using a mixture of n-alkanes C7 to C16 (Sigma-Aldrich, Steinheim, Germany); (ii) retention indices of standards; and (iii) comparison of mass spectra with those in the NIST 14 and Wiley 229 libraries. All analyses were performed in triplicate, and results were expressed as percentages of the total peak area.
2.7. Fatty Acid Content
The powdered macroalgae T. lutea (184 mg), T. weissflogii (115 mg), C. muelleri (100 mg), T. chuii (140 mg), and Nannochloropsis sp. (117 mg) were extracted overnight with 6 mL of dichloromethane/methanol (2:1 v/v). The extracts were centrifuged at 3000 rpm for 10 min and dried by rotary evaporator at 30°C, to obtain, respectively, 63.0, 18.0, 100, 20.0, and 32.0 mg. Then, 1 mL of each extract was dried with nitrogen and in the presence of methanol transmethylated with BF3. The extraction of the obtained fatty acid methyl esters (FAMEs) after evaporation of methanol was carried out with n-hexane. The analyses were performed using a gas chromatograph coupled to a Clarus 500 mass spectrometer model Perkin Elmer (Waltham, MA, USA), equipped with a flame ionization detector (FID). A Varian Factor Four VF-5 capillary column was housed in the GC oven [22]. 2 μL of the extract was injected into the column in splitless mode. The gas chromatographic conditions were as follows: The injector was set at 280°C and the oven temperature program started from 170°C and increased up to 260°C with a rate of 3°C/min and held constant for 15 min. The identification of the volatile compounds was performed first through the comparison of the mass spectra with those present in the Wiley 2.2 and Nist 11 mass spectral library database and then through the calculation of the linear retention indices (LRI) thanks to a series of alkane standards (C8-C24). The calculated LRIs were then compared with those reported in the literature. The areas of individual peaks of the FID signal were used to calculate the relative concentrations of the components compared to the total area. The analyses were performed in triplicate.
2.8. Biological Activities of Algal Extracts
2.8.1. Preparation of Microalgae Extract for Antioxidant and Antimicrobial Analyses
Powdered microalgae were used to prepare water extracts for subsequent analysis. To prepare the extracts, 50 g of powdered microalgae was placed into a 1-L glass bottle. To each bottle, 500 mL of distilled water was added. The mixture was incubated in a dark environment at 25°C for 24 h using a shaking incubator (GFL 3031, Burgwedel, Germany). Following incubation, the liquid was filtered through Whatman® Grade 2 filter paper (Germany). The microalgae powder was then subjected to a second extraction by soaking it in an additional 500 mL of distilled water and incubating for another 24 h under the same conditions. This extraction procedure was repeated two more times to ensure thorough extraction. Next, the extracts were processed using a vacuum rotary evaporator (Witeg Labortechnik, Germany). The evaporation was conducted in a water bath at 50°C under a pressure of 42 mBar, which corresponds to a boiling point of 30°C within the flask (KIMBLE®, DWK Life Sciences, Rockwood, TN, USA). The concentrated extracts were transferred to sealable glass containers using a metal spoon and stored in the dark at approximately 4°C until further analysis. On the day of analysis, a 500 mg/mL extract solution was prepared by diluting the concentrated extracts with ultrapure water.
2.8.2. Antioxidant Capacity
The antioxidant capacity of water microalgae extracts was evaluated using radical scavenging assays with 2,2-diphenyl-1-picrylhydrazyl (DPPH•) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+) radicals, sourced from Sigma-Aldrich, Taufkirchen, Germany [23]. The extracts were prepared in ultrapure water at a concentration of 500 mg/mL. For the DPPH• assay, a 0.025 g/L solution of DPPH• was made in methanol and adjusted to an absorbance of 0.8 at 515 nm using a Glomax spectrophotometer (Promega Inc., Madison, WI, USA). The ABTS+ radical cation was produced following standard procedures and diluted to achieve an absorbance of 0.7 at 744 nm. In each test, 190 μL of either DPPH• or ABTS+ solution was combined with 10 μL of microalgae extract in a 96-well microtiter plate. The plate was incubated in darkness at room temperature with continuous shaking at 1000 rpm for 30 min. Absorbance measurements were then taken at 744 nm for ABTS+ and 515 nm for DPPH•. The inhibition percentage for DPPH• or ABTS+ was calculated using the formula (A0 − AA)/A0 × 100, where A0 is the absorbance of the methanol control and AA is the absorbance of the sample. Trolox, dissolved in methanol (Uvasol® for spectroscopy, Merck, Darmstadt, Germany) at concentrations from 0 to 100 μg/mL, was used as a standard to create a Trolox equivalent antioxidant capacity (TEAC) calibration curve. This methodology provides a comprehensive assessment of the antioxidant activity of the water microalgae extracts, highlighting their potential health benefits.
2.8.3. Antimicrobial Activity
2.8.3.1. Preparation of Microorganisms
The bacterial strains used for the evaluation of antimicrobial activity were Xanthomonas arboricola CCM 1441, Pectobacterium carotovorum CCM 1008, Pseudomonas syringae CCM 2868, Agrobacterium radiobacter CCM 2926, and Priestia (Bacillus) megaterium CCM 2007. All bacterial strains were obtained from the Czech Collection of Microorganisms (Brno, Czech Republic) in lyophilized form and were stored at −18°C. For testing, lyophilized bacterial strains were reconstituted and cultured in Mueller–Hinton broth (MHB) (Oxoid, Basingstoke, UK) at 37°C for 24 h, except for P. syringae, which was incubated at 30°C. After incubation, bacterial cultures were adjusted to a 0.5 McFarland standard using a densitometer, which corresponds to approximately 1.5 × 108 colony-forming units (CFU) per milliliter. The prepared bacterial cultures were then ready for testing for antimicrobial activity.
The fungal strains used for assessing antimicrobial activity included the microscopic filamentous fungi Monilinia fructigena CCM F-300, Fusarium solani CCM 8014, Botrytis cinerea F-314, and Trichoderma harzianum CCM F-470. These strains were sourced from the Czech Collection of Microorganisms (Brno, Czech Republic). For the assays, the fungal strains were inoculated onto potato dextrose agar (PDA) (Oxoid, Basingstoke, UK) using a bacterial loop with three distinct inoculations. The fungi were then cultured at 21°C for 5 days. After incubation, the fungal cultures were adjusted to the 0.5 McFarland standard using a densitometer, which equates to approximately 1.5 × 108 CFU per milliliter. The prepared fungal cultures were then set for antimicrobial activity testing.
2.8.3.2. Disk Diffusion Method
Microorganisms, including bacteria and fungi, were cultivated, and their densities were adjusted following the procedure described in Section 2.8.3.1. For bacterial testing, 100 μL of the bacterial suspension was pipetted onto Mueller–Hinton agar (MHA) (Oxoid, Basingstoke, UK). For fungal testing, the fungal suspension was evenly spread on PDA plates using a sterile cotton swab, with three parallel streaks made on the agar surface. Sterile 6-mm disks (Oxoid, Basingstoke, UK) were then placed on the surface of the agar inoculated with microorganisms. Each disk was treated with 10 μL of the microalgae water extracts (500 mg/mL). Incubation was carried out for 24 h at 37°C for bacteria (30°C for P. syringae) and for 5 days at 21°C for the microscopic fungi. Positive controls included two antibiotics (e.g., cefoxitin, gentamicin; Oxoid, Basingstoke, UK) for gram-negative and gram-positive bacteria, and an antifungal agent (e.g., fluconazole; Oxoid, Basingstoke, UK) for filamentous fungi. Negative controls comprised disks impregnated with the solvent used to dissolve the extract (ultrapure water) and blank disks. The size of the inhibition zones (radius) around each disk was measured three times, and the mean and standard deviation (SD) in mm was calculated. All measurements were performed in triplicates [24].
