A Joint Analysis of Metabolomics, Network Pharmacology, and Molecular Docking Reveals the Efficacy Patterns in Various Medicinal Segments of Angelica sinensis (Oliv.) Diels Root
Abstract
Angelica sinensis (Oliv.) Diels root (ASR) is a medicinal and edible traditional Chinese herb medicine. Understanding the varying efficacies in different ASR segments and their associated pharmacological mechanisms at the metabolome level has been a largely unexplored research area. This study integrates metabolomics, network pharmacology, and molecular docking to investigate the characteristics and mechanisms underlying hemostasis, blood enrichment, and blood circulation promotion in distinct ASR medicinal segments. The distinguishable metabolic spectra were visually presented for the head (ASRH), body (ASRB), and tail (ASRT) in ASR, highlighting the dominant metabolites in each. Furthermore, a network linking components, targeted proteins, signaling pathways, and diseases was constructed. The combined analysis of metabolomics and network pharmacology confirms that ASRT primarily enhances blood circulation, whereas ASRH and ASRB lean toward hemostasis and blood enrichment. The dominant ingredients of ASRT mainly influence signaling pathways of calcium, PI3K-Akt, and arachidonic acid metabolism by modulating targeted proteins like EGFR, SRC, AKT1, and HSP90AA1, thus enhancing hemodynamics. In contrast, the dominant ingredients of ASRH and ASRB regulate PI3K-Akt, IL-17, and JAK-STAT signaling pathways via proteins, such as CTNNB1, AKT1, SRC, and EP300, playing a role in hemostasis and blood enrichment. These results were subsequently validated by molecular docking. This study innovatively combines metabolomics, network pharmacology, and molecular docking to preliminarily reveal the mechanisms governing hemostasis, blood enrichment, and blood circulation improvement regulated through multiple components, targeted proteins, and pathways in different ASR segments. These findings offer valuable insights for future investigations into the efficacies of distinct ASR segments.
1. Introduction
Angelica sinensis (Oliv.) Diels is a perennial herb belonging to Umbelliferae. The historical significance of A. sinensis (Oliv.) Diels root (ASR) as a medicinal herb traces back to its mention in Shennong’s Herbal Classic, China’s first pharmaceutical monograph [1]. ASR is one of the earliest Chinese herbal medicines (CHMs) classified as homologous medicine and food, offering a range of health benefits, including blood enrichment, blood circulation promotion, menstruation regulation, pain relief, and bowel relaxation [1, 2]. As a widely used bulk CHM, ASR has earned its place in traditional Chinese medicine prescriptions due to its versatility in regulating the cardiovascular system, liver, and spleen meridians [1–3]. ASR is slightly cylindrical, featuring a light brown or brown surface with longitudinal wrinkles, hole-like protrusions, and branch roots at the lower portion. The morphology of different medicinal parts in ASR is shown in Figure 1. The upper portion of ASR is called the head of the root (hereafter known as ASRH) and exhibits annular folds. The taproot, or body of the root (hereafter known as ASRB), has an uneven surface. The branch root, called the tail of the root (hereafter known as ASRT), is thicker at the top and gradually tapers toward the bottom, often bearing a few scars from fibrous roots [2]. The chemical composition of CHM serves as the foundation for its clinical efficacy and is a key indicator for quality assessment. The quality of CHM varies among different regions, with genuine medicinal materials being the most desirable [3, 4]. The researches have consistently highlighted Min County, Gansu Province, China, as a prime location for ASR production. Its unique natural conditions contribute to ASR’s superiority over herbs from other regions in terms of appearance, compound content, and trace element composition [3–6].
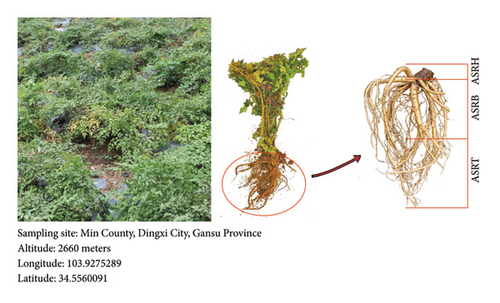
ASR is mainly used in medicine and dietotherapy in various forms, including ASRH, ASRB, ASRT, and whole ASR slices [2]. Since ancient times, medical experts have observed that different segments of ASR exhibit distinct therapeutic properties. They have proposed that ASRH effectively stops bleeding, ASRB enriches the blood, ASRT promotes blood circulation, and whole ASR both enriches and activates the blood [2]. Meanwhile, researchers have confirmed apparent differences in the effective ingredients among different medicinal segments. Li et al. conducted a multivariate analysis and found higher levels of certain compounds in ASRH, such as valine and isoleucine, whereas ASRT exhibited higher concentrations of Z-butylidenephthalide and cis-Z, Z′-3α,7α′,7α,3α′-dihydroxy-ligustilide. Additionally, ASRB contained elevated proportions of tryptophan and coniferyl ferulate, with both ASRH and ASRT showing greater abundance of aspartic acid and Z-ligustilide [1]. Yang et al. revealed that ASRH had significantly lower levels of ferulic, chlorogenic, caffeic, and cinnamic acids than ASRT. Among them, ferulic and caffeic acids were identified as key substances responsible for promoting blood circulation [4]. Overall, although all three medicinal segments exhibit overlapping pharmacodynamic aspects and corresponding ingredients, they differ in proportions and contents [1, 4], which are critical factors contributing to the varied therapeutic effects observed in different ASR segments [1, 2, 4]. These comparative studies on metabolic ingredients across ASR segments preliminarily confirm the principles of Chinese medicine, which suggest that ASRH, ASRB, and ASRT are associated with hemostasis, blood enrichment, and blood circulation promotion, respectively [1, 2, 4].
Furthermore, in recent years, metabolomics has been employed to uncover the diverse ASR ingredients [1, 4, 7–13]. However, most of these studies primarily focus on the effects of varieties, origins, processing methods, and product forms on its chemical composition [7–12]. There remains a relative paucity of comparative studies on the different medicinal segments of ASR at the metabolome level, particularly those that examine all three segments simultaneously [4, 8, 13]. Consequently, there is an urgent need to comprehensively investigate the distribution and content disparities of the pharmacodynamic material basis in ASRH, ASRB, and ASRT, as well as the corresponding pharmacological effects. This will help elucidate the relationship between active ingredients and medicinal efficacies in different segments and provide strong evidence for the accurate use of ASR in medicine. In terms of methodology, metabolomics technology, a comprehensive method for qualitative and quantitative analysis of small molecule metabolites using high-throughput detection, has been extensively applied in the modernization research of CHMs, offering a reliable means for studying the medicinal ingredients [1, 4, 7–15]. On this basis, network pharmacology allows for mapping interaction networks among active ingredients, targeted proteins, and diseases related to medicinal plants. Through network analysis and molecular docking, it becomes possible to further reveal the material basis of the CHM curative effect, the influence of active ingredients on disease target networks, and the modes of action among these ingredients at a holistic level [16]. Although metabolomic detection identifies crucial active small molecules for network pharmacology, a few studies have integrated metabolomics, network pharmacology, and molecular docking to uncover differential metabolites and their pharmacological mechanisms in various medicinal segments of CHMs.
In this study, ASR from its authentic producing region was taken as the research object. Initially, nontargeted metabolomics techniques were employed to identify and analyze the metabolic ingredients and their abundance levels in ASR’s distinct segments, namely, ASRH, ASRB, and ASRT. A set of logically structured mathematical and statistical models were applied to pinpoint the dominant metabolites within each segment of ASR. This allowed for a quantitative and visual representation of the chemical ingredients in different medicinal segments of ASR. With the help of relevant literature and databases, the efficacies of dominant metabolites were annotated. Subsequently, network pharmacology was employed to reveal the pharmacological mechanisms associated with the dominant active ingredients in varying medicinal segments. This approach also established connections among medicinal segments, metabolic ingredients, targeted proteins, signaling pathways, and diseases. Finally, a literature-based chemometric analysis was employed to verify the qualitative characterization of key components and preliminarily assess their actual contents. Molecular docking was performed to validate the results of the network pharmacological analysis, ensuring its reliability. The primary goal of this study is to provide a theoretical foundation for explaining the variations in the pharmacological effects observed in distinct segments of ASR and to advance the precision of medication choices.
2. Materials and Methods
2.1. Sample Collection and Identification
In September 2023, experimental materials were collected from the ASR production base located in Min County, Gansu Province. Six batches of fresh ASRs with healthy growth, uniformity, and representativeness were collected on-site and transported to the laboratory at a low temperature. Species identification of the samples was completed by Sun Ying, chief pharmacist of the Gansu Institute of Drug Inspection. Identification was based on morphological, odor, and molecular levels. The morphological characteristics and odor of the samples were completely consistent with the local main variety of A. sinensis (Mingui No. 1). Molecular identification was mainly realized by simplifying genome sequencing and analysis. In detail, specific-locus amplified fragment (SLAF) tag sequencing was performed on the samples to be tested and the samples of known variety (Mingui No. 1) [17]. The extracted genome was digested by HaeIII + HinCII, and the sequence with a length of 414–464 bp was defined as the SLAF tag. After the sequencing library was constructed and sequenced, the reads were aligned with the reference genome of A. sinensis. The average sequencing depth was over 10 ×, and single nucleotide polymorphism (SNP) and insertion and deletion (InDel) were detected. The results showed that more than 95% of the SLAF tags of the investigated samples and Mingui No. 1 samples could be completely mapped to the reference genome. Moreover, the SNP and small InDel from the investigated samples and Mingui No. 1 are relatively few compared with the reference genome, and their statistical results are highly consistent (Supplementary Table 1). Finally, the collected samples were identified as A. sinensis (Oliv.) Diels. The backup specimens are properly kept in the Tibetan Medicine Inspection Laboratory of Gansu Institute of Drug Control (No. 7 Yin’an Road, Anning District, Lanzhou City, Gansu Province). To distinguish ASRH, ASRB, and ASRT, morphology-based assessments were conducted (Figure 1). The external appearance of the samples was thoroughly cleaned with sterile water, followed by chopping into small pieces. These pieces were then distributed into centrifuge tubes, with approximately 1 g of sample per tube. In total, there were 18 test samples, including ASRH, ASRB, and ASRT. Additionally, quality control samples were created by combining equal portions of all sample types.
2.2. Extraction of Metabolites
All samples underwent grinding and extraction. The metabolites were extracted into a solution composed of methanol and acetonitrile in a 1 : 1 volume ratio, with an internal standard concentration of 2 mg/L. Methanol exhibits superior extraction efficacy for fat-soluble substances, whereas acetonitrile is more effective for extracting water-soluble substances. Employing a 1 : 1 ratio of these two solvents ensures comprehensive extraction of both fat-soluble and water-soluble compounds [1, 4, 7, 9–15]. After 10 min of ultrasonic treatment in an ice-water bath, the samples were left at −20°C for 1 h. The samples were then centrifuged at 4°C and 12,000 rpm for 15 min, and 500 μL of the supernatant was carefully transferred to Eppendorf tubes. The extract was dried in a vacuum concentrator, and 160 μL of extracting solution (with an acetonitrile-to-water volume ratio of 1 : 1) was added to the dried extract to redissolve them. After redissolving and vortexing for 30 s, the sample was subjected to 10 min of ultrasonication in an ice-water bath. Finally, the sample was centrifuged at 4°C and 12,000 rpm for 15 min, and 120 μL of the supernatant was collected in 2 mL injection bottles. To assess the repeatability of the entire analysis process, 10 μL from each sample was mixed to create quality control (QC) samples for detection.
