Advancements in Fluorescence Sensing: Carbon Quantum Dots for Acrylamide Detection in Food
Abstract
Acrylamide is a hazardous chemical mainly synthesized during the thermal processing of foods representing a significant concern within the broader issue of food contaminants and their impact on public health. Acrylamide can be absorbed by the human body through dietary intake, respiration, dermal contact, and mucosa. The metabolic conversion of acrylamide into mercapturic acid metabolites and glycidamide results in several adverse and toxic effects. Therefore, this review explores the formation, toxicity, and metabolism of acrylamide. Hence, it is crucial to detect and ensure product quality via risk evaluation. Traditional analytical techniques for acrylamide detection often require expensive instrumentation and complex sample preparation, prompting the exploration of alternative, cost-effective, sustainable methods. Here, we propose the utilization of carbon quantum dots (CQDs) synthesized through green approaches as a novel solution. CQDs display their immense potential for diverse applications due to their valuable properties such as biocompatibility, photocatalysis, and strong fluorescence. This review highlights the distinct potential of CQDs as a fluorescence probe for detecting acrylamide, showcasing their efficacy in addressing food safety concerns. In addition, various extraction and purification techniques for acrylamide such as QuEChERS, solid phase extraction, Carrez clarification, and dispersive liquid-liquid microextraction are comprehensively reviewed. QuEChERS is regarded as a most promising technique for the extraction of acrylamide owing to its cost-effective, rapid, and higher recovery rates.
1. Introduction
The global concern regarding food safety is increasing due to the constant occurrence of various hazardous substances in the food matrices. Regardless of strict industrial regulations, persistent outbreaks of various food safety issues including the development of toxins, and the presence of xenobiotics, pathogens, and heavy metals [1]. Several food processing techniques are extensively employed to eliminate or control these toxic compounds but also facilitate the synthesis of food-borne toxins such as acrylamide, heterocyclic aromatic amines, furans, and hydroxyl methyl furfural. However, it is important to utilize effective and efficient detection approaches for food and nutritional safety [2].
Acrylamide is a chemical contaminant that is primarily synthesized in starch-rich foods processed above 120°C due to the interaction between asparagine and reducing sugar present in the food matrix [3]. The composition of food matrices such as potato chips, coffee, bread, fried twists, French fries, cookies, biscuits, and crackers promotes the biosynthesis of acrylamide. It is a small unsaturated amide with the chemical formula of C3H5NO, exhibiting high water solubility and a white odorless substance possessing electrophilic properties [4]. It has diverse applications in various industries as a valuable raw material. Acrylamide is employed in the manufacturing of polyacrylamide membranes acting as flocculant material in the treatment of wastewater and sewage. This polymer is extensively used as a dry strength enhancer in the paper-making industry, gel electrophoresis, and cosmetic industries [5, 6]. Dietary intake is considered the primary route for acrylamide exposure whereas industrial application of acrylamide is regarded as a secondary route. This leads to the absorption of acrylamide in our body. Acrylamide is regarded as a potential neurotoxin, genotoxin, and reproductive toxin due to its adverse effects on the neurofilaments and induced mutation in genes. The reactive metabolites of acrylamide cause disrupted neurotransmitter functionality, resulting in neuronal cell damage [7]. The International Agency for Research on Cancer (IARC) classified acrylamide as a Group-2A carcinogen due to metabolic conversion into glycidamide. This reactive metabolite is considered to be highly mutagenic as compared to acrylamide The International Agency for Research on Cancer (IARC) classified acrylamide as a Group-2A carcinogen due to metabolic conversion into glycidamide. This reactive metabolite is considered to be highly mutagenic as compared to acrylamide. Group 2A means that the substance is “probably carcinogenic to humans.” This classification is based on evidence of carcinogenicity in humans but sufficient evidence of carcinogenicity in experimental animals. Glycidamide is the metabolite formed when acrylamide is metabolized by cytochrome P450 enzymes, particularly CYP2E1. This reactive metabolite is highly mutagenic due to its ability to form adducts with DNA, leading to mutations. These mutations can disrupt normal cellular processes and contribute to the development of cancer [8]. The assessment of acrylamide is important because of its impact on human health [9]. Wang [10] investigated the neurological effects of acrylamide on female rats. The study revealed significant neurological impacts, including an increase in methylglyoxal content, inhibition of 3-phosphoglyceraldehyde dehydrogenase (PG-D) and triphosphate isomerase (TP-1) expression and activity, reduced expression of pyruvate kinase, and decreased levels of glycolysis products. Thus, this review paper will highlight the toxicological effects of acrylamide on the human body with several epidemiological studies that showcased the link between acrylamide consumption and an increased risk of various types of cancers such as ovarian, kidney, and endometrial cancers. There are numerous analytical techniques employed in the determination of acrylamide such as chromatographic technique, carbon quantum dots (CQDs), biosensors, and electrochemical sensors.
Chromatography techniques such as high-performance liquid chromatography (HPLC) [9], gas chromatography-mass spectrometry (GCMS) [11], liquid chromatography-mass spectrometry (LCMS) [6], Fourier-transform infrared spectroscopy (FTIR) [12], and surface-enhanced Raman spectroscopy (SE-RS) [13] are already being employed in the detection of acrylamide. These methods give efficient and accurate results. There are several drawbacks associated with these techniques such as tedious sample preparation, time consuming, and expensive. At present, fluorescent spectroscopy has acquired the interest of the scientific world due to being a budget-friendly, rapid, precise, easy, and sensitive technique. Several fluorescence techniques such as carbon dots, plant-derived nanoparticles, and biosensors can be employed as promising fluorescence probes. Carbon quantum dots offer a promising solution with their simple synthesis methods, cost-effectiveness, and ability to be functionalized for selective binding to acrylamide.
CQDs represent nanomaterials with dimensions ranging from 1 to 10 nanometres [14]. These particles were first fabricated by Xu in 2004 [15]. Carbon nanoparticles exhibit excellent optical properties, negligible toxicity, high-water solubility, thermally stable, and fluorescent properties, thus making them ideal for the detection of food additives, heavy metals, food toxins, and pesticides [16]. Due to these properties and the diversity in raw materials for tailoring, carbon dots have gathered the attention of the scientific community globally. The synthesis method of carbon dots is classified into two approaches, i.e., top-down and bottom-up approaches (Figure 1). The top-down method involves the breakdown of large-size carbon precursors such as graphite, graphite nanotubes, and graphene into CQDs through arc discharge, chemical oxidation, laser ablation, and electrochemical pathways. Although these techniques are unable to provide precise control over size and morphology, the bottom-up approach is preferred for the synthesis of carbon dots owing to its numerous advantages such as simple operation, being sustainable and environment friendly, and control over size and structure [16]. In this method, small fragments are merged to fabricate CQDs from green precursors by utilizing methodologies such as hydrothermal, pyrolysis, ultrasound-assisted, and microwave-assisted syntheses. Hydrothermal treatment is primarily employed in the fabrication of CQDs credited to its unique properties such as being ecofriendly, cheap, and surface treatment [17]. CQDs can be modified to selectively bind to acrylamide through functionalization. The application of carbon dots for utilization as fluorescence probes has been extensively explored in food safety and quality management.
