The Inhibitory Effect of Bioactive Substances on Immunoreactivity of Gluten and Crustacean Protein by ELISA Method
Abstract
This study deals with the possibility of using bioactive substances to reduce the allergenic potential of food. In the first phase of the research, the work aimed to verify the inhibitory effect of bioactive substances on crustacean protein (tropomyosin) and gluten (gliadin) in model samples. In the next phase of the research, the inhibitory effect of selected antioxidants was demonstrated on samples from the market network. The ELISA method showed a decrease in the immunoreactivity of the kits (Veratox, Biocheck) by an average of 36% in the model samples. Model samples containing gluten and shellfish showed lower immunoreactivity due to the addition of p-coumaric acid (33.2%), β-carotene (26.5%), caffeic acid (33.3%), quercetin (26.4%), gallic acid (46.2%), lycopene (32.4%), epicatechin (43.5%), chlorogenic acid (32.7%), and rutin (46.8%). This study further demonstrated an inhibitory effect in samples from the market network with gluten content (breadcrumbs, tarragon) by an average of 61.1% and in samples with crustacean content (shrimp and surimi sticks) by 15.7% after the addition of lycopene, epicatechin, and caffeic acid. The results confirmed the impact of bioactive substances on the immunoreactivity of ELISA tests and provide a foundation for developing hypoallergenic foods and enhancing allergen detection accuracy in food safety applications.
1. Introduction
The use of food additives has long been perceived negatively by consumers. Manufacturers and retail chains are therefore looking for a suitable alternative in natural substances [1]. Antioxidants and other bioactive substances, which are able to partially or fully replace various additives in some cases, have a significant application in this area [2]. However, all the effects of these substances in food are not yet known in detail. Some antioxidants (e.g., polyphenols) have been shown to form soluble and insoluble complexes with proteins [3]. Some of these protein changes subsequently also lead to a change in their immunoreactivity [4], which may also affect the sensitivity of immunological tests that are a recognized method of detecting allergenic proteins in food [5]. Insufficient sensitivity, accuracy, and robustness of detection methods can cause false negative results of determined allergens. This fact is significant for sensitive consumers. The prevalence of allergic reaction to food is reported to be around 8% in children and 5% in adults [6]. Cereals containing gluten and crustaceans are listed at the top of the list of allergens found in food. Information about their content must be given on the packaging of the given foodstuff [7].
Celiac disease is considered one of the most common food intolerances (hypersensitivities) not only in Europe. It is an inflammatory immune-mediated disease of the upper part of the small intestine, where the only effective treatment is a gluten-free diet [8]. Foods with a gluten content of less than 20 mg/kg are considered gluten-free foods [9, 10]. Since a strict gluten-free diet is often the only effective treatment option, it is very important to develop an accurate, sensitive, and specific gluten detection method that ensures the absence of gluten in the body [11]. ELISA kits with the R5 monoclonal antibody primarily recognize the QQPFP epitope, which is present in gliadins, secalins, and hordeins and occurs in many peptides that are toxic or immunogenic to celiac patients [8].
Nutrient-rich seafood also plays an important role in the human diet. Seafood allergy is a global health problem as it can cause both mild and life-threatening adverse reactions in susceptible individuals [12]. The main shellfish allergen is tropomyosin [13, 14], a highly thermally and biochemically stable protein with a molecular weight of approximately 34–38 kDa [12]. This protein can form a homodimer while creating a larger protein complex that is present in both muscle and nonmuscle cells as part of the cytoskeleton [12, 14]. The homology between different crustacean species is 98%. In the food industry, seafood processing techniques that lead to reduced allergenicity are effective. Sensitive and rapid allergen detection methods are also being developed to identify and evaluate these allergenic components [12].
Recently, much attention has been paid to functional foods of plant origin containing polyphenols, which are characterized by their high bioactivity [15]. Polyphenols are the primary source of antioxidants for humans and are the most numerous and widespread group of bioactive molecules. Polyphenols can be divided into two basic groups: flavonoids and phenolic acids [15, 16].
Recent research indicates that the binding of an allergenic protein to a polyphenol can, under certain conditions, lead to a reduction in the allergenic potential of the food. However, this issue has not yet received sufficient attention in the scientific literature. The goal of this work was to verify the change in immunoreactivity for selected proteins with allergenic potential and clearly defined bioactive substances, which will bring new knowledge in the issue of allergen identification in food. For the purposes of this work, two commercial kits were selected for determining the content of gliadin and tropomyosin in food.