2.8.3.3. Minimum Inhibitory Concentration (MIC)
To determine the MIC of microalgae water extracts against bacteria, MHB (Oxoid, Basingstoke, UK) was used as the liquid culture medium. A 96-well microtiter plate was prepared by adding 150 μL of the bacterial culture to each well. To the first wells, 150 μL of microalgae extract (500 mg/mL) was added. Serial dilutions of the microalgae extract were prepared, with concentrations ranging from 250 to 0.122 mg/mL. Negative controls included wells with only MHB and MHB with the solvent used to dissolve the extract (ultrapure water). The growth control consisted of bacterial culture without any extract [25]. After 24-h incubation at 37°C for bacteria (30°C for P. syringae), the MIC was determined using a Glomax plate spectrophotometer (Promega Inc., Madison, WI, USA) at an absorbance of 570 nm. MIC values, representing the concentrations causing 50% (MIC50) inhibition of bacterial growth, were determined from dose–response curves fitted to the inhibition data using Microsoft Excel (version 2.73). The concentration–response data were plotted as scatter plots, and logarithmic trendlines were applied to the data. MIC50 and values were interpolated from these trendline equations, representing the concentrations at which 50% inhibition of bacterial growth occurred, respectively. The results are reported as the mean ± SD of triplicate experiments.
2.9. Antibiofilm Activity
2.10. Statistical Analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) to assess the differences between the values of each microalgal species. Tukey’s test for significant differences was performed to determine which specific values were significantly different from each other. The significance level was set at p < 0.05. All statistical analyses were performed using Astatsa software, and results were presented as mean ± SD for each microalgal species.
3. Results
3.1. NMR Spectroscopy
The NMR analysis of 50% water–methanol extracts of microalgae revealed 18 identified compounds, primarily organic acids, amino acids, carbohydrates, and other metabolites (Table 1).
| Microalgae | Nannochloropsis sp. | Tetraselmis chuii | Chaetoceros muelleri | Thalassiosira weissflogii | Tisochrysis lutea |
|---|---|---|---|---|---|
| Acetate | 2.41 | 9.51 | 2.56 | 1.08 | 0.43 |
| Alanine | 2.11 | 4.73 | 5.26 | 1.07 | 9.72 |
| Formate | 1.21 | 5.77 | 0.30 | 0.11 | 0.28 |
| Glutamate | 1.74 | 7.77 | 7.03 | 4.31 | 5.45 |
| Glutamine | 0.30 | 0.50 | 0.45 | 0.89 | 1.61 |
| Histidine | 0.07 | 0.05 | 0.04 | 0.02 | 0.15 |
| Isoleucine | 0.24 | 0.31 | 0.84 | 0.23 | 1.56 |
| Lactate | 9.00 | 1.02 | 0.11 | 0.93 | 16.19 |
| Leucine | 0.41 | 0.69 | 0.82 | 0.24 | 4.68 |
| Lysine | 0.14 | 0.87 | 0.43 | 1.12 | 4.10 |
| Methionine | 0.03 | 0.13 | 0.11 | 0.02 | 1.06 |
| Phenylalanine | 0.21 | 0.38 | 0.93 | 0.10 | 2.40 |
| Proline | 7.11 | 2.04 | 1.44 | 1.40 | 3.59 |
| Succinate | 3.32 | 5.75 | 1.33 | 4.91 | 3.69 |
| Sucrose | 0.09 | 0.04 | 0.04 | 0.11 | 1.08 |
| Threonine | 0.23 | 0.57 | 0.54 | 0.16 | 2.06 |
| Tryptophan | 0.17 | 0.20 | 0.14 | 0.02 | 0.88 |
| Valine | 0.50 | 0.73 | 1.11 | 0.59 | 2.97 |
Additionally, some samples exhibited strong signals of unknown carbohydrates, particularly in Nannochloropsis sp. and T. lutea, which were not identified and, therefore, are not included in the table. Among the organic acids, T. chuii had the highest concentration of acetate and glutamate. Alanine and lactate were most abundant in T. lutea. Proline was highest in Nannochloropsis sp., while lysine and leucine were prominent in T. lutea. These amino acids suggest the nutritional or biotechnological potential of T. lutea. Overall, T. lutea exhibited the highest concentrations of alanine, lactate, leucine, and unknown carbohydrates. Nannochloropsis sp. was characterized by high levels of proline and an unidentified carbohydrate, whereas T. chuii had elevated levels of organic acids and amino acids.
3.2. Risk Elements and Mineral Content in Various Microalgae Strains
Table 2 summarizes the concentrations of various trace elements and minerals in five microalgae strains (mg/kg). The highest calcium concentrations were recorded in T. chuii, while the other species exhibited significantly lower values. Sodium was most abundant in C. muelleri, followed by T. weissflogii and T. chuii. Potassium reached its highest levels in T. weissflogii.
| Element | Nannochloropsis sp. | Tetraselmis chuii | Chaetoceros muelleri | Thalassiosira weissflogii | Tisochrysis lutea |
|---|---|---|---|---|---|
| Co | ND | 0.18 ± 0.07a | 0.06 ± 0.03b | 0.10 ± 0.07b | 0.13 ± 0.04a |
| Ca | 2193 ± 97.53a | 32,907 ± 263.81b | 2209 ± 26.03a | 1575 ± 51.89a | 2964 ± 104.84a |
| Na | 11,37,217 ± 204.12a | 19,105 ± 2321.22b | 24,521 ± 1755.48c | 20,876 ± 187.62c | 17,804 ± 6508.56b |
| K | 9534 ± 290.69a | 14,676 ± 119.35b | 18,256 ± 258.15c | 21,965 ± 325.30d | 12,375 ± 205.90b |
| Mg | 218.58 ± 5.40a | 510.93 ± 2.37b | 510.14 ± 5.24b | 279.78 ± 7.51c | 332.29 ± 6.57d |
| Al | 2.35 ± 1.67d | 3.92 ± 0.96c | 20.11 ± 0.74a | 25.96 ± 0.77b | 1.41 ± 0.39e |
| Ag | ND | ND | ND | ND | ND |
| Ba | 1.69 ± 0.43c | 4.61 ± 0.14a | 2.88 ± 0.04b | 2.37 ± 0.06b | 1.08 ± 0.31d |
| Cd | ND | ND | ND | ND | ND |
| Cr | ND | ND | 0.50 ± 0.10a | 3.19 ± 0.12b | ND |
| Cu | 8.71 ± 0.16a | 20.95 ± 0.14c | 13.75 ± 0.27b | 12.03 ± 0.24b | 11.92 ± 0.22b |
| Fe | 477.55 ± 8.33a | 631.81 ± 5.77c | 668.67 ± 14.57b | 425.67 ± 9.17a | 191.37 ± 5.71d |
| Li | 0.10 ± 0.02c | 0.23 ± 0.01a | 0.30 ± 0.01b | 0.10 ± 0.01d | 0.16 ± 0.01e |
| Mn | 41.82 ± 0.73c | 73.28 ± 0.36a | 35.91 ± 0.43b | 50.17 ± 1.18d | 26.50 ± 0.57e |
| Ni | 0.74 ± 0.18d | 0.71 ± 0.18c | 1.23 ± 0.54b | 2.26 ± 0.12a | 0.72 ± 0.23e |
| Pb | ND | 0.29 ± 0.12b | 0.48 ± 0.19a | ND | ND |
| Sr | 8.98 ± 0.77c | 353.37 ± 3.73a | 14.20 ± 0.16b | 10.10 ± 0.32b | 10.06 ± 0.33b |
| Zn | 37.85 ± 0.58a | 41.62 ± 0.56a | 40.32 ± 1.32a | 30.42 ± 0.41b | 103.01 ± 1.46c |
| Se | 1.86 ± 1.14a | 1.89 ± 0.59a | 2.15 ± 1.10b | 1.78 ± 0.55a | 1.48 ± 0.65a |
| As | 0.87 ± 0.09a | 1.96 ± 0.48b | 0.42 ± 0.17a | ND | 0.69 ± 0.30a |
| Sb | 0.59 ± 0.37a | 0.98 ± 0.39b | 0.47 ± 0.25a | 0.44 ± 0.19a | 0.66 ± 0.47a |
- Note: Values marked with the same letter in rows do not differ significantly p > 0.05.
