Negative Effects of Cx43 during Denervated Skeletal Muscle Atrophy Can Be Attenuated by Blueberry Extracts via Regulating Akt Pathway
Abstract
The lack of effective treatments for alleviating irreversible skeletal muscle atrophy caused by peripheral nerve dysfunction is a clinical problem. To identify therapeutic targets, signaling pathways in muscle cells activated by denervation have been explored, including the important roles of Cx43, a kind of protein that forms gap junctions, in other types of pathological process. The present study investigated the negative role of Cx43 in aggravated muscle cell degeneration following denervation. The blueberry extract was applied orally on mice models, and its protective effect against denervated skeletal muscle atrophy was found by wet weight measurement and tissue staining observation. Cx43/Akt pathway in denervated muscle cells was modulated by blueberry extract. In conclusion, the negative effects of Cx43 on denervated skeletal muscle atrophy were attenuated by blueberry extract by regulating the Akt pathway.
1. Introduction
Denervated skeletal muscle atrophy is characterized by an irreversible decrease in skeletal muscle mass due to pathological changes in dominant nerves [1]. It can be one of the most severe sequelae of peripheral nerve diseases such as injury, neuritis, and neuroma. Although surgical techniques for nerve repair have greatly improved a lot now, there is still no satisfactory treatment that can be applied to alleviate targeted muscle atrophy.
Members of the connexin family of proteins that form gap junctions in vertebrates are recently found to be also important for skeletal muscle cells [2, 3]. Among connexins, Cx43 is considered to affect injured skeletal muscle cells and has more negative but key effects on skeletal muscle regeneration. Previous studies have reported that decreased Cx43 expression can protect skeletal muscle tissue from pathological conditions such as Duchenne muscular dystrophy [4, 5]. In denervation-induced muscle atrophy, Cx43 is expressed in fast muscle cells [6].
To identify possible therapeutic targets, previous studies have explored several signaling pathways involved in denervation-induced skeletal muscle degeneration. Among numerous signaling pathways, Akt-related pathways have been identified. Akt, a classic muscle cell regulator, is helpful for skeletal muscle growth [7, 8], and its activation helps alleviate and prevent muscle atrophy caused by sepsis [9], starvation [10], myocardial infarction [11], and chronic kidney disease [12]. Transcriptome sequencing and analysis results from Chen et al. [13] showed that Akt was also downregulated in denervated skeletal muscle cells. Recent studies have reported an interaction between Cx43 and Akt pathways in cardiac muscles [14, 15]. However, no study has explored the relationship between Cx43 and Akt in denervated skeletal muscle atrophy model.
It is widely accepted that blueberry has a strong antioxidant function, and its protective role in skeletal muscles has been reported recently [16, 17]. Additionally, in the study of Miyazaki et al. [18], blueberry diet prevented increased connexin 43 expression in bladder outlet obstruction model. Also, the protective role of blueberries is related to the Akt pathway in other pathological models [19, 20]. However, these relationships have not been reported in denervated muscle atrophy model. In this study, we verified the negative effects of Cx43 on denervation-induced skeletal muscle atrophy, which can be attenuated by blueberry extracts. Also, we explored the mechanisms behind the protective effect of blueberry extracts, which is achieved via regulating the Cx43/Akt pathway.
2. Materials and Methods
2.1. Human Muscle Tissue Sample Collection
All experiments of this study were performed in accordance with the guidelines of the Ethics Committee on Human and Animal Experiments (Huashan Hospital, Fudan University). Human atrophic and normal muscles were obtained from three donors diagnosed with upper trunk BPI (brachial plexus injury). Inclusion criteria were as follows: (1) upper trunk BPI 6 months ago diagnosed by preoperative physical examination and electromyography; (2) uninjured accessory nerve and second to fourth cervical nerve; (3) 18–50 years old; (4) no other diseases; and (5) willing to participate in this study. Pieces of denervated deltoid muscle and normal (non-denervated) sternocleidomastoid muscle about 2 cm in length and 1 cm in diameter were resected from each patient during surgery (under intravenous anesthesia with intubation) and preserved in 4% paraformaldehyde until use. Information of collected patients is listed in Supplementary Table 1.
2.2. Experimental Animals
Male C57BL/6 mice aged 6–8 weeks and weighing 22–25 g were purchased from the Vital River Laboratory Animal Technology Co. (Zhejiang, China). The mice were housed in standard cages in a room at 23°C and 50% relative humidity on a 12-h: 12-h light/dark cycle. The study was approved by the ethics committee of Fudan University (202107012S, Date: 2021/7/30). The whole experiment was conducted from 2021/8/1 to 2023/7/30.
2.3. Experimental Grouping
- (1)
All mice were numbered.
- (2)
Each mouse was distributed by a random number table in one experimental group.
- (3)
Mice number and distribution were conducted by one experimenter, operation and sample processing were conducted by another experimenter, and data analysis was conducted by a third experimenter.