2. Materials and Methods
2.1. Materials
Verification of the inhibitory effect of bioactive substances took place in two phases. First, the inhibitory effect of bioactive substances on crustacean protein and gluten was verified. Model samples were prepared from tropomyosin, the crustacean protein standard (Natural Shrimp Tropomyosin, VWR, CZE), and gliadin (Merck, USA) to which selected bioactive substances were added in concentrations of 0%, 0.5%, 1.5%, and 15%. Antioxidants that are commonly found in food and are also representatives of individual groups of bioactive substances (carotenoids, flavonoids, and phenolic acids) were selected. The tested bioactive substances are listed below.
2.1.1. Tested Bioactive Substances
-
PKU p-Coumaric acid (ROTH, Germany)
-
KGA gallic acid (MP Biochemicals, USA)
-
RUT Rutin (ThermoFisher Scientific, USA)
-
BCA Beta-carotene (Merck, USA)
-
LYK Lycopene (Merck, USA)
-
KKA Caffeic acid (Apollo Scientific Ltd, UK)
-
KCH Chlorogenic acid (MP Biochemicals, USA)
-
KVE Quercetin (Cayman Chemical, USA)
-
EPI Epicatechin (Merck, USA)
In the second phase of the research, real samples purchased in the market network were used instead of pure protein. For gluten determination, Breadcrumbs (Penam, Czech Republic) and Tarhonya (Ideal, Czech Republic) were chosen. For the determination of tropomyosin, samples of Pickled Shrimp (Mylord, Vietnam) and Surimi Sticks (Vici classic, Lithuania) were selected from the market network. The products were tested with the addition of selected bioactive substances—lycopene, epicatechin, and caffeic acid in concentrations of 0.0%, 0.5%, 1.5%, 5.0%, and 15%. Lycopene, epicatechin, and caffeic acid were chosen as the most suitable for the purpose of this work due to their suitable physicochemical properties (solubility). Another relevant reason was the comparison of bioactive substances of the carotenoid (lycopene) and polyphenol (epicatechin and caffeic acid) groups [16, 17]. All samples were prepared in duplicate, and each measurement was performed in triplicate.
2.1.2. Composition of Samples Obtained From the Market Network
-
Matrix A Breadcrumbs: wheat flour, water, yeast, rapeseed oil, salt with iodine (table salt, potassium iodate), barley malt flour, emulsifier: E471; acidity regulator E341; flour improver: L-ascorbic acid. The product may contain traces of eggs, milk, soy, nuts, and sesame.
-
Matrix B Tarhonya: wheat flour, pasteurized dried egg mass (2%).
-
Matrix C Shrimp: precooked shrimp (60% by weight without glaze), drinking water, table salt, acidity regulator: E450, E451; humectant: E452.
-
Matrix D Surimi: 41% (fish meat, stabilizers: sorbitol, E450, E451, E452; sugar), drinking water, starch (contains wheat), egg white, rapeseed oil, aroma (contains shellfish), table salt, sugar, soy protein, modified starch, egg yolk, dyes: E120, E160c. May contain traces of shellfish, milk, celery, and mustard.
2.2. Methods
2.2.1. Preparation of Solutions of Bioactive Substances
0.15 g of p-coumaric acid, β-carotene, caffeic acid, and quercetin were dissolved in ethanol and made up to 25 mL. The same amount of 0.15 g of gallic acid was dissolved in 25 mL of distilled water, and 0.15 g of lycopene was dissolved in methanol with a final volume of 25 mL. 0.15 g of epicatechin, chlorogenic acid, and rutin were first dissolved in 1 mL of dimethyl sulfoxide (DMSO) (Roth, Germany), and then the individual solutions were made up to 25 mL with distilled water. Furthermore, the antioxidant solutions were diluted with distilled water to concentrations of 0.5%, 1.5%, and 15%.
2.2.2. Preparation of Model Samples
2.2.2.1. Preparation of Gliadin and Tropomyosin Solution
Gliadin: 1 mL of DMSO solution was added to 4 mg of gliadin standard, and after dissolving, the solution was made up to 25 mL. 1 mL was taken from the solution prepared in this way and made up to 100 mL with distilled water.
Tropomyosin: 3.3 μL of tropomyosin standard was added to 1 mL of phosphate-buffered saline (PBS) and dissolved. The resulting solution was diluted with 4 mL of PBS buffer.
