Physicochemical and Functional Properties and In Vitro Digestibility of Green Banana Flour-Based Snacks Enriched With Mango and Passion Fruit Pulps by Extrusion Cooking
Abstract
Surplus banana crops that are not marketable, but still of suitable quality for consumption, are indeed a challenge in terms of underutilization as a post-harvest by-product. However, this situation also presents an exciting opportunity: these by-products can serve as a valuable raw material for the production of extruded snacks. This study examines the impact of incorporating passion fruit and mango pulp (6% w/w) into green banana flour on physicochemical properties, pre- and postextrusion cooking, and bioactive compound content, alongside their bioaccessibility during in vitro digestion. Proximate composition analysis revealed that there were no significant changes in lipid and protein content following extrusion. However, there was a notable decrease in fiber due to alterations in starch. Initial values for zinc, sodium, and calcium in banana flour were 0.678 mg/100 g, 9.237 mg/100 g, and 14.214 mg/100 g, respectively. Extrusion resulted in an increase in zinc content (1.032, 0.736, and 0.999 mg/100 g), sodium content (21.904, 17.608, and 18.787 mg/100 g), and calcium content (28.217, 16.884, and 58.207 mg/100 g) in the banana flour extrudates without pulp, with passion fruit pulp, and with mango pulp, respectively. The addition of fruit pulp significantly enhanced the total phenolic compounds and carotenoids, though thermal degradation reduced the compound content postextrusion. Texture analysis demonstrated no significant differences among the samples, which were found to have hardness and crispness values consistent with those reported in the literature, at 20–25 N for hardness and 100–117 for crispness. In vitro digestion demonstrated increased bioaccessibility of total phenolic compounds, flavonoids, and carotenoids in extrudate samples compared to raw mixtures. The enhancements were 41%–72% for total phenolics, 100% for flavonoids, and 22%–78% for carotenoids. The utilization of surplus green banana represents a valuable alternative to produce healthy and functional snacks enriched with fruit pulps.
1. Introduction
The contemporary concept of snacks as ready-to-eat, conveniently packaged foods meets the human need to satisfy hunger or cravings at any time of the day. However, the widespread availability of snacks high in oils, sodium (Na), and additives, particularly those made from conventional starches such as rice, corn, and wheat, poses a significant challenge to the food industry [1, 2]. The challenge is further compounded by the increasingly busy and stressful lifestyles of the population, which has led to an increased reliance on these products as a solution to alleviate hunger between meals [3]. In addition, the impact of the COVID-19 lockdown on snacking habits showed a significant increase in snack consumption, with a 45% increase in consumption observed [4].
The global snack market reached a value of USD 256.5 billion in 2023, representing a compound annual growth rate of 7.9% [5]. In this context, there is a prevalence of snacks characterized by a high energy content but diminished nutritional value [6, 7].
The consumption of ultraprocessed foods has emerged as a significant global public health challenge, with established links between this consumption and an increase in the risk of cardiovascular diseases and functional gastrointestinal disorders [8, 9]. In response to this issue, a proposed solution is oriented towards developing healthy snacks characterized by low caloric content, high nutrient density, and functional compounds, including carotenoids, flavonoids, and phenolic compounds. This strategy is manifested through the enrichment of foods with bioactive compounds, particularly fruits, which are known to possess properties that counter the risk of human diseases due to the presence of functional compounds and pleasant flavors [10]. Furthermore, starch is a carbohydrate that is also present in fruits and plays a key role in providing nutritional value and acting as fiber in the human body [11].
The growing interest in healthy foods has created a market niche for snacks that not only satisfy basic dietary needs but also position themselves as carriers of biologically active compounds. This has led to the conceptualization of functional foods, which are adapted to contemporary needs. The most prevalent technique employed in the production of these ready-to-eat foods is extrusion cooking [12], a practice that has been widely utilized for a diverse range of products, including crunchy snacks, breakfast cereals, instant soups, and meat analogs [13]. This thermomechanical process has the ability to modify the starch structure, the main component responsible for product expansion, through stages of gelatinization, fusion, and degradation [14].
Banana, a member of the Musa genus of the Musaceae family (Musa cavendishii), emerges as one of the most consumed fruits globally, especially in tropical climates. In 2022, global banana production reached 135.11 Mt, with India and China accounting for a significant portion of this output. Colombia, in particular, has emerged as a major exporter, with a production of 2.52 Mt and approximately 104,000 ha planted, according to data from the Food and Agriculture Organization [15]. Despite its relevance in the food chain, the banana value chain is affected by a significant amount of waste and products sold at lower prices due to mechanical and visual damage caused by improper postharvest handling.
In accordance with the prevailing trend towards sustainability and the utilization of food waste, the potential of utilizing surplus bananas as a nonconventional raw material for the extrusion process was previously investigated [16–18]. Green banana flour (BF) or immature BF has emerged as a promising raw material to produce healthy snacks, demonstrating a high content of total starch (73.4%), and dietary fiber (~14.5%) [19]. Additionally, research has demonstrated that BF retains some of its phenolic content during processing [20–22]. Considering the potential for this raw material to exhibit unfavorable flavors, such as bitterness or astringency, due to its phenolic compound content, a viable approach to addressing these concerns is the incorporation of other ingredients that can serve as natural flavor enhancers. Previous studies have indicated that the inclusion of passion fruit pulp [23, 24] and mango pulp [25, 26] in extruded products can lead to enhanced sensory acceptability and the potential improvement of their functional nutritional profile.