Mice in all denervation groups were subjected to unilateral sciatic nerve transection under anesthesia (each animal was anesthetized by an intraperitoneal injection of 50 mg/kg 1% sodium pentobarbital), as previously described [21]. In brief, after deep anesthetization by intraperitoneal injection with sodium pentobarbital 50 mg/kg, a 0.5 cm-long sciatic nerve portion in the right hind leg of the mouse was resected, the two nerve ends were buried in muscle, and the incision was closed using 4 − 0 absorbable sutures. After sciatic nerve transection, mice in the Gap-19 group were treated with Gap-19 every 2 d via tail vein injection [22]. After sciatic nerve transection, mice in the blueberry group were treated daily with blueberry extracts dissolved in 37°C distilled water by intragastric administration by gastric tube. The mice in the denervation group received the same amount of distilled water daily. The mice were sacrificed after materials were obtained by overdose intraperitoneal injection with sodium pentobarbital 180 mg/kg.
Operations were all done at 9–11 am. Also, operations and tissues collecting procedures were followed in the same order. We have to state that we did not control cage location and room brightness, which were under unified management in Fudan University.
2.4. Wet Weight
Seven days or fourteen days after denervation, with and without blueberry extract administration, the mice were anesthetized, and the gastrocnemius muscles of both the left and right hind legs were removed, washed with saline, and weighed. The loss of muscle weight ratio was defined as the weight of the contralateral side minus the muscle weight of the nerve injury side, divided by the weight of the contralateral side. The muscle samples were stored in 4% paraformaldehyde at −80°C until use.
2.5. Grip Strength
Grip strength meter was used to measure the hindlimb grip strength 14 d after operation. Each mouse was allowed to grasp the machine with their forelimb taped to the chest, and then its tail was gently pulled back and the tension was recorded automatically by the machine. Each mouse had three times to perform the test, and 30°s rest was given between each test. The remainder values were recorded and normalized with whole body weight for analysis [23].
2.6. Hematoxylin and Eosin (HE)
Gastrocnemius muscle samples from mice and patients were fixed in 4% paraformaldehyde in room temperature and embedded in paraffin. The samples were cut at a thickness of 5 μm. Before staining, the slices were dewaxed by xylene in room temperature for 2-3 min until the wax was completely dissolved. Then, orderly move the slices into 100%, 90%, 80%, and 70% ethanol, water, and distilled water for about 2 min. The sections were stained with hematoxylin and eosin (HE) (Beyotime, Shanghai, China) to evaluate histopathologic changes. An EclipseCi-L camera light microscope was used to select the target area of the tissue for 400x imaging so that the tissue could fill the whole field of view as much as possible to ensure that the background light of each photo was consistent. The mean diameter of myofibers was determined by blinded analysis using ImageJ software (National Institutes of Health, Bethesda, MD, United States) from five randomly captured images per mouse in each experimental condition [24].
2.7. Immunofluorescence
To reveal Akt and Cx43 proteins in denervated muscles, double immunostaining was performed. The sections were deparaffinized with xylene in room temperature for 2-3 min until the wax was completely dissolved and rehydrated with ethanol, and antigen retrieval was performed in a 0.01-M citrate buffer (pH, 6.0) in a 95°C pressure cooker for 10 min, followed by natural cooling to room temperature. The paraformaldehyde-fixed denervated muscle was incubated with the primary antibody, followed by the secondary antibody, and subsequently mounted with DAPI. The primary antibodies used were rabbit anti-Akt (1 : 100, cat. no. AF1789, Beyotime Institute of Biotechnology Co. Ltd.) and rabbit anti-Cx43 (1 : 100, cat. no. 52559, Cell Signaling Technology, Danvers, MA, USA). The primary antibodies were incubated in 37°C for 1 h. Samples were viewed using a fluorescence microscope at 400x magnification and analyzed by Huygens software.
2.8. Western Blot Analysis
Frozen gastrocnemius muscle samples were homogenized in radioimmunoprecipitation assay buffer containing 1 mM phenylmethylsulfonyl fluoride and a Protease Inhibitor Cocktail (Roche Applied Science). Lysates were centrifuged for 20 min at 12, 000 × g (4°C) and the protein level in the supernatant was quantified using a bicinchoninic acid assay kit (Beyotime). Proteins were separated by SDS-PAGE (Beyotime) and transferred to a polyvinylidene difluoride membrane (Beyotime), which was blocked with 5% non-fat dry milk in Tris-buffered saline at room temperature for 1 h, followed by incubation at 4°C for 20 h with primary antibodies: rabbit anti-Akt (1 : 1000, cat. no. AF1789, Beyotime Institute of Biotechnology Co. Ltd.), rabbit anti-p-Akt (1 : 1000, cat. no. ab38449, Abcam), rabbit anti-Cx43 (1 : 5000, cat. no. C6219, Sigma), and rabbit anti-p-Cx43 (1 : 1000; cat. no. PA5-104820, Invitrogen). After three washes by TBST, the membrane was incubated with the appropriate secondary antibody (Abcam) at room temperature for 1 h. An enhanced chemiluminescence detection reagent and X-ray film were used for protein visualization.