Model samples containing 100 μL of solution of individual bioactive substances in different concentrations and 100 μL solution of gliadin or tropomyosin were incubated for 1 h. The resulting concentration of tropomyosin and gliadin in the model samples was 200 ng/mL. These samples were subjected to ELISA quantification. The experimental procedure is shown in Scheme 1.

In the second phase of the research, the influence of the food matrix on products purchased in the market network was verified. Kits from two different companies (Bio-Check and Veratox) were used to determine the immunoreactivity of tropomyosin and gliadin.
2.2.2.2. Kits for the Determination of Gliadin
Gluten R5 Allergen-Check ELISA (Bio-Check, UK) and Veratox for Gliadin R5 Test (Neogen, USA) were used for the determination of gliadin.
2.2.2.3. Kits for the Determination of Tropomyosin
Crustacea Allergen-Check ELISA (Bio-Check, UK) and Veratox for Crustacea Allergen Test (Neogen, USA) were used for the determination of tropomyosin.
2.2.3. Preparation of Product Samples Obtained From the Market Network
The procedure for preparing samples obtained from the commercial network was the same for both tropomyosin- and gluten-containing products, following the Bio-Check sample preparation guide.
Brief summary: After homogenization of 100 g of the samples by blender, 1 g of homogenate was weighed into a plastic test tube with a volume of 50 mL. The corresponding amount of bioactive substance (0.0%, 0.5%, 1.5%, 5.0%, and 15%) (viz Scheme 1) and 10 mL of extraction buffer were added to the treated sample. For samples with gliadin content, a level 1-mL scoop of tannin was added. The samples were shaken thoroughly and then allowed to stand for 10 min; this procedure was then repeated once more. Then, 16 mL of ethanol was added, and the samples were vortexed and subsequently mixed on a mixer for 20 min at room temperature. After intensive mixing, the sample was centrifuged for 15 min at a speed corresponding to 1000 g. After centrifugation, 50 μL of the supernatant was used for the immunoassays. For dilution, 950 μL of the diluent solution was added.
2.2.4. ELISA Quantification Procedure
2.2.4.1. Veratox Procedure
-
Limit of Quantification: 2.5 ppm crustacea.
-
Range of Quantification: 2.5–25 ppm crustacea.
-
Limit of Detection: 2.5 ppm gliadin.
-
Range of Quantification: 2.5–40 ppm gliadin.
2.2.4.2. Biocheck Procedure
-
Limit of Detection: 1 μg/kg foods (tropomyosin).
-
Range of Quantification: 20–400 μg/kg foods (tropomyosin).
-
Limit of Detection: < 0.15 mg/kg foods (gluten).
-
Range of Quantification: 2.5–50 mg/kg foods (gluten).
Each sample as well as standard was measured in duplicate. The measurement was performed for each sample three times.
2.2.5. Statistics
The results were statistically processed by Pearson’s correlation coefficient at a significance level of p = 0.05 Xlstat 2024 (Addinsoft, USA). The Kruskal–Wallis test with post hoc Dunn’s method was used to compare the differences in inhibition between the bioactive substances. These data did not follow a normal distribution according to the Shapiro–Wilk normality test.
3. Results and Discussion
3.1. Effect of Benzoic Core Antioxidants on Immunoreactivity in a Model Situation
This study confirms the effect of bioactive substances on the immunoreactivity of both detection kits. As the addition of the bioactive substance increases, the immunoreactivity of both, the Veratox and Biocheck, detection kits decreases in most cases (Table 1).