In the context of an industry that is increasingly demanding gluten-free products, the versatility of green BF is a strategic matrix in this dietary evolution, as it allows for the complete absence of this component in foods for the celiac population. Celiac disease, affecting approximately 1% of the population [27, 28], characterized by symptoms spanning intestinal and extraintestinal manifestations, commonly known as irritable bowel syndrome [29], drives the adoption of gluten-free diets as the only means to mitigate its adverse effects [27, 30]. Adherence to a gluten-free diet has been documented not only to alleviate symptoms associated with celiac disease but also to bring additional benefits such as the reduction of schizophrenia symptoms, as asserted by Cascella et al. [31], as well as an increase in performance and endurance in athletes, attributed to the elimination of gastrointestinal symptoms and fatigue linked to gluten consumption. Furthermore, there is evidence of an association between weight loss and gluten-related effects on various health conditions [32]. However, the relevance of these products extends beyond the celiac realm and has permeated contemporary dietary trends. The food industry has made significant efforts to optimize gluten-free extruded snacks, focusing on improving macronutrients, fiber, and bioactive compounds through formulations that incorporate cereals, legumes, and, in the particular case of this study, fruits [33].
In this sense, the objective of this research is to assess the impact of extrusion cooking and enrichment with mango and passion fruit pulps on the physicochemical and functional properties of both raw blends and extruded snacks made from green BF. Additionally, the bioaccessibility of bioactive compounds is addressed using an in vitro digestion model.
2. Materials and Methods
2.1. Raw Material and Pretreatment
Bananas (M. cavendish) were obtained at Stage 2 of ripening (green color with yellow traces) according to the scale used by Nobre et al. [34]. At the same time, yellow passion fruit (Passiflora edulis) and mango (Mangifera indica L. cv. Tommy Atkins) fruits were obtained at Stages 3–4 of ripening, determined by evaluating their firmness according to the method described by Villalpando-Guzmán et al. [35]. These varieties were chosen because they are three types of fruits that are very common in Colombia and widespread in neighboring countries. All fruits were purchased from local supermarkets in Manizales, Caldas, Colombia.
The unripe bananas were washed and then soaked in a solution of bleach (50 ppm) for 3 minutes. They were then soaked in hot water (70°C) for 15 minutes, peeled, and cut into slices of 4 mm thickness. These slices were dried at 70°C for 12 hours in an oven (Thermo Electron Corporation, Model 6545, United States). The dried banana was ground in a mill (TE-650/1, Tecnal, Brazil) and sifted through a #20 mesh (850 μm). The resulting green BF was stored in sealed plastic bags at room temperature (20°C) for later analysis and use in experiments. The mango and passion fruit were disinfected with a solution of sodium hypochlorite (50 ppm) for a period of 3 minutes. They were then chopped, liquefied, and filtered to remove insoluble fibers and seeds. Their pulps were processed fresh before each extrusion process.
2.1.1. Preparation of Mixtures and Extrusion Process
The extrusion conditions and batch size were adjusted based on the results of preliminary experiments, which demonstrated that these modifications enabled the production of a product with the desired textural characteristics and prevented the clogging of the extrusion equipment.
The experimental batches for extrusion were prepared by combining appropriate quantities of BF with one of the two fruit pulps, with 6.0% ± 0.1% by weight of pulp added to the total mixture, resulting in a final moisture content of 16.0% ± 2.0%. The extrusion process was conducted using a single-screw extruder (EX 0113, Rap Engineering, Bogotá, Colombia) with the following specifications: a screw diameter of 50 mm, a length-to-diameter ratio of 15:1, a die orifice of 4.5 mm, and a feed rate to the extruder of 12 kg/h. The extruder barrel was subdivided into three distinct heating zones, each of which was independently heated by electric resistances: the feed zone, the central zone, and the die zone. A jacket containing ethylene glycol was employed to facilitate cooling at the outlet, maintaining a temperature of 3°C. The die temperature was maintained at a constant 90°C ± 3°C, and the screw speed was set to 175 rpm. The moisture content of the mixture [36] and the extrusion temperature [37] were similar to those reported in previous studies on single-screw extruders.
2.2. Proximal and Mineral Composition of Raw Mixtures and Extrudates
2.2.1. Proximal Composition of Raw Mixtures and Extrudates
The proximate composition assessment was conducted in accordance with the guidelines established by the AOAC (Association of Official Analytical Chemists) for BF, BFPM (green banana flour + passion fruit mixture), and BFMM (green banana flour + mango fruit mixture) as well as the extrudates BFE (banana flour extrudate), BFPE (green banana flour + passion fruit extrudate), and BFME (green banana flour + mango extrudate). The moisture content was determined gravimetrically by drying in a vacuum oven at 70°C until a constant weight was achieved, following AOAC method 926.12. Protein content was determined using the Kjeldahl method (AOAC-920.87), lipids were quantified using the Soxhlet extraction method (AOAC 920.85), ash or mineral residues were calculated through incineration at 550°C (AOAC 923.03), and total dietary fiber content was analyzed via enzymatic-gravimetric treatment using the Megazyme K-FDT kit (AOAC 991.43).
2.2.2. Mineral Composition of Mixtures and Extrudates
Mineral determination was carried out following the AOAC 53.923 method. Phosphorus (P) was quantified through visible spectroscopy in a spectrophotometer (UV/Vis LAMBDA 365, Korea), while iron, zinc (Zn), Na, and potassium (K) were analyzed through flame atomic absorption spectroscopy in an atomic absorption spectrophotometer (ThermoScientific iCE 3500, China).
2.3. Determination of Bioactive Compounds: Total Phenolic Content (TPC), Total Carotenoids (TC), Total Flavonoids (TF), and Antioxidant Activity (AA) of Raw Mixtures and Extrudates
2.3.1. Extraction of Bioactive Compounds
To quantify the bioactive compounds for TPC, TF, and AA, a previous ultrasound-assisted extraction method was employed, as outlined by Alvarez, Ospina, and Orrego in [38]. For the extraction of carotenoids, a hexane-acetone-ethanol solvent mixture (50:25:25, v/v/v) was employed, in accordance with the methodology delineated by Barba et al. [39]. All extractions were conducted in triplicate.
