Bioaccessibility and Speciation of Iron from Aqueous Extracts of Moringa oleifera Leaves
Abstract
Investigating the different chemical species of soluble iron in food digests provides more relevant information on the nutritional potential of an iron-rich food. The objective of this study was to assess the bioaccessibility and speciation of iron from various aqueous extracts of Moringa (Moringa oleifera) leaves. Aqueous extracts were prepared from fresh and dried Moringa leaves using infusion and decoction methods. Spectrophotometric assays were performed to quantify inhibitors and enhancers of iron absorption in the extracts, bioaccessible iron, and its different chemical species. The highest contents of inhibitors (239.43 mg/L for polyphenols and 2.92 mg/L for phytates) and enhancers of iron absorption (1.58 mmol/L for carotenoids and 488.00 mg/L for ascorbic acid) were found in the 5-minute decoction extract of fresh leaves, and the lowest in all infusion extracts (27.34 mg/L for polyphenols, 0.50 mg/L for phytates, 0.15 mmol/L for carotenoids, and 86.00 mg/L for ascorbic acid). The percentages of bioaccessible iron were higher for decoction extracts (42.57–52.70%) compared to infusion extracts (33.89–36.44%). Ferrous iron was the dominant inorganic species of bioaccessible iron and was more concentrated in the digests of decoction extracts (1.32–4.85 mg/L). The highest content of organic iron (5.33 mg/L) was found in the digest of the 8-minute decoction extract of dried leaves. Drinking decoction extracts of fresh and dried Moringa leaves could be recommended to alleviate iron deficiency in vulnerable groups of the population living in areas where this plant can grow.
1. Introduction
Iron deficiency represents a major public health burden in developing countries [1]. It affects nearly 2.15 billion people worldwide, mainly women from adolescence onwards, infants, and young children [2]. A recent meta-analysis of population studies estimated the pooled prevalence of anaemia among pregnant women in sub-Saharan Africa to be 35.6% [3]. In Cameroon, approximately 57% of children aged 6–59 months and 40% of women aged 15 to 49 years suffer from anaemia [4]. The consequences of iron deficiency on individual health are numerous and well-documented. These include stunted growth, lower intellectual performance, maternal, foetal, and neonatal mortality, decreased physical capacity and productivity, and decreased immune competence [5–7].
Various strategies have been recommended to combat iron deficiency and anaemia. A great emphasis is generally put on iron fortification and supplementation [8, 9]. However, these techniques are expensive, inaccessible to disadvantaged populations, and carry risks of toxicity [8–10]. Among the more recommended approaches to prevent iron deficiency in low-income countries is the consumption of affordable local foods rich in this trace element [11].
Moringa (Moringa oleifera) is a tree whose leaves are particularly rich in iron and enhancers of its intestinal absorption (ascorbic acid and carotenoids) [12–14]. The use of Moringa leaf powder for improving the iron content of food products and dishes has been reported [15, 16]. Consuming aqueous extracts of Moringa leaves could be a simpler and more direct way to valorise their nutritional potential. Previous studies revealed that these extract to be also rich in iron [17, 18]. But no emphasis was put on bioaccessibility, which refers to the fraction of a nutrient released from a food matrix in the gastrointestinal tract and available for absorption [19]. Also, iron speciation studies that identify and quantify the different iron species in digests of Moringa leaf extracts are still lacking. Speciation studies on soluble iron in food digests provide more relevant information on the nutritional potential of an iron-rich food. There is evidence demonstrating that ferrous iron (Fe2+) and iron complexed to small soluble organic molecules are better absorbed in the gastrointestinal tract [20–22].
The aim of this study was to generate more insights into the nutritional potential of Moringa oleifera by assessing the bioaccessibility and speciation of iron from various aqueous extracts of this plant’s leaves.
2. Materials and Methods
2.1. Biological Material
Mature Moringa (Moringa oleifera) leaves were harvested from a home garden. Part of these leaves was immediately used for the preparation of extracts from fresh leaves, while the other part was shade-dried for five days at room temperatures ranging from 22°C to 30°C. The difference between the fresh and dry weights of a precise quantity of leaves was calculated and used to determine the water proportion in fresh leaves, found to be around 70%. This information was used to consider 1 g of dried leaves to correspond to 3 g of fresh leaves, on a dry matter basis.
