Utilization of Deep Eutectic Solvent and Ethanol for the Extraction of Oil and Astaxanthin From Black Tiger Shrimp (Penaeus monodon) Shells
Abstract
The black tiger shrimp (Penaeus monodon) shell, a by-product of shrimp processing, is a valuable natural source of oil and astaxanthin (AXT). However, extracting these from the shell is difficult due to its tough cell walls. This study examined the use of deep eutectic systems (DESs) to extract oil and AXT from black tiger shrimp shells. Three DESs (choline chloride/glycerol, choline chloride/citric acid, and choline chloride/lactic acid) were tested, and their extraction efficiencies were compared with those of traditional solvents (hexane, ethanol, and acetone). Results indicated that the extraction efficiency of oil and AXT from DESs was lower than that of traditional solvents, but when ethanol was added to the DES as a co-solvent, it significantly improved the yield. Specifically, combining choline chloride/lactic acid with 45% ethanol resulted in the highest concentrations of oil (6.42/100 g) and AXT (39.10 μg/g), surpassing ethanol (5.12/100 g and 31.56 μg/g) or hexane (5.62/100 g and 33.47 μg/g), and acetone (4.46/100 g and 28.59 μg/g). The shrimp extracts were rich in polyunsaturated fatty acids (i.e., linoleic acid, α-linolenic acid, EPA, and DHA) and AXT with potent antioxidant properties.
1. Introduction
The shrimp processing industry plays a crucial role in Vietnam’s economy, producing approximately 1081 thousand tons of brackish water shrimp in 2022, which includes 337.4 thousand tons of black tiger shrimp and 743.6 thousand tons of white-leg shrimp [1, 2]. A large amount of by-products, such as cephalothorax, carapace, and tail, are generated during shrimp processing and are often used as animal feed or as supplemental food for aquaculture [3–5]. Several studies have demonstrated that shrimp oil is rich in astaxanthin (AXT) and polyunsaturated fatty acids (PUFAs), especially eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which are essential for cardiovascular health and brain function [5–8]. Moreover, AXT-rich shrimp oil is known for its antioxidant and anti-inflammatory properties, making it of interest to researchers, businesses, and industries looking to create new food and pharmaceutical products [6–8]. Thus, recovering lipids and AXT from shrimp processing by-products presents not only economic benefits but also an opportunity to mitigate environmental impacts associated with shrimp processing.
Different methods for extracting oil and AXT have been documented, including traditional organic solvent extraction, supercritical carbon dioxide extraction, and other approaches [4, 7–10]. The traditional organic solvent extraction method is often favored because it can efficiently dissolve hydrophobic compounds such as lipids and AXT [5, 8, 9]. However, organic solvents pose risks such as flammability, explosiveness, and toxicity, especially at high temperatures [11, 12]. Supercritical fluid extraction is considered a safer option but requires costly specialized equipment [7]. To address these issues, deep eutectic solvents (DESs) have emerged as alternative solvents to improve the extraction efficiency of bioactive compounds [13–15]. DESs are eco-friendly and similar to ionic liquids (ILs) but easier to prepare [8, 11–15]. They are typically formed by combining a hydrogen bond acceptor (HBA) like ammonium or phosphonium salts with a hydrogen bond donor (HBD) such as amides, amines, alcohols, or carboxylic acids [14–16]. By adjusting the molar ratio of their components, DESs can be tailored to have specific physical and chemical properties for various applications [13–16].
DESs offer several advantages over traditional solvents and ILs, including nontoxicity, low cost, easy production, and biodegradability [13–15]. Recent studies have successfully used DESs to extract lipids and AXT from various sources, including brown crab (Cancer pagurus), shrimp shells (Penaeus vannamei), mussels (Mytilus galloprovincialis), and microalgae (Haematococcus) [15–17]. These studies highlight the ability of DESs to disrupt cell walls by interacting with cellulose, chitin, and proteins, leading to improved yields of lipids and AXT compared to traditional solvents. However, the low vapor pressure of DESs poses challenges in the recovery and recycling of both lipids and solvents [17, 18]. Dos Santos et al. proposed replacing absolute ethanol with DES as the solvent, achieving up to a 43.1% increase in AXT yield from shrimp residue [18]. Nonetheless, the high viscosity of DES-rich extraction phases can hinder mass transfer and reduce extraction efficiency. To enhance extraction efficiency, combining DESs with antisolvents is recommended for lipid and AXT extraction.
Although DESs have shown significant potential, no studies have yet focused exclusively on their use in combination with ethanol for the simultaneous extraction of both lipids and AXT from crustacean shells. Previous research has only investigated the extraction of either AXT or lipids individually. Therefore, this study aimed to address this gap by investigating the impact of aqueous DES combined with ethanol on the efficiency of extracting oil and AXT from black tiger shrimp (Penaeus monodon) shells. Additionally, the study assessed the antioxidant characteristics of the extracted oil-enriched AXT through thorough in vitro analyses.