Among trace elements, iron was predominant in C. muelleri and T. chuii, whereas zinc was most abundant in T. lutea. Magnesium was evenly distributed between T. chuii and C. muelleri, with lower concentrations in the other species. Copper showed the highest concentration in T. chuii. Regarding potentially hazardous elements, cadmium and silver were not detected in any of the analyzed microalgae. Lead was present only in T. chuii and C. muelleri. Overall, the results indicate that T. chuii and C. muelleri contain the highest concentrations of minerals and trace elements, suggesting their potential for nutritional and industrial applications. At the same time, the low levels of risk elements highlight their safety suitability.
3.3. High-Performance Liquid Chromatography of Ethanol and Hexane Extracts
The concentrations of carotenoids and chlorophylls in microalgae extracts obtained using ethanol and hexane are presented in Table 3.
| Extraction solution | Standard compound | Rt | Concentration | |
|---|---|---|---|---|
| Nannochloropsis sp. | H | Zeaxanthin | 12.67 | 0.0021 |
| α-Carotene | 26.22 | 0.0322 | ||
| Nannochloropsis sp. | E | Zeaxanthin | 12.64 | tr |
| Chlorophyll a | 21.16 | tr | ||
| Tetraselmis chuii | E | Fucoxanthin | 6.69 | 0.0047 |
| Violaxanthin | 7.18 | 0.0060 | ||
| Lutein | 12.82 | 0.0152 | ||
| Chlorophyll b | 17.73 | 0.1322 | ||
| Chlorophyll a | 20.19 | 0.0990 | ||
| α-Carotene | 25.97 | tr | ||
| Tetraselmis chuii | H | Lutein | 12.73 | tr |
| Chlorophyll b | 18.72 | tr | ||
| α-Carotene | 26.12 | tr | ||
| Chaetoceros muelleri | E | Fucoxanthin | 6.43 | 0.0655 |
| Chaetoceros muelleri | H | Fucoxanthin | 6.62 | 0.0353 |
| Astaxanthin | 9.26 | tr | ||
| α-Carotene | 26.21 | 0.0187 | ||
| Thalassiosira weissflogii | E | Fucoxanthin | 6.81 | 0.1188 |
| Violaxanthin | 7.54 | 0.0967 | ||
| Astaxanthin | 9.54 | 0.0142 | ||
| Thalassiosira weissflogii | H | Fucoxanthin | 6.63 | 0.1983 |
| Lutein | 12.04 | 0.0189 | ||
| α-Carotene | 26.28 | 0.0502 | ||
| Tisochrysis lutea | E | Fucoxanthin | 6.92 | 0.0415 |
| Lutein | 12.2 | tr | ||
| Tisochrysis lutea | H | Fucoxanthin | 6.58 | 0.0104 |
| Lutein | 11.99 | 0.0009 | ||
| Chlorophyll b | 19.02 | 0.0070 | ||
| α-Carotene | 26.21 | 0.0027 | ||
- Note: tr, trace; E, ethanol extract, H, hexane extract.
In the hexane extract of Nannochloropsis sp. H, zeaxanthin and α-carotene were identified, while trace amounts of zeaxanthin and chlorophyll a were found in the ethanol extract of Nannochloropsis sp. In the ethanol extract of T. chuii, carotenoids such as fucoxanthin, violaxanthin, lutein, and α-carotene, along with chlorophyll a and b, were detected. The hexane extract of T. chuii contained trace amounts of lutein, chlorophyll b, and α-carotene. In the ethanol extract of C. muelleri, only fucoxanthin was present, whereas the hexane extract also contained fucoxanthin, astaxanthin, and α-carotene. In the ethanol and hexane extracts of T. weissflogii, fucoxanthin was dominant. However, the ethanol extract also contained violaxanthin and astaxanthin, while the hexane extract had lutein and α-carotene in lower concentrations. In the ethanol extract of T. lutea, fucoxanthin and trace amounts of lutein were detected. In the hexane extract, in addition to fucoxanthin and lutein, chlorophyll b and α-carotene were also present. Overall, fucoxanthin was the most abundant and frequently detected carotenoid in the studied microalgae.
3.4. Total Carotenoids
The TCC in various microalgae strains varied significantly (Table 4). T. lutea exhibited the highest TCC at 2.35 mg/g, following C. muelleri, Nannochloropsis sp. and T. chuii. The lowest TCC was observed in T. weissflogii.
| Microalgae | TCC |
|---|---|
| Nannochloropsis sp. | 1.24 ± 0.12a |
| Tetraselmis chuii | 1.08 ± 0.10a |
| Chaetoceros muelleri | 1.43 ± 0.15a |
| Thalassiosira weissflogii | 0.09 ± 0.02b |
| Tisochrysis lutea | 2.35 ± 0.20c |
- Note: Values marked with the same letter in columns do not differ significantly p > 0.05.
3.5. Volatile Compounds
The most prevalent chemical groups in the studied microalgae (Table 5) were alcohols. The profile of Nannochloropsis sp. contained 50.99% alcohols and also had a relatively high ester content, primarily octyl acetate. The profile of T. chuii similarly showed the highest proportion of alcohols, accounting for 66.25%. The second most abundant group in T. chuii was ketones. C. muelleri again exhibited the highest relative amount of alcohols (69.39%), mainly 2-ethylhexanol, but also contained a significant amount of aldehydes. T. weissflogii had the highest alcohol content (47.97%) and a notable amount of alkanes. Finally, T. lutea showed high levels of alcohols (45.45%) and aldehydes. In summary, the studied microalgae can be classified as alcohol-type profiles.