2.2. Preparation of Aqueous Extracts
2.2.1. Infusions
One hundred millilitres (100 mL) of boiling deionised water were added to 3 g of fresh leaves or 1 g of dried leaves of Moringa. The mixture was left to cool at room temperature for 15 minutes, stirring every 3 minutes. Extracts of fresh and dried Moringa leaves were obtained by filtration using a Whatman filter paper, grade 1.
2.2.2. Decoctions
One hundred millilitres (100 mL) of deionised water were added to 3 g of fresh leaves or 1 g of dried leaves in a heat-resistant glass container. The mixture of water and leaves was covered and boiled for 5 or 8 minutes. The decoction was left to cool at room temperature for 20 minutes. The extracts were obtained by filtering the mixture using Whatman filter paper, grade 1.
All the various extracts (described in Table 1) were stored under 0°C until further analyses.
| Extracts | Type of leaves | Quantity of leaves (g) | Type of extraction |
|---|---|---|---|
| FI | Fresh | 3 | Infusion |
| DI | Dried | 1 | Infusion |
| FD1 | Fresh | 3 | Decoction (5 min) |
| FD2 | Fresh | 3 | Decoction (8 min) |
| DD1 | Dried | 1 | Decoction (5 min) |
| DD2 | Dried | 1 | Decoction (8 min) |
- FI: fresh leaves infusion; DI: dried leaves infusion; FD1: fresh leaves decoction (5 min); FD2: fresh leaves decoction (8 min); DD1: dried leaves decoction (5 min); DD2: dried leaves decoction (8 min).
2.3. Quantification of Inhibitors of Iron Absorption in Extracts
2.3.1. Phytates
The phytate content of the studied extracts was determined using the method described by Bhandari and Kawabata [23] with slight modifications. In a test tube, 1 mL of extract diluted 10-fold with 2.4% HCl, 1 mL of distilled water and 1 mL of Wade’s reagent were introduced and vortexed for 5 seconds, and then centrifuged at 1000 g for 10 minutes. The absorbance was read at 500 nm, using a UV-Vis spectrophotometer (PRIM light 5551, France). A standard curve of phytic acid (0–40 mg/L, R2 = 0.96) was used to calculate the concentration of phytates in extracts.
2.3.2. Polyphenols
Polyphenols were quantified using the method described by Shi et al. [24]. In a test tube, 0.1 mL of extract, 7.9 mL of distilled water, and 0.5 mL of Folin–Ciocalteu reagent were introduced. The mixture was left to stand for 10 minutes. Then, 1.5 mL of sodium carbonate (20%) was added. The mixture was left stand for 1 hour in the dark at room temperature and the absorbance was read at 765 nm. A standard curve of gallic acid (0.0–0.5 mg/mL, R2 = 0.99) was used to calculate the concentration of polyphenols in extracts.
2.4. Quantification of Enhancers of Iron Absorption in Extracts
2.4.1. Ascorbic Acid
Ascorbic acid was measured according to the method described by Davies and Masten [25]. One millilitre (1 mL) of extract, 1 mL of EDTA 1 mmol/L and 1 mL of 2.6-dichlorophenolindophenol 1.7 mmol/L were introduced into a test tube and vortexed. EDTA was used to chelate iron and prevent the oxidation of ascorbic acid. The absorbance was immediately read at 520 nm. A standard curve of ascorbic acid (0–10 μg/mL, R2 = 0.90) was used to calculate the concentration of ascorbic acid in extracts.
2.4.2. Carotenoids
Carotenoids were measured using the method described by De Carvalho et al. [26]. In a test tube, 2 mL of extract and 3 mL of solvent (hexane: acetone, 3 : 2 Volume/Volume) were added. The mixture was vortexed for 3 minutes and left to settle for 1 hour. Carotenoids were quantified in the organic phase (supernatant) by reading the absorbance at 450 nm and using the molar extinction coefficient 2500 mol−1·cm−1. L.
2.5. Quantification of Total Iron in Extracts
Total iron content in extracts was determined by colorimetry using potassium thiocyanate (KSCN) according to the method described by Pauwel et al. [27]. This method is based on the principle that total iron in a mineralised and solubilised sample is oxidised by hydrogen peroxide in acidic conditions to ferric iron. Ferric iron ions are revealed by a solution of potassium thiocyanate (KSCN) through the formation of a red complex which has a maximum absorbance at 420 nm. The extracts were calcined in a muffle furnace at 450°C, and then dissolved in 1 N nitric acid. The digested solutions were centrifuged at 1800 g for 10 minutes. Five millilitres (5 mL) of supernatant were introduced into a test tube, and then few drops of hydrogen peroxide and 1 mL of KSCN were added. After 10 minutes of incubation, absorbance was read at 420 nm. A standard curve of ferric iron (0–10 ppm, R2 = 0.99) was used to calculate the total iron concentration in extracts.