2.9. Quantitative Real-Time PCR (qPCR)
An RNeasy kit (Qiagen, Valencia, CA, United States) was used to extract total RNA from the gastrocnemius muscle. cDNA was synthesized using a first-strand cDNA synthesis kit with oligo dT primers (Invitrogen, Carlsbad, CA, United States) and used for quantitative real-time PCR (qPCR). The thermal cycling conditions were as follows: 94°C for 5 min; 35 cycles of 94°C for 30 s, 55°C for 45 s, and 72°C for 1 min; and 72°C for 5 min. The relative expression levels of target genes were calculated using the cycle threshold (Ct) value. Akt and Cx43 expression levels were normalized to those of β-actin. We used Primer3 to design all primer sequences. The primer sequences were as follows: AKT: F, 5′ GAA CGA TGG CAC CTT TAT T 3′, R, 5′ AGT TGT TGA GTG GGG ACT CT 3′; Cx43: F, 5′ GAA AGG CGT GAG GGA AGT A 3′, R, 5′ TGA AGT CAA AGG AAA AGG AGA G 3′; β-actin: F, 5′-TCA TCA CTA TTG GCA ACG AGC-3′ and R, 5′-AAC AGT CCG CCT AGA AGC AC-3′.
2.10. Statistical Analysis of Data
All data are presented as mean ± SD. All statistical tests were performed using one-way t-test or ANOVA. All statistical analyses were conducted using SPSS software (version 17.0; SPSS Inc., Chicago, IL, United States). Statistical significance was set at p < 0.05.
3. Results
3.1. Denervation Leads to Increased Expression of Cx43 and Decreased Expression of Akt in Atrophied Skeletal Muscle of Human Body
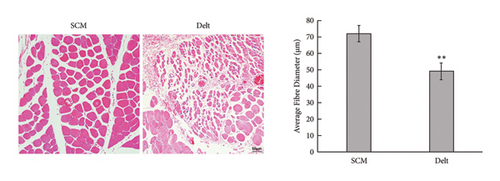
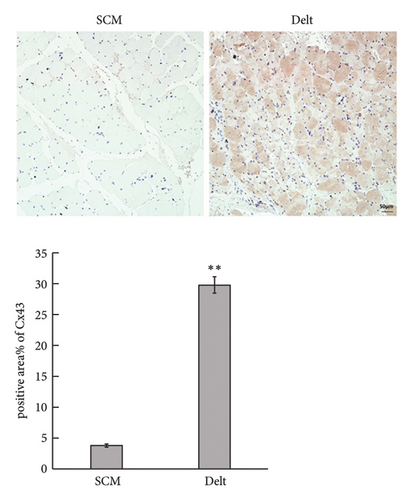
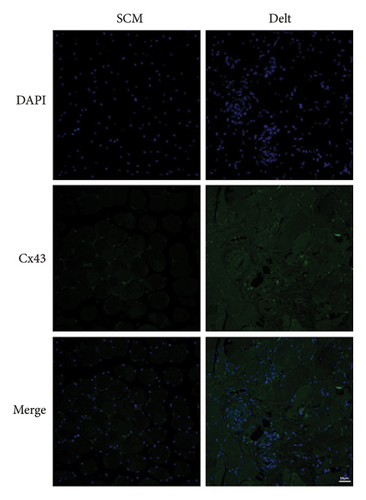
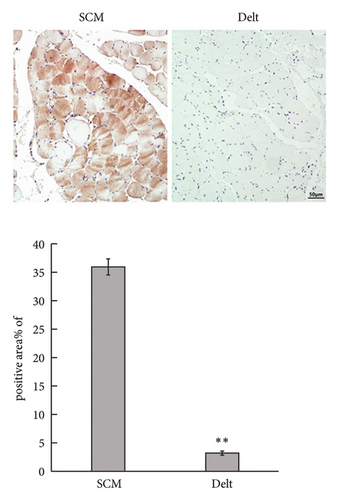
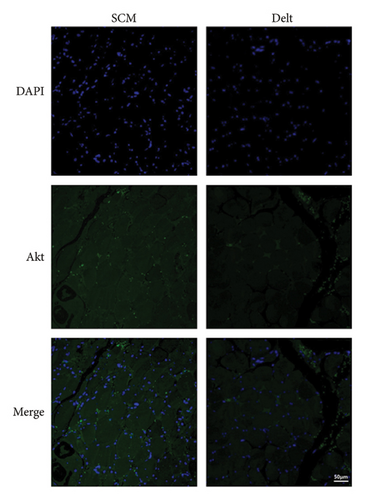
The HE staining also showed obvious atrophy of deltoid at 6 months postoperatively in histological level (Figure 1(a)). Compared with the sternocleidomastoid muscle, denervation induced increased expression of Cx43 in deltoid muscle, which was shown by both immunohistochemistry and immunofluorescence (Figures 1(b) and 1(c)). While, denervation induced decreased expression of Akt in deltoid muscle (Figures 1(d) and 1(e)). These results showed that the number of Cx43 increased and the number of Akt decreased after denervation.