2.3. Ultraperformance Liquid Chromatography Tandem-Mass Spectrometry Detection
The metabolomics detection was conducted using an ultraperformance liquid chromatography tandem-mass spectrometry (UPLC-MS) system consisting of PLUS UPLC (Acquity I-Class, Waters, Taunton, Massachusetts, USA) and high-resolution MS (Xevo G2-XS QTOF, Waters). The chromatographic column employed was an Acquity UPLC HSS T3 (1.8 μm 2.1 × 100 mm) from Waters. For both the positive and negative ion modes, mobile phase A consisted of a 0.1% formic acid aqueous solution, whereas mobile phase B was composed of a 0.1% acetonitrile formate. The injection volume for each sample was 1 μL. A gradient elution was applied as follows: 0–0.25 min with 98% A, 10–13 min with 2% A, and 13.1–15 minutes with 98% A. Primary and secondary mass spectrometry data were collected in the MSe mode using acquisition software (MassLynx V4.2, Waters). Dual-channel data were simultaneously collected for low (2 V) and high collision energy (10–40 V) at a scanning frequency of 0.2 s. Theoretical fragment identification was carried out using the MS-Fragmenter demo software (V2017.2) [4, 7, 9–14, 18]. To comprehensively describe the differential metabolic patterns of metabolites in various medicinal segments, an initial loose ion mass number deviation was set. This included a parent ion mass number deviation of 100 ppm and a fragment ion mass number deviation of less than 50 ppm. Subsequently, in the second stage, when identifying the dominant metabolites of different medicinal sites, our standard was that the deviation of parent ion mass number should not exceed 15 ppm. Finally, we examined the deviations in the parent ion mass numbers of active ingredients documented in the database that are capable of binding to key protein targets and found that most of them were less than 10 ppm (Tables 1 and 2). This strict threshold ensured the reliability of subsequent characterization of differential metabolites. The parameters of the ESI ion source included a capillary voltage of 2,500 V (positive ion mode) or −2,000 V (negative ion mode), a cone hole voltage of 30 V, an ion source temperature of 100°C, a desolvation air temperature of 500°C, a back-blowing air flow rate of 50 L/h, and a desolvation air flow rate of 800 L/h. Mass spectra were collected over a range of m/z 50 to 1,200 [4, 7, 9–14, 18].
| Serial number | Metabolite | Code name | Mass error (ppm) | m/z | Retention time (min) | Correlation degree | Betweenness centrality |
|---|---|---|---|---|---|---|---|
| 1 | Arachidonic acid | DM8 | 10.914 | 327.234 | 10.054 | 65 | 0.234 |
| 2 | Eicosapentaenoic acid | DM9 | 9.435 | 325.218 | 9.446 | 63 | 0.208 |
| 3 | Zingerone | DM12 | 8.333 | 239.095 | 5.557 | 54 | 0.289 |
| 4 | 4-Methylesculetin | DM15 | 7.753 | 193.052 | 3.531 | 38 | 0.202 |
| 5 | 8-8′-Dehydrodiferulic acid | DM11 | 7.978 | 369.100 | 2.760 | 34 | 0.162 |
| 6 | Amygdalin | DM6 | 12.353 | 475.201 | 4.059 | 28 | 0.127 |
| 7 | (S)-bilobanone | DM10 | −1.012 | 233.153 | 8.590 | 23 | 0.107 |
| 8 | Naringin | DM3 | 8.927 | 579.180 | 0.655 | 22 | 0.084 |
| 9 | Raffinose | DM1 | 3.142 | 503.163 | 3.116 | 16 | 0.064 |
| 10 | 6-Pentadecyl salicylic acid | DM4 | −7.556 | 390.298 | 5.457 | 12 | 0.055 |
| 11 | Coumarin | DM2 | 8.029 | 145.031 | 6.120 | 11 | 0.026 |
| 12 | Salidroside | DM14 | 3.664 | 345.120 | 2.082 | 11 | 0.022 |
| 13 | Echinacoside | DM5 | 13.865 | 787.284 | 6.207 | 7 | 0.009 |
| 14 | (−)-riboflavin | DM13 | 6.949 | 397.116 | 4.472 | 7 | 0.044 |
| 15 | p-Cymene | DM7 | 8.500 | 135.119 | 4.237 | 5 | 0.011 |
- The top 15 DMs were screened according to the degree of association.
| Serial number | Metabolite | Code name | Mass error (ppm) | m/z | Retention time (min) | Correlation degree | Betweenness centrality |
|---|---|---|---|---|---|---|---|
| 1 | Tyrosyl-glutamine | DM17 | −2.073 | 327.166 | 2.738 | 75 | 0.086 |
| 2 | Tryptophyl-glutamate | DM10 | 5.102 | 332.127 | 2.8677 | 73 | 0.049 |
| 3 | Trp-Gly-Phe | DM27 | 6.943 | 447.136 | 2.354 | 72 | 0.045 |
| 4 | Threoninyl-tryptophan | DM13 | −0.731 | 328.127 | 2.204 | 71 | 0.053 |
| 5 | Trp-Gly | DM25 | 5.753 | 262.120 | 2.653 | 70 | 0.050 |
| 6 | 4-Hydroxy-3-methoxycinnamic acid | DM22 | 0.826 | 177.055 | 3.581 | 65 | 0.099 |
| 7 | Lysyl-proline | DM19 | −1.672 | 531.314 | 9.538 | 59 | 0.042 |
| 8 | Lys-Ser-Gly | DM23 | 2.463 | 291.167 | 0.941 | 54 | 0.040 |
| 9 | Ser-Ala-Asn | DM24 | 1.664 | 291.130 | 0.769 | 36 | 0.030 |
| 10 | L-tryptophan | DM14 | 5.311 | 203.084 | 2.353 | 35 | 0.024 |
| 11 | Aloin A | DM30 | 5.173 | 881.255 | 0.634 | 34 | 0.046 |
| 12 | Lysyl-asparagine | DM20 | 9.103 | 557.280 | 7.633 | 31 | 0.052 |
| 13 | (+)-abscisic acid | DM28 | 8.593 | 265.144 | 2.439 | 31 | 0.015 |
| 14 | Nicotinamide adenine dinucleotide (NAD) | DM7 | 2.130 | 662.103 | 0.755 | 29 | 0.056 |
| 15 | Asn-Asp-Asn | DM26 | −1.051 | 362.130 | 2.532 | 28 | 0.020 |
| 16 | Melezitose | DM2 | 3.100 | 504.170 | 3.145 | 25 | 0.028 |
| 17 | Sucrose | DM21 | 3.369 | 683.224 | 0.697 | 24 | 0.025 |
| 18 | Folinic acid | DM11 | −6.458 | 508.132 | 2.810 | 21 | 0.033 |
| 19 | L-tyrosine | DM12 | 5.905 | 180.068 | 1.440 | 20 | 0.030 |
| 20 | Arginyl-valine | DM16 | 13.300 | 312.147 | 4.187 | 19 | 0.024 |
| 21 | Trans-2-phenylcyclopropylamine | DM18 | 4.456 | 156.079 | 2.082 | 19 | 0.010 |
| 22 | L-citrulline | DM1 | 8.448 | 174.092 | 0.620 | 16 | 0.008 |
| 23 | Citrulline | DM8 | 9.584 | 140.084 | 0.598 | 16 | 0.008 |
| 24 | Adenylsuccinic acid | DM9 | 2.958 | 481.109 | 1.554 | 15 | 0.020 |
| 25 | D-lysine | DM4 | 9.624 | 147.114 | 0.548 | 14 | 0.012 |
| 26 | L-lysine | DM6 | 8.629 | 145.100 | 0.555 | 14 | 0.012 |
| 27 | Glycyl-arginine | DM15 | 8.477 | 507.272 | 8.704 | 11 | 0.006 |
| 28 | Quinone | DM29 | −0.923 | 261.040 | 3.588 | 5 | 0.004 |
| 29 | DL-arginine | DM3 | 2.810 | 349.232 | 0.591 | 3 | 0.001 |
| 30 | L-arginine | DM5 | 2.286 | 387.187 | 0.591 | 3 | 0.001 |
- The top 15 DMs were screened according to the degree of association.
2.4. Identification, Quantification, and Annotation of Metabolites
Peak extraction and alignment were performed using Progenesis QI software 2.0 (Waters), with raw data collected using MassLynx V4.2. Peak identification was based on the online METLIN database attached to the Progenesis QI software and a self-built BioMarker database [7, 11, 13, 18]. Original peak areas for each metabolite were normalized by dividing the metabolite of each sample by the total peak area for that sample and then multiplying by the average of all the peak areas [13, 19]. The obtained values were used to characterize the expression intensities of the corresponding metabolites. Additionally, Q1 (parent ion) and Q3 (daughter ion), retention time, declustering potential, and collision energy were used for metabolite identification. Combining these parameters, a qualitative score and corresponding qualitative grade are output for each compound, which then serves as the basis for screening conservatively identified compounds [13, 20]. Only compounds with a score greater than 0.9 and known as phytochemical ingredients were retained to ensure the reliability of analysis results. According to the identification information, some metabolites were presumed to be structural analogues of synthetic drugs. Although the predictive efficacies of these metabolites were consistent with the representative functions of their main distribution segments, we finally filtered them out to ensure the rigor of the analysis. The QC and correlation analyses were conducted to verify instrument stability. All annotated metabolites were categorized using the Human Metabolome Database (HMDB) and LIPID MAPS Structure Database.
2.5. Screening of the Intersecting Targeted Proteins of Active Ingredients and Diseases, and Construction of Medicinal Segments, Active Ingredients, and Targeted Proteins Networks
The canonical SMILES formulas corresponding to the active ingredients in ASRH, ASRB, and ASRT were obtained from the PubChem (https://pubchem.ncbi.nlm.nih.gov) database. These were then imported into the SwissTarget Prediction platform (https://swisstargetprediction.ch) to predict targeted proteins. Proteins with a probability >0 were selected as targets, and duplicates were removed. The UniProt database (https://www.uniprot.org) was used for standardization. GeneCards (https://www.genecards.org) and OMIM databases (https://www.omim.org) were used to screen disease-targeted proteins. Keywords such as “blood stasis,” “thrombus,” and “coagulant” were employed to screen disease-related targeted proteins for blood stasis, and “bleeding” and “anemia” were employed to screen those for bleeding and anemia. After merging the results obtained from both databases, the duplicate values were removed and standardized using the UniProt database. Venn diagram analysis was carried out using Origin software to identify intersections of targeted proteins associated with active ingredients and diseases for each medicinal segment of ASR. A network connecting active ingredients and targeted proteins for various ASR medicinal segments was constructed using Cytoscape 3.9.1 software. We then conducted network topology analysis and employed the CytoNCA plug-in to identify the principal dominant ingredients in ASRH, ASRB, and ASRT based on degree values and betweenness centralities.