2.3.2. TPC, TC, TF, and AA
The TPC in the samples was estimated following the methodology described by Murillo-Franco, Galvis-Nieto, and Orrego [40], using the Folin–Ciocalteu method. TPC was expressed in gallic acid equivalents (mg GAE/g of extract or recalculated per 100 g of dry pulp after ultrasonic extraction, considering the extraction yield; mean values ± standard deviation, n = 3). A dose–response curve (50–500 ppm) for gallic acid was employed.
The flavonoid content was determined in accordance with the methodology outlined by Meneses et al. [41] with some modifications. Sequentially, 30 μL of the sample was added to 90 μL of methanol, 6 μL of aluminum chloride (10% w/v), 6 μL of potassium acetate (1 mol/L), and 170 μL of distilled water. The solutions were allowed to react in the dark for 30 minutes, and absorbance was measured at 415 nm using a SpectraMax ABS Plus microplate reader (Molecular Devices, San José, CA). The flavonoid content was expressed in milligram quercetin equivalent per dry weight of the sample (mg quercetin/g dry sample).
The carotenoid content was determined in accordance with the methodology described by Barba et al. [39]. One gram of the sample was agitated with a mixture of 25 mL hexane-acetone-ethanol (50:25:25, v/v/v). It was left to react in the dark for 30 minutes with constant agitation, followed by the addition of 2 mL of distilled water and further agitation for 5 minutes. Finally, it was allowed to settle for 15 minutes, and approximately 4 mL of the upper layer corresponding to the hexane phase was taken and measured at an absorbance of 446 nm in a spectrophotometer (UV-1150, Lab Science, China). Results were expressed as milligram β-carotene per 100 g of the dry sample (mg β-carotene/100 g dry sample), utilizing a dose–response curve (0.5 to 10 ppm) of β-carotene.
Consequently, for the assessment of AA utilising the ABTS+ radical cation, the methodology proposed by Re et al. [45] and Ozgen et al. [46] with modifications, 10 μL of the sample was mixed with 231 μL of 7 mM ABTS+ solution for 30 minutes. The absorbance was measured in a SpectraMax ABS Plus microplate reader (Molecular Devices, San José, CA). The calibration curve, analogous to the DPPH method, was established within a Trolox concentration range of 50–500 ppm.
2.4. Physical and Textural Properties of Extrudates
2.4.1. Expansion Index (EI)
2.4.2. Water Absorption Index (WAI) and Water Solubility Index (WSI)
2.4.3. Swelling Index (SWE)
The SWE was determined using the bed volume technique reported by Robertson et al. [49]. One gram of crushed sample was weighed and poured into a calibrated cylinder containing 10 mL of water. The samples were left to rest for 18 hours, and the SWE was recorded as the bed volume expressed in millimeters of swollen sample per gram of initial dry sample.
2.4.4. Hygroscopicity (Hy)
2.4.5. Bulk Density (ρb)
The methodology outlined by García-Segovia et al. [51] was employed to compute ρb. This was achieved by dividing the mass of the extrudate, recorded on a precision balance (AB204-S, Mettler Toledo, Switzerland), by its volume. The latter was determined by measuring its length (in centimeters) and diameter (in centimeters) using a digital caliper (Vernier, Comecta SA, Spain), thus enabling the calculation of the apparent volume of the cylinder.
2.4.6. Real Density (ρ)
The determination or ρ was conducted using a helium pycnometer (AccPyc 1330, Micromeritics, Norcross, United States) along with the corresponding weight recorded on a precision balance (AB204-S, Mettler Toledo, Switzerland) [51].
2.4.7. Porosity (ε)
2.4.8. Textural Analysis
The texture of the extrudates was assessed using a texture analyzer (TX.XT.Plus, Godalming, United Kingdom) equipped with Texture Exponent software (Version 6.1.12.0). A compression test was conducted at room temperature employing a cylindrical probe with a 5-mm diameter (SMS P/0-25), penetrating 50% of the sample height, and a test speed of 1 mm/s. Hardness was defined as the maximum force (N) of the initial compression required to rupture the sample, while crispness was measured by the total number of peaks observed in the curve. Texture parameters, including hardness and crispiness, were determined by averaging 30 repetitions for each sample [52].
2.5. Digestion In Vitro on Raw Mixtures and Extrudates
The general in vitro digestion methodology, standardized for food by the COST Infogest network [53], was applied to the samples of green BF and fruit blends BF MM and BF PM. Furthermore, the extrudates BFE, BME (banana–mango extrudate), and BPE (banana–passion fruit extrudate) were evaluated with the objective of assessing their digestibility and bioaccessibility. The polyphenolic content, carotenoids, and AA were evaluated. BF and BFE were considered the controls for comparisons.
2.6. Statistical Analysis
To assess the data analysis of blends and extrudates, an analysis of variance (ANOVA) test was employed (Microsoft Excel software) with a confidence level of 95% (p < 0.05). Mean differences were assessed using the Fisher test. Using R software Version 4.3.2, a high-level correlation analysis with a significance level of 95% was conducted among the textural characteristics of the extrudates. Pearson correlation was utilized to better elucidate the relationship between AA and other bioactive compounds. All samples were analyzed in triplicate, and the results were reported as means ± standard deviations.