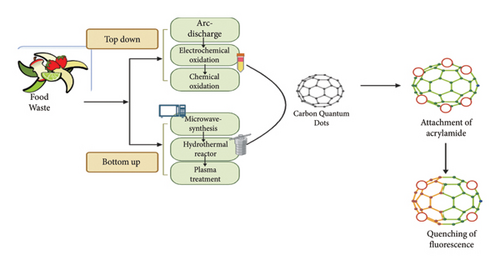
This review provides an overview of recent advancements in the fluorescence detection of acrylamide by employing CQDs. The paper is divided into different sections, assisted in providing a comprehensive overview regarding aspects related to acrylamide and its importance for determination. The initial section of the review outlines the biosynthesis of acrylamide and its hazardous impact on human health. The paper highlights synthesis methodologies of CQDs and extraction of acrylamide from various food matrices through several methods (QuEChERS, dispersive liquid-liquid microextraction, and solid phase extraction). Various sensing protocols are employed in the detection of acrylamide using carbon-based fluorescence material that helps in the development of a sustainable and economical approach by utilizing food waste as carbon. It also provides the complexities encountered in this promising technology, emphasizing its ability to encourage transformative changes in the field of allied sectors.
2. Mechanism of Acrylamide Formation
The synthesis of acrylamide primarily occurs in starch-rich foods (Table 1) such as potato chips, French fries, roasted nuts, coffee, meat, fish, and products derived from fruits and vegetables such as apple juice, green tea, and alcoholic beverages [23]. The biosynthesis of acrylamide occurs through various pathways including the Maillard reaction/Strecker pathway and non-Maillard reaction pathways. The Strecker pathway involves the interaction of asparagine with the carbonyl group of reducing sugar at a temperature above 120°C, leading to the formation of the Schiff base (Figure 2). Following that, Schiff base may undergo Amadori rearrangement reactions, resulting in the formation of Amadori products. These types of compounds undergo a β-elimination process shown in the synthesis of acrylamide [18, 24].
| Food matrix | Acrylamide level (µg/kg) | Benchmark level (µg/kg) | References |
|---|---|---|---|
| Roasted coffee | 566 | 400 | [18] |
| Crispbread | 443 | 350 | [18] |
| Chocolate powder | 2 | 350 | [18] |
| Biscuits | 200 | 350 | [18] |
| Wafers | 201 | 350 | [18] |
| Bread (wheat) | 37 | 50 | [18] |
| Instant coffee | 705–710 | 800 | [19] |
| Soft bread | 56 | 50 | [20] |
| Gingerbread | 1000 | 800 | [20] |
| Whole grain cereal | 151 | 300 | [20] |
| French fries | 323 | 500 | [20] |
| Roasted nuts | 93 | — | [21] |
| Crackers | 231 | 400 | [21] |
| Non whole grain cereal | 33 | 150 | [21] |
| Baby food | 100 | — | [22] |
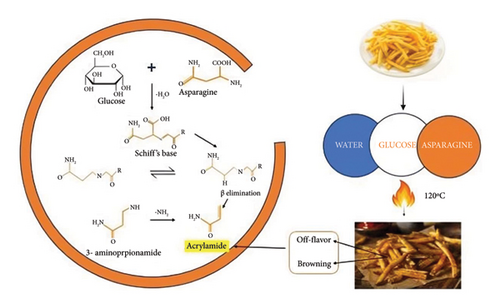
The non-Maillard reaction pathway involves several other mechanisms such as the acrolein pathway involving the thermal degradation of oils above their smoke point, and it also leads to the formation of acrylamide. In addition, the degradation of glycerol leads to the oxidation of acrolein, resulting in the formation of acrylic acid which provides carbon during interaction with asparagine. Moreover, in the presence of oxygen, the peroxyl group serves as a precursor in the polymerization of acrylamide [19, 25]. Another pathway involves the decarboxylation of organic acids such as malic acid, lactic acid, and citric acid, resulting in the formation of acrolein, which subsequently leads to acrylamide formation [26]. The biosynthesis of acrylamide in food products depends upon numerous factors such as temperature, pH, the concentration of amino acids and reducing sugar cooking technique, duration of cooking, metal ions, and oxygen levels. Also, the availability of damaged starch assisted in higher acrylamide formation because of the easy availability of sugars. The formation of acrylamide can also be influenced by genetic makeup, soil conditions, fertilizer dose, and storage conditions [27]. The comprehensive studies outline the assessment of acrylamide in various food products such as coffee, cereal-based products, and herbal products because of their significance in our daily lives. Food processing techniques can significantly impact acrylamide formation. Notably, frying causes the highest acrylamide formation in potatoes, while boiling and microwaving whole potatoes with skin on do not produce acrylamide. Soaking raw potato slices in water for 15–30 min before frying or roasting helps reduce acrylamide formation. Storing potatoes in the refrigerator can result in increased acrylamide during cooking, so it is recommended to store them outside the refrigerator, preferably in a dark, cool place. Cooking cut potato products to a golden yellow color rather than a brown color helps reduce acrylamide formation, as brown areas tend to contain more acrylamide.
CQDs are utilized in food processing techniques for various purposes. These carbon dots can be employed as fluorescent tracers for quality control and food safety assessment, enabling rapid and sensitive detection of contaminants or adulterants. In addition, CQDs can serve as antimicrobial agents when incorporated into food packaging materials, prolonging shelf life by inhibiting the growth of spoilage microorganisms. Furthermore, their antioxidant properties make them suitable additives for enhancing the nutritional value and stability of food products, reducing oxidative degradation, and extending freshness. Overall, CQDs contribute to improving food processing efficiency, safety, and quality.
Understanding the complex mechanisms primarily for acrylamide formation is crucial to optimize the utilization of CQDs in the determination of acrylamide. This understanding enables the tailoring of CQDs to selectively target specific molecular intermediates or byproducts along acrylamide formation pathways, thus enhancing their sensitivity and specificity in detecting acrylamide. Moreover, insight into how environmental and processing factors influence acrylamide formation facilitates the development of strategies suitable for diverse food matrices.