2.6. Protein-Protein Interaction (PPI) Network Analysis and Pathway Enrichment Analysis of Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO)
The intersecting targeted proteins of active ingredients and diseases were imported into the STRING database. The run was set to include “Multiple proteins,” with the species specified as “Homo sapiens,” and a minimum interaction threshold of “High confidence >0.7” was applied, while other settings remained at default values. Network parameters were exported in “TSV” format and visualized using Cytoscape 3.9.1 software, with targeted protein size and color depth reflecting degree values. Network topology and CytoNCA plug-in analyses were performed to identify core targeted proteins in each segment according to degree values and betweenness centralities. Enrichment analysis of GO and KEGG pathways was conducted using the Metascape platform (https://metascape.org) on intersecting targeted proteins associated with dominant active ingredients and diseases in different medicinal segments of ASR. “H. sapiens” was chosen as the species, “Custom analysis” was selected, and a significance threshold of p value <0.01 was applied for pathway and function selection. The top 20 enrichment pathways were selected for analysis, and the results were visualized via the R language tool (v4.0.5) (https://www.r-project.org/).
2.7. Molecular Docking between Active Ingredients and Targeted Proteins
To further verify the accuracy of network construction, molecular docking was used to assess the interaction between key target proteins and key compounds. According to the network topology analyses on different medicinal segments of ASR, the active ingredients with higher degree values and their targeted proteins were selected for molecular docking verification. Firstly, the structure of active ingredients was obtained through the PubChem database, stored as a “mol2” format file with minimized energy by Chem 3D software, and labeled as ligand small molecules. The structure of the core target protein was downloaded through the UniProt database and PDB database (https://www.rcsb.org). The original ligand and water molecules were removed by PyMoL 2.5.4 software, imported into AutoDock Tools 1.5.6 software for hydrotreatment, and labeled as protein receptors. The protein receptor and ligand molecules were introduced into AutoDock Tools 1.5.6 software, stored in “pdbqt” format, and then docked to obtain binding energy. The active ingredients with good docking effects with target proteins were selected and visualized by PyMoL 2.5.4 software.
2.8. Data Analysis
To assess the significance of differences among various medicinal segments, univariate and multivariate statistical analyses were conducted. We considered differences significant when meeting the following criteria: variable importance in projection (VIP) ≥ 1.0 and a p value <0.05. The fold change (FC) was filtered from large to small. Most of the log2FC values were greater than 1 for significantly differential metabolites. A dominant metabolite was defined as having higher content in one segment compared to the others.
3. Results and Discussion
3.1. Classification and Annotation Information of ASR Metabolites
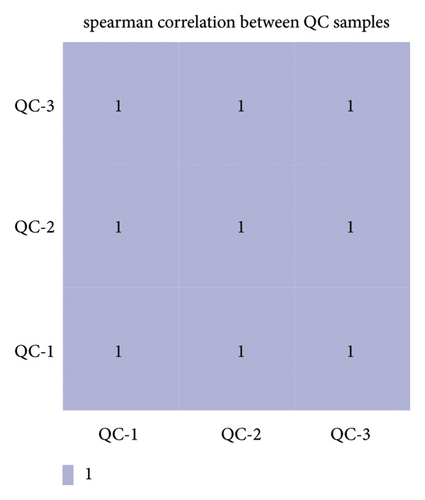
Initially, we confirmed the stability of the chromatography-mass spectrometry system. The base peak ion (BPI) peak retention times and peak areas of all QC samples exhibited complete reproducibility (Figures 2(a) and 2(b)). Spearman’s rank correlation was used to evaluate the correlation between QC samples, with the coefficient of determination equal to 1, indicating the reliability of the metabolomic results (Figure 2(c)).
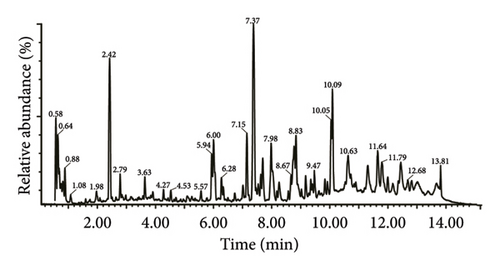
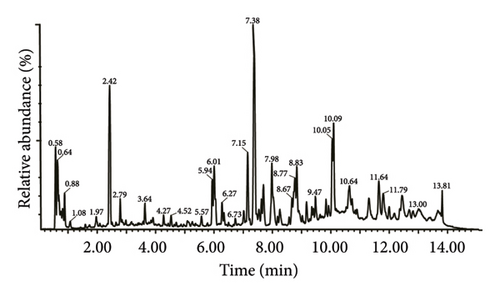
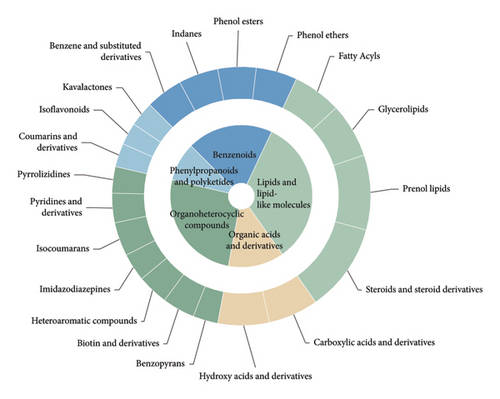
In total, 10,618 peaks were detected in the 18 ASR samples in the positive ion mode, with 778 metabolites being annotated. In the negative ion mode, 6,409 peaks were detected, and 313 metabolites were annotated. Because the positive ion mode provided richer metabolite information compared to the negative ion mode, our analysis primarily focuses on the metabolites detected in the positive ion mode. We initially annotated these metabolites using the HMDB database, and Figure 2(d) shows the top 20 classes with the most annotation information in the positive ion mode. The results showed that the five most frequently annotated metabolites were as follows: (1) lipids and lipid-like molecules, mainly comprising steroids and steroid derivatives, prenol lipids, glycerolipids, and fatty acyls, totaling 195 metabolites; (2) organic heterocyclic compounds, mainly comprising isocoumarins, biotin and derivatives, heteroaromatic compounds, pyridines and derivatives, imidazodiazepines, pyrrolizidines, and benzopyrans, totaling 194 metabolites; (3) benzenoids, consisting mainly of phenol ethers, phenol esters, indanes, benzene, and substituted derivatives, totaling 122 metabolites; (4) phenylpropanoids and polyketides, mainly comprising coumarins and derivatives, isoflavonoids, and kavalactones, totaling 78 metabolites; (5) organic acids and derivatives, primarily composed of carboxylic acids and derivatives, and hydroxy acids and derivatives, totaling 69 metabolites. The results showed that lipids and lipid-like molecules constituted the largest group among all ASR metabolites, followed by organ heterocyclic compounds and benzenoids. Lipids and lipid-like molecules were further classified using the LIPID MAPS database, and the top 20 categories with the most annotations were selected. Further classification of lipids and lipid-like molecules revealed seven categories: fatty acyls, polyketides, sterol lipids, prenol lipids, glycerophospholipids, glycerolipids, and sphingolipids (Supplementary Figure 1). The top 20 categories with the most annotated information in HMDB and LIPID MAPS databases under negative ion mode are shown in supplementary materials (Supplementary Figure 2 and Supplementary Figure 3). Previous studies using nuclear magnetic resonance metabolomics identified 36 primary and secondary metabolites in ASR, which can be broadly classified into amino acids and organic acids, saccharides, aromatic compounds, fatty acids, and other secondary metabolites [1]. Li et al. conducted nontargeted metabolomics detection on different varieties of A. sinensis (Oliv.) Diels and found that the key components were predominantly distributed among phenylpropanoids, lipids, and amino acids [7]. Additionally, the 30 compounds in ASR, characterized by Zhou et al. using nontargeted metabolomics, primarily belong to categories, such as phthalides, organic acids, amino acids, glycosides, esters, and coumarins [12]. These findings are consistent with the results of our metabolomic assay in characterizing and classifying ASR metabolites.
ASR is composed of numerous phytochemical ingredients, making it a valuable source of natural active products. Pharmacological studies have demonstrated that these ingredients exhibit various biological activities and play distinct roles in pharmacological actions, primarily influenced by their molecular structure [1, 4, 7–13, 21]. According to the classification information of metabolites based on UPLC-MS detection in this study, the characteristics of active ingredients of ASR were further supplemented. Specifically, many lipids and lipid-like molecules, such as steroids and their derivatives, fatty acids, and glycerophospholipids, exert positive effects on human health [7, 21]. Steroids and their derivatives, as important plant hormones, are prevalent in many CHMs. They form a crucial material basis for CHMs to regulate the endocrine system and enhance cardiovascular function [21]. Fatty acids play a vital role in maintaining human metabolism. Notably, ASR is rich in unsaturated fatty acids, such as linoleic acid, linolenic acid, and arachidonic acid [1, 7, 13], which are effective in protecting the liver and enhancing lipid metabolism. Furthermore, these fatty acids act as precursors for the synthesis of prostaglandins, the latter are also significant for improving lipid metabolism and hemodynamics [21]. Additionally, the role of glycerophospholipids in signal transduction and metabolic regulation is also noteworthy [7, 21]. The main subclasses of organic acid compounds included isocoumarin, biotin, indole, and their derivatives, which are typical active ingredients in CHMs [1, 4, 7, 13]. Isocoumarin and its derivatives have antibacterial, anticancer, and other biological activities. Biotin has a very important effect on the normal physiological activities of the human body and participates in the metabolism and synthesis of many substances. Indole and its derivatives have antitumor and anti-inflammatory effects [4, 7, 8, 13, 21]. Benzene compounds mainly include phenolic compounds, anthraquinones, and phenol esters. For example, ferulic and caffeic acids, as typical phenolic acids, widely exist in traditional Chinese medicine with the functions of promoting blood circulation and reducing blood stasis [4, 7, 8]. In contrast, anthraquinones and phenolic ether compounds such as catechol and other active ingredients of traditional Chinese medicine generally have anti-inflammatory and antitumor effects [21]. Among phenylpropanoids and polyketides compounds, coumarins and their derivatives have pharmacological effects such as antioxidant and anticoagulant activities, whereas isoflavones can lower cholesterol and prevent cardiovascular diseases [22]. Cinnamic acid and its derivatives regulate blood sugar levels and relieve pain. They are also the most common active ingredients in CHMs [4, 7, 8, 13, 21]. Amino acids, short-chain polypeptides, typical organic acids such as malic acid, and some lactones belong to the carboxylic acid group, and they usually play roles in improving general health, as well as bacteriostatic, hypoglycemic, antioxidant, and immune regulating effects. They can also increase coronary blood flow and prevent atherosclerosis [7, 8, 13, 21]. Overall, the biological activities characterized by the classification information of these metabolites are consistent with the multiple medicinal efficacies of ASR [4, 7, 8, 13].