2. Materials and Methods
2.1. Materials and Chemicals
Waste material from black tiger shrimp, which included the head and carapace, was sourced from Minh Phu Ltd. in Ca Mau Province, Vietnam. The waste was washed with tap water before being dried at 60°C in a vacuum oven for one day. Subsequently, the dried shrimp waste was finely ground into a 100-µm powder using a grinder and stored in plastic bags. Ethanol absolute (99.9%), hydrochloric acid (99%), and sodium hydroxide (> 95%) were purchased from Synth (São Paulo, Brazil). Chemicals such as 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) (98%) and 1,1-diphenyl-2-picryl-hydrazyl (DPPH) (> 98%) were obtained from Sigma-Aldrich (Saint Louis, MO, USA). Additionally, choline chloride (> 99%), lactic acid (LA) (99.5%), citric acid (CA) (99.5%), and glycerol (99.2%) were acquired from Isofar (Rio de Janeiro, Brazil) for DES synthesis. AXT (99.5%) and fatty acid ester standard (> 95%) were procured from Sigma-Aldrich (Saint Louis, MO, USA) as well. The analytical chemicals were imported through a Vietnamese import company.
2.2. The Formation of DES
The details for preparing DESs using different HBAs and HBDs are provided in Table 1. The DES mixtures were heated to 60°C in a thermostatic bath [17]. Then, 5%–25% water was added to the mixtures and stirred until a clear solution formed. The viscosity of all DESs was measured with a Brookfield rotational viscometer (DV II) using spindle RV-3 at 100 rpm. Viscosity readings were taken after 30 s and reported in Pascal seconds (Pa·s). Additionally, Fourier-transform infrared (FTIR) analysis was performed to confirm the formation of the DESs.
| Deep eutectic solvent | Molar ratio | Sample code | Water content (%) | Viscosity (Pa.s) | Oil extractability (g/100 g) | Astaxanthin content (μg/g) |
|---|---|---|---|---|---|---|
| Choline chloride-glycerol (Ch-Gl) | 1:1 | DES1 | 0 | 0.98cd ± 0.02 | 1.69f ± 0.09 | 8.51k ± 0.43 |
| DES2 | 5 | 0.74e ± 0.03 | 1.95f ± 0.06 | 9.58i ± 0.31 | ||
| DES3 | 15 | 0.51f ± 0.02 | 2.04f ± 0.04 | 10.01i ± 0.35 | ||
| DES4 | 25 | 0.47f ± 0.02 | 2.11f ± 0.11 | 10.55i ± 0.34 | ||
| 1:2 | DES5 | 0 | 1.03c ± 0.02 | 1.61g ± 0.05 | 8.44k ± 0.41 | |
| DES6 | 5 | 0.88d ± 0.01 | 1.85f ± 0.06 | 9.06i ± 0.36 | ||
| DES7 | 15 | 0.48 ± 0.04 | 1.97f ± 0.09 | 9.57i ± 0.31 | ||
| DES8 | 25 | 0.45f ± 0.03 | 1.81f ± 0.09 | 9.12i ± 0.40 | ||
| 1:3 | DES9 | 0 | 1.14c ± 0.04 | 1.55g ± 0.08 | 8.28k ± 0.30 | |
| DES10 | 5 | 0.90d ± 0.03 | 1.63f ± 0.01 | 8.51k ± 0.23 | ||
| DES11 | 15 | 0.55f ± 0.02 | 1.91f ± 0.04 | 9.05i ± 0.20 | ||
| DES12 | 25 | 0.50f ± 0.01 | 1.85f ± 0.03 | 9.35i ± 0.26 | ||
| Choline chloride-citric acid (Ch-CA) | 1:1 | DES13 | 0 | 1.11c ± 0.05 | 2.19f ± 0.12 | 10.56i ± 0.31 |
| DES14 | 5 | 1.03c ± 0.06 | 2.75ef ± 0.09 | 12.50h ± 0.44 | ||
| DES15 | 15 | 0.85d ± 0.03 | 3.09e ± 0.07 | 13.21h ± 0.34 | ||
| DES16 | 25 | 0.71e ± 0.03 | 3.11e ± 0.10 | 13.35h ± 0.43 | ||
| 1:2 | DES17 | 0 | 1.23b ± 0.06 | 2.31f ± 0.05 | 11.02 ± 0.34 | |
| DES18 | 5 | 1.15c ± 0.02 | 2.95e ± 0.04 | 13.16h ± 0.36 | ||
| DES19 | 15 | 0.88d ± 0.05 | 3.20e ± 0.11 | 14.85g ± 0.21 | ||
| DES20 | 25 | 0.74e ± 0.04 | 3.01f ± 0.09 | 13.42h ± 0.32 | ||
| 1:3 | DES21 | 0 | 1.34a ± 0.04 | 2.45f ± 0.08 | 11.58h ± 0.49 | |
| DES22 | 5 | 1.21b ± 0.03 | 3.05e ± 0.02 | 13.94g ± 0.41 | ||
| DES23 | 15 | 1.05c ± 0.02 | 3.09f ± 0.04 | 13.80f ± 0.30 | ||
| DES24 | 25 | 0.80e ± 0.20 | 3.11f ± 0.03 | 13.05f ± 0.29 | ||
| Choline chloride-lactic acid (Ch-LA) | 1:1 | DES25 | 0 | 0.25g ± 0.03 | 3.31de ± 0.03 | 17.17f ± 0.28 |
| DES26 | 5 | 0.17h ± 0.02 | 3.55d ± 0.06 | 18.24f ± 0.33 | ||
| DES27 | 15 | 0.13h ± 0.01 | 4.06c ± 0.07 | 24.80d ± 0.37 | ||
| DES28 | 25 | 0.09i ± 0.01 | 3.51d ± 0.05 | 22.10e ± 0.41 | ||
| 1:2 | DES29 | 0 | 0.18h ± 0.02 | 3.21e ± 0.03 | 15.69g ± 0.35 | |
| DES30 | 5 | 0.12h ± 0.02 | 3.45d ± 0.05 | 21.45e ± 0.43 | ||
| DES31 | 15 | 0.08i ± 0.01 | 4.51b ± 0.15 | 29.45b ± 0.39 | ||
| DES32 | 25 | 0.05i ± 0.01 | 3.95c ± 0.06 | 27.90c ± 0.39 | ||
| 1:3 | DES33 | 0 | 0.12h ± 0.02 | 3.21e ± 0.03 | 15.09g ± 0.42 | |