| Bioactive substance | Addition of bioactive substance | Correlation coefficient | |||||
|---|---|---|---|---|---|---|---|
| Detection kit | |||||||
| 0% | 0.50% | 1.50% | 15% | R | p | ||
| BCA (beta-carotene) | Veratox gluten | 69.94 ± 0.99 | 61.37 ± 0.13 | 52.93 ± 0.26 | 45.46 ± 3.38 | −0.733 | 0.010 |
| Biocheck gluten | 69 ± 0.42 | 60.94 ± 1.92 | 56.96 ± 0.92 | 56.4 ± 0.37 | −0.513 | 0.107 | |
| Veratox crustaceae | 106.71 ± 1.89 | 40.24 ± 29.02 | 89.13 ± 26.89 | 87.59 ± 28.49 | 0.176 | 0.605 | |
| Biocheck crustaceae | 58.56 ± 1.8 | 46.54 ± 0.15 | 44.82 ± 2.28 | 38.07 ± 1.32 | −0.636 | 0.035 | |
| EPI (epicatechin) | Veratox gluten | 69.94 ± 0.99 | 58.22 ± 0.06 | 52.42 ± 2.63 | 53.52 ± 0.32 | −0.483 | 0.226 |
| Biocheck gluten | 69 ± 0.42 | 59.81 ± 0.88 | 58.48 ± 1.21 | 55.09 ± 1.46 | −0.664 | 0.072 | |
| Veratox crustaceae | 106.71 ± 1.89 | 52.64 ± 5.46 | 39.86 ± 7.75 | 34.19 ± 1.21 | −0.545 | 0.162 | |
| Biocheck crustaceae | 58.56 ± 1.8 | 57.7 ± 1.04 | 52.79 ± 1.56 | 22.11 ± 16.21 | −0.931 | 0.001 | |
| KCH (chlorogenic acid) | Veratox gluten | 69.94 ± 0.99 | 58.72 ± 2.99 | 54.82 ± 3.24 | 49.01 ± 1.13 | −0.725 | 0.042 |
| Biocheck gluten | 69 ± 0.42 | 61.5 ± 3.39 | 56.78 ± 0.03 | 54.89 ± 0.63 | −0.647 | 0.083 | |
| Veratox crustaceae | 106.71 ± 1.89 | 40.89 ± 9.77 | 39.46 ± 23.02 | 40.73 ± 18.75 | −0.362 | 0.379 | |
| Biocheck crustaceae | 58.56 ± 1.8 | 55.44 ± 0.09 | 49.98 ± 1.27 | 47.67 ± 0.78 | −0.747 | 0.033 | |
| KGA (gallic acid) | Veratox gluten | 69.94 ± 0.99 | 54.51 ± 0.07 | 53.1 ± 3.38 | 49.28 ± 2.01 | −0.576 | 0.064 |
| Biocheck gluten | 69 ± 0.42 | 61.05 ± 9.05 | 55.34 ± 1.97 | 45.6 ± 0.14 | −0.782 | 0.004 | |
| Veratox crustaceae | 106.71 ± 1.89 | 37.94 ± 7.88 | 25.5 ± 0.6 | 19.58 ± 2.83 | −0.493 | 0.123 | |
| Biocheck crustaceae | 58.56 ± 1.8 | 48.19 ± 3.18 | 45.43 ± 1.58 | 35.42 ± 0.58 | −0.858 | 0.001 | |
| KKA (caffeic acid) | Veratox gluten | 69.94 ± 0.99 | 60.45 ± 0.93 | 58.29 ± 1.8 | 52.73 ± 0.16 | −0.752 | 0.031 |
| Biocheck gluten | 69 ± 0.42 | 51.39 ± 1.43 | 50.66 ± 0.7 | 61.37 ± 3.16 | 0.180 | 0.669 | |
| Veratox crustaceae | 106.71 ± 1.89 | 43.32 ± 18.6 | 36.34 ± 13.23 | 28.17 ± 24.35 | −0.498 | 0.209 | |
| Biocheck crustaceae | 58.56 ± 1.8 | 52.31 ± 0.12 | 51.98 ± 0.42 | 44.42 ± 0.19 | −0.877 | 0.004 | |
| KVE (quercetin) | Veratox gluten | 69.94 ± 0.99 | 48.22 ± 5.5 | 44.79 ± 6.24 | 42.21 ± 4.84 | −0.491 | 0.075 |
| Biocheck gluten | 69 ± 0.42 | 60.79 ± 5.43 | 57.54 ± 4.08 | 50.4 ± 1.41 | −0.789 | 0.001 | |
| Veratox crustaceae | 106.71 ± 1.89 | 104.86 ± 1.51 | 100.81 ± 0.53 | 91.96 ± 3.58 | −0.429 | 0.126 | |
| Biocheck crustaceae | 58.56 ± 1.8 | 48.8 ± 6.33 | 45.46 ± 4.72 | 43.77 ± 3.88 | −0.582 | 0.029 | |
| LYK (lycopene) | Veratox gluten | 69.94 ± 0.99 | 58.12 ± 1.89 | 51.47 ± 2.39 | 50.28 ± 3 | −0.450 | 0.165 |
| Biocheck gluten | 69 ± 0.42 | 61.13 ± 5.39 | 58.65 ± 1.93 | 54.76 ± 1.7 | −0.724 | 0.012 | |
| Veratox crustaceae | 106.71 ± 1.89 | 105.12 ± 1.21 | 88.48 ± 5.91 | 58.57 ± 1.79 | −0.629 | 0.038 | |
| Biocheck crustaceae | 58.56 ± 1.8 | 42.12 ± 0.53 | 38.16 ± 0.44 | 37.73 ± 0.43 | −0.568 | 0.068 | |
| PKU (p-coumaric acid) | Veratox gluten | 69.94 ± 0.99 | 54.3 ± 6.55 | 52.19 ± 9.09 | 50.13 ± 7.46 | −0.552 | 0.078 |
| Biocheck gluten | 69 ± 0.42 | 63.46 ± 8.15 | 59.15 ± 4.16 | 55.61 ± 7.45 | −0.542 | 0.085 | |
