Neptunea cumingii Extract Inhibits Adipogenesis and Stimulates Browning of Adipocytes in 3T3-L1 Preadipocytes
Abstract
This study investigates the potential antiobesity effects of Neptunea cumingii extract (NCE). Our findings illustrate that NCE effectively reduces lipid accumulation and triglyceride content, while simultaneously increasing free glycerol release. The reduction in lipid accumulation and induction of lipolysis were evidenced by the downregulation of lipogenesis proteins, such as fatty acid synthase and lipoprotein lipase, and the upregulation of hormone-sensitive lipase expression. Furthermore, the downregulation of adipogenic transcription factors, including peroxisome proliferator–activated receptor gamma, CCAAT/enhancer-binding protein α, and sterol regulatory element-binding protein 1, indicates the inhibition of adipocyte differentiation. Additionally, NCE treatment induced brown adipocyte phenotype by upregulating brown adipose tissue–specific proteins, such as uncoupling protein 1 and peroxisome proliferator–activated receptor-gamma coactivator 1α. Moreover, NCE led to the phosphorylation of AMPK, the master regulator of energy homeostasis. Pharmacological inhibition of AMPK using an AMPK inhibitor (Compound C) attenuated the lipogenesis inhibitory effect of NCE and reduced lipolysis and adipocyte browning. This suggests that AMPK activation is involved in these processes. GC–MS analysis reveals that NCE primarily consists of cholest-5-en-3-ol (27.15%) along with an array of fatty acids which possess favorable antiobesity properties. Collectively, these results highlight the potential of NCE as a lipid-lowering agent for the intervention of obesity.
1. Introduction
Adipose tissue serves as a pivotal regulator of systemic energy homeostasis. The chronic imbalance between energy intake and expenditure results in an unhealthy expansion of adipose tissue, which is the delineated feature of obesity [1]. To address obesity, it needs to reduce calorie intake first and change metabolic efficiency through inhibiting fat synthesis and augmenting energy expenditure in metabolic organs like adipose tissue. There are two forms of adipose tissue: white adipose tissue (WAT) and brown adipose tissue (BAT), in which WAT stores excess energy as triglyceride (TG), while BAT dissipates energy in the form of heat. AMP-activated protein kinase (AMPK), a proficient cellular energy sensor, is crucial in maintaining overall body energy balance. Activated AMPK has an important role in the metabolic regulation of both BAT and WAT. A deficiency of AMPKα in adipocytes leads to cold intolerance and obesity in mice. Conversely, AMPK agonist induces browning of WATs. AMPK directly influences peroxisome proliferator–activated receptor-gamma coactivator 1α (PGC-1α) activity which regulates mitochondrial biogenesis and thermogenic activity in BAT [2]. Uncoupling protein-1 (UCP-1) is also found in BAT and is responsible for heat generation. It has been discovered that with exposure to cold or β-adrenergic stimulation, adipocytes within WAT undergo a process called “browning,” wherein they adopt a brownish phenotype and begin expressing UCP-1, and these fat cells are termed as beige adipocytes [3]. This transformation enhances WAT’s capacity to generate heat, thereby elevating energy expenditure. WAT browning can be induced by exercise, pharmaceutical, and nutritional interventions. Consequently, targeting WAT browning has emerged as a promising strategy for combating obesity besides dietary restrictions and physical activities [4]. Therefore, these proteins have been widely monitored to confirm the effects of nutritional food compounds on WAT browning and BAT activation [5]. In adipogenesis, transcription factors, peroxisome proliferator-activated receptor gamma (PPARγ), CCAAT/enhancer-binding protein α (C/EBPα), and sterol regulatory element-binding protein 1 (SREBP-1), play a pivotal role in differentiating adipocytes from preadipocytes through activating lipogenic proteins such as FAS and LPL [6]. Therefore, effective regulation of both WAT inhibition and BAT activation could be beneficial for modulating obesity.