| DES34 | 5 | 0.09i ± 0.01 | 3.35d ± 0.07 | 17.05f ± 0.45 | ||
| DES35 | 15 | 0.07i ± 0.01 | 3.98c ± 0.09 | 26.53c ± 0.41 | ||
| DES36 | 25 | 0.05i ± 0.01 | 3.70d ± 0.10 | 25.84cd ± 0.47 | ||
| Organic solvents | Hexane | — | 0.0041 | 5.62a ± 0.09 | 33.47a ± 0.36 | |
| Acetone | — | 0.0033 | 4.46b ± 0.08 | 28.59b ± 0.41 | ||
| Ethanol | — | 0.0101 | 5.12a ± 0.14 | 31.56a ± 0.41 | ||
- Note: In each column, distinct lowercase letters attached to values denote a significant difference at p < 0.05.
2.3. DES for AXT and Oil Extraction
One gram of shrimp shell powder was weighed and placed into test tubes, followed by the addition of 20 mL of DES under optimal conditions determined from the preliminary survey. The tubes were kept away from light, shaken, and incubated at 60°C for 240 min. After incubation, the mixture was separated using an SL-706 centrifuge at 6000 × g for 5 min. The DES extract was stored at 4°C for further analysis, while the residues were washed twice with distilled water, dried at 100°C in an oven, and subjected to structural analysis (scanning electron morphology [SEM] and FTIR).
2.4. Extraction of AXT and Oil Using Ethanol Combined With DES
The experiment involved using DESs in combination with ethanol to extract oil and AXT from shrimp powder. The DES was prepared by mixing choline chloride and LA in a 1:2 molar ratio, following a method by Rodrigues et al. [17]. Test tubes containing 1g of powder and 20 mL of the DES with varying ethanol ratios (5% to 65% (wt)) were shaken and kept at 60°C for 240 min. The resulting two layers were separated by centrifugation at 6000 × g for 10 min to collect the upper layer containing oil, AXT, and DES solvent. To determine the shrimp oil yield extracted using ethanol and DES, 100 mL of hexane was added to the DES extract, placed in a 40°C water bath with agitation for 1 h, and then centrifuged again. The upper layer containing oil and hexane was collected, and the solvents were evaporated using a rotary evaporator (Buchi Rotavapor R-200, Essen, Germany) at 40°C. Control experiments using pure ethanol or hexane were also performed, and the oil yield was calculated and compared to the yields obtained with the control solvents.
2.5. Determination of AXT Concentration
AXT levels were analyzed using high-performance liquid chromatography (HPLC) on a Shimadzu system equipped with an SPD-10AV detector and a Shim-pack CLC-ODS (M) C18 column [18]. The mobile phase, comprised of dichloromethane, acetonitrile, and methanol in a 70:20:10 ratio, facilitated isocratic elution at a flow rate of 1.0 mL/min, with absorbance measured at 450 nm. A calibration curve for AXT was prepared using concentrations ranging from 0.05 to 30 μg/mL. Prior to analysis, the samples underwent filtration with a 0.22-μm membrane, and the results were based on three replicates.
2.6. Determination of Fatty Acid Profile
Fatty acids were examined using GC-FID (Agilent 7890C axis detector, England), following the method by Phan et al. [19] with minor adjustments. In the analysis process, a 20-mg oil sample was placed in a screw-top test tube and dissolved in 1 mL of 0.5 M KOH. The mixture was then heated in a water bath at 90°C for 30 min. Next, 1 mL of 0.6 M HCl was added for neutralization, followed by the introduction of 3.0 mL of BF3 in methanol. The resulting mixture underwent further heating at 90°C for an additional 15 min in the water bath. The methylated oil was extracted using n-hexane, and the solvent was evaporated using nitrogen gas before analysis. The GC-FID procedure involved setting the injector temperature at 180°C, the detector temperature at 250°C, and the ion source temperature at 230°C. The column temperature was programmed to increase from 50°C to 250°C at a rate of 40°C/minute. Nitrogen served as the carrier gas with a flow rate of 1.0 mL/min, and the injection volume was 1 µL. Identification of the fatty acid methyl esters was based on comparing the retention times with standard fatty methyl esters.