| Veratox crustaceae | 106.71 ± 1.89 | 103.16 ± 2.16 | 80.24 ± 6.54 | 42.24 ± 2.75 | −0.937 | < 0.0001 | |
| Biocheck crustaceae | 58.56 ± 1.8 | 53.8 ± 3.89 | 47.39 ± 8.77 | 44.15 ± 10.71 | −0.646 | 0.032 | |
| RUT (rutin) | Veratox gluten | 69.94 ± 0.99 | 47.96 ± 6.13 | 45.15 ± 5.4 | 33.05 ± 0.78 | −0.674 | 0.023 |
| Biocheck gluten | 69 ± 0.42 | 61.32 ± 1.22 | 56.38 ± 0.67 | 49.93 ± 0.74 | −0.810 | 0.003 | |
| Veratox crustaceae | 106.71 ± 1.89 | 34.52 ± 23.25 | 32.79 ± 22.34 | 30.47 ± 20.69 | −0.337 | 0.310 | |
| Biocheck crustaceae | 58.56 ± 1.8 | 55.48 ± 1.46 | 52.21 ± 2.72 | 37.74 ± 3.51 | −0.919 | < 0.0001 | |
- Note:p, p value at alpha = 0.05 significance level in bold; R, correlation coefficient.
On average, a decrease of 36% was noted for 15% additions of bioactive substances. The lowest decrease in immunoreactivity was found with test kits from the company of Bio-check for the determination of gluten in KKA (11.1%), while the highest decrease in immunoreactivity (81.7%) was determined with the Veratox kit for the detection of crustaceans in the addition of KGA (Table 2). The decrease in immunoreactivity after the addition of bioactive substances shows a negative correlation for all tested compounds. There were two exceptions: KKA-gluten (R = 0.18) and BCA–crustaceans (R = 0.18). For both, a significant decrease in immunoreactivity was observed at a 0.5% addition, but at other concentrations (1.5% and 15%), an increase in immunoreactivity was noted (p > 0.05). In general, the negative correlation indicates a relationship between the addition of bioactive substances and the reduction of immunoreactivity. However, in some cases, the correlation is not significant, and for significant correlations, the range is between −0.58 and −0.94. These results suggest that additional interactions occur between bioactive compounds and allergenic proteins, beyond those directly affecting immunoreactivity. Structural changes in proteins after the addition of bioactive compounds are expected, as reported in other studies [18, 19].
| Bioactive substance (15% addition) | Inhibition (%) |
|---|---|
| BCA | 26.5 ± 9.8 |
| EPI | 43.5 ± 25.1 |
| KGA | 46.2 ± 24.0 |
| KCH | 32.7 ± 20.0 |
| KKA | 33.3 ± 27.6 |
| KVE | 26.4 ± 10.6 |
| LYK | 32.4 ± 10.5 |
| PKU | 33.2 ± 18.5 |
| RUT | 46.8 ± 19.5 |
Commercial kits used for the immunochemical determination of prolamins in food were also discussed by Hulín, Dostálek, and Hochel [20]. The authors compared the commercial kits of Ridasreen Gliadin Kit and Transia Plate Gluten based on the antibody against ω-gliadins and with the R5 antibody (QQPFP). The difference in results was explained by the use of different standards in individual kits, the use of different extraction reagents, and the possibility of interference of some substances extracted from the complex sample matrix [20]. Considerable variability in the yield of gluten and wheat flour when using different commercial ELISA kits was also found by Sharma [21]. One of the reasons may be the different contents of bioactive substances in wheat varieties [22] or in the food matrix [23], which is also confirmed by this study (Table 3). Table 2 summarizes the average inhibitory effect for individual bioactive substances (Table 2). There were no significant differences between the inhibitory effects of the bioactive substances at a 15% concentration.