Among the variety of therapeutic options available to address obesity, pharmacotherapy is the most common approach. Dual-action GLP-1-based antiobesity drugs (e.g., oxyntomodulin and liraglutide) are now in the pipeline for urgent need [7, 8]. However, prolonged use of these drugs causes serious side effects such as nausea, pancreatitis, and acute kidney injuries [9, 10]. Hence, there is an indispensable need to evolve novel natural antiobesity agents to treat obesity effectively while minimizing side effects. The healthy and nutritious life of humans relies on the food and agricultural products they consume, and seafood occupies a major component of the human diet specifically in Asia. In lieu of this, the exploration of medicinal foods from marine environments is effective and ongoing [11]. Marine environments offer innumerable and diverse resources for developing new drugs to combat major diseases [12]. Therefore, the utilization of commonly available marine organisms can lead to optimal dietary practices for human [13–15].
Neptunea cumingii is a predatory snail widely distributed in the subtidal zone of South Korea and renowned as the most economically important shellfish in Asia [16]. It is esteemed as a popular, highly prized edible species for delicacy and nutritious meat. The edible part of N. cumingii is rich in amino acids, glycogen, proteins, and vital trace elements, which can provide numerous health benefits to humans [17]. To date, research on N. cumingii has primarily concentrated on ecological relevance by analyzing its distribution and genetic diversity [17–20]. Only one research has been reported on antioxidant peptides from N. cumingii [21]. Thus, it is worthwhile to investigate the health benefits of N. cumingii. The objective of this study is to investigate the anti-obesity effect of N. cumingii extract (NCE) in 3T3-L1 preadipocytes. To this end, this study focused on whether NCE can stimulate the browning of adipocytes. Additionally, the chemical composition of NCE was also analyzed.
2. Materials and Methods
2.1. Materials
3T3-L1 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Supporting reagents were sourced from Sigma-Aldrich and were up to analytical grade (St. Louis, MO, USA). Cell culture reagents were obtained from Life Technologies (Gibco BRL, Grand Island, NY, USA). All antibodies were products from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
2.2. Cell Culture and Adipocyte Differentiation
Cells were cultured in routine medium in a humidified atmosphere containing 5% CO2 at 37°C. Following confluence, referred to as Day 0, 3T3-L1 cells were exposed to differentiation initiation media (MDI) containing DMEM, 1% penicillin/streptomycin (P/S), 10% fetal bovine serum (FBS), 3-isobutyl-1-methylxanthine (IBMX-0.5 mM), dexamethasone (DEX-1 μM), and insulin (10 μg/mL, for 2 days. Then, the medium was replaced with DMEM containing 10% FBS and 1% P/S with insulin for another 2 days (Day 2). Subsequently, the medium was replaced with DMEM + 10% FBS + 1% PS (Day 4) for another 2 days. After 6 days, mature adipocytes were utilized for subsequent experiments. Various concentration of NCE was added alongside with differentiation medium. A schematic diagram of the differentiation schedule is shown in Figure 1(a). To examine the effects of the p-AMPK inhibitor, cells were treated with 10 μM Compound C (CC), dissolved in dimethyl sulfoxide (DMSO), in the differentiation medium and maintained until harvest. For the control wells, the desired amount of solvent control was added.
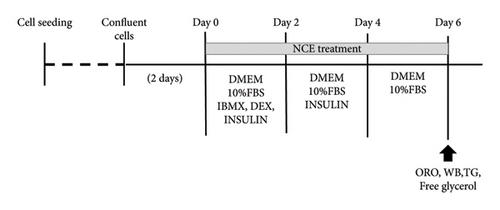
2.3. Preparation of NCE
NCE was generously provided by the National Marine Biodiversity Institute of Korea (MABIK). N. cumingii was collected from Goseong, Gangwon Province, Republic of Korea, and a voucher specimen (NP-0229) was deposited at MABIK. The extract was prepared according to our previously published work [22]. In brief, thoroughly washed N. cumingii was stowed at −80°C. With a grinder, the frozen samples were lyophilized and homogenized. The resulting powder was wrenched out with 70% (v/v) EtOH (1:10 w/v) for 1 h (five repetitions) by sonication. Vacuum evaporation was conducted to obtain the dried extract. The dried extract was stored at −80°C until the biological effect assessment. For the experiments, a stock solution was prepared by dissolving the dried extract in distilled water with continuous agitation to ensure complete solubility. For the control wells, an appropriate amount of solvent control was added.