| Family | Compound | Retention time (min) | ANOVA† | Nannochloropsis sp. | Tetraselmis chuii | Chaetoceros muelleri | Thalassiosira weissflogii | Tisochrysis lutea |
|---|---|---|---|---|---|---|---|---|
| Esters | Methyl hexanoate | 3.663 | NS | 0.43 | 0.14 | 0.06 | 0.12 | 0.07 |
| Methyl butyrate | 5.529 | NS | 0.38 | 0.07 | 0.27 | 0.24 | 0.23 | |
| Methyl octanoate | 8.170 | ∗∗∗ | 3.32a | 0.04b | 0.05b | 0.40b | 0.14b | |
| Octyl acetate | 8.920 | ∗∗∗ | 9.15a | 0.39c | 6.76b | 4.48b | 5.71b | |
| Ethyl octanoate | 10.609 | ∗∗∗ | 2.05a | 0.10b | 0.06b | 0.21b | 0.04b | |
| Neomenthyl acetate | 6.131 | NS | 0.39 | 0.32 | 0.23 | 0.29 | 0.29 | |
| ∑ | ∗∗∗ | 15.72a | 1.06c | 7.43b | 5.74b | 6.48b | ||
| Aldehydes | 2-Pentenal | 2.190 | ∗ | 1.59a | 0.06b | 0.51b | 0.41b | 0.43b |
| Hexanal | 2.449 | ∗ | 3.36a | 1.46b | 1.35b | 2.41a | 2.96a | |
| Safranal | 2.848 | NS | 0.12 | 0.01 | 0.10 | 0.17 | 0.26 | |
| 2-Hexenal | 2.888 | NS | 0.40 | 0.11 | 0.17 | 0.47 | 0.32 | |
| Heptanal | 3.405 | ∗ | 0.39b | 0.10b | 1.14a | 1.78a | 0.51b | |
| 2-Ethylhexanal | 4.179 | NS | 0.04 | 0.04 | 0.24 | 0.04 | 0.03 | |
| 2-Heptenal | 4.229 | ∗ | 0.06b | 0.01b | 0.12b | 0.26b | 1.76a | |
| Benzaldehyde | 4.403 | ∗ | 1.85b | 1.75b | 3.56b | 2.60b | 10.57a | |
| 2,4-Undecadienal | 4.848 | NS | 0.66 | 1.27 | 0.53 | 0.45 | 0.72 | |
| 2,4-Heptadienal | 4.966 | ∗ | 0.28b | 0.19b | 0.09b | 0.27b | 1.53a | |
| 2,6-dimethyl-5-heptenal | 5.901 | NS | 0.75 | 0.96 | 0.76 | 0.66 | 1.09 | |
| Phenylacetaldehyde | 6.065 | NS | 0.15 | 0.03 | 0.58 | 0.06 | 1.00 | |
| ∑ | ∗∗ | 9.65b | 5.99b | 9.15b | 9.58b | 21.18a | ||
| Alcohols | 1-Octen-3-ol | 4.594 | NS | 3.67 | 2.90 | 4.32 | 2.38 | 3.28 |
| 2-Ethylhexanol | 5.599 | ∗∗∗ | 34.02b | 58.30a | 58.07a | 36.92b | 33.52b | |
| 2-Decen-1-ol | 6.550 | NS | 0.18 | 0.44 | 0.72 | 0.49 | 0.36 | |
| 3,4-dimethylcyclohexanol | 7.903 | ∗ | 9.73a | 3.70c | 4.35bc | 5.08b | 5.95b | |
| 2-Pentenol | 2.240 | ∗∗∗ | 2.45a | 0.40b | 0.62b | 0.37b | 1.40a | |
| 1-Octen-3-ol | 2.303 | ∗ | 0.36b | 0.03b | 0.43b | 1.70a | 0.28b | |
| 1,5-Pentanediol | 2.649 | NS | 0.16 | 0 | 0.07 | 0.09 | 0.32 | |
| 1-Hexanol | 2.994 | NS | 0.42 | 0.48 | 0.81 | 0.94 | 0.34 | |
| ∑ | ∗∗∗ | 50.99ab | 66.25a | 69.39a | 47.97b | 45.45b | ||
| Acids | Acid Isovaleric | 2.565 | ∗ | 0.51b | 0c | 0.33b | 0.30b | 2.04a |
| Hexanoic acid | 2.933 | NS | 0.04 | 0.02 | 0.06 | 0.01 | 0.39 | |
| ∑ | ∗ | 0.55b | 0.02c | 0.39b | 0.31b | 2.43a | ||
| Ketones | 2-Octanone | 4.148 | NS | 0.24 | 0.5 | 0.09 | 0.2 | 0.15 |
| 2-Heptanone | 3.240 | NS | 0.19 | 0.4 | 0.12 | 0.11 | 0.17 | |
| 6-Methyl-5-hepten-2-one | 4.688 | ∗∗∗ | 0.98b | 10.6a | 1.09b | 1.05b | 1.32b | |
| 2-Nonanone | 4.796 | NS | 0.21 | 0.29 | 0.2 | 0.15 | 0.23 | |
| 2-Nonanone | 7.217 | NS | 0.14 | 0.32 | 0.16 | 0.22 | 0.09 | |
| 3,5-Octadien-2-one | 7.309 | ∗∗ | 1.93b | 3.30a | 0.83b | 1.43b | 1.15b | |
| ∑ | ∗∗∗ | 3.69b | 15.41a | 2.49b | 3.16b | 3.11b | ||
| Terpenes | Camphene | 5.018 | NS | 0.18 | 0.09 | 0.17 | 0.18 | 0.4 |
| Limonene | 5.783 | ∗∗ | 1.95b | 0.86b | 1.84b | 4.61a | 2.95ab | |
| Citronellol | 6.211 | NS | 0.14 | 0.02 | 0.03 | 0.04 | 0.29 | |
| ∑ | ∗∗ | 2.27b | 0.97c | 2.04b | 4.83a | 3.64ab | ||
| Alkanes | Pentadecane | 22.637 | ∗ | 1.91a | 0.02b | 0.22b | 2.14a | 0.93ab |
| Octadecane | 30.29 | ∗ | 1.19b | 0.03c | 0.01c | 14.48a | 1.31b | |
| ∑ | ∗ | 3.10b | 0.05c | 0.23c | 16.62a | 2.24b | ||
| Ionones | α-Ionone | 19.404 | ∗∗∗ | 0.08b | 2.19a | 0.39b | 0.13b | 0.07b |
| β-Ionone | 21.655 | NS | 1.74 | 1.33 | 1.38 | 2.52 | 1.01 | |
| ∑ | ∗ | 1.82c | 3.52a | 1.77c | 2.65bc | 1.08c | ||
| Pyrazines | 2,6-Dimethylpyrazine | 3.584 | ∗ | 3.11ab | 0.50c | 2.13b | 1.24bc | 4.20a |
| Trimethylpyrazine | 5.086 | NS | 1.26 | 0.58 | 0.90 | 1.14 | 2.00 | |
| 2,6-Dimethyl-3-ethyl pyrazine | 6.861 | NS | 1.15 | 0.35 | 1.00 | 0.68 | 1.71 | |
| ∑ | ∗∗ | 5.52ab | 1.43c | 4.03b | 3.06bc | 7.91a | ||
| Others | Benzylamine | 3.483 | NS | 0.39 | 1.21 | 0.32 | 0.13 | 0.21 |
| 2-Methyl-6-ethylpyridine | 4.332 | NS | 0.01 | 0.95 | 0.03 | 0.09 | 0.01 | |
| 3,5,5-Trimethyl-2-Hexene | 4.499 | ∗∗ | 5.74a | 2.54b | 2.63b | 5.38a | 5.62a | |
| α-Isophorone | 6.479 | NS | 0.54 | 0.62 | 0.12 | 0.47 | 0.63 | |
| ∑ | ∗∗ | 6.68a | 5.32ab | 3.1b | 6.07a | 6.47a | ||
- Note: Values (mean of 3 replications) followed by the same letter, within the same row, were not significantly different (p > 0.05), according to Tukey’s least significant difference test.
- †NS = not significant at p > 0.05.
- ∗, ∗∗, and ∗∗∗ significant at p < 0.05, 0.01, and 0.001, respectively.