3. Toxicity and Metabolism of Acrylamide
There are various routes for exposure of acrylamide to the body. The distribution of acrylamide in various body parts, such as the respiratory tract, brain, and reproductive organs, is majorly assisted with the absorption of acrylamide in the blood [28]. Acrylamide is considered a neurotoxin, carcinogen, genotoxin, and reproductive and developmental toxin [29]. Acrylamide possesses high solubility and low molecular weight, which facilitates its passive diffusion throughout the body. These properties significantly influence its toxicity and metabolism. Due to its high solubility, acrylamide is readily absorbed through the gastrointestinal tract, skin, and lungs. Its low molecular weight allows it to easily cross cellular membranes and distribute widely across various tissues. This extensive absorption and distribution increase the potential for acrylamide to exert toxic effects, including neurotoxicity and carcinogenicity, as it interacts with cellular components and disrupts normal biological processes. Furthermore, its rapid absorption can lead to higher concentrations in target organs, enhancing its overall toxicological impact. During the metabolism, acrylamide forms a glutathione adduct through conjugation resulting in formation of mercapturic acid metabolites such as N-acetyl-S-(2 carbamoyl-2-hydroxyethyl)-L-cysteine, N-acetyl-S-(L-carbamoyl-2-hydroxylethyl)-L-cysteine, and N-acetyl-S-(2 carbamoyl ethyl)-L-cysteine and this process is catalyzed by glutathione-S-transferase. These compounds are subsequently eliminated from the body through urine. The acrylamide transforms glycidamide through cytochrome P-450 CYP2E1, following conjugation with glutathione [30]. Glycidamide is a highly mutagenic metabolite towards DNA due to its ability to directly conjugate with DNA and result in the formation of DNA adducts. Exposure to acrylamide increases tumor prevalence in certain organs such as the thyroid, central nervous system, and male genitalia [31].
In the previous study, Bainmahfouz et al. [32] revealed the deterioration of the myelin sheath in the central and peripheral nervous system by administering a dose of 50 mg/kg/day for 7 days on male Wistar rats. This study outlines the reduction in dietary and water intake of rats. Acrylamide exposure leads to a reduction of norepinephrine levels in noradrenergic axons in the prefrontal cortex and hinders the function of kinesin in axonal transport. Tremors, balance impairment, and cognitive disruption are common symptoms of acrylamide exposure in the human body [33]. In another study, Wang et al. [34] administered an oral dosage of 1 µg/kg to the male mice for 4 weeks, resulting in a reduction of weight and dietary intake. This study highlights the reduction of gut microorganisms that resulted in making the host susceptible to the Salmonella typhimurium infection. In addition, results showcased the presence of Firmicutes, Burkholderiales, and Erysipelotrichales in abundance.
3.1. Neurotoxicity
The term is used to depict an agent that has the potential to disrupt neurological functions by affecting both the central and the peripherical nervous systems. The impact of neurological syndromes is influenced by the site and degree of damage. Several agents can disrupt the functioning of the nervous system such as food-borne toxins, drugs, and chemicals. Cytoskeletal protein aberration, reduction in the level of neurotransmitters, inhibition in kinesin fast axonal transport, generation of oxidative stress, ion reactions, nerve-end damages, and protein interaction have been identified as the mechanisms underlying neurotoxicity [35]. The formation of acrylamide-sulfhydryl linkages has been observed to impair nerve and axon regeneration activity. Acrylamide forms covalent bonds with sulfhydryl groups on proteins, leading to the disruption of essential enzymatic functions and structural proteins involved in neuronal repair. This biochemical interaction can inhibit the regeneration of nerve fibers and axons by interfering with critical processes such as axonal transport and the stabilization of cytoskeletal elements. Therefore, axon nerve transport disruption in animals may lead to musculoskeletal weakness, hind limb paralysis, and decreased mobility [28]. The disruption in forward and reverse fast axonal transfer is one of the fundamental mechanisms for acrylamide neurotoxic effects, owing to the susceptibility of kinesin towards covalent modifications. Acrylamide exposure also leads to the deterioration of nascent cerebral development and reduces the expression of astrocytic markers [33]. Wang et al. [10] explored the neurological effects of acrylamide on female rats for 24–48 hours by introducing a dosage of 1.91 mg, revealing an increase in methylglyoxal content, inhibition of 3-phosphoglyceraldehyde dehydrogenase (PG-D) and triphosphate isomerase (TP-1) expression and activity, limited expression of pyruvate kinase, and decreased glycolysis products levels (Table 2). Farouk et al. [36] also examined the effects of acrylamide on albino rats for 12 hours by administering a dosage of 25 mg/kg. This study discovered changes in RBC cells, increased malondialdehyde content in cerebral, cerebellar, and hippocampal regions, a decline in neurotransmitters (serotonin and dopamine), and lycopene’s effects against acrylamide-induced neurotoxicity. In another study, Zhao et al. [37] subjected BALB/C mice to acrylamide at concentrations that varied from 0 to 1.0 mM for 24–96 hours. This research discovered an increase in free radical formation, a reduction in glutathione levels, and an increase in 8-hydroxy-2-deoxyguanosine-adducts (HDG-A) and 4-hydroxynonenal-adducts (HN-A). The impact of acrylamide-induced neurotoxicity can be reduced with an understanding of the mechanism involved. This assisted in the implementation of preventive measures as well as the development of therapeutic approaches against the neurotoxic effects of acrylamide from its ability to evaluate reactive metabolites and disturbance in neurotransmitter levels. It also triggers neuroinflammation by disturbing the balance of antioxidants and free radicals generation. The effects of neurological syndromes induced by acrylamide can be reduced by the consumption of various phytochemicals such as flavones, flavanols, terpenoids, alkaloids, quinones, vitamin C, and vitamin E.