3. Results
3.1. Extrusion Cooking’s Effect on Proximal Composition and Mineral Content of Mixtures and Extrudates
Figure 1 shows the final extrudates obtained. Table 1 presents the results of the proximal composition both before and after the extrusion process. Initially, it is noteworthy that the lipid content shows no significant variations (p > 0.05) between green BF and BFPM. However, an increase in lipid content is observed when enriching the flour with mango pulp. Furthermore, when examining lipid content before and after the extrusion process, a significant decrease is evident. This phenomenon has been previously reported in similar studies, such as in extruded rice enriched with carob fruit and beans [55] and sorghum and cowpea extrudates [56]. The underlying explanation is attributed to the apparent disappearance of lipid content related to the formation of complexes with starch. These complexes resist conventional fat quantification methods used for proximal analyses [55].



| Mixtures | Extrudates | |||||
|---|---|---|---|---|---|---|
| BF | BFPM | BFMM | BFE | BFPE | BFME | |
| Fat [g/100 g] | 0.556 ± 0.003c | 0.631 ± 0.004b | 0.690 ± 0.014a | 0.101 ± 0.014e | 0.110 ± 0.004e | 0.209 ± 0.001d |
| Protein [g/100 g] | 4.622 ± 0.018b | 5.247 ± 0.107a | 5.173 ± 0.059ab | 4.737 ± 0.018b | 3.842 ± 0.197c | 4.845 ± 0.063b |
| Fiber [g/100 g] | 11.768 ± 0.287b | 9.869 ± 0.395c | 13.581 ± 0.395a | 12.954 ± 0903ab | 7.355 ± 0.045d | 11.536 ± 0.003b |
| Carbohydrate [g/100 g] | 71.910 | 79.731 | 76.026 | 78.756 | 85.070 | 80.019 |
| Moisture (%) | 5.441 ± 0.452a | 19.432 ± 0.525a | 19.090 ± 1.129a | 6.758 ± 0.420c | 9.782 ± 0.069b | 9.866 ± 0.010b |
| Ash [g/100 g] | 4.144 ± 0.001b | 4.522 ± 0.020a | 4.530 ± 0.039a | 3.452 ± 0.053cd | 3.623 ± 0.081c | 3.391 ± 0.065d |
| Zn [mg/100 g] | 0.678 ± 0.018b | 0.748 ± 0.037b | 0.806 ± 0.065b | 1.032 ± 0.018a | 0.736 ± 0.054b | 0.999 ± 0.004a |
| Na [mg/100 g] | 9.237 ± 0.023d | 8.997 ± 0.164d | 12.136 ± 0.004c | 21.904 ± 0.738a | 17.608 ± 0.699b | 18.787 ± 0.072b |
| Fe [mg/100 g] | 2.875 ± 0.211a | 2.930 ± 0.107a | 1.640 ± 0.3436b | 3.231 ± 0.157a | 3.008 ± 0.027a | 1.703 ± 0.054b |
| Ca [mg/100 g] | 14.214 ± 0.666d | 13.256 ± 0.137de | 18.476 ± 0.736c | 28.217 ± 2.368b | 16.884 ± 0.717ce | 58.207 ± 1.346a |
| K [mg/100 g] | 1308.076 ± 87.965a | 1278.847 ± 33.965ab | 1199.954 ± 11.415ab | 1240.764 ± 57.868ab | 1240.490 ± 29.579ab | 1131.619 ± 14.353b |
- Note: Means ± standard deviation (n = 3). The values that do not share the same lowercase letter in a row indicate significant changes between the samples, according to the Fisher test (p < 0.05) when comparing the samples in the mixtures or extrudates. All the results were expressed on a wet basis.
- Abbreviations: BF: green banana flour; BFE: green banana flour extrudate; BFME: green banana flour + mango extrudate; BFMM: green banana flour + mango mixture; BFPE: green banana flour + passion fruit extrudate; BFPM: green banana flour + passion fruit mixture.
The protein content of the extrudates demonstrated no significant differences (p > 0.05) in the presence or absence of fruit pulps or following the application of the extrusion process. It is widely recognized that most fruits are not significant sources of protein, thereby limiting their capacity to enhance the protein content of the product.
About fiber content, no significant differences (p > 0.05) are observed between BF and BFMM. However, significant differences (p < 0.05) in fiber content are recorded between BFPM and the other samples. It is noteworthy that the fiber content undergoes significant variations (p < 0.05) for BFPM after the extrusion process, demonstrating a decrease in fiber content. This phenomenon of decreased total dietary fiber content after extrusion has been documented in other studies. For example, Arribas et al. [55] observed a similar decrease in fiber content in extrudates based on rice, carob, and bean. Similarly, Sarawong et al. [17] reported a reduction in fiber content in green banana extrudates. Additionally, Arribas et al. [57] observed a decrease in fiber content in extrudates of mixtures of rice, pea, and carob flours. These changes are attributed to the partial solubilization of fiber compounds linked to alterations in starch resulting from the extrusion process [58].
Given the initial moisture content of BF, as shown in Table 1, it was necessary to achieve a moisture level of approximately 16% (w/w) for the material to undergo the extrusion process. Consequently, BFE exhibited a higher moisture loss after extrusion, which can be attributed to its initial fiber content. A higher fiber content results in greater water absorption, which in turn leads to an increased moisture loss at the nozzle exit due to the pressure drop [59]. The moisture content of the BFPE and BFME samples exhibited a similar trend, with moisture losses ranging between 49% and 48%.
The ash content showed no significant differences (p > 0.05) between the raw flour and the enriched variants. This may be attributed to the relatively low percentage of fruit pulp addition (6%). However, upon a more detailed analysis of the minerals present in the ashes of the extruded products, a notable increase (p < 0.05) in Zn content was observed in BFPE and BFME in comparison to their raw flours.