3.2. Denervation Leads to Increased Expression and Activated Function of Cx43 in Atrophied Skeletal Muscle
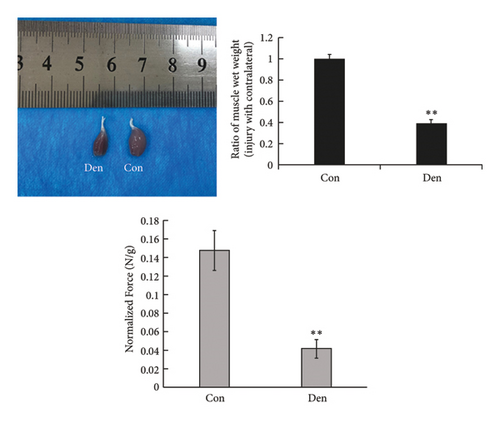
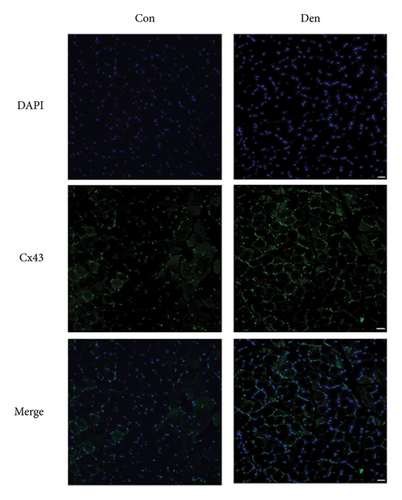
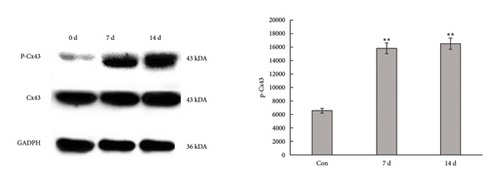
The unilateral sciatic nerve was transected in mice in the denervated group. The wet weight ratio and grip strength decreased significantly at 14 d postoperatively (Figure 2(a)). The ipsilateral gastrocnemius also showed obvious atrophy at 14 d postoperatively in gross and histological examinations (Figures 2(a) and 2(b)). All these changes at 14 d were more obvious than those at 7 d (Supplementary Figures 1(A)–1(C)), and materials at 14 d postoperatively were used for further study. Compared with the control group, denervation induced increased expression of Cx43 in skeletal muscle cells at the level of transcription (Figures 2(c) and 2(d)) level. These results showed that the number of Cx43 increased after denervation. To explore if their function was activated, p-Cx43/Cx43 was analyzed by western blotting and it was found that p-Cx43/Cx43 increased continuously after denervation until 14 d (Figure 2(e)).
3.3. Gap-19 Inhibited the Function of Cx43 and Attenuated Denervated Muscle Atrophy
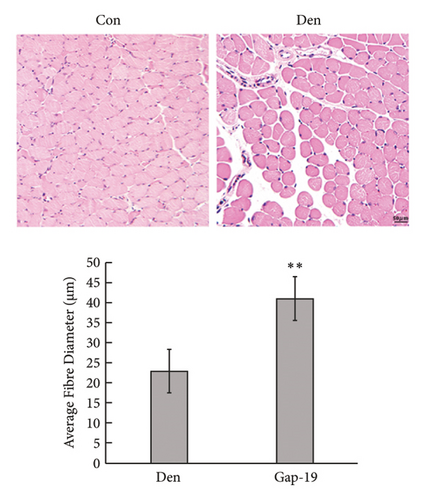
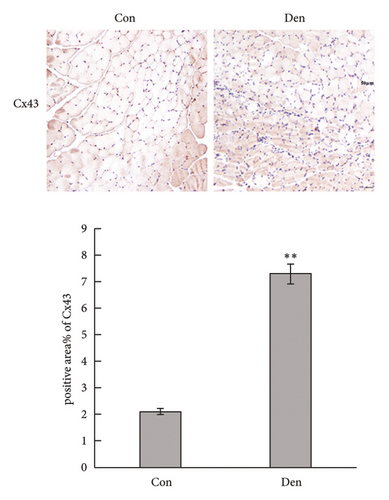
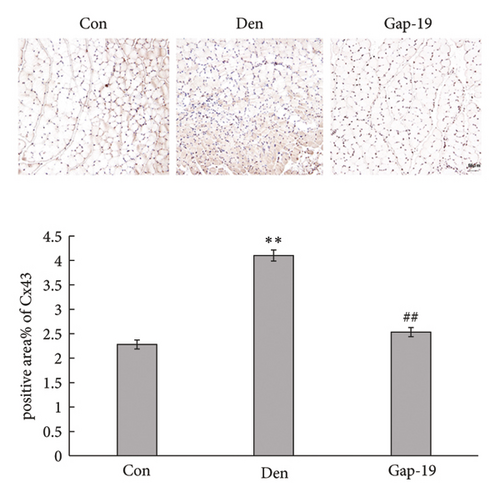
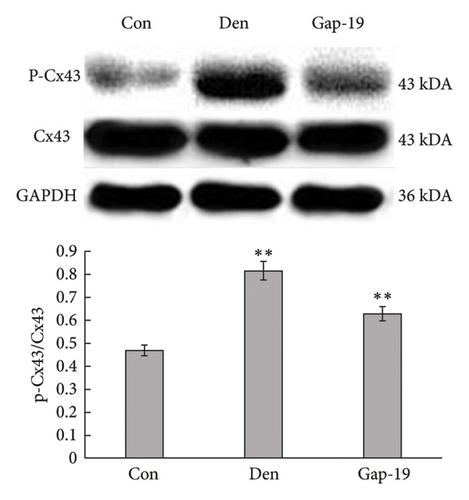
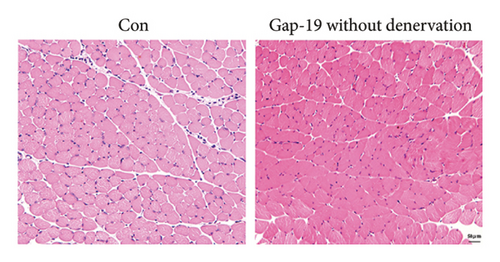
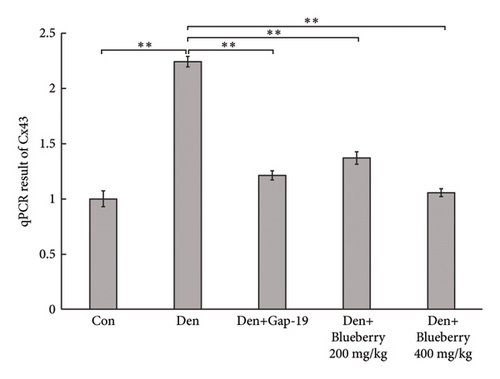
To determine the effects of Cx43 on skeletal muscle cells after denervation, we administered Gap-19 by tail intravenous administration, a selective Cx43 inhibitor, in mice in the Gap-19 group. Gap-19 reduced Cx43 expression in denervated skeletal gastrocnemius muscle during translation (Figures 3(b) and 3(c)) level and inhibited Cx43 activation (Figure 3(c)). In terms of histology (Figure 3(d)) and gross levels (Figure 3(a)), Gap-19 attenuated skeletal muscle atrophy 14 d after denervation. Gap-19 showed no obvious effects on skeletal muscle without denervation (Figure 3(e)). Combined with previous results, we conclude that Cx43 activation promotes denervation-induced skeletal muscle atrophy. Also, Gap-19 reduced denervation-induced Cx43 expression increase in skeletal gastrocnemius muscle during transcription level (Figure 4(f)).