| Type of die | Bioactive substance | Addition of bioactive substance (%) | Correlation coefficient | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Kit used | |||||||||
| 0% | 0.5% | 1.5% | 5% | 15% | R | p | |||
| Gluten matrix A | Biocheck | Lycopene | 69.80 | 67.55 | 68.47 | 65.43 | 62.70 | −0.939 | 0.018 |
| Biocheck | Epicatechin | 78.36 | 71.44 | 69.68 | 67.32 | 63.94 | −0.799 | 0.105 | |
| Biocheck | Caffeic acid | 73.07 | 67.68 | 66.75 | 64.75 | 63.67 | −0.717 | 0.173 | |
| Veratox | Lycopene | 63.54 | 64.49 | 64.52 | 64.57 | 60.50 | −0.877 | 0.136 | |
| Veratox | Epicatechin | 71.04 | 59.70 | 62.62 | 65.88 | 60.34 | −0.411 | 0.272 | |
| Veratox | Caffeic acid | 63.25 | ∗ | 58.74 | 56.33 | 60.24 | −0.152 | 0.848 | |
| Gluten matrix B | Biocheck | Epicatechin | 61.02 | 57.72 | 57.94 | 57.20 | 55.99 | −0.727 | 0.164 |
| Biocheck | Caffeic acid | 62.12 | 57.24 | 59.32 | 58.51 | 64.29 | 0.672 | 0.214 | |
| Biocheck | Lycopene | 73.07 | 67.68 | 66.75 | 64.75 | 63.67 | −0.717 | 0.173 | |
| Veratox | Epicatechin | 60.38 | 62.62 | 60.46 | 61.43 | 57.11 | −0.840 | 0.075 | |
| Veratox | Caffeic acid | 71.04 | 59.70 | 62.62 | 65.88 | 60.34 | −0.411 | 0.491 | |
| Veratox | Lycopene | 60.99 | 63.74 | 61.64 | 60.26 | 60.10 | −0.611 | 0.274 | |
| Crustacea matrix C | Biocheck | Caffeic acid | 43.01 | 46.96 | 46.38 | 46.68 | 41.81 | −0.601 | 0.432 |
| Biocheck | Lycopene | 39.48 | ∗ | 40.57 | 42.85 | 44.87 | 0.953 | 0.016 | |
| Biocheck | Epicatechin | 46.44 | 42.95 | 38.43 | 41.16 | ∗ | −0.475 | 0.696 | |
| Veratox | Caffeic acid | 21.34 | 16.59 | ∗ | 22.56 | 21.91 | 0.484 | 0.516 | |
| Veratox | Lycopene | 20.73 | 24.60 | 22.52 | 25.50 | 18.67 | −0.563 | 0.423 | |
| Veratox | Epicatechin | 23.57 | 26.08 | 23.80 | 20.99 | 23.55 | −0.272 | 0.734 | |
| Crustacea matrix D | Biocheck | Lycopene | 7.80 | ∗ | 5.71 | 8.83 | 4.75 | −0.570 | 0.346 |
| Biocheck | Epicatechin | 7.80 | 2.81 | 5.71 | 8.83 | 4.75 | −0.102 | 0.430 | |
| Biocheck | Caffeic acid | 4.45 | 3.43 | 7.63 | 5.13 | 2.91 | −0.459 | 0.311 | |
| Veratox | Lycopene | 6.83 | ∗ | 6.30 | 6.87 | 5.86 | −0.752 | 0.248 | |
| Veratox | Epicatechin | 5.40 | 5.42 | 5.42 | 4.10 | 4.67 | −0.580 | 0.487 | |
| Veratox | Caffeic acid | 5.52 | 5.17 | 5.57 | 5.71 | 4.41 | −0.798 | 0.101 | |
- Note: ∗value removed as extreme (Dixon test); p, p value at alpha = 0.05 significance level in bold; R, correlation coefficient.
The differences in results may have been due to the use of different antibodies. For gluten, the ELISA kits from Veratox (Veratox for Crustacea Allergen, Product 8520, 2018) contained R5 antibodies, while the ELISA kit producer Biocheck (Gluten-Check ELISA, R6099, 2018) used detection antibodies “Mendez R5.” The CRUSTACEA Allergen-Check ELISA kits (R6026, 2021) from Biocheck use polyclonal antibodies to detect crustacean muscle protein (tropomyosin; Pen a1) as a marker for the presence of crustacean proteins in food extracts, and the results are expressed as micrograms of tropomyosin per kilogram of food (μg/kg). This test is intended for the detection of tropomyosin in fresh, processed, and heat-treated foods. While the test from the producer Veratox (Veratox for Crustacea Allergen, Product 8520, 2018) detects crustacea proteins and the results are expressed as ppm of crustacea. The result can be expressed by conversion as crustacea proteins. The producer of the Veratox for Crustacea Allergen kits further states in the instructions that the ELISA kit Veratox for Crustacea Allergen (Product 8520, 2018) is intended for the quantitative analysis of crustacea proteins in food products.