Similarly, both Na and calcium show an increase after the extrusion process, with significant differences (p < 0.05) between raw flours and extruded products. On the other hand, iron shows no significant differences between extruded products and their raw flours, as does K. These results suggest that, although the total ash content does not vary significantly (p > 0.05), there are notable changes in specific mineral composition, highlighting increases in Zn, Na, and calcium in the extruded products compared to their raw forms. This result of increased content of some minerals has already been reported for extrudates from other raw materials, such as corn extrudates enriched with Rosa canina [60], wheat extrudates enriched with insect flour (larvae) [61], rice and organic corn flour extrudates enriched with spirulina [62].
Additionally, it is essential to highlight the K content present in the extrudates BFE, BFPE, and BFME—1240.764, 1240.490, and 1131.619 [mg/100 g], respectively. These values substantiate and corroborate the assertion that banana is a rich source of K [63, 64].
3.2. Extrusion Cooking’s Effect on Bioactive Compounds’ Content
The objective is to enhance the level of bioactive compounds in extruded foods. This is being pursued through the incorporation of ingredients derived from fruit and vegetable by-products [65]. This approach allows for the valorization and improvement in quality. The bioactive compounds present in raw flours and extrudates are presented in Table 2. The inclusion of fruit pulps led to a significant increase (p < 0.05) in the content of phenolic compounds in the extrudates compared to BF. In this regard, BFPM exhibited the highest TPC, showing significant differences (p < 0.05) from all other samples. On the other hand, no significant differences (p > 0.05) in TPC were observed between BFMM and BF.
| Mixtures | Extrudates | |||||
|---|---|---|---|---|---|---|
| BF | BFPM | BFMM | BFE | BFPE | BFME | |
| TPC [mg gallic acid equivalent/100 g] | 2115.134 ± 102.250 | 3463.356 ± 217.715 | 2393.553 ± 115.515 | 782.590 ± 15.518 | 1458.450 ± 107.926 | 972.809 ± 22.709 |
| TF [mg quercetin/100 g] | 218.067 ± 12.248a | 68.667 ± 2.867c | 70.667 ± 6.549c | 177.310 ± 8.524b | 55.427 ± 2.051c | 57.407 ± 0.241c |
| TC [mg β-carotene/100 g] | n.d | 2.741 ± 0.158b | 5.276 ± 0.043a | n.d | 0.689 ± 0.030c | 1.416 ± 0.119d |
| AA DPPH [μmol Trolox equivalent/100 g] | 829.587 ± 8.687b | 1133.372 ± 2.279a | 1169.301 ± 11.393a | 553.190 ± 42.544c | 632.182 ± 7.325d | 563.902 ± 6.436cd |
| AA ABTS [μmol Trolox equivalent/100 g] | 1171.467 ± 11.287b | 921.078 ± 57.660c | 1367.972 ± 94.995a | 1079.460 ± 63.747bc | 1059.359 ± 0.749bc | 1324.068 ± 12.640ab |
- Note: Means ± standard deviation (n = 3). The values that do not share the same lowercase letter in a row indicate significant changes between the samples, according to the Fisher test (p < 0.05) when comparing samples in the mixtures or extrudates. All of the results were expressed on a dry basis.
- Abbreviations: BF: green banana flour; BFE: green banana flour extrudate; BFME: green banana flour + mango extrudate; BFMM: green banana flour + mango mixture; BFPE: green banana flour + passion fruit extrudate; BFPM: green banana flour + passion fruit mixture; n.d.: not detected.
Although BFPE exhibited the highest TPC value among the products obtained through extrusion, significant differences (p < 0.05) were observed concerning the TPC content before extrusion, resulting in a decrease of 57.88% compared to its initial mixture content. This trend of TPC reduction after extrusion cooking was replicated in BFME and BFE, with losses of 59.53% and 63.02%, respectively, compared to the initial content, highlighting the highest TPC loss in BF. These losses fall within the reduction ranges reported by Sarawong et al. [17], for extruded green BF, attributed to the combination of shear force and temperature during the extrusion process, given the presence of thermosensitive phenolic compounds. Extrusion conditions can cause the breakdown of phenol molecular structures, induce reactions during the extrusion cooking process, and generate matrices that limit the extractability of compounds [60, 66].
Previous research, such as that by Nadeesha et al. [67], has confirmed the decrease in phenolic compounds with increasing temperatures. This suggests that temperatures above 80°C may lead to the decomposition of these compounds. In this study, the extrusion temperatures applied were close to 100°C, which could explain the identified decrease in TPC.
In contrast, a reduction in TF was observed when the product was enriched with both pulps, with not significant differences (p > 0.05) between them. This reduction could be attributed to the fact that the fruits used for enrichment are not considered to be particularly rich sources of flavonoids [68].
After the extrusion process, the flavonoid content in the samples enriched with fruit pulps was not affected, as no significant differences (p > 0.05) were identified between the enriched extruded products BFPE and BFME and their respective initial blends. However, losses were observed in the case of BF, which are linked to the decomposition of thermosensitive flavonoids, polymerization of phenolic compounds, and their oxidation due to high temperature [69], as previously explained for TPC. This outcome of loss following extrusion was also reported by Igual et al. [11] for corn extrudates enriched with alfalfa (Medicago sativa).
In terms of TC content, an increase in composition was observed in samples enriched with pulps. It is well established that both passion fruit and mango pulp exhibit an orange coloration, which is attributed to the presence of carotenoids [70–72]. This results in an enrichment of these compounds in the final product.