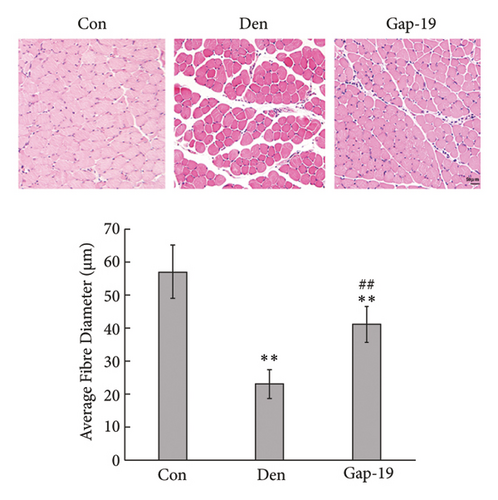
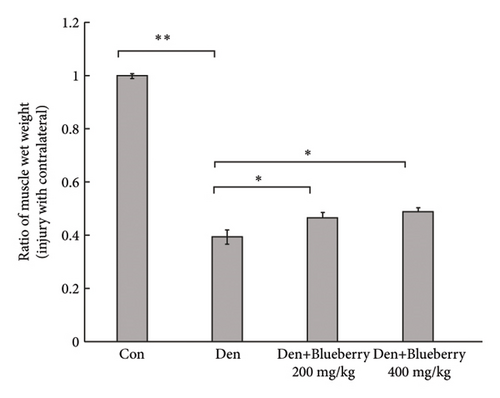
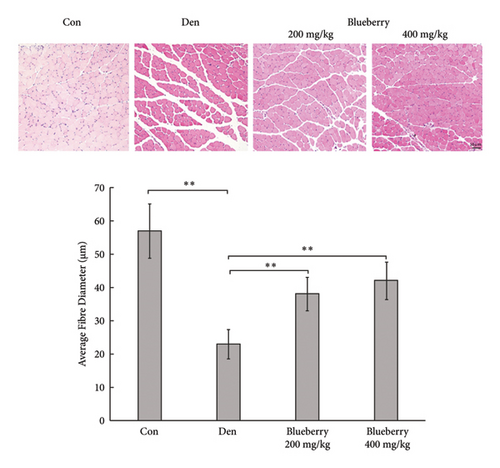
3.4. Blueberry Extracts Attenuated Denervated Muscle Atrophy and Downregulated Cx43 Expression
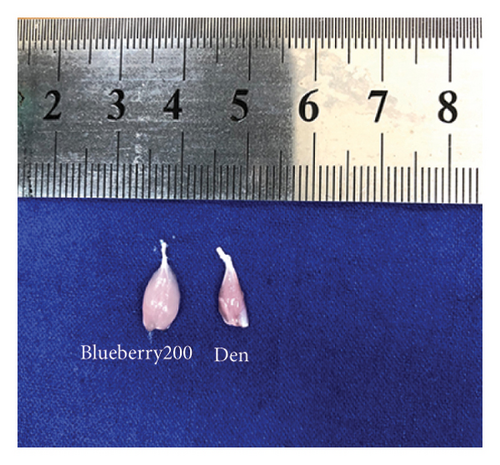
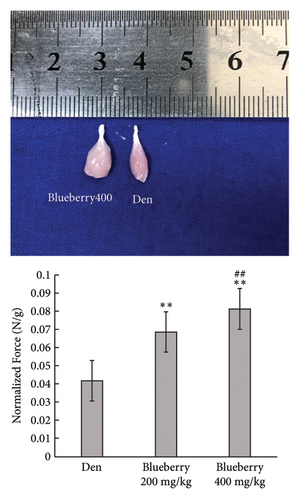
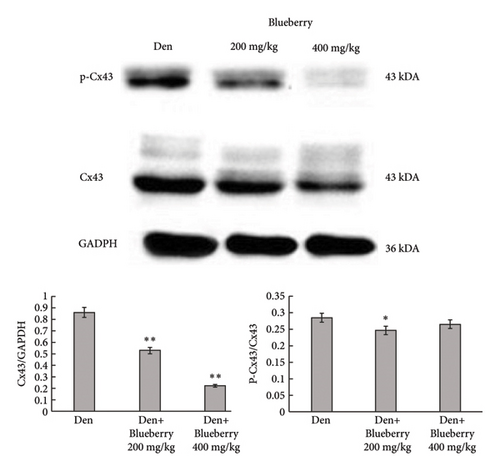
Mice in the blueberry groups were treated daily with blueberry extracts dissolved in 37°C distilled water by intragastric administration from the day of operation. Figure 4(a) and Figure 4(b) show that, compared with gastrocnemius in denervation group (Figure 4(a) and 4(b) right), 200mg/kg (Figure 4(a) left), and 400 mg/kg (Figure 4(b) left) blueberry extracts treatment could improve the muscle mass of denervated gastrocnemius. Wet weight ratio comparison (Figure 4(c) also showed that denervation-induced skeletal muscle atrophy could be partially reversed by both doses of blueberry extracts treatment. Additionally, in histology (Figure 4(d)), skeletal muscle tissue showed a larger stained area of myocytes and a narrower intercellular space in both blueberry groups. Moreover, higher blueberry extract doses showed stronger protective effects. Next, we explored whether Cx43 was involved in the protective role of blueberry extracts against denervated muscle atrophy. Cx43 expression and p-Cx43/Cx43 were upregulated in denervated skeletal muscle, whereas blueberry extracts downregulated Cx43 expression in both transcriptions (Figure 4(f)) and translation (Figure 4(e)), and p-Cx43/Cx43 (Figure 4(e)) also decreased in the blueberry 200 group. However, the p-Cx43/Cx43 ratio did not decrease significantly in the blueberry 400 group.