3.2. Analysis of Metabolite Distribution Patterns across Various Medicinal Segments of ASR
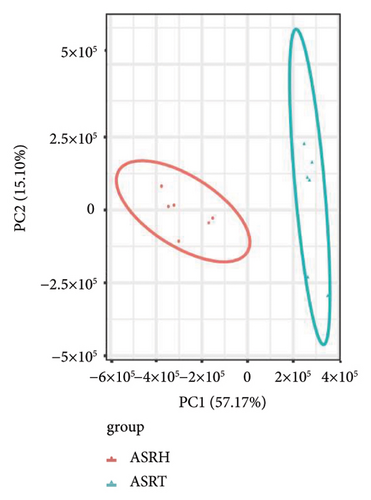
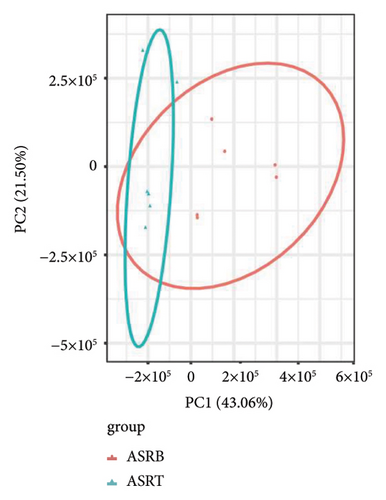
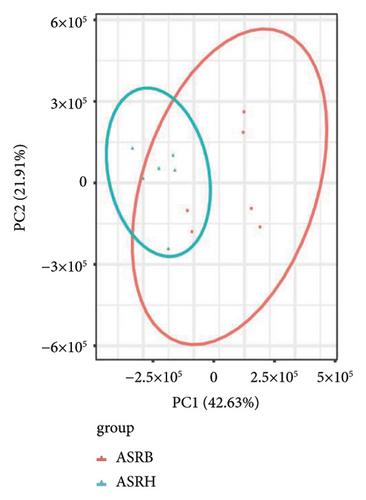
To assess the variations in overall metabolism between groups and the correlations among samples within each group, we conducted PCA on the identified metabolites. The cumulative contribution rate of PC1 and PC2 exceeded 60%, as shown in Figures 3(a), 3(b), and 3(c). The PCA scores of samples from different segments exhibited significant differences and fell within the 95% confidence interval. In this diagram, the metabolites in ASRH and ASRT have the most apparent separation trend. In contrast, the metabolites in ASRB, ASRH, and ASRT have a partial intersection, which indicates that ASRH and ASRT may have the greatest difference in pharmacodynamics, whereas ASRB is partially similar to ASRH and ASRT. The results of the analysis of metabolite distribution patterns across various medicinal segments of ASR are consistent with the spatial relationship among ASRT, ASRH, and ASRB.
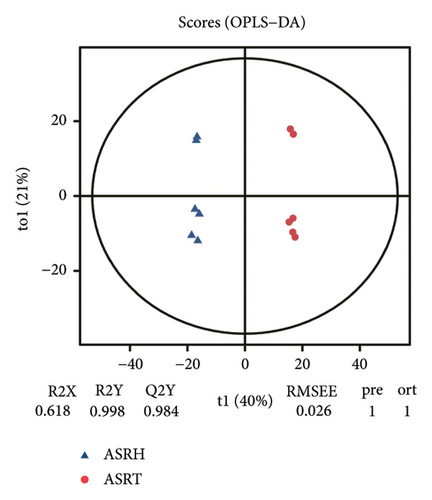
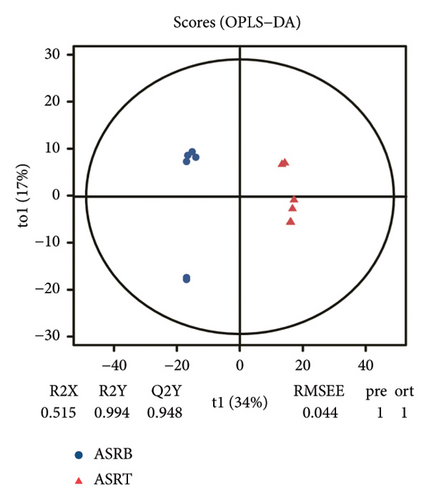
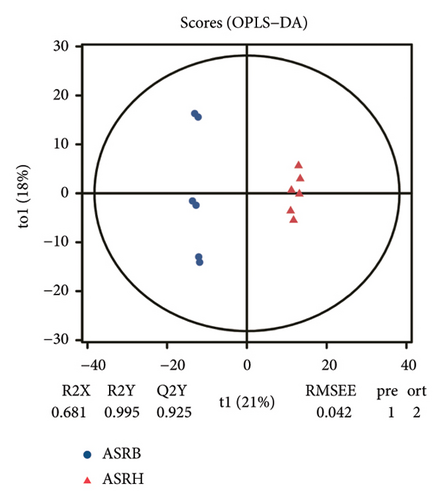
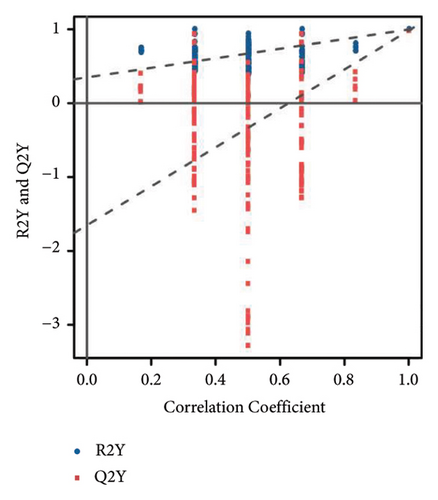
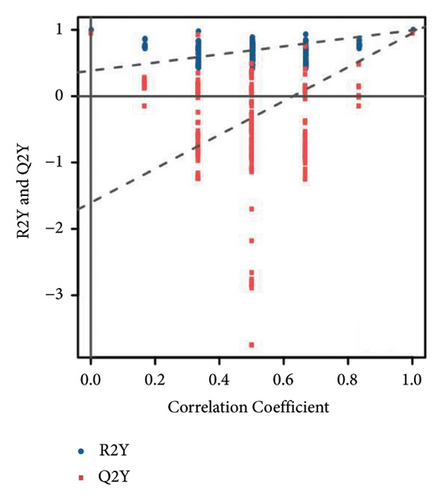
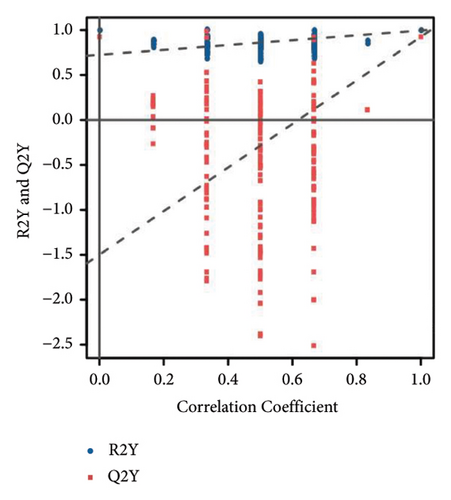
To further clarify the metabolite differences between medicinal segments and eliminate the influence of irrelevant variables, we subjected the experimental results to the orthogonal projections to latent structure-determinant analysis (OPLS-DA). This method helped screen for variables that contributed to the observed differences. The parameters R2X and R2Y represent the interpretation rates of X and Y matrices, respectively. Here, the X matrix represents the model input (metabolite quantitative matrix), whereas the Y matrix represents the model output (sample grouping matrix). Additionally, Q2Y represents the predictive ability of the model, indicating whether it can accurately distinguish sample groups through metabolic expression. The model scores of each group are shown in Figures 3(d), 3(e), and 3(f). Q2Y for each group exceeded 0.9, R2X was greater than 0.5, and R2Y was close to 1. These values collectively suggest that the model is both stable and reliable. We further validated the model’s reliability through a permutation test, obtaining the corresponding permutation test diagram as shown in Figures 3(g), 3(g), and 3(i). In this test, the positive slope of the Q2Y fitting regression line and R2Y > Q2Y demonstrates good independence between the modeling training set and the test set, confirming the absence of overfitting. Consequently, the model is deemed meaningful and serves as a solid foundation for subsequent model analysis. The OPLS-DA model fitting of metabolomic data revealed significant between-group differences and within-group consistency of samples, all within the 95% confidence interval. This clearly demonstrates that metabolites from different medicinal segments of ASR exhibit a separation trend and notable differences. Existing reports involving discriminant analyses based on multiple ingredients of ASR segments have reached similar conclusions [1, 4, 13]. For instance, Yang et al. performed a comparative metabolomics study of ASRH and ASRT. Both PCA and partial least squares discriminant analysis demonstrated a clear separation trend between the two segments, with significant differences between groups [4].
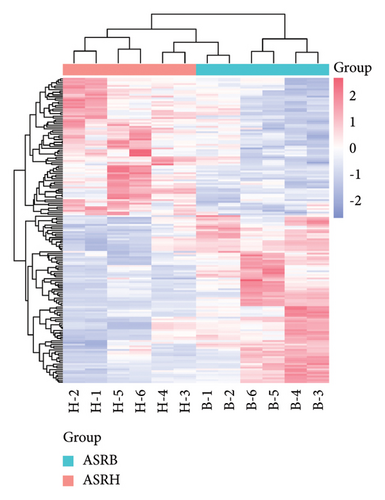
3.3. Analysis of Differential Metabolites between Medicinal Segments of ASR
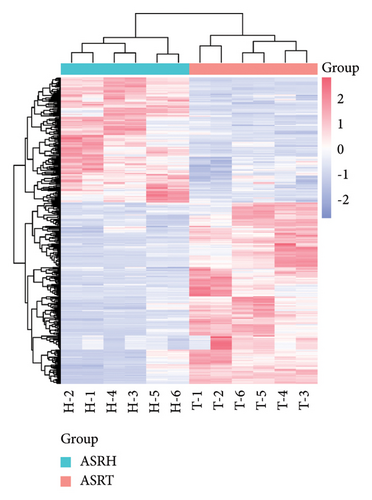
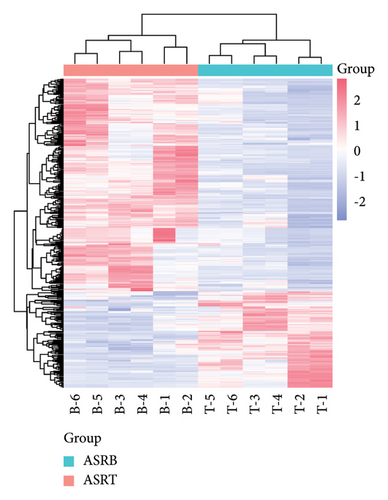
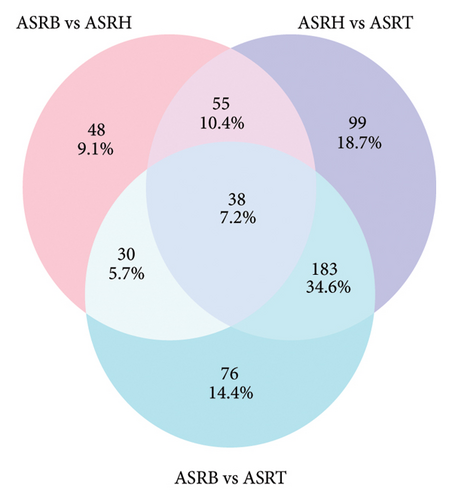
To explore the differential metabolites between medicinal segments of ASR, we conducted a comparative analysis of ASRH, ASRB, and ASRT. In the comparison between ASRH and ASRT, 375 differential metabolites were identified. Among these, 222 were downregulated metabolites, and 153 were upregulated metabolites. Similarly, in the ASRH vs. ASRB comparison, we identified 171 metabolites, with 94 being downregulated and 77 upregulated. There were 327 differential metabolites in the ASRB vs. ASRT comparison, with 225 downregulated and 102 upregulated metabolites. To visualize the differential metabolites and their abundance, the hierarchical clustering heatmap of samples was drawn based on all pairwise comparisons of medicinal segments (Figures 4(a), 4(b), and 4(c)). The results showed that samples from different segments tend to separate from each other, indicating considerable differences in the distribution of differential metabolites among different segments. Conversely, samples from the same segment cluster together, indicating similarities in the distribution patterns of differential metabolites. Further classification of the differential metabolites using a Venn diagram (Figure 4(d)) revealed 38 common differential metabolites and 48, 99, and 76 unique differential metabolites in the three comparison groups (ASRH vs ASRT, ASRH vs ASRB, and ASRT vs ASRB, respectively). Previous comparative studies on the metabolome profiles of ASRH and ASRT have shown that the proportion of differential metabolites relative to all identified ingredients is approximately 30% [4]. This proportion closely aligns with the findings of this study. Overall, a comprehensive comparison of the number of differential metabolites and the magnitude of differences revealed that ASRH and ASRT exhibit the most considerable difference, ASRB and ASRT fell in the middle, and ASRH and ASRB had the smallest discrepancy. This observation aligns with the spatial relationship and functional differences among ASRT, ASRB, and ASRT, with ASRB appearing to be in a transitional state.