The extrusion process had a notable effect on carotenoids, with a reduction (p < 0.05) observed. Previous research, such as that by Ortak et al. [66], has reported similar reductions in corn flour extrudates enriched with carrot pulp. This phenomenon may be attributed to the thermosensitivity of carotenoids present in pulps subjected to hot extrusion, as heat has been demonstrated to promote oxidation reactions associated with carotenoid degradation [73].
The DPPH assay was employed to analyze the behavior of AA through a series of experiments. The results indicated a significant increase in AA content when the product was enriched with pulps. Furthermore, no differences were observed between pulp-enriched flours and BF regarding AA. In contrast, the AA content of BFME was comparable to that of BFPE and BFE, with no significant differences (p > 0.05).
However, discrepancies were observed between the flours and their extruded products, with a significant decrease (p < 0.05) in AA. This result is linked to the breakdown of phenolic compounds, which affects their antioxidant capacity. It has been demonstrated that the depolymerization of flavonoids results in a reduction in electronic delocalization, which subsequently leads to a decline in AA [74]. On the other hand, when examining AA through the ABTS assay, it was observed that the extrusion process did not generate significant differences in AA between any of the extruded products and their respective flours.
Furthermore, a Pearson correlation analysis was carried out between various measures of bioactive compounds. In this context, the variables that showed significant correlations (p < 0.05) were TPC, TC, TF, and AA.
In detail, a notable positive correlation (0.902, p < 0.05) was identified between TPC and AA, measured by the DPPH assay. Phenolic content, known for its ability to reduce the impact of free radicals, has previously shown a similar correlation in extruded samples. This was reported by Anton et al. [75], who examined extruded corn fortified with beans, and Dlamini et al. [76], who studied sorghum extrudates.
Furthermore, AA also showed a positive correlation (0.794, p < 0.05) with carotenoids. It is suggested that this association could be linked to the stabilizing effect of AA on carotenoids, as noted by Choi, Kim, and Lee [77]. This kind of positive correlation has been previously documented by other studies, as evidenced by Arilla et al. [78] and Igual et al. [6].
3.3. Extrusion Cooking’s Effect on Physicochemical and Textural Properties
The performance of a new extruded snack product is directly related to its sensory attributes, which are primarily defined by its quality and especially its texture. The objective is to provide a desirable palatability experience [65]. Among these attributes, crispy texture is one of the most crucial and desirable aspects for extruded foods [79, 80]. The physicochemical and textural parameters of foods are inherently linked to their structural properties and composition. Table 3 provides a detailed overview of the physicochemical and textural parameters of the produced extrudates.
| Mix | Extrudates | |||
|---|---|---|---|---|
| BF | BFE | BFPE | BFME | |
| EI | — | 6.350 ± 0.238a | 5.520 ± 0.458b | 4.223 ± 0.071c |
| WAI | 1.730 ± 0.206c | 3.740 ± 0.078a | 3.435 ± 0.217ab | 2.054 ± 0.077c |
| WSI [%] | 5.501 ± 0.283c | 21.800 ± 1.061b | 20.706 ± 3.654b | 33.651 ± 1.515a |
| SWE [mLswollen/gdry solid] | — | 1.230 ± 0.021ab | 1.183 ± 0.049a | 0.647 ± 0.167b |
| Hy [gw/100gdry solid] | — | 21.300 ± 0.010a | 20.626 ± 0.002a | 21.796 ± 0.010a |
| ρb [g/cm3] | — | 0.190 ± 0.018a | 0.261 ± 0.033a | 0.266 ± 0.028a |
| ε [%] | — | 87.143 ± 0.014a | 82.767 ± 0.421b | 83.705 ± 0.937b |
| Hardness [N] | — | 14.88 ± 3.99a | 15.74 ± 2.34a | 14.22 ± 0.70a |
| Crispness | — | 139.332 ± 10.563a | 118.333 ± 10.137a | 133.931 ± 11.480a |
- Note: Means ± standard deviation. The values that do not share the same lowercase letter in a row indicate significant changes between the samples, according to the Fisher test (p < 0.05) when comparing samples in the mixtures or extrudates.
- Abbreviations: BF: green banana flour; BFE: green banana flour extrudate; BFME: green banana flour + mango extrudate; BFPE: green banana flour + passion fruit extrudate.
Regarding the WAI of the extrudates, significant differences (p < 0.05) were found, especially for BFME compared to the other two, which showed no notable differences (p > 0.05) between them. It is relevant to note that the higher WAI value corresponded to the BFE, which can be explained by a higher flour proportion, reducing gelatinization and starch degradation, leading to increased WAI to promote more flexibility, as explained by Enríquez-Castro et al. [81], Sarawong et al. [17], Hagenimana, Ding, and Fang [82] and Pavani et al. [2].
As for EI, significant variations (p < 0.05) were demonstrated among the three extruded samples, with a lower value for BFME. This result could be related to the presence of a higher fiber content in mango, specifically insoluble dietary fiber (IDF), as suggested by Brennan et al. [83] in their research. Given that the IDF content in mango pulp is 4.35%, as reported by Madalageri, Bharati, and Kage [84], and is undetectable in passion fruit pulp according to Adeyeye and Aremu [85], the observed reduction in BFME can be attributed to this difference. A higher presence of IDF is linked to increased material plasticization and weaker gelatinization, hence lower EI, as reported by Leonard et al. [86]. A negative Pearson correlation (−0.731, p < 0.05) was identified between EI and ρb; this finding is explained by the fact that as EI increases, ρb tends to decrease [2, 87]. As a more expanded product has a higher apparent volume for the same mass, this causes a decrease in the quotient represented by the ρb value. This inverse correlation pattern between EI and ρb has been previously reported in other studies [51, 60].
ρb shows no significant differences among its samples (p > 0.05). However, it has a significant negative Pearson correlation (−0.586, p < 0.05) with ε. This difference is associated with a desired and expected attribute in expanded snacks, as an increase in pores leads to an increase in product volume, which in turn results in a lower ρb value.