3.6. Fatty Acid Content
By GC/MS analyses of the dried microalgae extract, 10 fatty acids were detected and identified (Table 6). The saturated fraction prevailed over the unsaturated one in T. weissflogii and C. muelleri and Nannochloropsis sp., while it was reversed in T. lutea and T. chuii. Myristic acid reached higher average percentage values ranging from 20.6% to 32.0% in T. lutea, T. weissflogii, and C. muelleri. Palmitic acid was the major component in T. weissflogii (54.4%) and Nannochloropsis sp. (43.0%). On the other side, oleic acid (46.8%) was the principal fatty acid in T. chuii and palmitoleic acid (47.9%) was in C. muelleri (47.9%). Qualitative differences between the microalgae samples were found; in particular, stearidonic acid (12.0%; 3.2%) was present only in T. lutea and in T. chuii, respectively, while elaidic (1.6%) only in C. muelleri was detected.
| N | Component1 | LRI2 | LRI3 | (%)4 | (%)5 | (%)6 | (%)7 | (%)8 |
|---|---|---|---|---|---|---|---|---|
| 1 | Myristic acid, C14:0 | 1740 | 1748 | 32.0 ± 2.10 | 36.7 ± 2.50 | 20.6 ± 1.09 | 0.9 ± 0.04 | 7.8 ± 0.15 |
| 2 | Pentadecanoic acid, C15:0 | 1842 | 1844 | — | 4.0 ± 0.06 | 0.6 ± 0.03 | — | 0.3 ± 0.02 |
| 3 | Palmitoleic acid, C16:1n7 | 1928 | 1930 | 6.4 ± 0.08 | 2.7 ± 0.05 | 47.9 ± 3.20 | 0.8 ± 0.03 | 34.2 ± 2.02 |
| 4 | Palmitic acid, C16:0 | 1935 | 1940 | 17.5 ± 0.12 | 54.4 ± 5.10 | 20.4 ± 2.30 | 42.3 ± 3.15 | 43.0 ± 3.52 |
| 5 | Stearidonic acid, C18:4n3 | 1945 | 1951 | 12.0 ± 0.10 | — | — | 3.2 ± 0.09 | — |
| 6 | Linoleic acid, C18:2n6 | 2145 | 2143 | 4.5 ± 0.06 | — | 1.1 ± 0.08 | 6.0 ± 0.11 | 3.3 ± 0.10 |
| 7 | Oleic acid, C18:1n9 | 2155 | 2152 | 26.4 ± 0.15 | 2.2 ± 0.09 | 2.0 ± 0.10 | 46.8 ± 4.15 | 9.6 ± 1.10 |
| 8 | Elaidic acid, C18:1n9 | 2168 | 2175 | — | — | 1.6 ± 0.06 | — | — |
| 9 | Stearic acid, C18:0 | 2183 | 2180 | 0.3 ± 0.02 | — | 1.4 ± 0.08 | — | 1.6 ± 0.09 |
| 10 | Arachidonic acid, C20:4n6 | 2330 | 2324 | 0.8 ± 0.04 | — | 4.3 ± 0.07 | — | 0.2 ± 0.02 |
| SUM | 99.9 | 100.0 | 99.9 | 100.0 | 100.0 | |||
| Saturated FAs | 49.8 | 95.1 | 56.9 | 43.2 | 52.7 | |||
| Unsaturated FAs | 50.1 | 4.9 | 43.0 | 56.8 | 47.3 |
- Note: —, not detected.
- 1The components are reported according to their elution order on apolar column (VF-5 ms).
- 2Linear retention indices measured on apolar column.
- 3Linear retention indices from literature.
- 4Percentage mean values of T. lutea.
- 5Percentage mean values of T. weissflogii.
- 6Percentage mean values of C. muelleri.
- 7Percentage mean values of T. chuii.
- 8Percentage mean values of Nannochloropsis sp.
3.7. Evaluation of the Antioxidant Activity of Water Extracts of Microalgae
Table 7 provides a detailed overview of the antioxidant activity of various microalgae extracts assessed using the DPPH assay. The results, expressed in terms of IC50 values and TEAC equivalents, reveal significant variations in antioxidant effectiveness among the tested microalgae.
| Microalgae | IC50 (mg/mL) | TEAC equivalent (10−4) |
|---|---|---|
| Nannochloropsis sp. | 10.09 ± 1.69b | 2.94b |
| Tetraselmis chuii | 14.64 ± 1.76c | 2.03c |
| Chaetoceros muelleri | 1.61 ± 0.43a | 18.48a |
| Thalassiosira weissflogii | 13.63 ± 0.65c | 2.18c |
| Tisochrysis lutea | 18.66 ± 1.02d | 1.59d |
- Note: Values marked with the same letter in columns do not differ significantly from p > 0.05.
Water extract of C. muelleri exhibited the highest antioxidant activity, with the lowest IC50 value (1.61 mg/mL) and the highest TEAC equivalent (18.48 × 10−4). In contrast, T. lutea showed the weakest antioxidant capacity, with the highest IC50 (18.66 mg/mL) and the lowest TEAC equivalent (1.59 × 10−4). The remaining species displayed intermediate antioxidant activities, with Nannochloropsis sp. demonstrating a moderate effect. Statistical analysis indicated significant differences between the species (p < 0.05). When compared to Trolox, which is a standard antioxidant with an IC50 of 2.97 μg/mL, all tested microalgae extracts exhibit lower antioxidant activity.
Table 8 presents the antioxidant activity of water extracts from various microalgae as measured by the ABTS assay. The results, displayed as IC50 values and TEAC equivalents, provide insights into the efficiency of each microalgae extract in neutralizing ABTS radicals.
| Microalgae | IC50 (mg/mL) | TEAC equivalent (10−4) |
|---|---|---|
| Nannochloropsis sp. | 0.29 ± 0.01a | 86.58a |
| Tetraselmis chuii | 0.30 ± 0.09a | 82.22a |
| Chaetoceros muelleri | 0.13 ± 0.01a | 191.55a |
| Thalassiosira weissflogii | 0.26 ± 0.01a | 95.01a |
| Tisochrysis lutea | 4.95 ± 0.83b | 5.01b |
- Note: Values marked with the same letter in columns do not differ significantly from p > 0.05.
C. muelleri demonstrates the highest antioxidant activity with the lowest IC50 value of 0.13 mg/mL and a TEAC equivalent of 191.55 × 10−4. This indicates that C. muelleri is the most effective at scavenging ABTS radicals among the tested microalgae, requiring the least amount to achieve 50% inhibition of the radicals. Nannochloropsis sp. and T. chuii show comparable antioxidant activity with IC50 values of 0.29 mg/mL and 0.30 mg/mL, respectively. Their TEAC equivalents are 86.58 × 10−4 and 82.22 × 10−4, reflecting their moderate effectiveness in radical scavenging. In contrast, T. lutea shows the lowest antioxidant activity with an IC50 value of 4.95 mg/mL and a TEAC equivalent of 5.01 × 10−4. This suggests that T. lutea is the least effective among the tested microalgae in neutralizing ABTS radicals. Compared to Trolox, which serves as a standard antioxidant with an IC50 of 2.48 μg/mL, all microalgae extracts exhibit lower antioxidant activities.
3.8. Evaluation of Antimicrobial Activity
Table 9 presents the antimicrobial activity of water extracts (500 mg/mL) from various microalgae against different microorganisms using the disk diffusion method.
| Microorganism | Nannochloropsis sp. | Tetraselmis chuii | Chaetoceros muelleri | Thalassiosira weissflogii | Tisochrysis lutea |
|---|---|---|---|---|---|
| Bacteria | |||||
| Priestia megaterium | 3.56 ± 0.53a | 2.44 ± 0.53b | 3.22 ± 0.83a | 3.00 ± 0.50a | 2.00 ± 0.50b |
| Pseudomonas syringae | 2.89 ± 1.05a | 4.11 ± 0.60b | 4.11 ± 0.60b | 1.89 ± 0.60c | 3.22 ± 0.39b |
| Xanthomonas arboricola | 0.33 ± 0.50a | 2.33 ± 0.50b | 1.67 ± 0.50b | 2.33 ± 0.50b | 3.89 ± 0.53c |
| Agrobacterium radiobacter | 0.33 ± 0.50a | 1.56 ± 0.53b | 2.33 ± 0.87b | 2.00 ± 0.37b | 2.22 ± 0.44b |
| Pectobacterium carotovorum | 2.67 ± 0.50a | 2.56 ± 0.53a | 4.89 ± 0.60b | 2.33 ± 0.50a | 1.89 ± 0.60c |
| Fungi | |||||
| Fusarium solani | ND | 0.56 ± 0.53 | ND | ND | ND |
| Monilinia fructigena | 3.33 ± 0.50a | ND | 3.67 ± 0.50a | 4.11 ± 0.60b | 4.22 ± 0.67b |
| Trichoderma harzianum | ND | 1.22 ± 0.44 | ND | ND | ND |
| Botrytis cinerea | 0.89 ± 0.60a | ND | 1.11 ± 0.60a | 1.11 ± 0.60a | ND |
- Note: Values marked with the same letter in rows do not differ significantly from p > 0.05.