2.6. Determination of the Bioaccessibility of Iron
The bioaccessibility of iron from the studied extracts was determined using the method described by McGee and Diosady [28]. Three millilitres (3 mL) of extracts were introduced in a glassware previously rinsed with deionised water. The pH was adjusted to 2 with a solution of concentrated HCl (2.4%). The solution was then incubated at 37°C for 2 hours under regular moderate shaking. The pH was then adjusted to 7 with NaOH (0.1 mol/L) and the solution was incubated at 37°C for 2 hours under regular moderate shaking. Digests were finally centrifuged at 1400 g for 15 minutes and the supernatants were kept below 0°C until further analyses.
The quantification of iron-polyphenol complex was carried out directly in the supernatants of digests. Polyphenols can chelate iron to form a nonabsorbable complex in the gastrointestinal tract. This complex has a maximum absorption at 565 nm for neutral solutions (pH 7) [29]. A standard curve for iron-gallic acid complex (0–0.5 mmol/L, R2 = 0.88) was used to calculate the concentration of iron-polyphenol complex in the supernatants of digests.
2.7. Study of Iron Speciation
The iron speciation study was carried out using the method described by da Silva et al. [30], and modified by Mawouma et al. [31]. This method is based on the principle that ferric iron, in the presence of hydroxylamine hydrochloride, is reduced to ferrous iron which form a stable-coloured complex (red orange) with 1-10-phenanthroline having a maximal absorbance at 510 nm.
Two millilitres (2 mL) of digest were mixed with 0.16 mL of sodium nitrite (0.39%), 1 mL of protein precipitating solution (5 g TCA + 5 mL of concentrated HCl for 50 mL of solution) and 6.84 mL of deionised water. The mixture was incubated at 100°C for 10 minutes, and then centrifuged at 1400 g for 15 minutes.
2.7.1. Quantification of Inorganic Iron
Two millilitres (2 mL) of supernatant were introduced into a test tube. Then 1 mL of hydroxylamine hydrochloride (1%) and 1 mL of 1,10-phenanthroline (0.25%) were added. The homogenised mixture was allowed to stand for 15 minutes, then the absorbance was read at 510 nm. A standard curve (0–5 mg/L of FeSO4 solutions, R2 = 0.99) was used to calculate the concentration of total ferrous iron, corresponding to the total inorganic iron found in the samples.
2.7.2. Quantification of Ferrous Iron
Two millilitres (2 mL) of supernatant were introduced into a test tube. Then 1 mL of 1,10-phenanthroline (0.25%) was added. The homogenised mixture was left to stand for 15 minutes, then the absorbance was read at 510 nm. The same standard curve (0–5 mg/L of FeSO4 solutions, R2 = 0.99) was used to calculate the concentration of ferrous iron in the samples.
2.7.3. Determination of Organic and Ferric Iron Contents
2.8. Statistical Analyses
Analyses were done in triplicates and the data were reported as mean ± standard deviation. Differences between means were revealed via post hoc Duncan’s multiple$ range test (p < 0.05).
The principal component analysis (PCA) was performed to investigate relationships among the studied parameters.
3. Results and Discussion
3.1. Contents of Inhibitors of Iron Absorption in Extracts
The contents of inhibitors of iron absorption in aqueous extracts of Moringa leaves are shown in Table 2. Phytates and polyphenols were less concentrated (p < 0.05) in all extracts obtained by infusion, and in the extract obtained by 5-minute decoction of dried leaves. This could be explained by the short extraction time applied to these samples, and the rapid cooling of the solvent, unfavourable to the diffusion of phytochemicals in solution. Also, 5 minutes would have not been enough to allow the rehydration of dried leaves, and a subsequent diffusion of phytochemicals in the extraction solvent.
| Extracts | Number of replicates | Polyphenols | Phytates |
|---|---|---|---|
| FI | 3 | 32.43a± 0.51 | 0.50a ± 0.13 |
| DI | 3 | 27.34a ± 0.26 | 0.60a ± 0.01 |
| FD1 | 3 | 239.43d ± 20.25 | 2.92d ± 0.04 |
| FD2 | 3 | 176.12c ± 3.51 | 1.60b ± 0.70 |
| DD1 | 3 | 40.46a ± 0.64 | 0.38a ± 0.12 |
| DD2 | 3 | 152.70b ± 2.57 | 2.25c ± 0.15 |
- FI: fresh leaves infusion; DI: dried leaves infusion; FD1: fresh leaves decoction (5 min); FD2: fresh leaves decoction (8 min); DD1: dried leaves decoction (5 min); DD2: dried leaves decoction (8 min). Mean values in the same column with different superscript letters are significantly different (p < 0.05).