In the comparison between ASRH and ASRT, the active ingredients with high content in ASRT included arachidonic acid, (±)7-epi jasmonic acid, amygdalin, corchorusoside E, and zingerone. In ASRH, the active ingredients with high content included melezitose, L-citrulline, folinic acid, salannin, and abscisic acid. In comparing ASRH and ASRB, the active ingredients with high content in ASRB included lysyl-proline, quinone, Gly-Ile-Lys-Arg, Gly-Asn-Gly, and lysyl-proline. Conversely, ASRH contained high levels of glycyl-arginine, malic acid, Ser-Ala-Asn, abscisic acid, and aloin A. When comparing ASRB and ASRT, it was found that ASRT had higher levels of arachidonic acid, echinacoside, zingerone, amygdalin, and genkwanin, while ASRB had higher levels of virginiamycin S1, L-citrulline, DL-arginine, L-arginine, and D-lysine.
Among these active ingredients, arachidonic acid, zingerone, and amygdalin are known to promote blood circulation [23–25]. Gly-Ile-Lys-Arg, Gly-Asn-Gly, and lysyl-proline are short-chain polypeptides, whereas L-citrulline, DL-arginine, L-arginine, and D-lysine are amino acids known for their blood-enriching properties [7, 8, 13, 21]. Abscisic acid and aloin A have hemostatic effects [26–28]. Therefore, the main differences in metabolites among segments highlighted the respective roles of ASRT in promoting blood circulation, ASRB in enriching blood, and ASRH in stopping bleeding.
3.4. Construction of Medicinal Segments-Active Ingredients-Targeted Proteins Network
Based on the differential distribution patterns of metabolites among ASRH, ASRB, and ASRT, the dominant metabolites in different segments were identified. These dominant metabolites reflect the specific and quantitative advantages of each segment relative to other segments. The screening results showed that there were 46, 27, and 154 dominant metabolites in ASRH, ASRB, and ASRT in the positive ion mode, respectively, and 9, 7, and 76 dominant metabolites in ASRH, ASRB, and ASRT in the negative ion mode, respectively. ASRB has the fewest dominant metabolites, which illustrates that in terms of ingredient composition, it is in a transitional state and has some common efficacies with ASRH and ASRT. According to the results of cluster analysis and pairwise comparison (Figures 3 and 4), the difference between ASRH and ASRB was the smallest, and both of them show fewer dominant metabolites, which indicates that their metabolite distribution patterns and corresponding efficacy characteristics are similar. Therefore, we treated ASRH and ASRB as a combined entity and compared their dominant metabolites with those of ASRT. Finally, we found 230 metabolites where ASRT had an advantage over ASRH and ASRB and 254 metabolites where ASRH and ASRB had an advantage over ASRT.
Through the comprehensive statistical analyses and database annotations, the dominant active ingredients in different segments of ASR were highlighted. The dominant ingredients in ASRT are mainly annotated as promoting blood circulation and removing blood stasis, whereas those in ASRH and ASRB highlighted their hemostatic and blood-enriching effects (Supplementary Table 2 and Supplementary Table 3). Arachidonic acid, eicosapentaenoic acid, and zingerone were the main active ingredients within the dominant metabolites of ASRT. Arachidonic acid, as an important unsaturated fatty acid, plays a crucial role in blood and liver functions, promoting cholesterol esterification, enhancing vascular elasticity, lowering blood viscosity, and regulating blood cell functions. It inhibits platelet aggregation, releases vasodilators, and relaxes vascular smooth muscle, leading to vasodilation under specific stimuli [7, 21, 23]. The unsaturated fatty acid-rich property of ASR was also reflected in previous metabolomic studies [1, 7, 13]. Metabolites of eicosapentaenoic acid, such as prostacyclin, contribute to inhibiting platelet aggregation and vasodilation, acting synergistically against inflammation [21]. Zingerone is an antithrombotic compound with FXa inhibitory and antiplatelet aggregation activities [24]. Furthermore, significant active ingredients in ASRT included 4-methylesculetin, amygdalin, and salidroside. 4-Methylesculetin, a coumarin-type compound, has protective effects on blood vessels and can help prevent atherosclerosis. Coumarin compounds are recognized for their effective anticoagulant properties and have been highlighted in both routine detections and metabolomic tests of ASR [4, 7, 13, 21, 22, 29]. Amygdalin and salidroside are glycoacetal derivatives. Previous metabolomic studies have demonstrated that glycosides are significant metabolite components of ASR. Amygdalin can reduce blood viscosity, promote vasodilation, and enhance normal blood flow [25]. Salidroside inhibits platelet function and thrombus formation through the AKT/GSK3β signaling pathway, promoting blood circulation [30]. Other compounds, such as naringin, riboflavin, and (S)-bilobanone, also contribute to blood activation [31].
Among the dominant active ingredients in ASRH and ASRB (Supplementary Table 3), aloin A promotes the exfoliation of necrotic cells and promotes wound healing and recovery [26], whereas abscisic acid affects the number of hematopoietic stem and progenitor cells, megakaryocytes, and vitro-differentiated platelets, benefiting the induction of differentiation of megakaryocytes and platelets by human induced pluripotent stem cells [27, 28]. These active ingredients collectively exhibit potential hemostatic effects, with ASRH containing the highest content, emphasizing its hemostatic properties. Quinone, a dominant component in ASRB, is particularly significant in traditional Chinese medicine. It promotes platelet production, increases fibrinogen levels, shortens coagulation time, reduces capillary permeability, and enhances blood vessel contraction, thereby promoting blood coagulation [31]. Both ASRH and ASRB were revealed to contain abundant amino acids, short-chain polypeptides, and their derivatives, which play crucial roles in enhancing body function, improving immunity, and promoting health. For instance, many of these ingredients can serve as effective nutritional supplements. L-arginine has notable hemostatic efficacy [21], with its content in ASRH and ASRT being substantial, and ASRH slightly higher than ASRT. L-citrulline, the metabolite with the highest upregulation in ARB, is an α-amino acid with health benefits that can also improve immunity and alleviate fatigue and stress [7, 21]. The short-chain polypeptides have been extensively reported to promote wound healing, enhance hematopoietic function, treat anemia, prevent platelet aggregation, improve red blood cell oxygen-carrying capacity, and repair cells [7, 8, 21]. Studies on amino acids and metabolome of ASR predominantly detected abundant amino acids and their derivatives, such as tryptophan, tyrosine, leucine, isoleucine, proline, valine, arginine, and citrulline [1, 4, 7, 8, 13]. The amino acid contents in different segments were ranked as ASRH > ASRB > ASRT [8], consistent with the trends observed in this study, supporting the reliability of further analyses based on these qualitative and quantitative characteristics. For other dominant components in ASRH and ASRB, nicotinamide adenine dinucleotide primarily promotes nutrient metabolism and energy biosynthesis. Melezitose has been shown to increase glucose and fructose in the bloodstream, helping maintain blood sugar levels [31]. Sucrose contributes to blood enrichment and promotes blood circulation. Folinic acid addresses megaloblastic anemia caused by folinic acid deficiency [7, 21]. In terms of the preliminary function investigation on these dominant ingredients, ASRT primarily focuses on promoting blood circulation, and ASRH and ASRB share similar effects, with those of ASRH emphasizing hemostasis and those of ASRB focusing on blood enrichment.