- Abbreviation: ND = not detected.
Among the bacteria tested, Nannochloropsis sp. exhibited the highest antimicrobial activity against Priestia megaterium with an inhibition zone of 3.56 mm. T. lutea showed the least activity among the microalgae tested, with an inhibition zone of 2.00 mm. For P. syringae, T. chuii and C. muelleri were the most effective, each producing an inhibition zone of 4.11 mm. Nannochloropsis sp. displayed a moderate inhibition zone. Regarding X. arboricola, T. lutea showed the highest inhibition with a zone of 3.89 mm. A. radiobacter was most effectively inhibited by C. muelleri, with an inhibition zone of 2.33 mm. For P. carotovorum, C. muelleri showed the highest inhibition with an inhibition zone of 4.89 mm.
F. solani was inhibited only by T. chuii, showing a slight inhibition zone of 0.56 mm, while no activity was detected for the other extracts. M. fructigena was most inhibited by T. weissflogii and T. lutea. T. harzianum showed inhibition only when treated with T. chuii, which produced an inhibition zone of 1.22 mm. B. cinerea was weakly inhibited by Nannochloropsis sp., C. muelleri, and T. weissflogii, while no activity was observed for T. chuii and T, lutea.
Table 10 summarizes the MIC values of water extracts from various microalgae against different microorganisms. The MIC values, expressed in mg/mL, indicate the lowest concentration of each microalgal extract that effectively inhibits the growth of the respective microorganism.
| Microorganism | Nannochloropsis sp. | Tetraselmis chuii | Chaetoceros muelleri | Thalassiosira weissflogii | Tisochrysis lutea |
|---|---|---|---|---|---|
| Priestia megaterium | 1.44 ± 0.05a | 6.37 ± 0.98b | 2.12 ± 0.85a | 1.18 ± 0.18a | 174.68 ± 28.16c |
| Pseudomonas syringae | 2.60 ± 0.08a | 0.80 ± 0.20b | 27.42 ± 6.86c | 1.65 ± 0.07a | 167.17 ± 15.71d |
| Xanthomonas arboricola | 1.97 ± 0.20a | 0.79 ± 0.03b | 1.24 ± 0.26a | 1.51 ± 0.16a | 166.15 ± 13.07c |
| Agrobacterium radiobacter | 1.64 ± 0.14a | 0.86 ± 0.05b | 0.95 ± 0.01b | 1.24 ± 0.11a | 199.77 ± 42.91c |
| Pectobacterium carotovorum | 1.84 ± 0.21a | 0.58 ± 0.02b | 1.10 ± 0.03a | 1.39 ± 0.12a | 177.76 ± 28.67c |
- Note: Values marked with the same letter in rows do not differ significantly from p > 0.05.
- Abbreviation: ND = not detected.
For P. syringae, X. arboricola, A. radiobacter, and P. carotovorum, microalgae T. chuii demonstrated the lowest MIC, indicating its strong antibacterial activity against these pathogens. In contrast, T. weissflogii showed the most potent activity against P. megaterium, while Nannochloropsis sp. and C. muelleri exhibited moderate effectiveness. T. lutea had the highest MIC values across all microorganisms, suggesting lower antimicrobial activity.
The antibiofilm activity of microalgae extracts against P. megaterium (Figure 1) was evaluated at various concentrations. At the highest tested concentration (250 mg/mL), T. weissflogii and T. lutea exhibited the highest activity, reaching 69.20% and 69.89%, respectively. Nannochloropsis sp., T. chuii, and C. muelleri showed lower activity at this concentration, with values of approximately 60.77%, 42.05%, and 43.19%. As the concentration decreased, a general trend of declining activity was observed. At 125 mg/mL T lutea maintained relatively high activity (46.46%), while T. weissflogii showed a significant drop to 15.58%. At concentrations of 62.5 mg/mL and lower, all species exhibited reduced activity, with most showing negative values or minimal effects at the lowest concentration (7.81 mg/mL). Overall, the results indicate that the effectiveness of the extracts decreased with lower concentrations, with T. weissflogii and T. lutea demonstrating the most pronounced activity at higher concentrations. At lower concentrations, the effects were inconsistent or minimal, suggesting a limited impact at low doses.

The antibiofilm activity of microalgae extracts against P. syringae was evaluated at various concentrations (Figure 2). At the highest concentration (250 mg/mL), Nannochloropsis sp. (74.74%) and T. lutea (71.65%) showed the highest activity, followed by T. chuii (64.57%), T. weissflogii (58.08%), and C. muelleri (46.37%). At 125 mg/mL, Nannochloropsis sp. (68.52%) remained effective, while other species showed reduced activity. As the concentration decreased, activity generally declined, with negative values or minimal effects observed at 31.25 mg/mL and lower. Overall, Nannochloropsis sp. exhibited the strongest activity at higher concentrations, with diminished effects at lower doses.
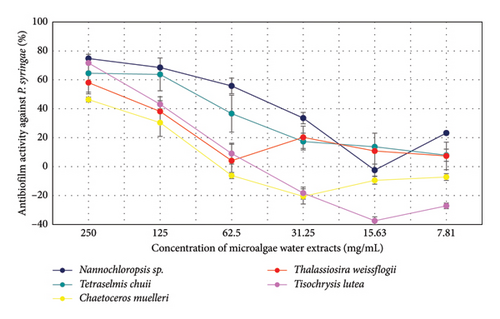
The antibiofilm activity of microalgae extracts against P. carotovorum was evaluated at various concentrations (Figure 3). At 250 mg/mL, T. lutea showed the highest activity (76.16%), followed by Nannochloropsis sp. (70.74%) and T. weissflogii (58.95%). At 125 mg/mL, Nannochloropsis sp. (44.44%) maintained moderate activity, while T. chuii (37.44%) and T. lutea (43.92%) showed reduced effects. At 62.5 mg/mL, all species demonstrated lower activity, with T. chuii (9.52%) and T. weissflogii (15.12%) showing the least effectiveness. At concentrations of 31.25 mg/mL and lower, activity declined further, with negative values observed for most species, except for T. weissflogii (17.29%) at 7.81 mg/mL. Overall, the extracts exhibited the most pronounced activity at higher concentrations, particularly T. lutea and Nannochloropsis sp.
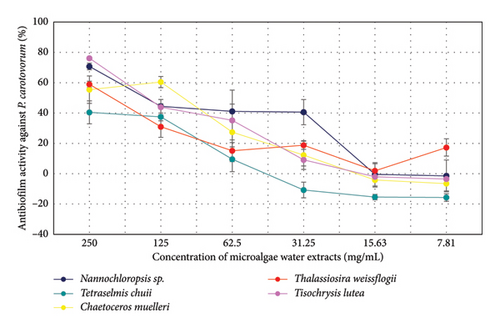
The antibiofilm activity of microalgae extracts against X. arboricola was evaluated at various concentrations (Figure 4). At the highest concentration (250 mg/mL), Nannochloropsis sp. (69.00%) and T. weissflogii (68.69%) demonstrated the highest activity, followed by T. lutea (60.96%) and C. muelleri (51.36%). At 125 mg/mL, a general reduction in activity was observed across all species, with Nannochloropsis sp. (53.36%) maintaining relatively higher activity. Further decreases in activity were noted at lower concentrations (62.5 mg/mL and below), with T. chuii and T. weissflogii showing the highest remaining effects. At concentrations below 31.25 mg/mL, most species exhibited minimal or negative activity. These results suggest a general decline in antibiofilm activity with decreasing concentrations, with Nannochloropsis sp. and T. weissflogii demonstrating more consistent performance at higher concentrations.