2.7. Determination of Physicochemical Parameters of Shrimp Oils
The acid value (AV) and peroxide value (PV) of shrimp oils were determined using the methods outlined in AOAC [20].
2.8. Antioxidant Activity
2.8.1. ABTS•+ Radical Scavenging Activity
2.8.2. DPPH Radical Scavenging Activity
2.9. FTIR
FTIR spectroscopy was used to analyze the functional groups in both the DESs and shrimp shell powder before and after being treated with various extraction methods. Initially, the samples were mixed with potassium bromide (KBr), ground, and formed into pellets. They were then examined in the transmittance and mid-infrared region, ranging from 400 to 4000 cm−1, using a Shimadzu FTIR-8400S spectrometer equipped with IRAFFINITY-1 series and IR solution software Version 1.60. The analysis included 32 scans and a resolution of 4 cm−1.
2.10. SEM
SEM was used to examine the morphological changes of shrimp shell powder before and after treatment. The samples were prepared with adhesive tape and coated with gold before imaging. Scanning was done at 15 kV voltage using an XL 30 ESEM FEG electron microscope, and the images were captured at a magnification of 10 k.
2.11. Statistical Analysis
The statistical analysis of the experimental design was conducted using Version 25 of the SPSS statistics software. All measurements were done three times, and the data were presented as mean ± SD at a significance level of p < 0.05.
3. Results and Discussion
3.1. Characterization of DES
The research focused on developing DESs using various combinations of HBA and donor monomers (HBA/HBD), different molar ratios, and varying levels of water content. FTIR analysis was conducted to examine the composition of the DES and the interactions among functional groups in each DES. The FTIR results (Supporting Figure S1) identified specific peaks in pure choline chloride (Ch) between 1100 and 1300, 1415 and 1480, 2800 and 3050, 3100 and 3800 cm−1, corresponding to various stretching vibrations such as the C-N, C-H, N-O, and O–H [22–26]. The DES binary mixtures still exhibited these peaks, indicating the presence of Ch+ in the DESs. Additionally, broader peaks in the 1600–1800 and 2800–3500 cm−1 range, particularly in DESs with high water content (15%–25%), were observed to be wider compared to LA, CA, or glycerol, or DES without water. The strong peaks in DESs with high water content suggested increased hydrogen bond interactions between HBA, HBD, and H2O. Similar findings were reported by Deng et al. and Chandra Roy et al. in their research on utilizing DES for extracting bioactive compounds from shrimp shell powder [27, 28]. Our study also demonstrated that all DESs, regardless of water content (ranging from 5% to 25%), formed clear and homogeneous solutions without phase separation or crystallization, even after a week at room temperature (Figure S2). Consequently, our results indicate that all mixtures exhibited absorption profiles similar to the individual components, except for the O−H stretching vibration bands, indicating complete dissolution of HBAs/HBDs in DESs, resulting in uniform solutions.
3.2. Selection of Solvent Extraction
The research evaluated and compared the extraction abilities of various solvents (i.e., Ch-LA, Ch-CA, Ch-Gl, hexane, acetone, and ethanol) on oils and AXT from shrimp shell powder. The highest levels of oil and AXT (5.62/100 g and 33.47 μg/g, respectively) were achieved by the hexane, followed by ethanol (5.12/100 g and 31.56 μg/g, respectively), while acetone had the lowest values at 4.46/100 g and 28.59 µg/g. Similar findings were noted by Xie et al., who reported that hexane and ethanol resulted in greater oil and AXT yields compared to acetone extractions from Antarctic krill (Euphausia superba) [29].
In Table 1, the oil and AXT content in DESs varied from 1.55 to 4.51/100 g and 8.28 to 29.45 μg/g, respectively, depending on the types of HBA/HBD, molar ratio, and amount of water added. Among the three DESs studied, Ch-LA and Ch-CA had a significant impact on the yields of oils and AXT extracted from shrimp shells compared to Ch-Gl at all molar ratios and water contents (p < 0.05). Additionally, the increase mole of LA and CA in Ch-LA and Ch-CA mixtures also altered the efficiency of extraction. This effect was likely because LA and CA in DESs promoted the hydrolysis inside the cell wall between AXT, oil, and cell membrane, leading to improved extraction efficiency of AXT and oil. Furthermore, DES-LA showed significantly higher extraction efficiency compared to DES-CA at equivalent molar ratios of HBA/HBD and water content.