| Effects | Study model | Dosage | Experiment duration | Findings | References |
|---|---|---|---|---|---|
| Neurological | Female rats | 1.91 g/kg | 24 to 48 hours | (i) Increase in intracellular methylglyoxal content | [10] |
| (ii) Inhibition expression and activity of triphosphate isomerase and 3-phosphoglyceraldehyde dehydrogenase | |||||
| (iii) Limited expression of pyruvate kinase | |||||
| (iv) Decrease pyruvate and lactate content of glycolysis products | |||||
| Neurological | Albino rats | 25 mg/kg | 12 hours | (i) Change in RBC cell | [36] |
| (ii) Increase in malondialdehyde content in cerebral, cerebellar, and hippocampal | |||||
| (iii) Decline in neurotransmitters (serotonin and dopamine) | |||||
| (iv) Lycopene’s antioxidative efficacy in reducing acrylamide-induced neurotoxicity in rat cerebral tissues | |||||
| Neurological | BALB/c mice | 0–1.0 mM | 24−96 h | (i) Increase in reactive oxygen species (ROS), a decrease in glutathione levels, and an elevation in the formation of 4-hydroxynonenal-adducts and 8-hydroxy-2-deoxyguanosine-adducts | [37] |
| Liver, kidney, heart, brain | Albino rats | 38.27 mg/kg | 12-hours | (i) Increase in malondialdehyde levels in the kidney | [38] |
| (ii) Decrease in antioxidants markers i.e. superoxide dismutase and glutathione | |||||
| (iii) Damage to brain, heart, kidney tissue | |||||
| (iv) Damage in testicular tissues | |||||
| Reproductive system | BALB/c mice | 25 mg/kg/day | 7 days | (i) Slows down the development of the oocytes | [39] |
| (ii) Reduces the size of the meiotic spindle within the oocytes | |||||
| (iii) Decreases the chances of chromosomal abnormalities in the oocytes | |||||
| Kidney and liver | 50 ICR mice (half male and half female) | 50 mg/kg | 12 hours | (i) Reduced the weight gain of all mice by 98% | [40] |
| (ii) 8-hydroxy-2-deoxyguanosine in testes was increased 22.0% | |||||
| (iii) Decreases Glutathione by 18.8% | |||||
| (iv) Decreases Glutathione peroxidase by 22.3% | |||||
| (v) Decreases total superoxide dismutase by 17.1% | |||||
| (vi) Reduced RBC count by 14.2% | |||||
| (vii) Decreases MCH concentration by 7.5 | |||||
| Neurological | Male sprague-dawley rats | 20 mg/kg | 24 hours | (i) Modified metabolites in kidney fat, serum, heart, kidney, cortex, and hippocampus was 6, 5, 17, 15, 21, 21 | [41] |
| Liver and kidney | Wistar rats | 25 and 75 mg/kg c | 24 h | (i) Increased levels of urea nitrogen and creatinine in the blood | [42] |
| (ii) Increase levels of bilirubin | |||||
| (iii) Decreased levels of red and white blood cells | |||||
| (iv) Platelets decreases | |||||
3.2. Carcinogenic and Genotoxicity
The carcinogenic and genotoxicity effects of acrylamide are primarily associated with its metabolic conversion into glycidamide (GLA). This reactive metabolite has a significantly higher mutagenic potential than acrylamide [43]. The primary mechanism for the action of acrylamide’s carcinogenicity and genotoxicity is its ability to act as a DNA alkylating agent. Thus, it can cause alteration in DNA by attaching an alkyl group to nucleotide bases, especially guanine (G). The covalent bonding between acrylamide and DNA results in the formation of DNA adducts due to alkylation [44]. DNA adducts can disrupt DNA’s normal structure and functioning, leading to errors during the replication and repair processes. Therefore, a mutation in certain genes can develop, which can lead to the initiation or progression of cancer development. In addition, these acrylamide-induced DNA damages can result in a cellular response like increased cell proliferation, apoptosis (programmed cell death) inhibition, and activation of tumor-related signaling mechanisms [45]. Acrylamide has also been associated with inducing genotoxicity, which causes chromosomal abnormalities and gene mutations. The generation of oxidative stress in conjunction with disruption in the antioxidant mechanism results in cell redox imbalance, subsequently leading to cell death. These chromosomal aberrations involve structural changes in chromosomes including translocations, inversions, and deletion, which could lead to genetic instability of cancer cells [46]. In a recent study, Neophytou et al. [47] investigated the consumption of acrylamide with the progression of colon cancer due to overexpression of genes related to protein and RNA metabolism. This study utilized male mice as experiment models for 28 days along with a dosage of 0.1 mg/kg per day.
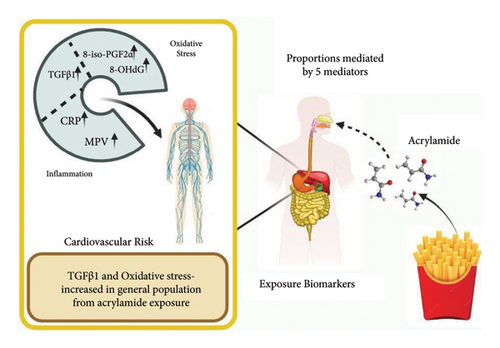
Acrylamide mitigation plays a vital role in evaluating risk assessment and formulating a systematic approach to reduce the genotoxic and cariogenic effects of acrylamide. Acrylamide tends to generate oxidative stress and epigenetic alteration and interfere with cell signalling pathways, biomarkers (Figure 3), and production of DNA adducts resulting in adverse effects on individuals. Biomarker monitoring assisted in the evaluation of these alterations in the human body.
3.3. Reproductive Toxicity
Acrylamide has been classified as a reproductive toxin owing to its association with the reduction in sperm cell quality and disruption in oocyte development. It also leads to hindrance in hormone secretion and menstruation resulting in decreased fertility among men and women [48]. Acrylamide can disturb the balance between free radicals and antioxidant defenses. This disturbance results in the generation of oxidative stress leading to infertility in individuals. Acrylamide affects prenatal development by causing hindrance in the development of the limbs and other fetal abnormalities as this chemical crosses through the placental membrane [49]. Albino rats were exposed to a dose of acrylamide, around 39 mg per kg for 12 hours. This study revealed the reduction of antioxidant markers such as glutathione and superoxide dismutase, along with the increase in the concentration of malondialdehyde (MA) in the kidney. In addition, damage to reproductive organs, heart, and brain tissues was observed [38]. The research by Aras et al. [39] applied a dose of 25 mg/kg/day to BALB/c mice for 7 days. The core insights of the study were a reduction in the size of the meiotic spindle and a delay in oocyte development along with chromosomal abnormalities.
Acrylamide disrupts hormonal imbalance triggers reproductive disorders and the development of reactive derivatives that can disrupt reproductive functionality. By increasing public awareness as well as influencing the regulatory discussion, individuals can effectively minimize the harmful impact of acrylamide, ensuring the protection of reproductive wellbeing and global health status.