With regard to WSI (p > 0.05), no significant differences were observed between BFE and BFME. However, in the case of BF and the extruded samples, significant differences were identified (p < 0.05). This is due to the fact that extrusion cooking results in the degradation of starch, which in turn increases the amount of soluble molecules, thereby leading to an increase in WSI value in the extrudates [88].
Significant differences (p < 0.05) were observed between the enriched extrudates BFPE and BFME. In contrast, no significant differences were found in the samples after the addition of pulps with respect to Hy. It is noteworthy that extruded products exhibit a high degree of Hy, which affects their texture when exposed to a humid atmosphere [89].
Moreover, hardness may be related to the fiber content present in the mixture, meaning that higher concentration could increase hardness and decrease crispy texture, as fiber interferes with air bubble formation and increases cell wall thickness [90]. This finding aligns with the results reported by Medina et al. [91] where the addition of mango peel increased the hardness of the extrudate, particularly at high fiber content.
The hardness values for the extrudates are consistent with those reported by Lucas et al. [92] for rice and corn extrudates enriched with açaí pulp and Lucas et al. [62] for rice and corn extrudates enriched with spirulina. Moreover, the present findings are consistent with those by Oliveira, Alencar, and Steel [93], who studied the crispiness of rice and corn extrudates with whole wheat and corn flour enriched with jabuticaba peel powder (Myrciaria cauliflora).
3.4. Extrusion Cooking’s Effect on Bioactive Compounds’ Content on In Vitro Bioaccessibility in Mixtures and Extrudates
The expression of bioactivity in phenolic compounds requires, in the initial stage, the liberation of these compounds from the food matrix and solubilization following the digestive process. This can be achieved through the application of in vitro gastrointestinal models, which permits the faithful replication of the in vivo physiological environment and facilitate the critical examination of the interactions between phenolic compounds and the complexities of digestion [94].
The extrusion process has been highlighted as a treatment alternative for foods, improving their bioaccessibility—it means the body’s capacity to assimilate the compounds [33, 95]. In this context, Table 4 presents the content of bioactive compounds in the blends and extrudates after in vitro digestion, as determined by the in vitro digestion model of the present research. Regarding the blends, it was evident that the enrichment with fruit pulps had a positive effect on both the flours and the extrudates. Specifically, BFPM exhibited a 1.532-fold increase in TPC, while BFMM demonstrated a 5.461% increase in contrast to BF.
| Mixtures | Extrudates | |||||
|---|---|---|---|---|---|---|
| BF | BFPM | BFMM | BFE | BFPE | BFME | |
| TPC [mg gallic acid equivalent/100 g] | 408.670 ± 15.255de | 1032.262 ± 52.770a | 434.171 ± 42.454d | 329.054 ± 21.050e | 596.550 ± 5.624c | 704.511 ± 6.129b |
| TF [mg quercetin/100 g] | 80.296 ± 2.182c | 181.240 ± 3.561a | 165.396 ± 12.120ab | 162.353 ± 0.371b | 59.310 ± 2.849d | 68.890 ± 2.355cd |
| TC [mg β-carotene/100 g] | n.d | n.d | n.d | n.d | 0.151 ± 0.019b | 1.0910 ± 0.074a |
| AA DPPH [μmol Trolox equivalent/100 g] | 517.045 ± 0.874c | 523.352 ± 9.798bc | 585.515 ± 15.363a | 514.948 ± 12.411bc | 202.290 ± 3.712d | 255.330 ± 6.743d |
| AA ABTS [μmol Trolox equivalent/100 g] | 888.065 ± 4.934a | 858.789 ± 18.131a | 888.64 ± 32.45a | 533.706 ± 4.642b | 328.300 ± 6.047c | 462.240 ± 6.551b |
- Note: Means ± standard deviation. The values that do not share the same lowercase letter in a row indicate significant changes between the samples, according to the Fisher test (p < 0.05) when comparing samples in the mixtures or extrudates. All of the results were expressed on a dry basis.
- Abbreviations: BF: green banana flour; BFE: green banana flour extrudate; BFME: green banana flour + mango extrudate; BFMM: green banana flour + mango mixture; BFPE: green banana flour + passion fruit extrudate; BFPM: green banana flour + passion fruit mixture; n.d.: not detected.
In TPC, statistically significant differences (p < 0.05) were observed among the samples, both before and after the extrusion process, with the exception of BF with BFE and BF with BFME. Furthermore, TF records significant differences (p < 0.05) after extrusion in all samples, showing an increase in flavonoid content, as seen in BF with a value of 80.296 [mg quercetin/100 g], and its extrudate BFE increased its TF content by 1.2 times, reaching 162.353 [mg quercetin/100 g], respectively. This finding contrasts with the previous results obtained prior to the digestion process, suggesting a possible dissociation of the flavones. These results align with those of previous research, such as that conducted by Herrera et al. [96] on corn starch samples enriched with mango bagasse.
In the case of TC, significant differences (p < 0.05) are observed in the digests of enriched samples BFPE and BFME, as they were the only ones that showed detection of this bioactive compound. Tonyali, Sensoy, and Karakaya [97] reported increases in the bioaccessibility of carotenoids in tomatoes when subjected to pretreatments with heating and exposed to high temperatures. This could be attributed to the breakdown of fiber compounds in the cell wall structure, thus increasing the accessibility of carotenoids at the end of the in vitro digestion process. Considering the decrease in TPC in the extruded samples after in vitro digestion, it was expected that there would be no significant increase in AA measured by DPPH, especially in the enriched samples. Additionally, AA measurements by ABTS reveal significant variations (p < 0.05) among all samples. The extruded samples exhibited elevated AA, which may be attributed to the presence of bioactive compounds, such as flavonoids.