Our research also discovered that the viscosity of DESs was significantly influenced by the amount of water present, impacting the extraction efficiency of oils and AXT. It was observed that, at a consistent HBD/HBA molar ratio, the viscosity of all DESs notably decreased as the water content increased from 5% to 25%, leading to higher yields of oil and AXT, as indicated in Table 1. This observation aligns with the findings of Meifeng Denga et al. [27] and Tomislav Bosiljkov et al. [30], who stated that the addition of water reduces DES viscosity, thereby improving mass transfer and extraction efficiency. Mária Vilková et al. also noted that DES–water systems are more effective for extraction compared to pure DES, possibly due to water’s ability to form hydrogen bonds with the target extraction compounds [31]. The most favorable results in terms of oil and AXT yields were achieved with Ch-LA at a molar ratio of 1:2 with 15% water, followed by Ch-CA at a molar ratio of 1:2 with 15% water, as shown in Table 1. Excessive addition of water (above 25%) in this study led to a decline in oil and AXT yields, approximately 1.1 times lower compared to DES with 15% water content. This reduction in yields may be attributed to decreased DES stability and weakened hydrogen interactions with the target compounds, as reported by Vilková, Płotka-Wasylka, and Andruch [31]. Consequently, a water content of 15% was chosen for the subsequent oil and AXT extraction experiment.
| Deep eutectic solvent | Cosolvent content (%) | Oil extractability (g/100 g) | Astaxanthin content (μg/g) | Acid value (mgKOH/g oil | Peroxide value (meq O2/kg oil) | |
|---|---|---|---|---|---|---|
| Choline chloride-lactic acid (Ch-LA) | Ethanol | 0 | 4.46d ± 0.03 | 29.59c ± 0.35 | 0.90 ± 0.04 | 0.59 ± 0.01 |
| 5 | 4.95d ± 0.02 | 31.45bc ± 0.40 | 0.89 ± 0.02 | 0.60 ± 0.02 | ||
| 25 | 5.46bc ± 0.04 | 32.59b ± 0.47 | 0.86 ± 0.03 | 0.58 ± 0.02 | ||
| 45 | 6.42a ± 0.05 | 39.10a ± 0.60 | 0.90 ± 0.01 | 0.59 ± 0.01 | ||
| 65 | 5.88b ± 0.04 | 34.04b ± 0.45 | 0.84 ± 0.03 | 0.61 ± 0.01 | ||
| Organic solvents | Hexane | — | 5.62b ± 0.09 | 33.47b ± 0.36 | 0.83 ± 0.02 | 0.57 ± 0.03 |
| Ethanol | — | 5.12c ± 0.14 | 31.56bc ± 0.41 | 0.90 ± 0.02 | 0.62 ± 0.02 | |
- Note: In each column, distinct lowercase letters attached to values denote a significant difference at p < 0.05.
However, in this study, the amount of oils and AXT extracted by DESs consistently remained lower than that extracted by hexane and ethanol, indicating that these DESs are less effective in penetrating shrimp shell cells for oil and AXT extraction. Some previous study showed that by combining DESs with hexane or tetrahydrofuran improves the efficiency of extraction of oils and DESs with ethanol for AXT [18, 32]. The addition of cosolvents can alter the solubility of target compounds, improve mass transfer rates, and enhance the extraction yield [18, 32]. However, there have been no studies on using Ch-LA with ethanol as a cosolvent for extracting oil and AXT from shrimp shells. Considering the flammability and toxicity of hexane, selecting appropriate DES (Ch-LA)-combined green organic solvent, ethanol as cosolvents, is crucial to balance oil and AXT yields’ performance with safety considerations.
3.3. DES-Combined Ethanol as Cosolvent Extraction
A study was conducted to improve extraction efficiency by comparing a mixture of Ch-LA and ethanol with individual solvents like hexane and ethanol. As detailed in Table 2, the ethanol content had a significant impact on the oil and AXT yields. Using Ch-LA alone resulted in relatively low yields, with 4.46% oil and 29.59 μg/g AXT. However, combining Ch-LA with ethanol at varying concentrations (5% to 65% wt) led to a marked increase in yields. The highest levels, 6.42 g/100g for oils and 39.10 μg/g for AXT, were obtained with a mixture of Ch-LA containing 45% ethanol. As shown in Table 2, the yields from DES combined with ethanol were approximately 1.2 times higher for both oil and AXT compared to extraction with hexane, and about 1.25 times higher than those extracted with ethanol. These results align with previous studies using different extraction methods on shrimp shells [9, 29, 33]. The variance in oil and AXT yields in this study may be attributed to Ch-LA breaking down cell walls, enabling improved ethanol penetration for better extraction, as supported by SEM results (Figure 1) and FTIR results (Figure 2). Previous studies have suggested that DES treatment aids in breaking down cell walls to facilitate the extraction of valuable components [33, 34]. Santos et al. noted that DES can enhance cell permeability, decrease resistance to mass transfer, enhance ethanol penetration, and promote the dissolution of bioactive compounds in cells [18]. However, in this study, increasing the ethanol content from 45% to 65% in Ch-LA resulted in reduced extraction efficiency. This could be due to the instability of H+ ions at higher ethanol levels, which hindered Ch-LA’s ability to break down the shrimp shell and impede ethanol penetration for dissolving oil and AXT. Nevertheless, these results are unprecedented in the literature and suggest a potential synergistic effect between ethanol and DES.