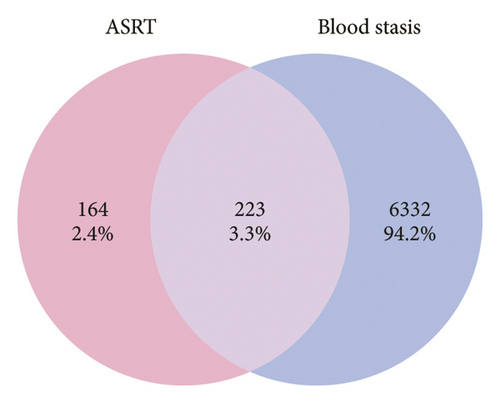
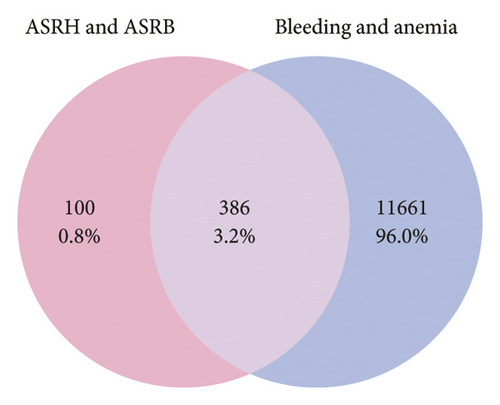
We further conducted a network pharmacology analysis, resulting in 15 eligible main active ingredients obtained from ASRT, along with 387 targeted proteins, after standardization and removal of duplicate values. Similarly, we obtained 30 main active ingredients from ASRH and ASRB and their 486 targeted proteins. Using GeneCards and OMIM databases, 6,555 targeted proteins associated with blood stasis diseases corresponding to the blood-activating properties of ASRT were identified. Furthermore, 12,047 targeted proteins related to bleeding and anemia were collected considering the hemostatic and blood-enriching effects of ASRH and ASRB. There were 223 intersecting targeted proteins between the dominant active ingredients in ASRT and blood stasis diseases, and 386 intersecting targeted proteins between the active ingredients in ASRH and ASRB and bleeding disorders and anemia (Figures 5(a) and 5(b)). These data sets and their connections allow us to construct a “medicinal segments-active ingredients-targeted proteins” network, in which triangles represented ASR medicinal segments, circles represented active ingredients, and rectangles represented targeted proteins corresponding to active ingredients. The connection between nodes indicated interactions between active ingredients and targeted proteins. The network diagram of dominant active ingredients in ASRT and blood stasis diseases consists of 235 nodes and 396 edges (Figure 5(c)), whereas the network of dominant active ingredients in ASRH and ASRB and targeted proteins related to hemorrhage and anemia consists of 392 nodes and 988 edges (Figure 5(d)). Active ingredients were sorted according to their degree values and betweenness centralities, with higher degree values signifying greater importance in terms of their impact on diseases. As shown in Tables 1 and 2, the dominant active ingredients recorded in the pharmacology database that binds to key target proteins exhibit highly favorable qualitative scores. Most parent ion mass deviations are less than 10 ppm, the mass-to-charge ratios are accurate, and the retention times are stable and reasonable [4, 7–14]. This may be because the extensively studied components documented in the database tend to be relatively stable under common instrument conditions and operating parameters, which probably also serves as a guarantee for their extensive research. Meanwhile, these active ingredients, which will be analyzed subsequently, belong to the primary ingredient categories of ASR identified in previous studies, including organic acids, coumarins and their derivatives, amino acids and their derivatives, saccharides and their derivatives, and flavanones (Supplementary Table 4). Furthermore, the compounds characterized in this study significantly overlap with the qualitative results of prior research utilizing chromatography and mass spectrometry (Supplementary Table 4) [4–13, 32–41]. According to the degree values and betweenness centralities indicated by the network topology structure, the top 50% of the ASRT active ingredients that interact widely with disease-related proteins included arachidonic acid, eicosapentaenoic acid, zingerone, 4-methylesculetin, 8-8′-dehydrodiferulic acid, amygdalin, (S)-bilobanone, and naringin. According to the function annotation aforementioned, most of these ingredients have been revealed to show the activities of improving cardiovascular system (mainly promoting blood circulation), enhancing immunity, antibacterial, anti-inflammatory, and antiviral, which is consistent with the representative efficacies of ASRT. For ASRH/ASRB, amino acids and short-chain polypeptides are the most active ingredients with a high degree value and betweenness centrality, which have potential blood-enriching effects as important nutritional supplements, hematopoietic activator, and immunopotentiators [7, 8, 13, 21]. In addition, the contents of folinic acid and nicotinamide adenine dinucleotide (NAD) in ASRH and ASRB are higher than those in ASRT. They are important coenzymes in biochemical reactions, playing a healthcare effect [4, 7, 21]. As the dominant ingredient of ASRH, aloin A has a hemostatic effect [21]. It is worth noting that the content of 4-hydroxy-3-methoxycinnamic acid, as a typical blood-activating ingredient, in ASRB is slightly higher than that in ASRT. Previous studies have shown that the content of 4-hydroxy-3-methoxycinnamic acid in ASRH is the lowest, but there is no significant difference in other parts [4, 8]. On the whole, most of the dominant ingredients in ASRH/ASRB, which are closely related to disease targets, mainly highlighted their blood-enriching and hemostatic effects.

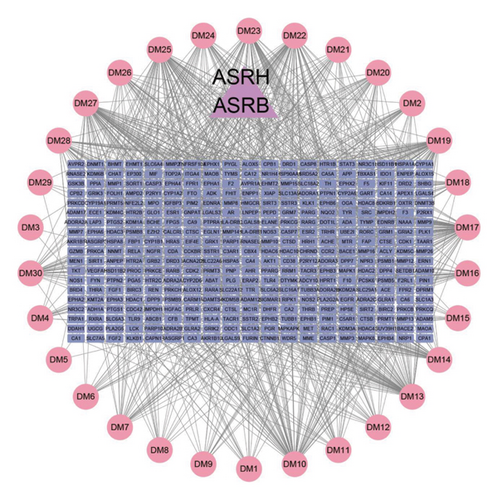
3.5. Construction of PPI Network and Screening of Core Targeted Proteins with a Significance Level
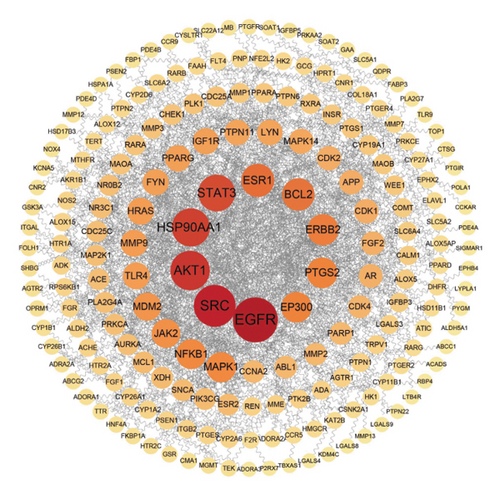
The intersection of disease-related targeted proteins and active ingredients-acting targeted proteins underwent standardization process through the STRING database and were then imported into Cytoscape 3.9.1 software to create PPI network diagrams, where the size and color depth of targeted proteins corresponded to their importance degree. Larger circles and darker colors indicated higher degree values. These targeted proteins were sorted based on topological properties. The results show that the PPI network of ASRT comprised 197 nodes and 772 edges (Figure 6(a)), with EGFR, SRC, AKT1, HSP90AA1, STAT3, ESR1, BCL2, ERBB2, PTGS2, and EP300 emerging as core targeted proteins in treating blood stasis. The PPI network of ASRH and ASRB included 340 nodes and 3,356 edges (Figure 6(b)), with CTNNB1, AKT1, SRC, EP300, STAT3, EGFR, ESR1, CASP3, HSP90AA1, and HDAC1 identified as core targeted proteins for the treatment of hemorrhage and anemia. For the targeted proteins associated with the dominant active ingredients in ASRT, Zhang et al. emphasized the importance of SRC, AKT1, EGFR, and other targeted proteins in the efficacy of Notoginseng radix et rhizoma and Salviae Miltiorrhizae radix et rhizoma for treating traumatic blood stasis syndrome [42]. Li et al. found that AKT1 and CASP3 are the key targeted proteins of Danggui Buxue Decoction in treating aplastic anemia [43]. Furthermore, Gu et al. found that ATK1, CASP3, STAT3, and other targeted proteins were crucial in the treatment of abnormal uterine bleeding using Sanguisorba officinalis and Rubia cordifolia herbs [44]. Thus, these target proteins that we have obtained based on network pharmacology were mostly reported to play a role in the process of improving cardiovascular system, which is consistent with the most concerned efficacy of ASRT.
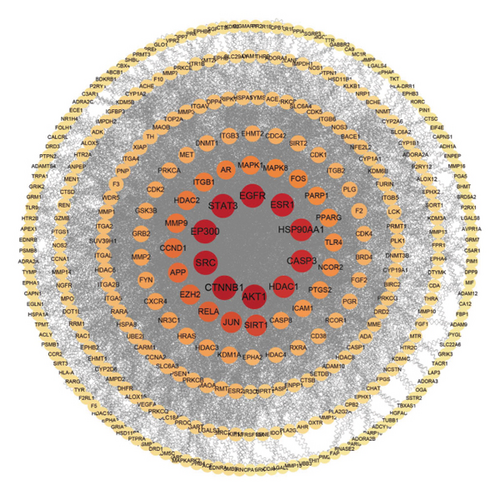
For the proteins targeted by the dominant active ingredients in ASRH and ASRB, SRC is involved in various signal pathways controlling biological activities, including gene transcription, immune response, cell adhesion, and apoptosis. Activation of SRC can significantly increase the content of vascular endothelial growth factor, enhancing vascular permeability [45, 46]. AKT1, present in vascular cells, impacts numerous regulatory pathways, including antiangiogenesis, vascular permeability regulation, angiogenesis response, and vascular maturation [47]. AKT1 is the core factor in the PI3K/AKT signaling pathway, which enhances the hematopoietic function and proliferation and differentiation of hematopoietic stem cells and progenitor cells after activation [48]. Proteins encoded by CTNNB1 play an essential role in adhesion junctions, which contribute to epithelial cell layer construction and maintenance. STAT3 is involved in many biological processes, such as cell proliferation, survival, differentiation, and angiogenesis, thus regulating wound healing [49]. Phosphorylation of STAT3 regulates the activity of the TXA2 receptor, thereby regulating platelet activation [50]. ESR1 contributes to vascular system regulation, injured blood vessel recovery, and central nervous system protection [51]. For the targeted proteins of the dominant active ingredients in ASRH/ASRB, previous studies have revealed that most of them can promote hematopoietic and hemostatic functions, which is consistent with the main efficacies of ASRH and ASRB.
3.6. Biological Pathways Enriched by Active Ingredients-Targeted Proteins for Varying Medicinal Segments
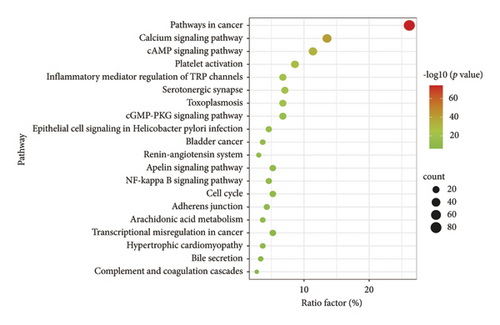
To gain insights into the biological pathways involved in the active ingredients-targeted proteins for varying medicinal segments, GO and KEGG enrichment analyses were performed (Figures 7 and 8). For potential targeted proteins responsible for the blood-activating effect of ASRT, GO enrichment analysis yielded a total of 2,360 items, comprising 148 cellular components (CC), 223 molecular functions (MF), and 1,989 biological processes (BP). The top 20 enrichments within each process are meticulously detailed in Figure 7(a). Enriched CC mainly includes the perinuclear region of cytoplasm, neuronal cell body, side of the membrane, and membrane raft. Enriched MF mainly includes phosphotransferase activity, oxidoreductase activity, kinase binding, protein domain-specific binding, and protein homodimerization activity. Pertaining to BP, pathways relevant to blood stasis predominantly involve responses to hormones, circulatory system process, regulation of inflammatory response, inflammatory responses, regulation of hormone levels, and responses to wounding. Subsequently, we conducted the KEGG enrichment analysis on potential targeted proteins for ASRT, identifying 189 items (Figure 8(a)). The top 20 items encompass numerous pathways related to blood circulation, including the calcium, PI3K-Akt, cAMP, apelin, cGMP-PKG, IL-17, and NF-kappa B signaling pathways, as well as arachidonic acid metabolism, the renin-angiotensin system, platelet activation, and vascular smooth muscle contraction. The enrichment results for intersecting target pathways in ASRT align with the biological mechanisms underpinning blood circulation promotion. In the KEGG analysis, by regulating calcium concentrations in smooth muscle cells, the calcium signaling pathway promotes smooth muscle relaxation, thereby dilating blood vessels and enhancing blood circulation [52]. The PI3K/Akt signaling pathway mediates various apoptosis factors through downstream targets to achieve signal regulation, which can protect myocardial cells and inhibit their apoptosis, thus slowing down the progress of heart failure [53, 54]. Activation of the cAMP signaling pathway can interfere with thrombus formation, dilate blood vessels, and increase myocardial blood supply and oxygen delivery [55]. The cGMP-PKG signaling pathway plays a role in regulating physiological processes, such as myocardial contraction, relaxation, and vasodilation, whereas the renin-angiotensin system participates in vasodilation and blood pressure regulation [56]. Furthermore, the apelin signaling pathway, a part of the adipocytokine signaling pathway, regulates cellular physiological functions via its receptor APJ. This pathway impacts the cardiovascular system by modulating myocardial contractility, vasomotor responses, and blood pressure. It also plays a vital role in regulating energy metabolism, inflammatory responses, and immune activities [57]. IL-17 mediates autoimmune and inflammatory responses. As a mediator of immune response, IL-17 can damage vascular endothelial cells. The combination of IL-17 and TNF-α can increase platelet aggregation, inhibit anticoagulation, and lead to thrombosis [58]. The NF-kappa B signaling pathway and arachidonic acid metabolism are pathways related to inflammation, making them instrumental in promoting blood circulation and removing blood stasis [57]. Additionally, signaling pathways such as platelet activation and vascular smooth muscle contraction are also associated with blood stasis [57]. For ASRT, the biological pathways enriched by the targeted proteins of dominant active ingredients were consistent with its efficacies, especially promoting blood circulation.