Extracts obtained by decoction of fresh leaves exhibited the highest (p < 0.05) polyphenols and phytates contents. This could be attributed to the promoting effect of heat on the diffusion of phytochemicals in the extraction solvent. The yield of aqueous extractions increases with temperature, because of an important diffusion of molecules caused by the disruption of cells [32, 33]. However, a longer extraction duration (8 minutes) resulted in a decrease (p < 0.05) of these contents. This decrease could be explained by the thermolability of the studied phytochemicals, as they are sensitive to heat [33]. In longer decoction duration of fresh leaves, the thermolabile effect of heat would have taken advantage on its promoting effect on the diffusion of polyphenols and phytates in the extraction solvent. In contrast, dried leaves could require a rehydration period before the start of the diffusion, explaining the significant increase of phytates and polyphenols contents with a longer decoction duration (8 minutes).
The polyphenol contents of decoction extracts (4.05–15.27 mg/g of dried leaves) are lower than those obtained by Bui-Phuc et al. for an aqueous extract of Moringa leaf powder (23.9 mg/g of powder) [34]. The difference may be due to the initial polyphenols content of the leaves used, and the drying processing methods which are not the same. Also, the leaves we used for preparing our extracts were entire and not ground, as it is the case in the study of Bui-Phuc et al. [34]. This could explain the limited diffusion of polyphenols and other phytochemicals observed in our study. Coz-Bolaños et al. obtained total soluble phenols contents ranging from 243.3 to 292.4 mg/L for infusions, and from 241.2 to 251.7 mg/L for decoctions. It can be noticed that these authors also used Moringa leaf powder in their experiment [35]. Size reduction of a plant material prior to extraction increases the surface area, which in turn enhances the mass transfert of soluble compounds from the plant material to the solvent [36].
3.2. Contents of Enhancers of Iron Absorption in Extracts
Table 3 displays the contents of enhancers of iron absorption in various aqueous extracts of Moringa leaves. The carotenoids and ascorbic acid contents of decoction extracts were higher (p < 0.05) compared to those of infusion extracts. These phytochemicals were more concentrated in extracts obtained by decoction of fresh leaves. Bui-Phuc et al. demonstrated that increasing extraction temperature enhanced the solubility of molecules in the solvent, thereby facilitating the extraction process [34]. Despite the thermolability of ascorbic acid and carotenoids, the promoting effect of heat on their diffusion in the extraction solvent would have been important, so that the remaining quantity after thermal destruction were still higher than the concentrations observed for infusion. The contents of carotenoids and ascorbic acid decreased (p < 0.05) with a longer extraction duration (8 minutes). Fratianni et al. reported that heating can result in a differential reduction in carotenoids concentration, depending on the duration and heating temperature [37].
| Extracts | Number of replicates | Carotenoids (mmol/L) | Ascorbic acid (mg/L) |
|---|---|---|---|
| FI | 3 | 0.07a ± 0.01 | 120.67a ± 3.51 |
| DI | 3 | 0.07a ± 0.02 | 73.67a ± 12.50 |
| FD1 | 3 | 1.33c ± 0.06 | 830.00c ± 50.00 |
| FD2 | 3 | 1.09b ± 0.18 | 471.67b ± 14.43 |
| DD1 | 3 | 0.05a ± 0.00 | 86.00a ± 5.00 |
| DD2 | 3 | 1.32c ± 0.09 | 471.66b ± 38.19 |
- FI: fresh leaves infusion; DI: dried leaves infusion; FD1: fresh leaves decoction (5 min); FD2: fresh leaves decoction (8 min); DD1: dried leaves decoction (5 min); DD2: dried leaves decoction (8 min). Mean values in the same column with different superscript letters are significantly different (p < 0.05).