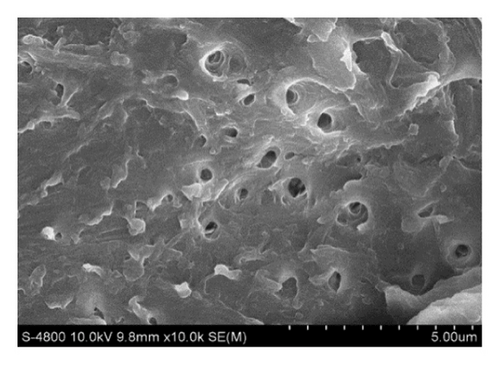

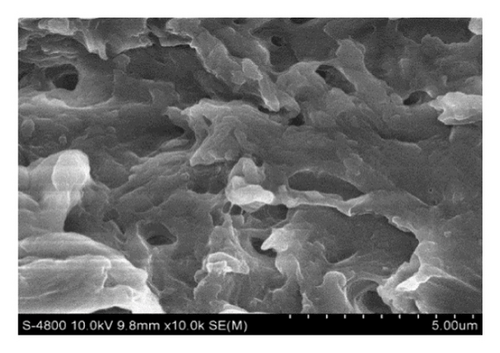

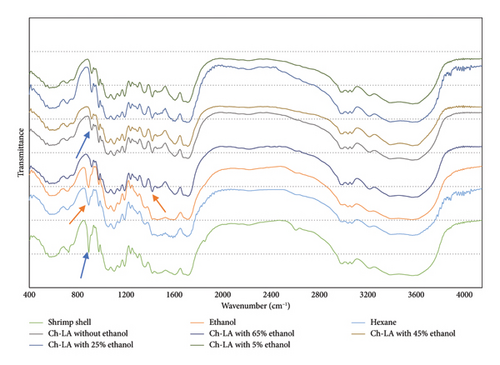
Figure 1(a) displays untreated shrimp shells that have a smooth surface without pores. Treatment with organic solvents such as ethanol and hexane, and Ch-LA without ethanol, results in the formation of some holes on the shrimp cell surface, illustrated in Figures 1(b), 1(c), and 1(d). Treatment involving Ch-CA combined with ethanol effectively alters the microstructure and morphology of Penaeus monodon cells, as shown in Figures 1(e), 1(f), 1(g), and 1(h). The utilization of Ch-LA along with ethanol as a cosolvent causes a more eroded appearance on the cell wall surfaces. The enhanced rupturing of shrimp cells facilitates the penetration of ethanol into the shrimp cell, thereby enhancing the efficiency of extracting AXT and oil. Wang et al. [34] also observed similar outcomes when treating shrimp cells with Ch-LA, suggesting that treating shrimp powder with Ch-LA modifies the hydrogen bonds of the cell walls, leading to increased erosion on the shrimp cell. However, incorporating a high ethanol concentration (45%–65% wt) in Ch-LA significantly decreases the yields of oil and AXT (p < 0.05). This is attributed to the reduced erosion and damage of the shrimp cell surfaces under DES treatment containing high ethanol compared to other treatments, as depicted in Figure 1(h). Consequently, the extraction yield of compounds like AXT and oil may be reduced.
The FTIR analysis (Figure 2) showed that the absorption peaks of shrimp shell residue changed depending on the extraction solvent used. The absorption peak at 874 cm−1, which was found in the raw material, slightly decreased with hexane and ethanol extraction and significantly decreased with Ch-LA combined with ethanol extraction, especially with ethanol content between 25% and 45%. This decrease may be linked to the removal of mineral salts like CaCO3 from the shrimp cells, as suggested in previous studies [18, 22, 34]. Furthermore, strong absorption peaks between 1331 and 1550 cm−1 were observed in shrimp residue treated with Ch-LA-combined ethanol or ethanol, indicating successful protein removal from shrimp cells. Wang et al. highlighted that the presence of free H+ in DES-LA played a crucial role in decalcification, aiding in the removal of CaCO3 and enhancing protein dissolution bound to chitin through covalent bonding [34]. In conclusion, the combination of Ch-LA with 45%wt ethanol disrupts the cell wall structure, facilitating better ethanol penetration into shrimp cells, thereby improving oil and AXT extraction from shrimp residue.
3.4. Physicochemical Characterization and Fatty Acids’ Composition
Table 2 presents the physicochemical properties of oils extracted from black tiger shrimp shells. PV and AV are crucial indicators of oil quality. PV measures oil oxidation, while AV indicates hydrolysis-induced oil degradation, leading to rancidity. The AVs ranged from 0.83 to 0.90 mg KOH/g oil, and PVs varied between 0.57 and 0.6 meq O2/kg oil, as shown in Table 3. According to CODEX standards [35], acceptable AV and PV for oils are below 4 mg KOH/g and 15 meq O2/kg oil, respectively. The PV and AV of the oil from shrimp shell powder were below these thresholds, indicating suitability for human consumption concerning oxidation and hydrolysis products. Some previous studies reported that the shrimp oil is low PV and AV due to the presence of AXT, which are a powerful antioxidant that protect the unsaturated fatty acid degraded due to oxidation [9, 19]. These authors also reported that shrimp oil is indeed rich in PUFAs, making it a valuable addition to a healthy diet.