3.5. Cx43/Akt Pathway Was Involved in the Effect of Blueberry Extracts on Denervated Muscle Atrophy
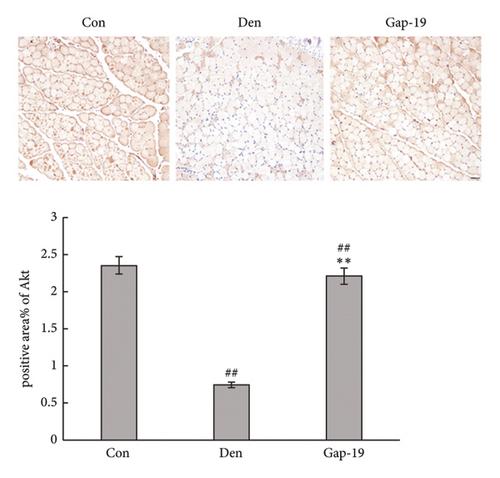
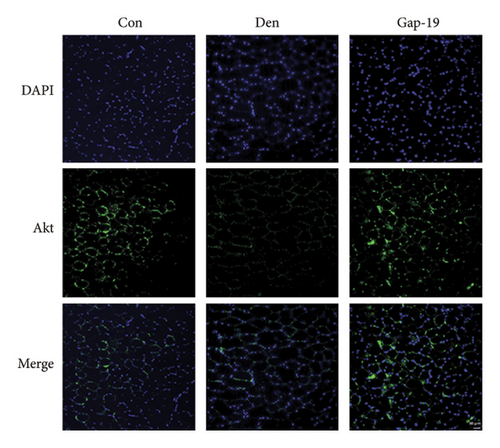
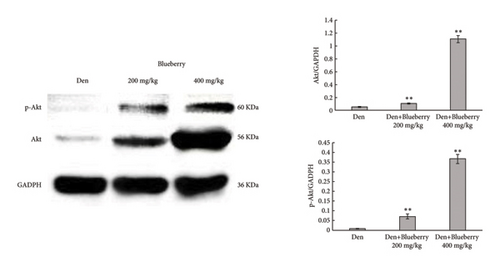
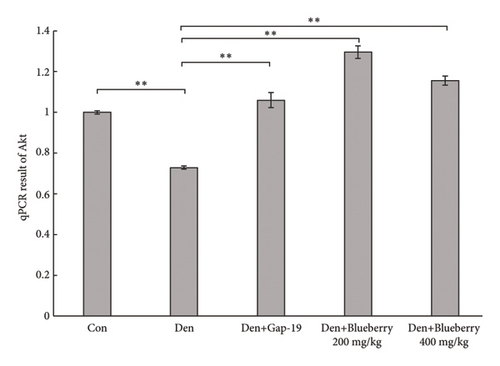
We then explored whether Akt contributes to the protective effect of blueberries and whether Cx43 interacts with Akt. HE staining (Figure 5(a)) and immunofluorescence (Figure 5(b)) showed that Gap-19-upregulated Akt expression was downregulated in denervated skeletal muscle tissue compared with the control group, which was upregulated by Gap-19. To explore whether Akt was involved in the protective effects of blueberry extracts on denervated skeletal muscle, we used western blot analysis and found that Akt expression was upregulated in the blueberry group compared to the denervation group (Figure 5(c)). Meanwhile, p-Akt expression was also upregulated in the blueberry group compared to the denervation group, which showed that activity of Akt was also restored in the blueberry group. Additionally, the relative Akt gene expression was downregulated in the denervation group, whereas Gap-19 and blueberry extracts reversed this change and even upregulated Akt expression compared to the control at the transcription level (Figure 5(d)). These results demonstrated that the Cx43/Akt interaction is involved in the effect of blueberry extracts on denervated muscle atrophy.