2.4. Cell Viability
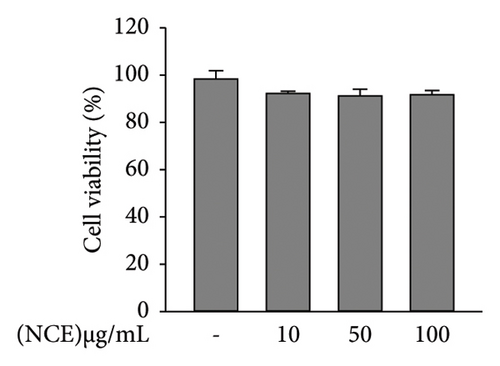
Cellular toxicities of NCE in 3T3-L1 cells were assessed by MTT assay. Cells were treated with varying concentrations (0–100 μg/mL) of NCE for 48 h and incubated with MTT working reagent. Formazan production, indicative of cell viability, was quantified by a microplate reader (Multiskan GO, Thermo Fisher Scientific, Finland).
2.5. Oil Red O Staining
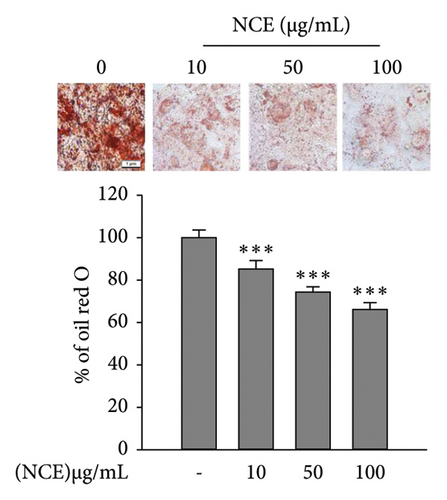
Oil Red O staining was performed to assess the lipid accumulation. After fixing cells with 10% formalin, an Oil Red O working solution was added. The unbound stain was washed off with distilled water. An inverted microscope (DMI6000, Leica, Wetzlar, Germany) was used to capture the images. Subsequently, the stain was extracted in isopropanol for quantitative analysis.
2.6. TG Assay
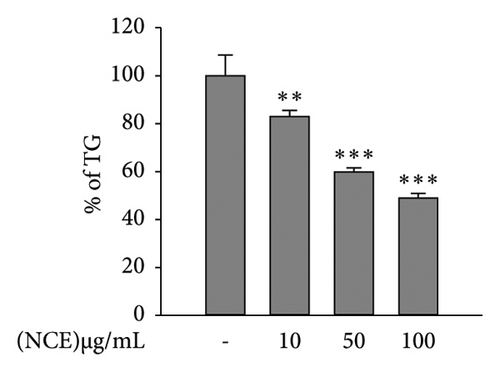
Cellular TG content was quantified by using a commercial colorimetric TG assay kit (Biomax, Seoul, Korea) following the manufacturer’s instructions.
2.7. Free Glycerol Release Assay
Free glycerol release into the culture medium by mature adipocytes was assessed by using a free glycerol reagent (F6428 Sigma-Aldrich St. Louis, MO, USA) following the manufacturer’s instructions.
2.8. Western Blot Analysis
Adipocytes were lysed with RIPA buffer. Equal amounts of protein were allowed for separation, electro-transferred to membranes, and probed with indicated primary antibodies at 4°C overnight. A 1:200 dilution was used for PPARγ (sc-7273), C/EBPα (sc-166258), SREBP-1 (sc-3655-13), FAS (sc-55580), LPL (sc-373759), HSL (sc-74489), p-HSL (sc-139656, AMPK (sc-74461), UCP-1 (sc-518024), PGC-1α (sc-517380), and β-actin (sc-47778) and 1:1000 dilution for p-AMPK (#2535). Then, the membranes were washed and incubated with an HRP-conjugated secondary antibody at room temperature at a 1:2000 dilution for 2 h. All primary and secondary antibodies were obtained from Santa Cruz Biotechnology, except for p-AMPK, which was purchased from Cell Signaling Technology, USA. The bands were visualized using a chemiluminescence (ECL) assay kit, and images were taken using a Davinch-Chemi Imager (CAS400SM, Core Bio, Seoul, Korea). Densitometry analysis was conducted by using ImageJ analysis software.