3.4. Other Toxicities
Several in vitro and in vivo studies have associated acrylamide exposure with hepatotoxicity, nephrotoxicity, thyrotoxicity, and immunotoxicity. The metabolism of hazardous compounds results in damage to the liver. Acrylamide exposure leads to an increase in alanine aminotransferase, alkaline phosphatase, aspartate aminotransferase, bilirubin, and glutamyl transferase in the liver. Foroutanfar et al. [50] monitored the effects of polyphenols against acrylamide-induced hepatotoxicity in Wistar rats by administering different dosages. In addition, there was a significant increase in the production of interleukin 1 beta and tumor necrosis factor-alpha because of acrylamide intake. This study further outlined the increase of redox potential by increasing lipid peroxide, nitric oxide, and carbonyl content. In the summarized view several toxicities associated with acrylamide exposure primarily affect the biomarkers, enzyme production, antioxidants mechanism, increased oxidative stress, and production of reactive metabolites. However, the utilization of carbon quantum dots CQDs offers promising pathways for improving the detection of acrylamide in the context of its toxicity and metabolism. CQDs can be engineered to selectively target and detect acrylamide metabolites, such as N-acetyl-S-(2 carbamoyl-2-hydroxyethyl)-L-cysteine, in biological samples like urine. By conjugating specific functional groups onto the surface of CQDs, their affinity towards these metabolites can be enhanced, thereby increasing the sensitivity and specificity of detection assays. Moreover, CQDs have shown potential for detecting acrylamide adducts formed with DNA, such as those resulting from the metabolism of glycidamide. This capability enables new possibilities for monitoring acrylamide-induced DNA damage and assessing the associated health risks. CQDs can be employed in various body fluids, including blood and cerebrospinal fluid, to provide real-time monitoring of acrylamide exposure and its effects on different organ systems. By integrating CQD-based detection methodologies into biomedical research as well as deeper insights into the toxicity and metabolism of acrylamide can be increased, ultimately contributing to improved risk assessment and mitigation strategies.
4. Extraction and Purification Techniques for Acrylamide
4.1. QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe)
The QuEChERS is a widely utilized rapid technique for the extraction of various analytes and organic pollutants such as pesticide residue, heavy metals, and food additives. This sustainable methodology aids in quick sample preparation and subsequently highly efficient analysis [51]. There are primarily 2 stages involved in this approach, i.e., extraction of analyte and dispersive solid phase extraction.
In the first step, organic solvent is utilized in the extraction of acrylamide from complex food structures. There are various organic solvents such as hexane and acetonitrile that serve as extraction solvents. Subsequently, the combination of sorbents is utilized in variable amounts depending upon the complex structure of food for purification [52]. Anhydrous magnesium sulfate acts as a dehydrating agent whereas primary secondary amines eliminate any matrix impurities by removing constituents such as organic acid and sugar. The incorporation of C18 powder in QuEChERS pouches helps in the removal of excess amounts of fats and pigments such as chlorophyll [53].
Various alternative methods among QuEChERS techniques such as unbuffered QuEChERS, buffered QuEChERS, and citrate-buffered QuEChERS. The unbuffered QuEChERS also known as a part of AOAC official method 2007.01 recommends the use of ethanoic acid in extracting solvent and sodium acetate in purification sorbents, as it provides strong buffer capacity. The utilization of trisodium citrate dihydrate (TS-CDH) and disodium citrate dihydrate (DS-CDH) in combination with sodium chloride and anhydrous magnesium sulfate in citrate-buffered QuEChERS pouch helps in the adjustment of pH [54].
A recent study by Kumari et al. [55] utilized the acetonitrile-extracting solvent and MgSO4, NaCl, PSA, and C-18 powder as salts in the detection of acrylamide from potato chips by the UHPLC-MS/MS technique (Table 3). The study observed the recovery rate and relative standard deviation (RSD) of range 91.0%–109.16% and 1.87%–10.61%, respectively. Long et al. [56] highlight the detection of acrylamide using the UHPLC-MS/MS technique. This method utilizes the QuEChERS approach for extraction of acrylamide from cookies sample and facilitates the recovery rate and RSD of 70.67%–104% and 0.29%–12.49%, respectively. Xu et al. [57] fabricated an aptasensor for the determination of acrylamide in biscuits and fried twists by employing acetonitrile as extraction solvent. This study obtained recovery rate and RSD of 95.30%–101.90% and 3.97%–10.97%, respectively. Liu et al. [58] employed acetonitrile as an extracting solvent in a colorimetric approach for the detection of acrylamide in the water sample, thus attaining a recovery rate of 87.82%–112.87%.
| Food matrix | Detection method | Extracting solvent | Extracting salts | Dispersive phase/solvent | Recovery | RSD (%) | References |
|---|---|---|---|---|---|---|---|
| Cake | GC-MS/MS | Tetrachloroethylene (80 µL) | — | Ethanol (450 µL) | 93 | 9.72 | [11] |
| Nuts and seeds | GC-MS | Tetrachloroethylene (80 µL) | — | Ethanol (300 µL) | 95 | 8.9 | [23] |
| Biscuit, cakes, cookies, wafers, bread, cracker | Tetrachloroethylene (80 µL) | — | Ethanol (300 µL) | 95 | 4.5 | [23] | |
| Potato cutlet | UHPLC-MS/MS | 0.1% Formic acid in acetonitrile (10 ml) | 3.0 g of MgSO4 + 0.5 g of NaCl | 450 mg PSA + 150 mg MgSO4 + 150 mg C-18 powder | 91.0–109.16% | 1.8–10.60 | [55] |
| Sample cookie | UHPLC-MS/MS | 0.1% Formic acid in acetonitrile (10 ml) | 4.0 g of MgSO4 + 1.0 g of NaCl | 500 mg MgSO4 + 250 mg PSA + 250 mg C-18 powder | 70.67–104.88% | 0.29–12.49 | [56] |
| Fried twist | Aptasensor | Acetonitrile (10 ml) | 4.0 g of MgSO4 + 0.5 g of NaCl | 150 mg MgSO4 + 50 mg PSA | 95.30–101.09% | 3.97 to 10.97 | [57] |
| Potato chips | Colorimetric sensor | Acetonitrile (20 ml) | 4.0 g of MgSO4 + 0.5 g of NaCl | 150 mg MgSO4 + 50 mg PSA | 87.72–112.87% | [58] | |
| Youtiao | |||||||
| Bread | |||||||
| Biscuit | |||||||
| Soy milk | |||||||
| Coffee | |||||||
| Powder infant formula | GC-MS | Tetrachloroethylene (60 µL) | — | Ethanol (500 µL) | >85 | 2.9 | [59] |
| Confectionery beverages | SERS | Acetonitrile | 4.0 g MgSO4 + 1.0 g NaCl + 1 g C6H5Na3O7⋅2 H2O + 0.5 g C6H6Na2O7⋅1.5 H2O | Citrate extraction tube | — | 0.6–2.0 | [60] |
| Grains | |||||||
| Brown bread | NAS-CQD | Acetonitrile and water (1 : 1) | — | 150 mg MgSO4 + 50 mg PSA + 50 mg C-18 powder | 99.13–103.7% | 0.84 | [61] |
| Pork | LC-FLD | 0.1% Acetic acid in acetonitrile (10 ml) | 4.0 g MgSO4 + 1.0 g NaCl + 1 g C6H5Na3O7⋅2 H2O + 0.5 g C6H6Na2O7⋅1.5 H2O | 600 mg MgSO4 + 200 mg PSA | 68.5–119% | <12 | [62] |
| Bread | HPLC | Trichloromethane (180 µL) | — | Acetonitrile (1.5 ml) | 90 | 9 | [63] |
| Biscuit | |||||||
- 1RSD: relative standard deviation.