4. Discussion
Overall, our study indicates that blueberry extract protects against denervation-induced skeletal muscle atrophy. Additionally, Cx43 is activated by denervation and aggravates skeletal muscle atrophy, while blueberry extracts can downregulate the number and function of Cx43 in human and mice, which could be the target of the protective effects of blueberry extracts. Blueberry extracts and the Cx43 selective inhibitor Gap-19 reversed the Akt expression inhibition by denervation in skeletal muscle cells. Therefore, this study provides proof from human body and mice samples of concept that blueberry extracts attenuate denervated skeletal muscle atrophy by regulating the Cx43/Akt pathway (Figure 6).
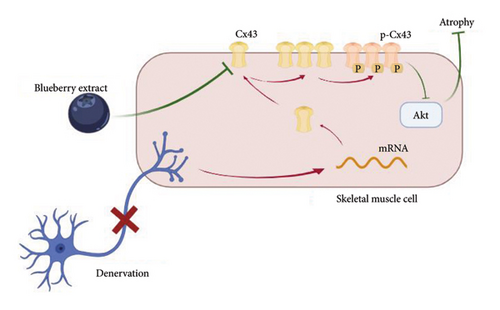
Skeletal muscle denervation can be caused by acute nerve injury, chronic neuritis, or many other pathological conditions. After denervation, the dominant skeletal muscle undergoes irreversible atrophy, which results in muscle mass and function loss [25]. Clinically, patients suffer from reduced functional status, quality of life, and even reduced survival caused by denervated muscle atrophy. Sciatic nerve transection is one of the most commonly used methods for creating animal models of denervated muscle atrophy. We performed unilateral sciatic nerve transection in C57BL/6 mice and observed the related changes in the ipsilateral gastrocnemius muscle. Obvious atrophy was observed in both gross and histological findings.
Connexins are a family of proteins that form connexons at the cell membrane, which mediate molecule exchange between cells [26]. Their extracellular part, called hemichannels, forms gap junction channels between neighboring cells [27]. Previous research has found that Cx43, a connexin family member, critically contributes to the skeletal muscle atrophy progression caused by denervation [6, 28, 29]. Ion channel imbalance may be the mechanism underlying its harmful role in skeletal muscle atrophy [30]. In this study, we used the Cx43 selective inhibitor Gap-19 to successfully attenuate denervated skeletal muscle atrophy, which further proved that augmented Cx43 expression induced by denervation contributed to skeletal muscle atrophy. Beyond changes in the quantity of Cx43, qualitative changes such as phosphorylation could better reflect the functional activation of Cx43 [31]. Here, we found that p-Cx43/Cx43 increased significantly after denervation, and that Gap-19 could also reduce this ratio. Based on these results, we concluded that denervation enhanced Cx43 quantity and quality, which further resulted in skeletal muscle atrophy.
Blueberries are one of the most beneficial fruits worldwide for their health effects. A recent clinical trial found that blueberry extracts can enhance muscle strength, power, and endurance [32]. Similarly, Sato et al. [16] found that Montgomerym [Mont], a blueberry cultivar, protects against skeletal muscle loss induced by sex steroid deficiency. Parra-Paz et al. [33] demonstrated that raw blueberries attenuated dopaminergic denervation and partially reversed motor disorders in rats. Inspired by previous discoveries, in this study, we tested blueberry extracts on mice that underwent sciatic nerve transection and found obvious protective effects on the gastrocnemius muscle. Combined with these results, we explored whether Cx43 is involved in the potential mechanism by which blueberry extracts protect against denervation-induced skeletal muscle atrophy. We found that blueberries could partially reverse the increase in Cx43 and p-Cx43/Cx43 caused by denervation, which indicated that blueberries attenuated denervation-induced skeletal muscle atrophy, possibly by inhibiting the function of Cx43. It should be noted that p-Cx43/Cx43 did not decrease significantly in the blueberry 400 group, which may due to the amount of total Cx43 being reduced by nearly 80% in the blueberry 400 group. Because of this, even though the amount of p-Cx43 alone was reduced significantly, the ratio decline was not remarkable.