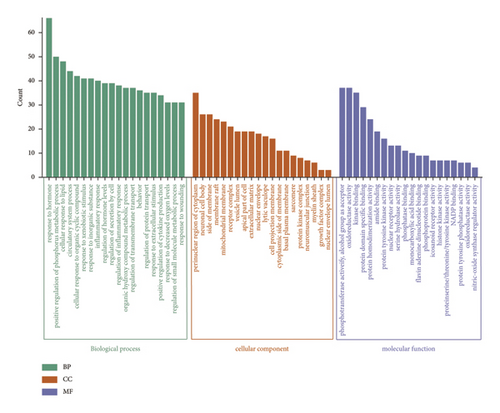
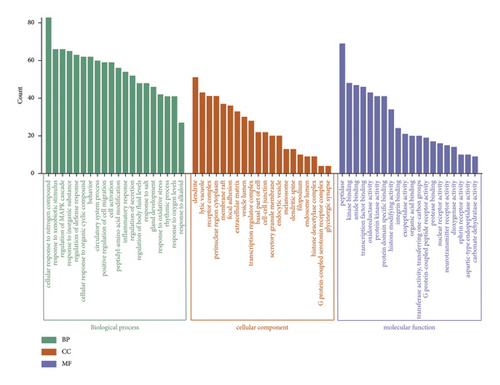
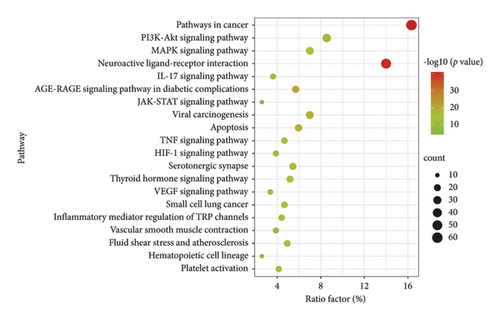
Potential target proteins of the dominant active ingredients in ASRH and ASRB were also substituted into a GO enrichment analysis, yielding 2,972 items. These encompassed 183 CC, 333 MF, and 2,456 BP items. From Figure 7(b), the enriched CC mainly included dendrites, membrane rafts, receptor complexes, and focal adhesions. Enriched MF comprised activities, such as peptidase activity, amide binding, histone-modifying activity, and transcription factor binding. The pathways related to hemostasis and blood enrichment enriched in BP included circulatory system processes, regulation of the MAPK cascade, positive regulation of cell migration, regulation of body fluid levels, inflammatory responses, cell activation, gland development, and secretion regulation. In the KEGG enrichment analysis, 222 items were obtained. Among the top 20 items (Figure 8(b)), many pathways were related to hemostatic and blood enrichment effects, including the PI3K-Akt, MAPK, IL-17, AGE-RAGE in diabetic complications, JAK-STAT, TNF, HIF-1, and VEGF signaling pathways, as well as fluid shear stress and atherosclerosis, hematopoietic cell lineage, and platelet activation [59–66]. The pathway enrichment results of the ASRH/ASRB active ingredients-targeted proteins align with the biological mechanisms underpinning hemostasis and blood enrichment. In the KEGG pathway enrichment analysis, the PI3K-Akt signaling pathway, a pivotal regulator of inflammation and angiogenesis, is involved in platelet activation, platelet aggregation, and various inflammatory reactions [59], activating related growth factors and playing an important role in wound angiogenesis [60]. The MAPK signaling pathway is involved in treating aplastic anemia and is mainly involved in platelet activation and aggregation induced by stimuli, such as ADP and thrombin [61]. The IL-17 and IL-6 signaling pathways promote angiogenesis, whereas the AGE-RAGE promotes wound healing [62]. Specifically, the interaction between AGE and the RAGE receptor can affect the proliferation of fibroblasts and inhibit their ability to synthesize collagen fibers, thus affecting wound healing [63]. The JAK-STAT pathway, activated by interleukins, interferons, and hemopoietin growth factors, regulates bone marrow cell development and differentiation, thereby enhancing hematopoietic function [64]. TNF, an important inflammatory factor, can induce the production of IL-2 and IL-6, enhance neutrophil adhesion to vascular endothelium, and affect coagulation and tissue factors, thereby regulating hemostasis [65]. The HIF-1 signaling pathway is directly involved in hematopoietic function. HIF is a transcription factor, which is very important to the body’s oxygen level. It can activate genes encoding proteins involved in hypoxic homeostasis and induce the expression of proteins controlling cell proliferation and angiogenesis [66]. The main function of the VEGF pathway is to promote angiogenesis while preserving existing blood vessels. Upon VEGF binding to VEGFR-2, diverse cascade signaling pathways are initiated. This process mediates endothelial cell proliferation, migration, and increased vascular permeability [57]. Overall, hematopoietic cell lineage activation, vascular protection, and platelet release and aggregation are closely associated with blood-enriching and hemostasis effects in ASRH and ASRB.
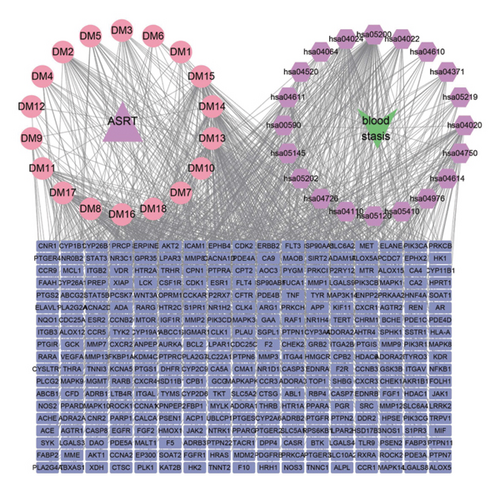
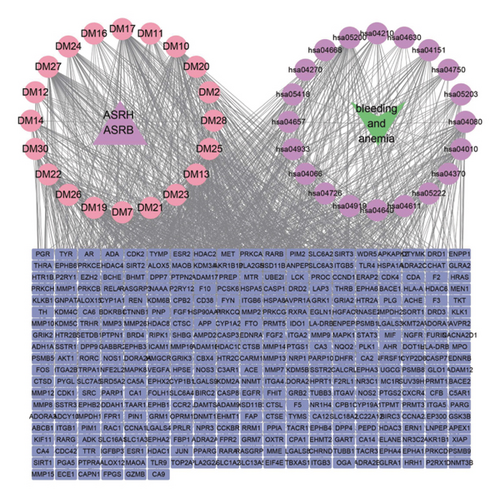
3.7. Networks Construction among Medicinal Segments, Active Ingredients, Targeted Proteins, Signaling Pathways, and Diseases
To further clarify the interactions between metabolic ingredients and diseases in various medicinal segments, the top active ingredients, disease-related targeted proteins, and enriched biological pathways for ASRT and ASRH/ASRB were further screened. Using Cytoscape 3.9.1 software, an interaction network among these elements was constructed (Figure 9). The network analysis revealed that 15 main active ingredients, including arachidonic acid, eicosapentaenoic acid, and zingerone found in ASRT, primarily function by regulating the calcium, PI3K-Akt, and arachidonic acid metabolism signaling pathways. They targeted key proteins such as EGFR, SRC, AKT1, HSP90AA1, and STAT3, thereby promoting blood circulation. The 30 main active ingredients in ASRH and ASRB, such as aloin A, abscisic acid, quinones, short-chain polypeptides, and amino acids, mainly regulate the PI3K-Akt, IL-17, and JAK-STAT signaling pathways. They act on targeted proteins such as CTNNB1, AKT1, SRC, EP300, and STAT3, thus exerting hemostatic and blood-enriching effects. These insights into the pharmacological mechanisms underscores how the active ingredients in ASRT exhibit blood circulation-enhancing effects (Figure 9(a)), whereas how those in ASRH and ASRB play a role in blood-enriching and blood stasis effects (Figure 9(b)). This visual representation of the interaction modes on ASR medicinal segments-active ingredients-targeted proteins-signaling pathway-diseases unveils a complex web that multiple active ingredients targeting the same or multiple targeted proteins, influencing various signaling pathways. In the study of the mechanism of Chinese medicine in preventing and treating diseases, collaboration among multiple ingredients, targeted proteins, and pathways is vital. Ma et al. found that Zanthoxyli Radix plays a role in promoting blood circulation and removing blood stasis through the multiple ingredients, targeted proteins, and pathways, and its mechanism may be related to VEGF, platelet activation, and other signaling pathways [55]. Gong et al. found that isorhamnetin, quercetin-3-methyl ether, and other chemical ingredients in Sophora japonica can act on many target proteins, such as MAPK1, TNF, and JUN, and regulate multiple signaling pathways, such as TNF and hepatitis B, thus exerting hemostatic effect [65]. Therefore, the pharmacological mechanism of each ASR segment has a same characteristic as other representative CHMs, that is, multiple ingredients with multiple targets and pathways.