2.9. Total Flavonoid Content (TFC) and Total Phenol Content (TPC)
TFC and TPC in NCE were determined according to previously reported methods [23, 24]. TFC was expressed as mg rutin equivalents per gram of dried extract (mg RE/g), and TPC was denoted as mg gallic acid equivalents per gram of dried extract (mg GAE/g).
2.10. Identification of Chemical Composition of NCE
The chemical composition of NCE was determined by GC–MS analysis as mentioned in our previous work [22]. Two microliters of sample dissolved in methanol were injected into a Shimadzu QP-2010 Ultra GC–MS with an Agilent DB-5MS UI column. The acquired spectra of the unknown constituents of the N. cumingii fraction were compared with the standard mass spectra of known constituents saved in the NIST V 11 data library, National Institute of Standard and Technology (NIST).
2.11. Statistical Analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) and Duncan’s test using SigmaPlot 12.0 (Systat Software Inc., San Jose, CA, USA). Shapiro–Wilk test and Levene′s median test were used for normality and homogeneity of variables. Results were means ± SD (n = 3), and p < 0.05 denotes significant differences among the groups.
3. Results
3.1. Cell Viability by NCE
Cell viability was assessed at the outset to investigate the effect of NCE during adipogenesis. None of the NCE concentrations tested affected cellular viability. Thus, proceeding experiments were performed at 0–100 μg/mL of concentrations (Figure 1(b)).
3.2. Effect of NCE on Adipocyte Differentiation and Lipolysis
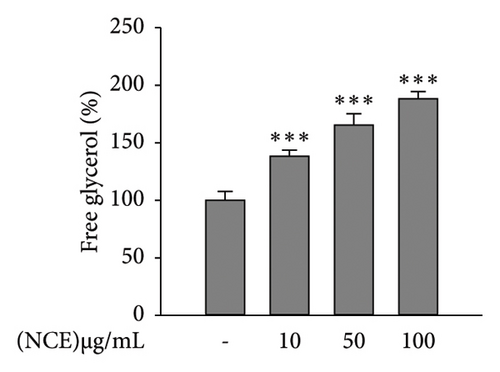
Matured adipocytes at Day 6 were stained with Oil Red O to observe lipid accumulation in the presence or absence of NCE. As shown in Figure 2(a), the control adipocytes tend to accumulate more lipids than the NCE-treated adipocytes. NCE treatment effectively reduced the level of lipid accumulation. Furthermore, NCE at 100 μg/mL shows a nearly 35% reduction in lipid accumulation compared to the control adipocytes. According to the results of the TG assay, significantly higher TG content was observed in the control adipocytes. In contrast, NCE treatment reduced TG content by 51%. The glycerol content in the culture supernatant served as an indicator of adipocyte lipolysis; thus, free glycerol release assay was performed to observe the NCE effect on lipolysis. As expected, free glycerol release by control adipocytes was low, and NCE comparatively increases free glycerol with increasing concentrations (Figure 2(c)).