Akt, also known as protein kinase B, plays a critical role in many cellular activities. Recent studies have also found that Akt signaling is involved in skeletal muscle cell responses to denervation [34, 35]. Also, including all mechanisms of muscle atrophy, Akt was involved in pathways of maintaining the balance between muscle protein synthesis and degradation [25]. When it was activated, muscle protein synthesis increases and muscle protein degradation decreases. Strong interactions between Akt signaling and Cx43 have been found in many other disease models [14, 20, 36, 37], and previous evidence indicates that they negatively regulate one another. In our study, we found that Gap-19 enhanced Akt expression, indicating that Cx43 interacts with Akt signaling during skeletal muscle atrophy denervation. Additionally, we investigated whether this type of interaction was involved in the effects of blueberries on denervated skeletal muscles. After treatment with the blueberry extract, Akt expression was enhanced. Therefore, Cx43/Akt interaction plays an important role in blueberry extract-mediated protection against denervated muscle atrophy (Figure 5). Furthermore, as discussed before, Akt is involved more in anabolic signals while there were also catabolic signals during skeletal muscle atrophy such as c-Jun N-terminal kinase (JUN) and nuclear factor-κB (NF-κB) pathway [38]. Besides inhibition of Akt expression and activation, phosphorylation of Cx43 had been reported to trigger catabolic signals in muscle cells [39, 40] which could aggravate muscle atrophy on the other side. Also, it had been reported that berries had an effect on inhibiting proinflammatory cytokine IL-6 which could trigger STAT and NF-κB pathways and other protein degradation pathways factors such as Atrogin-1 and MurF1 [41–43]. So, our study focused on protective effect of blueberry extracts towards denervated muscle atrophy by Akt signal, which is the one of the anabolic signals, while if blueberry extracts had effects on catabolic signals in denervated skeletal muscle was still unknown. Further studies should pay more attention to this topic, which could give us more comprehensive understanding of protective role of blueberry extracts for denervation-induced skeletal muscle atrophy.
Our study had some limitations. First, the effects of the chemical composition of blueberry extracts remain unclear and need to be analyzed and confirmed to facilitate reproducibility and for further medical research. Second, we only determined the effect of blueberry extracts at 14 d after denervation in mice, which is not sufficient for exploring the molecular events during the whole process, and the effect of blueberry extracts and their regulation on Cx43/Akt interaction at other time points is still unknown. Also, there was some potential bias such as operation time, tissue collection skills, and staining skills that could affect the result of immunohistochemistry and immunofluorescence. To minimize these potential biases, only one experimenter in our group did all the operations, and another experimenter did all staining processes to keep skills on the same level. Last but not least, our animal model used gastrocnemius muscle at 14 d after sciatic nerve transection as denervated atrophic muscle. However, muscle atrophy period was different between human body and mice models, 14 days post-denervation could be too short for muscles in human body to be obviously atrophied. But in mice models, we could observe obvious muscle atrophy on gross and histological level at this time point. More observation on time-dependent atrophy on mice model is required in further study. Moreover, follow-up experiments are required to determine how Cx43 and Akt signaling interact during denervation and after treatment with blueberry extracts at a more detailed level. Higher doses of blueberry extracts should be applied to observe stronger efficacy or toxicity. Blueberry extracts’ effective component analysis was also required to be clear in the future. Besides these limitations and further expectation, our study provides evidence for the potential medicinal value of blueberry extracts in denervated skeletal muscle atrophy and provides an initial attempt to explore the mechanisms underlying its protective effect. We used HE staining, immunohistochemistry, and immunofluorescence for multiple validations. Drug research and development and clinical trials can be expected after more follow-up research is conducted.
5. Conclusion
Our study demonstrates that blueberry extracts protect against denervation-induced skeletal muscle atrophy by regulating Cx43 expression and activation and interacting with the Akt signaling pathway. These results suggested that blueberry extracts may be an effective treatment for denervated skeletal muscle atrophy.
Abbreviations
-
- BPI:
-
- Brachial plexus injury
-
- HE:
-
- Hematoxylin and eosin
-
- DAPI:
-
- 4′,6-Diamidino-2-phenylindole
-
- SDS-PAGE:
-
- Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
-
- PCR:
-
- Polymerase chain reaction
-
- SD:
-
- Standard deviation.
Ethical Approval
The animal study was approved by the ethics committee of Fudan University (202107012S). The human study was approved by Huashan Hospital Institutional Review Board (2021486). The studies involving human participants were reviewed and approved by the Ethical Committee, Huashan Hospital, Fudan University. The animal study was reviewed and approved by the Institutional Animal Care and Use Committee of Huashan Hospital, Fudan University.
Consent
The patients/participants provided their written informed consent to participate in this study.
Disclosure
This manuscript was submitted as a preprint in the link “https://www.researchgate.net/publication/362407807_Negative_Effects_of_Cx43_during_Denervated_Skeletal_Muscle_Atrophy_can_be_Attenuated_by_Blueberry_Extracts_via_Regulating_Akt_Pathway/fulltext/64f8385387d7f830e8055c7e/Negative-Effects-of-Cx43-during-Denervated-Skeletal-Muscle-Atrophy-can-be-Attenuated-by-Blueberry-Extracts-via-Regulating-Akt-Pathway.pdf” [44].
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
XH conceptualized the study, collected and analyzed the data, and wrote the manuscript. JZ interpreted the data and collected and edited the figures. JL acquired the data and wrote the manuscript. ZY analyzed the data. YX analyzed and interpreted the data. JD wrote and edited the manuscript. LX visualized the data and edited the figures. JJ conceptualized the study, analyzed the data, and edited the manuscript. JX conceptualized and supervised the study. XH and JZ confirm the authenticity of all the raw data. All authors have read and approved the final manuscript. XH, JL, and JZ contributed equally to this study. XH, JL, and JZ contributed equally.
Acknowledgments
This study was sponsored by Shanghai Municipal Key Clinical Specialty (shslczdzk05601). The authors would like to thank Shanghai Municipal Key Clinical Specialty for helpful funding related to this work.
Open Research
Data Availability
Some or all data, models, and codes generated or used during the study are available in a repository or online in accordance with funder data retention policies.