As far as dried leaves are concerned, there was an increase of carotenoids and ascorbic acid contents with a longer decoction duration. This result is similar to what was observed with phytates and polyphenols and could be explained by the same reasons.
The ascorbic acid content of infusion extract from dried Moringa leaves (73.67 mg/L) is slightly higher compared to the one obtained by Madukwe et al. (62.6 mg/L) for an infusion$ extract of Moringa leaf powder [38]. The initial ascorbic acid content of the leaves and the longer infusion duration used by these authors (30 minutes) could explain this difference.
3.3. Bioaccessibility of Iron in Extracts
Table 4 displays the total iron contents and its bioaccessibility in the studied extracts. The total iron contents of decoction extracts were higher (p < 0.05) compared to those of infusion extracts. In the latter, these contents ranged from 3.16 to 4.75 mg/L, with no significant difference (p > 0.05). Focusing on decoction extracts, iron contents were higher (p < 0.05) in extracts from dried leaves compared to extracts from fresh leaves. In both cases, the iron contents significantly increased with a longer decoction duration (8 minutes). Also here, the promoting effect of heat would have enhanced the solubilisation of iron in the extraction solvent. The thermostability of iron could explained the absence of a decrease in its concentration with a longer decoction duration.
| Extracts | Number of replicates | Total iron (mg/L) | Bioaccessible iron (%) |
|---|---|---|---|
| FI | 3 | 4.75 ± 2.82 | 36.64a ± 0.13 |
| DI | 3 | 3.16a ± 1.94 | 33.89a ± 4.96 |
| FD1 | 3 | 12.93b ± 6.75 | 52.70c ± 11.54 |
| FD2 | 3 | 14.77b ± 8.85 | 49.07bc ± 0.29 |
| DD1 | 3 | 11.27b ± 0.97 | 49.49bc ± 0.04 |
| DD2 | 3 | 29.37c ± 3.77 | 42.57ab ± 0.00 |
- FI: fresh leaves infusion; DI: dried leaves infusion; FD1: fresh leaves decoction (5 min); FD2: fresh leaves decoction (8 min); DD1: dried leaves decoction (5 min); DD2: dried leaves decoction (8 min). Mean values in the same column with different superscript letters are significantly different (p < 0.05).
Regarding the bioaccessibility of iron, extracts obtained by decoction exhibited higher percentages of bioaccessible iron (p < 0.05) compared to those obtained by infusion which did not show a significant difference (p > 0.05). The low bioaccessibility of iron in extracts obtained by infusion could be attributed to their lower contents of enhancers of iron solubility and absorption (ascorbic acid and carotenoids). Extracts obtained by decoction were richer in bioaccessible iron, as they were richer in ascorbic acids and carotenoids. Ascorbic acid promotes the bioaccessibility of iron either by forming a soluble absorbable complex with iron, or by reducing Fe3+ to Fe2+ (better absorbed) [39]. The enhancing mechanism of carotenoids is not well elucidated. However, the formation of a soluble complex with iron has been suggested [40].
3.4. Speciation of Bioaccessible Iron
The contents of inorganic iron, ferrous iron (Fe2+), ferric iron (Fe3+), and organic iron are presented in Table 5. The contents of inorganic iron in the digest of extracts obtained by decoction were significantly higher (p < 0.05) than those of extracts obtained by infusion. Also, the contents of inorganic iron were higher (p < 0.05) than those of organic iron. Inorganic iron refers to the sum of ferrous iron (Fe2+) and ferric iron (Fe3+). The contents of Fe2+ were higher in the digests of extracts obtained by decoction. This could be attributed to the higher contents of ascorbic acid (with a reducing action) in these extracts.
| Digestates | Number of replicates | Inorganic iron (mg/L) | Fe2+ (mg/L) | Fe3+ (mg/L) | Organic iron (mg/L) |
|---|---|---|---|---|---|
| FI | 3 | 1.09a ± 0.12 | 0.44a ± 0.26 | 0.65a ± 0.14 | 0.17a ± 0.10 |
| DI | 3 | 0.36a ± 0.12 | 0.26a ± 0.09 | 0.10a ± 0.03 | 0.42a ± 0.11 |
| FD1 | 3 | 1.81c ± 0.60 | 1.32ab ± 0.44 | 0.49a ± 0.16 | 2.60a ± 0.61 |
| FD2 | 3 | 5.05a ± 0.91 | 2.20bc ± 0.44 | 2.84b ± 0.49 | 0.22b ± 0.19 |
| DD1 | 3 | 3.87b ± 0.48 | 3.26c ± 0.44 | 0.60a ± 0.39 | 1.76b ± 0.01 |
| DD2 | 3 | 7.25d ± 2.41 | 5.33c ± 0.80 | 2.40b ± 1.09 | 5.33c ± 0.80 |
- FI: fresh leaves infusion; DI: dried leaves infusion; FD1: fresh leaves decoction (5 min); FD2: fresh leaves decoction (8 min); DD1: dried leaves decoction (5 min); DD2: dried leaves decoction (8 min). Mean values in the same column with different superscript letters are significantly different (p < 0.05).