3.3. Effect of NCE on Adipocyte Differentiation
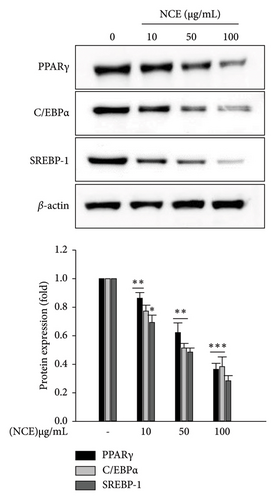
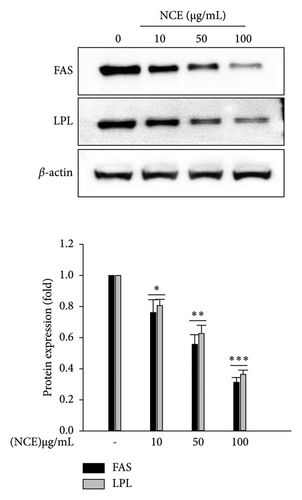
To elucidate the mechanism of the repressive effects of NCE on lipid accumulation, adipogenic transcription factors were detected by the western blotting technique. As depicted in Figure 3(a), significantly high expression of adipogenic transcription factors including PPARγ, C/EBPα, and SREBP-1 was detected in the control adipocytes. While significant dose-dependent inhibition by NCE was observed, the expression of three transcription factors was suppressed nearly 0.7-fold at 100 μg/mL of NCE. Furthermore, the effects of NCE on lipogenic enzymes such as FAS and LPL were investigated. As expected, expression of FAS and LPL was highly detected in the control adipocytes, whereas this increase was inhibited to nearly 0.7-fold by NCE treatment when used at 100 μg/mL.
3.4. NCE Stimulates Lipid Catabolism via Lipolysis and Adipocyte Browning
Lipid degradation in adipocytes is associated with lipolysis and/or thermogenesis. Therefore, we first observed the effect of NCE on lipolysis. For this, the phosphorylation of hormone-sensitive lipase (HSL), which is the rate-limiting enzyme of lipolysis, was determined via western blotting. The phosphorylation of HSL was comparatively higher upon NCE treatment compared to the control adipocytes. Furthermore, at 100 μg/mL of NCE, there was a 4.6-fold increase in p-HSL levels (Figure 4(a)).
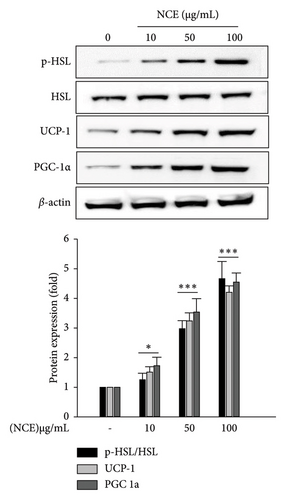
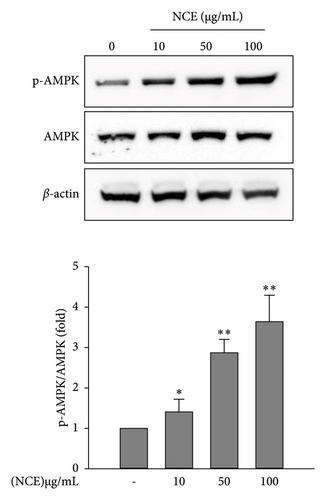
In this study, the development of beige adipocytes, which reduces lipid accumulation through thermogenesis, was further investigated in the presence of NCE. This was explored by detecting the expressions of adipocyte browning-specific markers PGC-1α and UCP-1. As shown in Figure 4(a), the control adipocytes showed negligible expression of PGC1α and UCP-1. On the contrary, NCE treatment significantly increased the expression of PGC-1α and UCP-1, confirming the formation of beige-like adipocytes. At last, since the activation of AMPK by phosphorylation is firmly associated with the activation of HSL, PGC-1α, and UCP-1, the phosphorylation of AMPK was observed. As shown in Figure 4(b), the control adipocytes demonstrated a relatively low level of p-AMPK. However, NCE treatment increased p-AMPK levels in a dose-dependent manner.