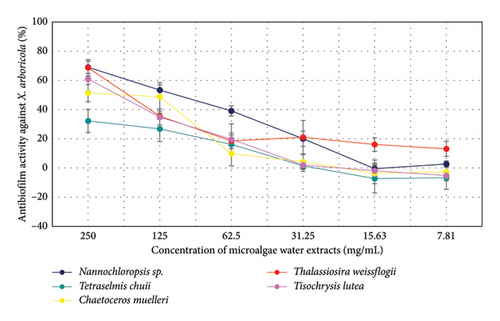
The antibiofilm activity of microalgae extracts against A. radiobacter was evaluated at various concentrations (Figure 5). At 250 mg/mL, T. lutea (71.87%) and Nannochloropsis sp. (68.35%) exhibited the highest activity, followed by T. weissflogii (60.93%) and T. chuii (49.18%). At 125 mg/mL, a decline in activity was observed for all species, with Nannochloropsis sp. (57.69%) showing the highest remaining effect. At 62.5 mg/mL, activity further decreased, with T. chuii (15.30%) and T. weissflogii (25.47%) showing the most significant remaining activity. At concentrations of 31.25 mg/mL and lower, activity was minimal or negative for most species, except for T. weissflogii (16.28%) at 31.25 mg/mL. These results suggest that higher concentrations of microalgae extracts are more effective against Agrobacterium radiobacter, with decreased activity observed at lower concentrations.
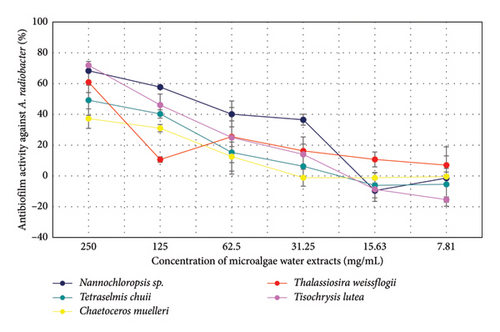
4. Discussion
By 2050, the world’s population is expected to surpass 10 billion, which will bring significant challenges like food security, energy needs, extreme weather, changing climates, and limited arable land [27]. To address these issues, microalgal biotechnology has advanced, allowing for the large-scale cultivation, which is rich in diverse bioactive compounds with many potential uses [28]. Additionally, NMR technology has become one of the most effective methods in the past 20 years for analyzing the composition, structure, and functions of different metabolites in various microalgal strains [29]. Microalgae, particularly, the diatom C. calcitrans, offer significant health benefits. Researchers [30] utilized 1H NMR spectroscopy and multivariate data analysis to identify essential metabolites from different solvent extracts, revealing 11 amino acids, cholesterol, 6 fatty acids, and various carotenoids. Notably, compounds like fucoxanthin and astaxanthin demonstrated strong antioxidant properties. In our study, we analyzed the metabolite profiles of various microalgae species, including Nannochloropsis sp., T. chuii, C. muelleri, T. weissflogii, and T. lutea. Our results indicated the presence of several amino acids, including alanine, glutamate, and proline, alongside fatty acids and carbohydrates. For example, T. lutea exhibited the highest levels of alanine (9.72 mg/g) and lactate (16.19 mg/g), suggesting its potential as a source of these bioactive compounds. In our study, we extracted microalgae samples using a 1:1 v/v mixture of MeOD-D2O, while Bustamam et al. [31] analyzed I. galbana using five solvents of varying polarities, including hexane and ethyl acetate. They identified 21 metabolites, with water extracts yielding the highest amounts of polar metabolites, while ethyl acetate was most effective for fatty acids and carotenoids. In contrast, our results show varying concentrations of amino acids and other compounds across different microalgal species, indicating that the choice of solvent and extraction method significantly impacts the metabolite profile.
Organisms require trace amounts of certain risk elements, including Co, Cu, Fe, Mn, Mo, V, Sr, and Zn, for proper functioning [32]. However, when these elements are present in excessive amounts, they can be harmful. Industrial development has led to an increase in contamination by risk elements in water and wastewater, often due to improper disposal into sewage systems or direct release into water bodies [33]. Microalgae absorb trace elements like B, Co, Cu, Fe, Mo, Mn, and Zn for enzymatic functions and cellular metabolism. In contrast, other risk elements, such as As, Cd, Cr, Pb, and Hg, are harmful to microalgae. Interestingly, the hormesis effect indicates that low concentrations of toxic elements can actually promote the growth and metabolic activity of microalgae [34]. In our study, we observed that microalgae, such as Nannochloropsis sp. and T. chuii, accumulated trace amounts of essential elements like Co, Cu, Fe, Mn, and Zn. This supports the idea that these metals are crucial for enzymatic functions and cellular metabolism [35]. High concentrations of iron were found in C. muelleri (668.67 mg/g) and T. chuii (631.81 mg/g), which is consistent with its role in photosynthesis. Although microalgae can absorb beneficial elements, they may also encounter harmful elements such as As and Cd. Our results showed no detectable amounts of cadmium and limited amounts of arsenic. This is also because our tested microalgae were grown in fully controlled conditions and not in the wild. In our study, we also found remarkable concentrations of minerals such as Ca, Na, and Mg in different types of microalgae. Our results are in agreement with the study of Santhakumaran et al. [36] who reported Ca ranging from 0.04 to 1.91 mg/g, suggesting that microalgae can serve as a good source of calcium. The sodium content varied in both studies, with Santhakumaran et al. [36] reporting a range from 2.31 mg/g to 25.33 mg/g, indicating variability among species. Magnesium content was also significant in our findings, supporting its importance as a nutraceutical mineral. Overall, both studies confirm that microalgae are rich in essential minerals, increasing their potential in nutraceutical applications.
Microalgae, as photosynthetic organisms foundational to the aquatic food chain, are among the most abundant and diverse sources of carotenoids [37]. The health benefits and properties of carotenoids have been documented for many years, primarily in relation to those found in fruits, vegetables, and other higher plant parts. However, there is limited research on carotenoids derived from marine sources like seaweeds, microalgae, and marine animals, which have garnered increasing interest in recent decades [38]. Our study identified key carotenoids like fucoxanthin, lutein, zeaxanthin, and α-carotene in various microalgae species. While our concentrations were generally lower than those in other studies [37, 39, 40], they still indicate the potential of these microalgae as nutraceutical sources. In comparison with previous research, our findings show a simpler carotenoid profile and lower chlorophyll levels. These differences may arise from growth conditions or species variations.