The QuEChERS technique is regarded as the most promising approach owing to its quick, higher recovery rate and economical. The QuEChERS technique is regarded as the most promising approach owing to its quick processing time, high recovery rate, and cost-effectiveness. However, it is important to critically evaluate the limitations and potential drawbacks of QuEChERS. For instance, its effectiveness can vary across different food matrices, and the composition of the sample can significantly impact recovery rates. Certain complex matrices may require additional cleanup steps to achieve accurate results, which can diminish the method’s efficiency. Moreover, some studies have reported variability in recovery rates for different pesticides and contaminants, indicating that the QuEChERS method may not be universally applicable without modifications. These factors must be considered when selecting QuEChERS for specific analytical purposes to ensure reliable and accurate outcomes. This technique can be paired better with the CQDs approach due to their detection mechanism.
4.2. Extraction of Acrylamide Using Dispersive Liquid-Liquid Microextraction (DL-LM)
The dispersive liquid-liquid microextraction involves three different phases, i.e., dispersant phase, aqueous phase, and extractant phase. In DL-LM, a suitable dispersant solvent is first added to the sample to facilitate the dispersion of the extractant solvent into fine droplets. The aqueous phase contains the analytes of interest dissolved in the sample matrix, while the extractant phase is typically a nonpolar organic solvent that selectively extracts the target analytes. Commonly used extractant solvents include chlorinated solvents such as chloroform or dichloromethane, as well as nonchlorinated solvents such as ethyl acetate or hexane. The selection of these solvents depends on factors such as the polarity of the analytes, the compatibility with the sample matrix, and the desired extraction efficiency. However, challenges may arise in choosing appropriate solvents, especially when dealing with complex food matrices that contain a wide range of compounds with different polarities and solubilities. Both the extractant and dispersant phases are organic solvents. The extractant phase is insoluble in water and has a high density, whereas the dispersant phase is miscible in both the extractant and aqueous phases. The dispersant phase assists to solubilize the targeted compound in the extractant phase. The analyte is highly soluble in phase as compared to the aqueous phase; therefore, there will transfer of the analyte from the aqueous phase to the extractant phase. In the last step, there is a separation of the analyte from the solvent with the help of the centrifugation process. Acrylamide is a highly polar compound that has less affinity towards organic solvents whereas it is readily soluble in water. Therefore, in the extraction process, acrylamide is chemically modified by covalent bonding with another moiety, resulting in a reduction in water solubility and increase in molecular weight. Xanthyl-AA and 2,3-dibromopropanamide are commonly used for the derivatization of acrylamide extraction. Aghvam et al. [11] extracted acrylamide from the cake sample by utilizing tetrachloroethylene and ethanol as extraction and dispersive solvents. This study achieved a recovery rate and RSD value of 98% and 5.4% respectively. In a previous study, Ghiasi et al. [59] summarized the extraction of acrylamide from infant powder using ethanol (500 µL) and tetrachlorethylene (60 µL) as dispersive and extracting solvents. This research utilized ethanol and KOH in 80 : 20 as an aqueous phase, resulting in a recovery rate of more than 85%. Elahi et al. [64] employed 80 µL of carbon tetrachloride along with 650 µL of ethanol as extracting and dispersive solvents for the extraction of acrylamide from a cookie sample. This study achieved a recovery rate of 89–95% and an RSD (%) of 9.2.
In another study, Altunay et al. [65] utilized an alkanol-based supramolecular dispersive liquid-liquid microextraction technique for the extraction of acrylamide from chocolate, coffee, French fries, roasted nuts, biscuits, bread, and chips. The research investigates the utilization of three organic solvents, namely, tetrahydrofuran, undecanol, and heptanol for the preparation of supramolecular solvents. These solvents help in attaining a recovery rate of 93.0–99% and RSD of 1.5–3.7%. DLLM provides higher analyte efficiency along with less solvent use. This technique is widely used in sample preparation for selective isolation of acrylamide from complex food matrices. Still, further studies are crucial to validate its reproducibility among several food samples.
4.3. Solid-Phase Extraction (SPE)
SPE mainly employs the concentration of analyte along with cleaning the sample. The first stage entails conditioning of column and dissolving the sample into an organic solvent [66]. Various types of SPE sorbents, including reversed-phase, normal-phase, ion-exchange, and mixed-mode sorbents, offering different selectivity and retention mechanisms suited to specific analyte classes and sample matrices. Following centrifugation of the sample, the supernatant is loaded onto the SPE column while discarding pallets. The unwanted components will pass through the column whereas the analyte is adsorbed. However, challenges may arise in SPE, particularly regarding sample matrix effects and the recovery of polar compounds like acrylamide. The presence of matrix components can interfere with analyte retention and elution, necessitating additional cleanup steps or method modifications. Furthermore, the adsorption and desorption characteristics of polar compounds can vary depending on the sorbent used, impacting their recovery efficiency. A recent study by Feng et al. [67] determined acrylamide and heterocyclic aromatic amines from French fries, bread, and cakes. This research emphasizes the use of magnetic solid-phase extraction (M-SPE) for the extraction of an analyte utilizing a magnetic framework. This approach provides a recovery rate and limit of detection of 90.4–102.8% and 0.012–0.210 g/kg.
In this context, Gómez et al. [68] extracted acrylamide from insect-based products using mesostructured silica SPE cartridges functionalized with amino groups, resulting in a recovery rate of 70–101% and RSD of less than 9%.