3.5. AMPK-Mediated Antiadipogenesis Effect of NCE
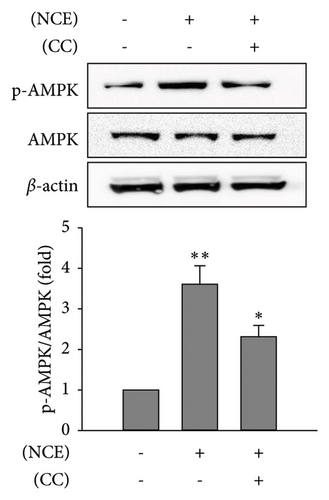
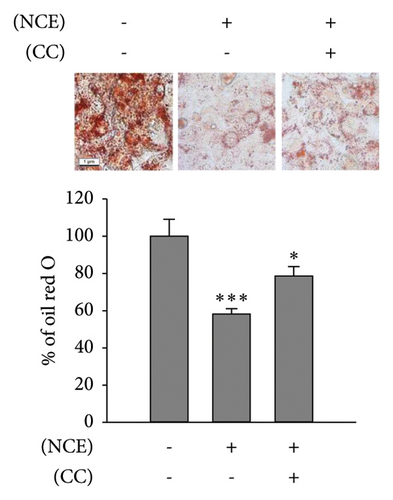
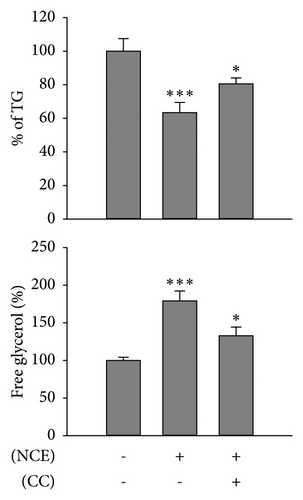
Recognizing the importance of AMPK’s role in inhibiting lipogenesis and stimulating adipocyte browning, CC, an AMPK antagonist, was employed. Subsequently, observations were made on lipid accumulation, TG content, free glycerol release, and expressions of adipocyte browning markers (Figures 5(a), 5(b), and 5(c)). As anticipated, the increased p-AMPK expression by NCE was significantly reduced (p < 0.05) with pretreatment of CC. Moreover, the reduced lipid accretion, TG content, and increased free glycerol release were markedly reversed by CC. In line with this, the increased expressions of UCP-1, PGC-1α, and p-HSL were significantly suppressed with CC exposure (Figure 5(d)).

3.6. TPC, TFC, and Chemical Composition of NCE
Phenolic and flavonoid contents, widely recognized for their direct contribution to biological activity, were quantified in NCE. The amounts of TFC and TPC were 0.15 ± 0.1 mg RE/g and 9.53 ± 0.04 mg GAE/g, correspondingly. To analyze the compounds in NCE, GC–MS was conducted. Out of 45 peaks, only 19 compounds were detected as major peaks (peak area > 0.27%) in the chromatogram (Supporting Figure 1). These compounds were characterized and identified by comparing the mass spectra with the standard library database. The detailed bioactive compounds are tabulated in Table 1. Based on peak areas, cholest-5-en-3-ol constituted 27.15% of NCE. Additionally, NCE exhibited a distinctive profile marked by the existence of fatty acids, including hexadecanoic acid, cis-vaccenic acid, and cis-11-eicosenoic acid.
| R. time | Compound name | Molecular formula | Peak area (%) |
|---|---|---|---|
| 11.767 | 3-Aminopiperidin-2-one | C5H10N2O | 0.61 |
| 12.363 | DL-proline, 5-oxo-, methyl ester | C6H9NO3 | 0.60 |
| 14.151 | Phenol, 2,4-bis(1,1-dimethylethyl) | C14H22O | 0.27 |
| 17.357 | Tetradecanoic acid | C14H28O2 | 2.11 |
| 17.470 | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- | C7H10N2O2 | 0.31 |
| 18.671 | Pentadecanoic acid | C15H30O2 | 0.35 |
| 19.599 | Hexadecenoic acid, methyl ester | C17H34O2 | 0.25 |
| 19.845 | 9-Hexadecenoic acid | C16H30O2 | 2.59 |
| 20.172 | Hexadecenoic acid | C16H32O2 | 11.70 |
| 21.038 | Heptadecenoic acid | C17H34O2 | 0.61 |
| 22.705 | Linoeladic acid | C18H32O2 | 0.84 |
| 22.824 | Cis-vaccenic acid | C18H34O2 | 5.01 |
| 26.005 | Cis-11-eicosenoic acid | C20H38O2 | 7.53 |
| 28.579 | Cis-5,8,11,14,17-eicosapentaenoic acid | C20H30O2 | 0.87 |
| 28.935 | 1,2-Benzenedicarboxylic acid, dioctyl ester | C24H38O4 | 3.1 |
| 37.467 | Cholest-5-en-3-ol (3.beta) | C27H46O | 27.15 |
| 38.810 | Campesterol | C28H48O | 0.28 |
| 39.922 | Gamma-sitosterol | C29H50O | 0.39 |
4. Discussion
Recent studies on obesity have gained a keen interest in identifying dietary compounds capable of boosting energy expenditure to combat obesity [25]. In this study, we demonstrated that NCE treatment during adipocyte differentiation of 3T3-L1 adipocytes reduces lipid accumulation by promoting lipid metabolism and augments energy expenditure via lipolysis and adipocyte browning.