A comparison of the effect of bioactive substances on individual potential allergens is shown in Figure 1.
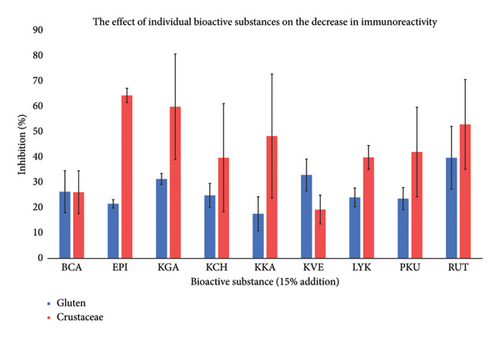
In their work, Dai et al. demonstrated the presence of hydrogen bonding between KGA and rice gluten proteins, primarily at the amino acids of lysine, tryptophan, arginine, and threonine [24]. Another study confirms the binding of zeaxanthin and echinenone, that is, bioactive substances from the carotene group, to the photosensitive proteins of bacteria [25]. The effect of phenolic substances (KGA, KCH, PKU, KKA) on the reduction of protein reactivity was also found in a study by [26] where the authors confirmed the reduction of enzyme activity in the intestine due to protein–phenol bonds. Our results confirmed a 36.3% decrease in reactivity that could be due to binding to the crustacean protein. According to Lasekan, the allergenic capacity of tropomyosin in heat-stable extracts, as assessed by inhibition ELISA analysis of differently processed samples (except for high-pressure steaming), was comparable to that of the raw sample [27]. Zhang et al. summarized findings from other authors, showing that polyphenols with high molecular weight are more likely to bind efficiently to proteins. However, when the conformation of the polyphenol is restricted, its ability to interact with proteins is significantly reduced, regardless of molecular weight. The formation of covalent bonds between proteins and polyphenols is an irreversible reaction. Nevertheless, data from the literature suggest that most interactions between proteins and polyphenols occur via noncovalent binding. It is likely that both covalent and noncovalent interactions occur simultaneously (e.g., binding of chlorogenic acid to proteins). The ability of proteins to bind to polyphenols also depends on their secondary and tertiary structures as well as their amino acid composition. Proteins that interact strongly with polyphenols typically have a high content of basic residues and proline, are hydrophobic, and exhibit a conformationally open and flexible structure [24].
In this study, a decrease in the immunoreactivity of the used kits was further confirmed by an average of 29.4% for BCA and LYK carotenes, which may be related to protein binding. The binding of bioactive substances from the group of flavonoids to protein is described, for example, in the work by [28, 29]. Rawel, Meidtner, and Kroll studied the effect of noncovalent bonds of selected phenolic compounds (chlorogenic, ferulic and gallic acids, quercetin, rutin, and isoquercetin) on various proteins and found that the binding parameters were affected by increasing temperature and ionic strength and decreasing pH, which resulted in reduced binding. The protein structure also indicated changes in the tertiary structure without disturbing the secondary structure [29]. In this study, a decrease in the immune response of the used kits was demonstrated by an average of 38.9% in RUT, EPI, and KVE. The correlation between the addition of the bioactive substance and the decrease in immunoreactivity was on average −0.6 (p < 0.05). In one case, the opposite situation was confirmed, where the correlation was 0.18 for the Veratox Crustaceae test kit and the addition of the bioactive substance BCA; however, in this case, the correlation was not statistically significant (p > 0.05).
3.2. Effect of Benzoic Core Antioxidants on Immunoreactivity in Food Matrix
After verifying the inhibitory effect of bioactive substances on model samples, the inhibitory effect was verified on selected samples from the market network (Table 3). It is clear from the results that the effect of bioactive substances on immunoreactivity in the food matrix is not as clear as in the case of model samples.