| Oil extract sample code | Hexane | Ethanol | Ch-LA with 0% ethanol | Ch-LA with 5% ethanol | Ch-LA with 25% ethanol | Ch-LA with 45% ethanol | Ch-LA with 65% ethanol | |
|---|---|---|---|---|---|---|---|---|
| Palmitic acid (C16:0) | 18.92q ± 0.23 | 17.71a ± 0.21 | 17.71a ± 0.30 | 17.73a ± 0.29 | 17.73a ± 0.25 | 16.01b ± 0.28 | 16.11b ± 0.31 | |
| Stearic acid (C18:0) | 13.50a ± 0.15 | 12.47a ± 0.16 | 12.47a ± 0.21 | 12.52a ± 0.19 | 12.50a ± 0.20 | 11.21ab ± 0.21 | 11.45 ab ± 0.19 | |
| Oleic acid (C18:1-Cis) | 15.32b ± 0.50 | 16.30ab ± 0.64 | 16.30ab ± 0.39 | 16.20ab ± 0.56 | 16.20ab ± 0.35 | 17.65a ± 0.57 | 17.24a ± 0.49 | |
| Linoleic acid (C18:2) | 21.62a ± 0.32 | 22.56ab ± 0.41 | 22.56ab ± 0.45 | 22.52ab ± 0.55 | 22.53ab ± 0.45 | 23.48a ± 0.39 | 23.59a ± 0.34 | |
| α-Linolenic acid (ALA) (C18:3n3) | 1.8b ± 0.01 | 2.00a ± 0.04 | 2.01a ± 0.02 | 2.08a ± 0.03 | 2.05a ± 0.03 | 2.12a ± 0.02 | 2.12a ± 0.01 | |
| cis-11,14-Eicosadienoic acid (C20:2) | 2.71 ± 0.01 | 2.71 ± 0.02 | 2.70 ± 0.01 | 2.71 ± 0.03 | 2.73 ± 0.02 | 2.79 ± 0.02 | 2.76 ± 0.04 | |
| cis-8,11, 14-Eicosatrienoic acid (C20:3n6) | 5.41 ± 0.07 | 5.49 ± 0.05 | 5.49 ± 0.06 | 5.50 ± 0.07 | 5.50 ± 0.06 | 5.70 ± 0.08 | 5.60 ± 0.06 | |
| cis-5,8,11,14,17- Eicosapentaenoic (EPA) (C20:5n3) | 10.81 ± 0.04 | 10.76 ± 0.03 | 10.75 ± 0.03 | 10.70 ± 0.05 | 10.74 ± 0.06 | 10.79 ± 0.06 | 10.82 ± 0.07 | |
| cis-4,7,10,13,16,19- Docosahexaenoic acid (DHA) (C22:6 (n3)) | 9.91 ± 0.05 | 10.00 ± 0.4 | 10.01 ± 0.05 | 10.05 ± 0.06 | 10.01 ± 0.04 | 10.25 ± 0.05 | 10.31 ± 0.07 | |
| Total saturated fatty acid (SFAs) | 32.42 | 30.18 | 30.18 | 30.25 | 30.23 | 27.22 | 27.56 | |
| Total unsaturated fatty acid (USFAs) | MUFAs | 15.32 | 16.30 | 16.30 | 16.20 | 16.20 | 17.65 | 17.24 |
| PUFAs | 52.26 | 52.62 | 53.52 | 53.55 | 53.57 | 55.13 | 55.20 | |
- Note: Different letters in each column denote statistically significant differences between treatments (p < 0.05). The values are mean of three replications ± standard deviation.
The fatty acid composition of shrimp oils extracted using Ch-LA-combined 45% ethanol, ethanol, or hexane is compared in Table 3. The main fatty acids found were linoleic, stearic, oleic, and palmitic acids, along with smaller amounts of α-linolenic acid (ALA), cis-11,14-eicosadienoic acid, and cis-8,11,14-eicosatrienoic acids. The study found that the use of different solvents did not alter the types of fatty acids present in oils from black tiger shrimp shells but did affect their quantities. As presented in Table 3, it can be seen that shrimp oils extracted with DES–ethanol or ethanol had higher levels of mono-unsaturated fatty acids (MUFAs; 16.20% to 17.65%) and PUFAs (53.52% to 55.20%) compared to hexane-extracted oils (MUFAs: 15.32% and PUFAs: 52.26%). Additionally, significant amounts of EPA (10.70%–10.82%) and DHA (9.91%–10.31%) were detected in the analyzed shrimp oils, aligning with literature values for EPA and DHA in shrimp oil extracted from P. borealis and red-spotted shrimp [36, 37]. Previous research by Jiao et al. [36] indicated that shrimp oil from shrimp (P. borealis) processing water is abundant in n-3 PUFAs and low in n-6 PUFAs, whereas the shrimp oil from Penaeus monodon shells in this study exhibited similar levels of n-3 and n-6 PUFAs. This discrepancy in fatty acid compositions and quantity may be attributed to variations in extraction solvents and types of shrimp shells used. These unsaturated fatty acids are known for their health benefits, such as reducing cardiovascular disease risk and improving brain and heart health [29, 36]. Therefore, the use of Ch-LA-combined ethanol as a solvent extraction method significantly influenced the extraction efficiency of unsaturated fatty acids from black tiger shrimp shells, according to the results.