In another study, Desmarchelier et al. [69] monitored the acrylamide dosage in coffee, biscuits, and baby food using the GC-MS/MS approach. This project utilized Isolute multimode and ENV + cartridge for the extraction providing a recovery rate of 94–104% along with RSD (%) of less than 12.
4.4. Carrez Clarification (CC)
The use of Carrez clarification (CC) is employed significantly for the isolating of acrylamide from food matrices by precipitation of proteins, breakdown in emulsions, and removal of turbidity [70]. This method utilized two essential chemicals, namely, Carrez reagents I and Carrez reagent II.
The Carrez reagent I consists of potassium hexacyanoferrate trihydrate (15 g/100 mL of solution) whereas Carrez reagent II is composed of zinc sulfate heptahydrate (30 g/100 mL of solution). The incorporation of these two reagents results in the precipitation of interference and the formation of zinc hexacyanoferrate. The following centrifugation assists in the separation of analyte efficiently. Adergani et al. [71] revealed the determination of acrylamide by utilizing Carrez reagents for the separation of acrylamide from traditional popcorn. This approach resulted in providing a recovery rate of 97–100% and a RSD of 2.8%. In another study, the Carrez reagents were utilized for the isolation of acrylamide from cookie and potato chip samples in a photoelectrochemical approach. This research underscores a recovery rate ranging from 93–106% and an RSD of 2.3% [72].
The current extraction and purification techniques for acrylamide are complex and have limitations. Traditional methods involve coextraction and copurification coupled with HPLC-DAD SPE and other techniques. These methods are time consuming, require multiple steps, and involve the use of potentially harmful solvents [73]. In addition, the recovery of acrylamide in food samples can be low, leading to inaccurate results. However, the utilization of CQDs for the extraction and purification of acrylamide offers a simpler and more efficient alternative. CQDs can be synthesized from sustainable organic precursors and are nontoxic, making them a promising technique for replacing metal-based additives for polymer reinforcement and functionalization [74]. CQDs can be easily dispersed in a polymer matrix, significantly altering the polymer properties at a 1-2% concentration. Furthermore, CQDs offer an extremely high surface area, making them ideal for use in extraction and purification processes. Despite the potential benefits of CQDs, their limited production yield and complex purification methods are currently hindering commercial-scale manufacturing [75]. However, with further research and development, CQDs could revolutionize the extraction and purification of acrylamide, making the process simpler, more efficient, and more environmentally friendly.
5. Carbon Quantum Dots (CQDs)
Food waste is one biggest concerns of the world as it contributes to ecological imbalance. Food waste still comprises essential and beneficial components that can be further utilized to benefit mankind [76]. Carbon is one of the most abundant components present in this food and industrial waste, which can be utilized in tailoring carbon dots. CQDs are fluorescent materials with a diameter of less than 10 nm, which were discovered in 2004 [77]. The carbon dots primarily comprise amorphous carbon and sp2 hybridized graphitic carbon. CQDs aroused attention due to their strong fluorescence and control over visible spectra. However, the fluorescence properties of CQDs are influenced by various factors, including synthesis methods, size, shape, and surface passivation. For instance, different synthesis techniques, such as hydrothermal, microwave-assisted, or solvothermal methods, yield CQDs with distinct structural and optical properties. Moreover, the size and shape of CQDs can profoundly impact their quantum confinement effects and bandgap, thereby influencing their fluorescence emission wavelengths. There are several advantages of these carbon nanoparticles over other nanoparticles such as budget friendly, low toxicity, tuneable properties, easy surface modification, biocompatible, and thermal stability [78]. The properties of CQDs can be modified with the help of doping, surface treatment, and different time and temperature combinations to carbon sources. The utilization of nitrogen, sulfur, and boron for the surface modification of CQDs helps enhance the sensing ability, providing optimum yield and purity, and increasing the quantum yield [79]. Consequently, CQDs derived from food waste assisted in maintaining quality management and human wellbeing. These multifaceted properties help in the utilization of these ideal particles in various applications such as bioimaging [80], drug delivery [81], detection of heavy metals and pathogens [82], packaging material [83], and biosensors [84].
5.1. Fabrication Approaches of CQDs
The tailoring of carbon nanoparticles can be classified into the following two techniques: “bottom-up” and “top-up.” The top-down technique involves the breakdown of large carbon structures with the help of various methodologies to synthesize CQDs [85]. This method emphasizes the reduction in size and decomposition of carbon macromolecules into carbon nanoparticles from electrochemical means and physical methods. The method is rapid, easy, and efficient but there is no control over the size and structure of carbon dots [86]. On another side, the bottom-up approach gained attention and trust within the scientific community owing to its properties including a wide range of raw materials, ecofriendly, and control over size and morphology. The bottom-up method involves small fragments merged to fabricate carbon dots [87].
5.1.1. Top-Down Synthetic Approach
This technique utilizes physical and chemical methods for the decomposition of carbon material (graphene nanotubes, activated carbon, and graphene electrodes) into CQDs by employing different methodologies such as laser ablation, chemical oxidation, electrochemical, and acid oxidation [88]. The top-down provides mass production and is quick and easy to use whereas there are certain disadvantages associated with this route such as difficulty in the controlling size and morphology and high energy requirements.
The laser ablation was first reported by employing irradiation to cement and graphite powder to fabricate CQDs [89]. This method involves continuous irradiation on the carbon surface, resulting in exfoliation of carbon nanoparticles using high temperature and pressure combinations. The laser ablation involves two steps for the fabrication of carbon dots. The initial stage involves pyrolysis of a precursor followed by irradiation using different wavelengths. Zhu et al. [90] highlighted the fabrication of carbon nanoparticles using a laser of 350 mJ/pulse. This approach assisted in attaining a particle size of 2.0–4.0 nm. The laser ablation route provides carbon nanoparticles in quick time but there are certain drawbacks such as complex and expensive operation which can impact the sensitivity and specificity of acrylamide detection.
The chemical oxidation route fabricates CQD by treating carbonaceous sources with strong oxidizing agents such as hydrochloric acid, hydrogen peroxide, nitric acid, and sulfuric acid. This technique facilitates the mass production of the carbon dots in short intervals. However, there are certain disadvantages associated such as size control, morphology, and surface modification are difficult which can affect the sensitivity and specificity of acrylamide detection [90]. CQDs were fabricated by utilizing 0.5 M HCl on Q235 steel, resulting in carbon dots having an average diameter of 10 nm [91].