Figure 2 illustrates the mean values and standard deviations of the bioaccessibility of TP, TC, and TF in flours and extrudates. It can be observed that in BF, the TPC does not show significant differences (p > 0.05) following extrusion. However, in blends and extrudates enriched with fruit pulps, extrusion generated significant differences (p < 0.05) for TPC, with the most pronounced increase in bioaccessibility observed in BFME with a value of 72%. In this regard, the bioaccessibility value of TPC for the blends ranged between 18% and 30%, and for the extrudates, it ranged between 40% and 70%. These values are comparable to those reported by Zeng et al. [94] for blends and extrudates of whole rice, wheat, and oats.
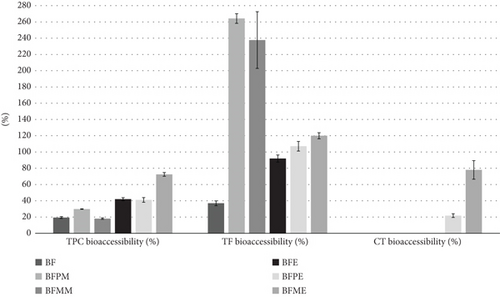
The bioaccessibility values for TF were 37% and 92% for BF and BFE, respectively, indicating an increase in bioaccessibility after the extrusion process. Moreover, both enriched blends and their extruded counterparts exhibited accessibility levels exceeding 100%. This enhanced bioaccessibility may be attributed to the nondissociation of flavonoids, which are bound components released during digestion. Similar results were reported by Duque et al. [98], who investigated olive leaf extracts and found that quercetin had a bioaccessibility of 108%. Similarly, another study conducted by Gomez et al. [99] examined various types of tea and observed a significant increase in flavonoid content after biodigestion, with biodigestibility values reaching 180% and 167% for oolong and oolong milk, respectively. Furthermore, it has addressed potential interactions between flavonoids and food matrices, as well as high molecular weight molecules such as simple carbohydrates, which may interfere with predigestion detection [98].
With regard to TC, significant differences (p < 0.05) are observed among all samples, with a notable increase in bioaccessibility in BFME. Tonyali, Sensoy, and Karakaya [97] propose that the temperature and shear during extrusion generate a cell-breaking effect, thereby facilitating greater accessibility of TC.
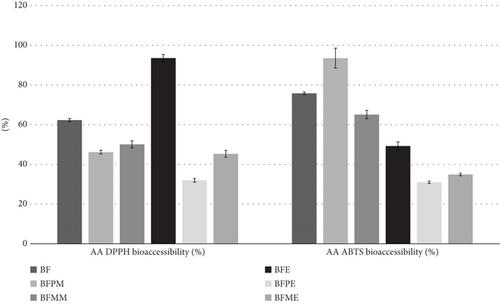
Figure 3 presents the percentage bioaccessibility of AA determined by both DPPH and ABTS, with bioaccessibility ranges found between 32%–94% and 30%–75%, respectively. With regard to AA, the highest value was obtained through DPPH by BFE, while for ABTS, the digestion of its raw material BFM (green banana flour + mango) yielded the highest value. With the exception of BFE and its AA through the DPPH method, extrusion significantly reduced the AA content of the extruded samples. This reduction may be attributed to the breakdown of bound phenols into lower molecular weight compounds, which could affect the antioxidant capacity and result in the observed reductions in the absorption of ABTS and DPPH [96].
4. Conclusions
The incorporation of fruit pulps into green BF significantly enhances the content of bioactive compounds and AA in both blends and extrudates. The novel gluten-free formulation developed in this study is a ready-to-eat product with increased levels of bioactive compounds and dietary fiber, thus demonstrating that surplus green bananas can be valorized as a valuable resource for producing healthy, functional snacks. The extrusion cooking process facilitates the release and solubilization of bioactive compounds, thereby improving their bioaccessibility during in vitro digestion. This demonstrates the efficacy of the technology in enhancing the starch matrix of green bananas. Furthermore, the incorporation of fruit pulps, even at low concentrations, enhances the sensory, textural, and functional properties of the extrudate. Despite a reduction in the absolute number of bioactive compounds during extrusion, their improved bioaccessibility suggests promising avenues for future research and process optimization.
Disclosure
The funding source, the Government of Colombia, as well as any government official or other person or organization had no role in the commissioning, conception, planning, design, execution or analysis, preparation or editing of the manuscript, or the decision to publish.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Luisa Fernanda Sarmiento-Torres: conceptualization, formal analysis, investigation, visualization, validation, writing—original draft. Sarha Lucía Murrillo-Franco: formal analysis, investigation, visualization, writing—original draft. Juan David Galvis-Nieto: investigation, writing—review & editing. L. Joana Rodríguez: conceptualization, supervision, review & editing. Marta Igual: conceptualization, formal analysis, validation, investigation writing—review & editing. Purificación García-Segovia: conceptualization, resources, supervision, writing—review & editing. Carlos E. Orrego: conceptualization, resources, supervision, project administration, writing—review & editing.
Funding
This research was funded by the Ministry of Science, Technology, and Innovation of Colombia, Minciencias, Grant Number Convocatoria 935-2023, Orchids Program, Women in Science: Agents for Peace, Contract Number: 271-2023, with the project titled “Strategy to Reduce the Environmental Footprint as a Contribution to the Challenge of Ending All Forms of Violence in Colombia: Production of Dried Fruits”, and it is funded with resources from the National Fund for Science, Technology, and Innovation, Francisco José de Caldas Fund.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