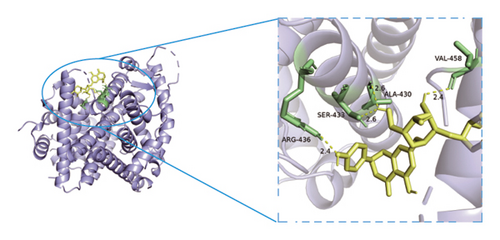
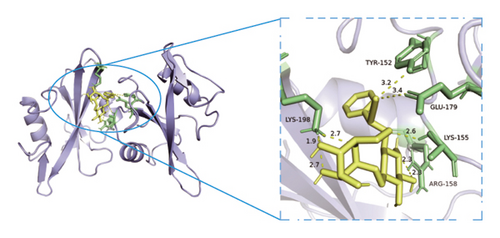
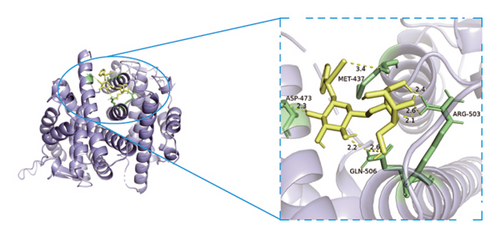
3.8. Verification of Key Nodes in the Network of Dominant Ingredients-Targeted Proteins-Signaling Pathways through Molecular Docking
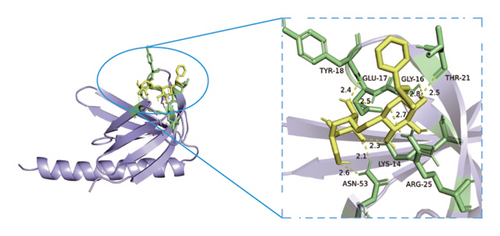
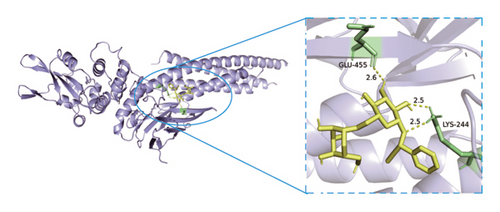
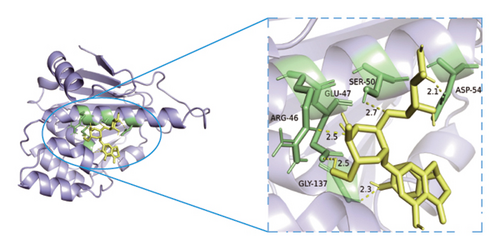
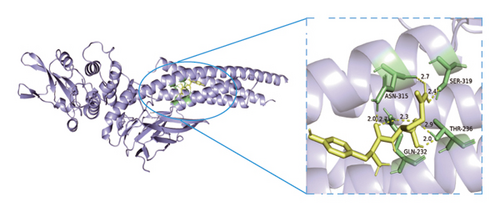
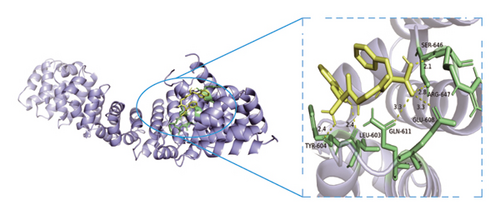
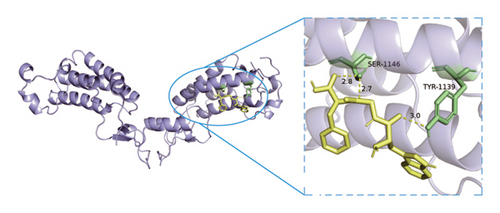
Based on metabolomics and network pharmacology quantification results, eight important active ingredients and six core target proteins in ASRH, ASRB, and ASRT were docked by AutoDock Tools 1.5, respectively. “Affinity” indicates the binding force, and the binding energy between the compound and the target is ≤−1.2 kcal/mol (−5.0 kJ/mol, 1 kcal/mol = 4.18 kJ/mol), which indicates that the compound has good affinity with its target [67]. The lower the binding energy, the more stable the interaction between them. The docking results of key ingredients and core targeted protein in ASRT are shown in Table 3, and all groups have good affinity. The first six groups with good docking results according to binding energy are ESR1-DM3, SRC-DM6, ESR1-DM6, AKT1-DM6, STAT3-DM6, and HSP90AA1-DM3. The optimal docking conformation is visualized by PyMoL 2.5. 4 software, and its conformational relationship is visually displayed (Figures 10(a), 10(b), 10(c), 10(d), 10(e), and 10(f)). The docking results of active ingredients in ASRH and ASRB with targeted proteins are shown in Table 4. The top five docking results are STAT3-DM27, STAT3-DM17, SRC-DM17, CTNNB1-DM27, EP300-DM27, and AKT1-DM27. DM17 and DM27 are tyrosyl-glutamine and Trp-Gly-Phe, respectively, belonging to short-chain polypeptides and derivatives. The docking details in an optimal conformation were visualized, demonstrating efficient binding between these active ingredients and their target proteins (Figures 10(g), 10(h), 10(i), 10(j), 10(k), and 10(l)). Notably, ingredients with hemostatic functions, such as (+)-abscisic acid (DM28) and aloin A (DM30), formed multiple hydrogen bond interactions with their target proteins, enabling stable binding.
| Targeted proteins | Affinity (kcal·mol−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| DM8 | DM9 | DM12 | DM15 | DM11 | DM6 | DM10 | DM3 | |
| EGFR | −2.4 | −3.1 | −3.5 | −4.9 | −3.9 | −5 | −4.6 | −5.2 |
| SRC | −3.8 | −3.1 | −4.3 | −5.4 | −5.0 | −7.8 | −5.1 | −6.6 |
| AKT1 | −3.0 | −3.6 | −4.0 | −5.5 | −4.8 | −7.1 | −4.8 | −6.7 |
| HSP90AA1 | −3.6 | −4.0 | −3.9 | −5.1 | −5.0 | −6.7 | −5.7 | −6.8 |
| STAT3 | −4.3 | −4.1 | −4.9 | −5.7 | −5.7 | −7.1 | −6.5 | −6.5 |
| ESR1 | −3.6 | −4.0 | −4.5 | −5.4 | −5.8 | −7.8 | −5.9 | −7.9 |



| Targeted proteins | Affinity (kcal·mol−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DM17 | DM10 | DM27 | DM13 | DM25 | DM22 | DM19 | DM23 | DM28 | DM30 | |
| CTNNB1 | −5.8 | −6.0 | −6.2 | −5.6 | −5.6 | −5.4 | −4.8 | −4.8 | −5.4 | −6.6 |
| AKT1 | −5.7 | −5.0 | −6.1 | −4.0 | −4.5 | −4.0 | −4.3 | −4.1 | −5.1 | −7.0 |
| SRC | −6.4 | −5.5 | −5.6 | −4.8 | −5.6 | −5.7 | −4.3 | −5.7 | −5.1 | −7.0 |
| EP300 | −5.8 | −5.7 | −6.2 | −5.0 | −5.6 | −4.8 | −4.7 | −4.2 | −5.6 | −7.1 |
| STAT3 | −6.5 | −6.0 | −7.5 | −5.5 | −6.0 | −5.3 | −5.6 | −5.0 | −5.7 | −7.6 |
| EGFR | −4.4 | −4.5 | −4.4 | −4.1 | −4.4 | −3.5 | −4.0 | −3.4 | −4.1 | −4.7 |
The results of molecular docking further highlighted the conserved interaction between key active ingredients and their core targeted proteins in each ASR segment and verified the reliability of the network topology relationship among medicinal segments, active ingredients, targeted proteins, signaling pathways, and diseases obtained in this study. The details of the binding between the active ingredients and target proteins will be helpful to understand the mechanisms of ASRT promoting blood circulation and ASRH/ASRB stopping and enriching blood.
Another critical aspect closely related to medicinal efficacy is the actual content of key active ingredients. To partially assess the absolute content of these qualified ingredients, a literature-based chemometric analysis was conducted (Supplementary Table 4) [4, 7–13, 32–41]. Through metabolomics, the relative content of numerous ingredients can be determined, while chemometrics based on literature can provide some absolute content information for specific components. The integration of these two methods enables a comprehensive evaluation of the actual content of various significant active ingredients. For instance, previous studies reported that the content of coumarin-type compounds in ASR is approximately 16.73 mg/g [38]. This study identified that 8-8′-dehydrodiferulic acid, 4-methylesculetin, and zingerone, which exhibit advantages in ASRT and stably bind to protein targets related to blood circulation, are all coumarin derivatives. Regarding amino acids, short-chain polypeptides, and their derivatives, which play roles in blood supplementation and hemostasis in ASRH and ASRB, earlier studies indicated that the Arg content in ASR is high, with average mass fractions ranging from 2.0 to 2.9% [39, 40]. L-arginine, DL-arginine, and arginyl-valine, identified in this study, are dominant metabolites in ASRH and ASRB and serve as crucial nodes in the topological network derived from network pharmacological analysis (Table 2).
4. Conclusions
In summary, we identified dominant metabolic components aligning with representative efficacy tendencies in distinct ASR medicinal segments through metabolome profiling. Networks illustrating medicinal segments, dominant active ingredients, targeted proteins, signaling pathways, and associated diseases were visually presented, offering preliminary insights into the pharmacological mechanisms of differential active metabolites among ASRT, ASRB, and ASRH. These detailed findings are expected to serve as a crucial reference for precise ASR medication, further pharmacological research, and the new drug discovery. Additionally, while the main targeted proteins and signaling pathways identified in this study align with the efficacy characteristics of different ASR medicinal segments as determined by network pharmacology and molecular docking, future validation at cellular, animal model, and clinical levels is necessary. Our results will also serve as a reference for these future studies.
Abbreviations
-
- ASR:
-
- Angelica sinensis (Oliv.) Diels root
-
- CHM:
-
- Chinese herbal medicine
-
- ASRH:
-
- The head of Angelica sinensis (Oliv.) Diels root
-
- ASRB:
-
- The body of Angelica sinensis (Oliv.) Diels root
-
- ASRT:
-
- The tail of Angelica sinensis (Oliv.) Diels root
-
- UPLC-MS:
-
- Ultraperformance liquid chromatography tandem-mass spectrometry
-
- PCA:
-
- Principal component analysis
-
- OPLS-DA:
-
- Orthogonal projections to latent structure-determinant analysis
-
- SLAF:
-
- Specific-locus amplified fragment
-
- SNP:
-
- Single nucleotide polymorphism
-
- InDel:
-
- Insertion and deletion
-
- QC:
-
- Quality control
-
- Q1:
-
- Parent ion
-
- Q3:
-
- Daughter ion
-
- PPI:
-
- Protein-protein interaction
-
- GO:
-
- Gene ontology
-
- KEGG:
-
- Kyoto encyclopedia of genes and genomes
-
- FC:
-
- Fold change
-
- VIP:
-
- Variable importance in projection
-
- BP:
-
- Biological processes
-
- CC:
-
- Cellular components
-
- MF:
-
- Molecular functions
-
- DM:
-
- Dominant metabolites.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
Xiaopeng Guo and Xuee Li contributed to this work equally and should be regarded as co-first authors.
Acknowledgments
We would like to thank our comrades at the Minxian Danggui Research Institute for their help in the collection of fresh Angelica sinensis (Oliv.) Diels root samples. This work was supported by the Gansu Province Technology Innovation Guidance Program Project in 2024 (Special Project for Science and Technology Commissioner), the Scientific Research Project of Drug Supervision of Gansu Provincial Drug Administration (2023GSMPA030), the Hongliu Excellent Youth Support Program in Lanzhou University of Technology (Fourth Batch), and the Longyuan Youth Innovation and Entrepreneurship Talent (Individual) Project in 2023.
Open Research
Data Availability
All the important data involved in this study are included in the Supporting Information section. The other data that support the findings of this study are available from the corresponding author upon reasonable request.