As far as the contents of organic iron in the digests are concerned, they were higher (p < 0.05) in extracts obtained by decoction. Organic iron could have been generated by the chelation of iron by polyphenols, ascorbic acid, or carotenoids [29, 39, 40].
3.5. Principal Components Analysis (PCA) of Experimental Data
A principal component analysis was carried out to visualise the relationships between the different extracts and the studied variables. The eigenvalues were 7.50, 1.74, 1.03, 0.73, and 0.01 for factors F1 to F5, respectively. The first two principal components or factors (F1 and F2) together explained 83.95% of the total original variance in the data set. A biplot projection of the observations (extracts) and measured variables on the plane defined by F1 and F2 is shown in Figure 1.
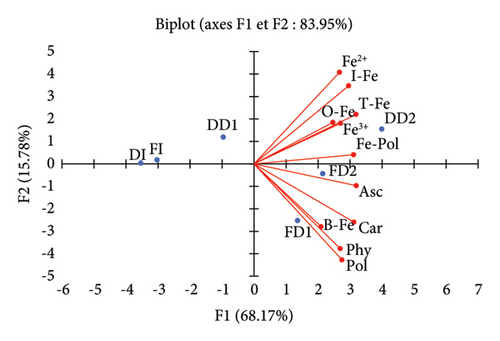
The first principal component, F1, which explained 68.17% of the total experimental variability, clustered most of the studied variables. The upper part of the axis aggregated iron species, while the lower part aggregated mainly modulators of iron absorption and bioaccessible iron. The location of extracts obtained by a longer decoction duration is in accordance with their richness in modulators of iron absorption, and bioaccessible iron. There is an apparent paradoxical positive correlation between bioaccessible iron and inhibitors of iron absorption (phytates and polyphenols). The important diffusion of both enhancers and inhibitors of iron absorption during decoction could explain this aggregation. Carotenoids are closer to bioaccessible iron, compared to ascorbic acid, suggesting a greater effect on the solubilisation of the iron pool of fresh leaves-decoction extracts. Previous studies reported carotenoids to be more thermostable than ascorbic acid [41, 42], suggesting a greater enhancing effect on iron bioaccessibility. According to the biplot, both carotenoids and ascorbic acid were not related to bioaccessible iron species. This contradicts the known chelating or reducing effect of these phytochemicals on iron. However, ascorbic acid and carotenoids could spatially better aggregate with iron species if other principal components (F3, F4, and F5) were considered.
It can be seen from Figure 1 that both inhibitors and enhancers of iron absorption aggregated with bioaccessible iron. Ascorbic acid and carotenoids have the advantage of being efficient as iron solubility enhancers, even at high pH and in the presence of significant amounts of antinutritional factors such as phytates and polyphenols [40, 43, 44].
The isolated aggregation of extracts FI, DI, and DD1 by the second principal component F2 is of interest. Indeed, these samples displayed the lowest concentrations of the assessed phytochemicals, clearly indicating that the infusion conditions used in our study were inappropriate for gaining advantage of the iron content of Moringa leaves.
4. Conclusion
The objective of this work was to investigate the bioaccessibility and speciation of iron from different aqueous extracts of Moringa leaves. Findings revealed that decoction allowed a greater diffusion of iron and modulators of its absorption in the extraction solvent, resulting in a more important iron bioaccessibility. The digests of decoction extracts were richer in iron species and better absorbed in the gastrointestinal tract. Drinking decoctions of fresh or dried Moringa leaves could be an effective and less expensive way of combatting iron deficiency in regions of the globe where Moringa is grown.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The authors express their sincere gratitude to Mr. Ayouba Toumba, a freelance editor and translator, for his assistance in the preparation of the manuscript and copyediting.
Open Research
Data Availability
All the data are presented in the manuscript.