Obesity development entails adipocyte hyperplasia, wherein preadipocytes are converted into mature adipocytes through the adipogenesis process which is principally controlled by transcription factors PPARγ and C/EBPα [26, 27]. Following the induction of these transcription factors, subsequent transcriptional activation of numerous genes establishes adipocyte phenotype. Moreover, lipid accumulation takes place as adipocyte hypertrophy during lipogenesis process, characterized by fatty acid and TG synthesis. This necessitates elevated expression of key enzymes including FAS and LPL alongside the transcription factor SREBP-1 [28]. FAS is the key enzyme responsible for fatty acid biosynthesis and is highly expressed in adipose tissue. LPL plays a central role in governing lipid accumulation by facilitating the uptake of fatty acid from circulating lipoproteins into adipose tissue [29]. Therefore, regulated expression of these biomarkers is essential for modulating adipogenesis. Our results show that NCE efficiently reduces lipid accumulation and TG content in adipocytes by regulating adipogenic transcription factors and lipogenic enzymes.
Adipose tissue acts as a metabolically flexible reservoir and is important for storing and releasing energy through anabolic and catabolic processes. Adipocyte lipolysis is a catabolic process in which TG stored in adipocytes is hydrolyzed into fatty acids and glycerol, which will efflux from cells in due course. This process is governed by lipases, including HSL, the rate-limiting enzyme that hydrolyzes diglycerides [30]. Furthermore, HSL exhibits a greater degree of promiscuity than other lipases and readily hydrolyzes various lipid substrates, including TG and monoglycerides, in vitro [31]. Phosphorylated HSL also activates UCP-1 directly by supplying fatty acids and indirectly by boosting fatty acid oxidation [32]. Many studies suggest that enhancing lipolysis activity by natural compounds is an effective strategy to achieve weight loss [33, 34]. Therefore, the next step was to evaluate whether NCE could enhance lipolysis in adipocytes. As expected, NCE treatment augmented spontaneous free glycerol release with increased phosphorylated HSL levels in 3T3-L1 adipocytes. Thus, the potential of NCE to inhibit adipogenesis was supported by enhanced lipolysis.
To further elucidate the antiobesity potentials of NCE, its effect on inducing adipocyte browning was investigated. This was performed by observing UCP-1 and PGC-1α expressions, the hallmark biomarkers of beige adipocyte formation [35]. The inner-mitochondrial membrane protein UCP-1 is responsible for the thermogenesis effect of beige-like adipocytes, where heat is generated instead of ATP via disrupting the proton gradient of the membrane. PGC-1α serves as a crucial regulator of mitochondrial biogenesis and function, and its presence in WATs promotes the acquisition of beige adipocyte traits [2]. In addition, the induction of PGC-1α promotes UCP-1 expression. In this study, it was confirmed that the NCE treatment effectively upregulated expression of these key proteins, suggesting that NCE triggers the induction of adipocyte browning in 3T3-L1 adipocytes.