The effect of the matrix was verified on foods available in the market network with a natural allergen content detected by the kits used. In this case too, a negative correlation was confirmed due to the addition of a bioactive substance, which demonstrates that despite further interactions between the bioactive substances and the components of the matrix, the immune response of the used kit decreases with the increasing concentration of the bioactive substance. On average, this was a 14.3% decrease. But the influence of the matrix differed significantly. For gluten, the decrease was 12.3% for matrix A and 7.6% for matrix B. For crustacean proteins, the decrease was 16.1% for matrix A, while it was 26.8% for matrix B. Differences were also confirmed between the individual kits, where a decrease of 9.8% was recorded for gluten determined using the Bio-check kit and 7.8% for the Veratox test kit. Crustaceans analyzed with the Bio-check kit showed a decrease of 28.6%, while immunoreactivity decreased by 10.9% with the Veratox detection kit. Interactions between bioactive substances and allergens can also be influenced by other food components such as emulsifiers or stabilizers [30, 31]. Interactions where the complexation of phenolic compounds with protein has emulsifying or stabilizing effects are also described [32].
The change in immunoreactivity was also confirmed using an ELISA kit by other authors, for example, for β-lactoglobulin, when immunoreactivity was reduced by 40.8% in KVE [33]. Although our study confirmed a lower decrease in immunoreactivity (26.4%) in the case of KVE, it should be taken into account that these were detection systems for the proof of other allergens. The authors of Lv et al. (2021) investigated the potential allergenicity of shrimp tropomyosin after conjugation with chlorogenic acid and (−) -epigallo-catechin 3-gallate, and using an indirect ELISA method, it was shown that complexes of tropomyosin and polyphenols caused a conformational change in the tropomyosin structure, with a decrease in immunoglobulinG binding capacity/immunoglobulinE (IgG/IgE). The authors of this study conclude that the complexes of tropomyosin with caffeic acid and catechin gallate could lead to a reduction of the allergenic potential of tropomyosin in shrimp, which could be used in the food industry in the production of hypoallergenic foods [34], a case for production foods with a reduced content of allergens as reflected in the European Regulation No. 828/2014 [10]. The results of our work are also in line with this study, indicating that it would be most appropriate to use RUT (46.8%), KGA (46.2%), and EPI (43.5%) for the production of hypoallergenic foods.
4. Conclusions
The results of this study confirmed the established hypothesis that there is a change in the immunoreactivity of ELISA tests for the detection of food allergens depending on the addition of bioactive substances to food. Using Veratox and Biocheck ELISA kits for gluten and crustacean allergen determination, the change in immunoreactivity was confirmed first on model samples where p-coumaric acid (33.2%), β-carotene (26.5%), caffeic acid (33.3%), quercetin (26.4%), gallic acid (46.2%), lycopene (32.4%), epicatechin (43.5%), chlorogenic acid (32.7%), and rutin (46.8%) were used as bioactive substances. In most cases, the addition of the bioactive substance in the model samples led to a decrease in immunoreactivity. Furthermore, a change in immunoreactivity was confirmed on products purchased in the market network. Using the same detection systems utilized for the model samples, samples purchased from the market were tested for shellfish allergen and gluten content. Selected bioactive substances—representatives of carotenoids (lycopene) and polyphenols (epicatechin and caffeic acid) in different concentrations were added to the samples from the market network. In matrices A and B containing gluten (breadcrumbs and tarhonya), a reduction in immunoreactivity was confirmed when using all bioactive substances—lycopene (59.1%), epicatechin (62.2%), and caffeic acid (61.9%). Matrices C and D (shrimp and surimi) containing crustaceans in combination with lycopene (18.6%), epicatechin (18.2%), and caffeic acid (10.3%) showed lower immunoreactivity compared to the matrix containing gluten.
The results confirmed that bioactive substances have an effect on the immunoreactivity of the used control tests (Veratox and Biocheck). As the concentration of the bioactive substance increases, the test response decreases by an average of 36%. Differences between individual tests were also confirmed (Veratox Gluten R = −0.6; Biocheck Gluten R = −0.59, Veratox Crustaceae R = −.45, Biocheck Crustaceae R = −0.75) with increasing concentration of added bioactive substance. The variability in the inhibitory effects of bioactive substances may have been caused by the different antibodies used or by variations in the interactions between bioactive substances and proteins. These interactions warrant further investigation in future research. Nonetheless, when analyzing allergenic proteins using ELISA kits, it is important to consider the potential addition of these proteins to food products.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This research was funded by ITA VETUNI from the University of Veterinary Sciences Brno, Czech Republic, grant number 2023ITA23.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