3.5. Antioxidant Properties
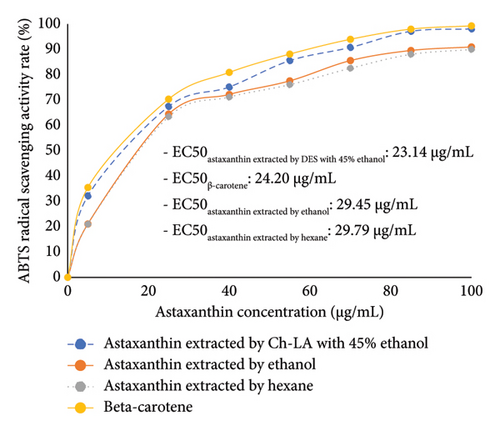
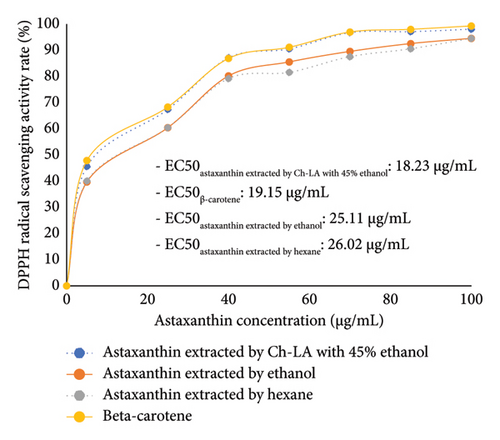
The antioxidant activity of shrimp extracts was evaluated using the ABTS and DPPH tests. The results showed that all samples tested at various concentrations of AXT demonstrated significant scavenging abilities on DPPH and ABTS radicals in a dose-dependent manner (Figure 3). A lower EC50 value indicates a stronger antioxidant capacity [37–39]. In the ABTS test, the Ch-LA-combined ethanol extract exhibited notable antioxidant potential with an EC50 value of 23.14 μg/mL, similar to the positive control, β-carotene (24.20 μg/mL), as depicted in Figure 3(a). Furthermore, the results in Figure 3(a) indicated that the ABTS radical scavenging activity of the Ch-LA-combined ethanol extract was superior to that of the ethanol extract (EC50: 29.45 μg/mL) or the hexane extract (EC50: 29.79 μg/mL). Similar trends were observed in the DPPH results (Figure 3(b)). The AXT extracted by Ch-LA-combined ethanol exhibited significantly greater ABTS scavenging activity and potency compared to the ethanol extract (EC50: 25.11 μg/mL) or the hexane extract (EC50: 26.02 μg/mL). These outcomes can be attributed to the specific molecular structure of AXT, which contains polyenes with conjugated double bonds and hydroxyl (OH) and keto (C=O) groups on each ionone ring, providing AXT with hydrogen-donating capability [38]. These results align with previous studies that found AXT from shrimp waste exhibited potent antioxidant activity similar to α-tocopherol [40].
4. Conclusion
The study used three different DESs (Ch-Gl; Ch-LA; and Ch-CA) to extract oil and AXT from black tiger shrimp shells. Results showed that Ch-LA, with a molar ratio of 1:2% and 15% water content, was the most effective solvent among the DESs tested. However, the extraction efficiency of Ch-LA was lower compared to traditional solvents such as ethanol and hexane. Adding ethanol as a co-solvent in the DES extraction process significantly improved the extraction efficiency of oil and AXT from shrimp shells compared to using pure hexane or ethanol alone. Specifically, combining Ch-LA and ethanol with a 45% ethanol content in the DES resulted in the highest concentrations of oil and AXT (6.42/100 g and 39.10 μg/g), surpassing the results obtained with ethanol (5.12/100 g and 31.56 μg/g) or hexane (5.62/100 g and 33.47 μg/g) alone. The shrimp extracts had excellent characteristics, including low PV and AV, and contained significant levels of PUFAs like linoleic, α-linoleic, EPA, and DHA. Furthermore, the shrimp extracts demonstrated potent antioxidant activity, as evidenced by ABTS and DPPH assays. By adding ethanol as a cosolvent with DESs, improved extraction efficiency for oil and AXT can be achieved while reducing the flammability and environmental risks associated with using ethanol or hexane alone. These results support the use of DESs for extracting oil and AXT from shrimp by-products, which can be valuable supplements in the food and pharmaceutical industries.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Tran Chi Hai worked in the conceptualization (equal), investigation (equal), methodology (equal), supervision (equal), data curation (equal), writing – review and editing (equal). Phan Van Man was assigned in conceptualization (equal), investigation (equal), supervision (equal), writing – review and editing (equal). Le Thi Hong Anh worked in the visualization and supervision (equal), writing – review and editing (equal). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Industry and Trade, Vietnam, under Contract No. 038.2023.ĐT.BO/HĐKHCN, dated August 31, 2023.
Acknowledgments
The authors are grateful for the kind support and funding from the Ministry of Industry and Trade, Vietnam, which has been extremely helpful for this study. AI was not involved in the preparation of the manuscript.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