AMPK plays a role in regulating lipid metabolism, orchestrating various processes such as the stimulation of catabolic ATP-generating pathways, the induction of mitochondrial biogenesis, and the activation of BATs. Additionally, AMPK inhibits adipogenesis by downregulating PPARγ and C/EBPα and regulates lipogenesis by modulating SREBP-1 and decreasing TG synthesis in adipocytes [36]. Numerous reports have consistently demonstrated that the browning of WATs is facilitated through AMPK signaling [37–39]. Therefore, an investigation was conducted to determine whether the observed NCE-induced adipocyte browning effect was mediated by AMPK activation. The data show that NCE positively increases AMPK phosphorylation. However, the inhibition of AMPK by CC results in the suppression of PGC-1α, UCP-1, and lipolytic marker HSL. Additionally, the reduced lipid accumulation, TG content, and increased free glycerol release were alleviated upon AMPK inhibition. These observations indicate that the phosphorylation of AMPK by NCE plays a central role in antiadipogenesis and browning of adipocytes.
N. cumingii has been reported to be rich in saturated, monounsaturated, and polyunsaturated fatty acids [40]. In the current study, we also confirmed that NCE was rich in several saturated and unsaturated fatty acids. A recent study showed that marine lipids rich in long-chain monounsaturated fatty acid (MUFA) can alleviate risk factors for metabolic syndrome by lowering plasma glucose and lipid levels in obese mice [41, 42]. Cis-9 vaccenic acid, an omega-7 MUFA, has demonstrated significant effects in reducing body weight, liver size, and adipose tissue when administered to mice [43]. Cis-11-eicosenoic acid has been observed to reduce adipogenesis. This is achieved by downregulating the expressions of PPARγ, CEBPα, SREBP-1, and lipid accumulation in 3T3-L1 adipocytes [44]. n-Hexadecenoic acid (palmitic acid, PA) is the most prevalent saturated fatty acid and is widely available in our diet from a variety of food sources. A PA-rich extract of the green algae Caulerpa lentillifera has been demonstrated to reduce lipid accumulation in Caenorhabditis elegans obesity model, suggesting that it may be a possible dietary supplement for obesity treatment [45]. Furthermore, NCE has large amounts of cholest-5-en-3-ol, which is commonly referred to as cholesterol. Marine organisms have been reported to contain cholest-5-en-3-ol as a common bioactive compound [46, 47]. Research suggests that taking cholesterol-rich foods does not elevate plasma or hepatic cholesterol levels [48, 49]. Several studies have shown that marine extracts consisting of cholest-5-en-3-ol as a major active compound provide therapeutic benefits such as anti-inflammatory and antioxidant activities [46, 50]. Further, our previous study showed that cholest-5-en-3-ol rich Strongylocentrotus intermedius extract showed an antiobesity effect in 3T3-L1 adipocytes [51]. These observations suggest that NCE rich in cholest-5-en-3-ol may be an efficacious ingredient in the treatment of obesity.
While this study reports the antiobesity effect of NCE, it does have some limitations. The chemical profiling of NCE requires further identification through isolation and purification to identify the promising compounds for antiobesity effects. Additionally, although the present results provide foundational data suggesting potential anti-obesity effects of NCE through 3T3-L1 cell model, additional studies using in vivo obesity model are necessary to extrapolate these results.
5. Conclusion
The present study provides compelling evidence that NCE treatment may be beneficial in alleviating obesity by modulating proteins associated with adipocyte differentiation and lipogenesis. Furthermore, NCE induces lipid catabolism by increasing lipolysis and adipocyte browning. Collectively, NCE plays a multifaceted role in exhibiting antiobesity effects in 3T3-L1 cell model. Therefore, it is suggested that NCE holds promise as a nutraceutical intervention, potentially serving as an active functional compound.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
L.A.D.: conceptualization, methodology, investigation, and data curation; S.-C.K., M.-J.Y., J.M.L., J.-Y.K., G.-W.O., D.-S.L. W.-K.J., and S.-J.L.: investigation, methodology, and funding acquisition; J.-Y.J.: supervision, conceptualization, methodology, writing–original draft, and review and editing.
Funding
This research was supported by the National Marine Biodiversity Institute of Korea (MABIK) Research Program 2024M00500 and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2021R1A6A1A03039211).
Supporting Information
Supporting Figure 1: GC–MS chromatogram of NCE.
Open Research
Data Availability Statement
The datasets of the current study are available from the corresponding author on reasonable request.