Analysis of volatile compounds in microalgae is essential to understand their bioactivity and potential uses. These compounds, known as volatile organic compounds (VOCs), are natural secondary metabolites that may provide benefits such as antimicrobial, antioxidant, and anti-inflammatory properties [41]. This area is largely untapped, and some volatile secondary metabolites could have significant bioactive effects. Overall, understanding the profile of these compounds is key to exploiting the full potential of microalgae in a variety of industries [42]. The volatile profiles of microalgae vary significantly by species and extraction methods. In our study, T. chuii primarily contained alcohols, particularly 2-ethylhexanol (58.3%), contrasting with previous findings [41] that highlighted N-based compounds. This suggests that extraction techniques impact volatile composition. Nannochloropsis sp. showed a strong presence of esters and alcohols, while Monilinia gaditana was noted for its high alkane content (58%) [41]. Differences in volatile profiles between our results and those for Chlorella vulgaris and P. tricornutum may stem from variations in cultivation and extraction methods. The distinctive aroma of microalgae, akin to that of seafood, arises from a blend of volatile compounds produced by the degradation of polyunsaturated fatty acids. This mixture includes alcohols, aldehydes, and esters, which can emit either pleasant or unpleasant scents based on their molecular structure. For example, certain aldehydes and branched-chain alcohols contribute fruity notes, whereas long-chain carboxylic acids are generally odorless [43].
Free radicals, including reactive oxygen species (ROS) and reactive nitrogen species (RNS), are produced during normal cellular metabolism. However, at elevated concentrations, they can induce oxidative stress, which leads to cell damage and death by oxidation of proteins, lipids, and DNA [44]. Antioxidants play an important role in protecting against this free radical-induced damage. Natural compounds such as vitamin C, tocopherol, and various plant extracts are marketed as antioxidants to combat oxidative stress [44]. The use of antioxidants to extend the shelf life of food products is widespread, with synthetic antioxidants being the most common. While most natural antioxidants on the market are sourced from terrestrial plants such as rosemary, tea, grape seeds, pine bark, and cocoa, unicellular microalgae are considered a promising alternative source of antioxidants [45, 46]. Goiris et al. [47] evaluated the antioxidant capacity of microalgae extract, phenolic, and carotenoid content in 32 microalgae biomasses. Their findings revealed that carotenoids and phenolic compounds play a role in the antioxidant capacity of microalgae, although the potency varied depending on the species, growth conditions, and extraction solvent used. Furthermore, fatty acids, including those found in our samples, have been reported to exert an antioxidant effect [48]. The results of Choochote et al. [49] suggest that the microalgae used in their study could be potential sources of natural antioxidants, with some of their activities being comparable to synthetic antioxidants such as butylated hydroxytoluene. These claims are supported by our results, where antioxidative activity was found for all tested water extracts of microalgae in both DPPH and ABTS assays.
Contemporary agriculture requires effective strategies to address the ongoing reduction in the use of pesticides and fertilizers. One promising approach to facilitate this transition is the adoption of bioproducts that are more environmentally sustainable and less harmful to human health. In particular, blue biotechnology, especially the utilization of seaweeds and microalgae, is gaining increasing attention in the scientific community each year [50]. Certain microalgae have demonstrated notable antimicrobial properties. For example, extracts from Spirulina sp. and Nannochloropsis sp. were found to be effective against the Fusarium graminearum species complex, due to their high levels of chlorogenic acid [51]. Furthermore, microalgae can boost the effectiveness of existing biocontrol agents, as evidenced by a study showing that Chlorella vulgaris extracts enhanced the performance of Trichoderma against Cephalosporium maydis, which causes late wilt disease in maize [52]. This synergy demonstrates the potential benefits of integrating microbial and algal biocontrol strategies for more effective disease management. The findings of our study align with the existing literature regarding the antimicrobial properties of microalgae, but they also highlight specific differences. While Spirulina and Nannochloropsis are noted for their effectiveness against Fusarium species, our results suggest that the efficacy of Nannochloropsis sp. against other pathogens was relatively limited. On the other hand, T. chuii and C. muelleri exhibited promising antibacterial activity, indicating that different microalgal species may have unique profiles of antimicrobial efficacy. The ability of T. chuii to inhibit fungal pathogens like M. fructigena and B. cinerea suggests its potential role in integrated pest management, complementing the findings about C. vulgaris enhancing Trichoderma’s effectiveness. This synergy indicates that combining specific microalgae with established biocontrol agents could provide a more robust strategy for managing plant diseases. Although our study confirms the antimicrobial potential of microalgae, it also highlights the importance of exploring diverse species and extraction methods to fully understand their potential in agriculture. Further research could help to elucidate the mechanisms of their antimicrobial effects and optimize their use.
While the antimicrobial activity of microalgae and cyanobacteria is well-known, their antibiofilm activity, especially against plant pathogens, is less studied. Biofilms play a major role in infections, and though microalgae produce bioactive compounds like quorum-sensing inhibitors, research on their effect on biofilms in plant diseases is limited. Given the importance of biofilms in plant pathogen persistence, exploring microalgae’s antibiofilm potential could offer new solutions for crop protection [53]. Microalgal-derived antimicrobial compounds hold significant promise for various industries, including pharmaceuticals, functional foods, aquaculture antibiotics, animal health, agricultural biopesticides, and wastewater treatment. However, despite the vast biodiversity and potential of microalgae as a source of new compounds, only a limited number of species are currently cultivated for commercial purposes [54]. In our study, Nannochloropsis sp., T. lutea, and C. muelleri showed potent antibiofilm activity against various pathogens. T. lutea was particularly effective, with up to 76.16% inhibition against P. carotovorum biofilm. Nannochloropsis sp. consistently showed high activity against P. syringae and X. arboricola biofilms, achieving more than 74% inhibition. Even at lower concentrations, C. muelleri showed remarkable activity, especially against P. carotovorum biofilm. These results suggest that microalgal extracts are promising for biofilm control, comparable to plant antibiofilm agents [55], and could be valuable in agricultural disease management.
5. Conclusion
This study highlights the diverse biochemical profiles of five microalgae species—Nannochloropsis sp., Tetraselmis chuii, Chaetoceros muelleri, Thalassiosira weissflogii, and Tisochrysis lutea—revealing their unique metabolic capabilities and potential applications. Through the analysis, we found significant variations in the types and concentrations of metabolites, underscoring the potential of these microalgae in various fields. Nannochloropsis sp. emerged as a strong candidate for biotechnological applications, particularly due to its high levels of carbohydrates and esters, which are valuable for biofuel production and functional foods. T. chuii showcased promising capabilities for bioenergy, while T. lutea’s ability to produce essential amino acids positions it as a potential source for nutritional supplements. The mineral and fatty acid content indicated that certain species could address global nutritional issues, with specific strains exhibiting high levels of important minerals. Additionally, the carotenoid content, particularly fucoxanthin, points to the potential for developing functional foods and nutraceuticals with antioxidant properties. Our evaluation of antimicrobial and antibiofilm activities revealed that some microalgae could serve as effective agents in controlling biofilm formation, which is significant for both medical and industrial applications. Furthermore, the antioxidant capacities of these species suggest their potential roles in food preservation and health supplements. Overall, this study emphasizes the importance of these microalgae as valuable sources of bioactive compounds, contributing to advancements in nutrition, biotechnology, and environmental sustainability. Future research should aim to optimize cultivation techniques and explore the full range of applications for these microalgae, maximizing their benefits in addressing global challenges.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Natália Čmiková: methodology, investigation, writing – original draft, formal analyses, writing – review & editing, and visualization. Milena D. Vukić, Nenad L. Vukovic, Jaroslav Havlík, Luis Noguera-Artiaga, Ángel A. Carbonell-Barrachina, Ivona Jančo, and Vittorio Vinciguerra: formal analyses and writing – review & editing. Stefania Garzoli: formal analyses, writing – original draft, writing – review & editing, visualization, and supervision. Miroslava Kačániová: methodology, investigation, writing – original draft, formal analyses, supervision, project administration, funding acquisition, and visualization. All authors reviewed the manuscript.
Funding
This work has been supported by the grant of the KEGA 023SPU-4/2024.
Open Research
Data Availability Statement
All the data in the article are available from the corresponding author upon reasonable request.