The electrochemical approach involves the use of a specific voltage to the conductive working electrode, primarily made up of carbon [92]. The application of electrical stimulation to the anode employed oxidation and also led to the synthesis of carbon nanoparticles. Subsequently, these carbon dots are further obtained by the centrifugation process [93]. Carbon nanoparticles were tailored by exfoliation of graphite rods as cathode and anode at 1.5 V. This study utilized electrolytes having 0.75 M NaOH, 3 g of urea, and 500 µL H2O2 in 100 mL of water. The multiwalled carbon nanotubes were operated as the working electrode platinum metals as the counter electrode and AgClO4 as the electrode material. The experiment was performed at a rate of 0.5 V/s, with potentials between −2.0 and 2.0 V [94]. The electrochemical methods have certain advantages such as low cost and a simple purification process.
However, the limitation of this method is that it only allows a small amount of molecule precursor for the synthesis of CQDs and nonuniform size distribution, which can impact the sensitivity and specificity of acrylamide detection [95]. The top-down method provides large-scale fabrication of CQDs along with a wide range of carbon sources. However, there are several drawbacks associated such as complex approach, control over size, and surface morphology. This approach assists in the production of functional nanoparticles with diverse applications.
5.1.2. Bottom-Up Approach
The bottom-up approach refers to the method for tailoring carbon dots by assembling smaller molecular or nanoscale components, mainly utilizing precursor molecules to prepare CQDs. It includes techniques such as microwave-assisted approach, hydrothermal approach, and ultrasound-assisted approach [96].
Pyrolysis is a process carried out at high temperatures and in an isolated environment. There are several factors affecting the fabrication and structures of CQDs such as time, temperature, pH, and heating rate [97]. There are mainly two types of pyrolysis, i.e., slow and fast. Wang et al. [98] tailored CQDs using citric acid and urea at 200°C for 2 hours; thus, this approach provided a particle size of 2.09 nm with a quantum yield of 49.55%.
The microwave synthesis route involves the utilization of electromagnetic waves to disrupt bonds of raw material, resulting in the synthesis of CQDs. This method synthesizes CQDs rapidly with uniform size and morphology. Başoğlu, Ocak, and Gümrükçüoğlu [99] applied 350 W for 120 seconds for the fabrication of CQDs using chickpea as raw material resulting in an average diameter of 8.7 nm and a quantum yield of 1.85%. Tohamy, El-Sakhawy, and Kamel [100] fabricated CQDs using sugar bagasse for 750 W for 135 minutes. This procedure provided a particle size of 8-9 nm and a quantum yield of 12.57%.
The hydrothermal method involves dissolving the carbon source into an aqueous solution and kept in a Teflon-lined stainless steel hydrothermal reactor for different durations and temperature combinations during this technique based on the raw material used [101]. This procedure is carried out at temperature and time ranging from 120 to 260°C for 5 minutes to 12 h. This interaction between the carbon source and aqueous solution results in the fabrication of carbon quantum dots. This technique is commonly employed due to several benefits such as economical, easy to perform, green technology, and easy surface modifications, which can enhance the sensitivity and specificity of acrylamide detection. Low production yield is one of the major constraints associated with the route.
Coffee ground waste was utilized to synthesize carbon quantum dots via the hydrothermal method at 300°C for 4 h. This study provides a sustainable approach to the removal of ibuprofen and, carbamazepine from water [102]. In a recent study, Zhou et al. [103] fabricated CQDs from tobacco leaves by utilization of a hydrothermal approach for 4 hours at 180°C. The primary purpose of tobacco-derived carbon dots was to detect borax in flour, bread, and noodle samples. Cui et al. [104] executed a hydrothermal method at 200°C for 6 hours to tailored carbon dots from safranine T. This research study examines the viability of carbon nanoparticles as a fluorescent dye. In another study, Devi et al. [105] applied 200°C for 3 hours for the fabrication of CQDs from walnut shells to detect the imidacloprid and its antimicrobial application. Hence, the bottom-up is mainly utilized owing to its advantages such as size control, surface modification, inexpensive, high surface area, and easy and sustainable approaches.
5.2. Utilization of CQDs as Fluorescence Probe in Food Safety and Quality Management
Carbon quantum dots are widely utilized for fluorescence sensing due to their unique properties (Table 4). Carbon dots display strong and tunable fluorescence properties, thermally stable, extended shelf life, low toxicity, and biocompatibility. The application of carbon dots as fluorescence probes in different types of fields includes gene therapy, heavy metal detection, targeted drug delivery, bioimaging of cancer cells, packaging material, and detection of food pathogens and toxins [102]. The detection of food additives by utilizing fluorescence quenching or fluorescence enhancement offers a sensitive and selective method for identifying and quantifying various substances. The quenching mechanism is classified into static quenching, fluorescence resonance energy transfer (FRET), dynamic quenching, inner filter effects (IFEs), and photoinduced electron transfer (PET) [114]. The formation of a fluorescent complex among the quenching and fluorescent molecule is known as static quenching. This results in the excitation of electrons and once the complex is formed and then it becomes nonfluorescent [115].
| Source | Method | Application | Particle size (nm) | Quantum yield (%) | References |
|---|---|---|---|---|---|
| Coffee ground | Hydrothermal (200°C for 4 h) | Detection of ascorbic acid | 1.68 | 4.18 | [91] |
| Plumbago indica leaves | Hydrothermal (200°C for 6 h) | Detection of picric acid | 3.6 | 16 | [106] |
| Okra | Hydrothermal (200°C for 7 h) | Dichlorvos detection in foods | 2.1 | 18.6 | [107] |
| Orange peel | Hydrothermal (220°C for 12 h) | Detecting folic acid, Fe3+, Ca2+ | 3.70 | 28.14 | [60] |
| Orange peel | Pyrolysis (180°C for 1 h) | Nitric oxide in meat | 7 | 13 | [108] |
| Sugarcane waste | Hydrothermal (200°C for 12 h) | Mercury ion | 2 to 8 | 14.12 | [109] |
| Ginkgo kernel | Hydrothermal (220°C for 12 h) | Nitrite ions | 2.7 | 37.8 | [110] |
| Banana peel | Hydrothermal (200°C for 24 h) | Bioimaging application (nematode) | 5 | 20 | [111] |
| Orange peel | Microwave assisted (900 W for 1 min) | Escherichia coli in milk | 4.2 | 16.2 | [112] |
| Citric acid | Hydrothermal (180°C for 20 h) | Tannic acid | 4.9 | 24 | [113] |
The interaction between the two light-sensitive molecules occurs in an excitation state. This interaction initiates the energy transfer, causing CQDs to return to the ground level without emitting electrons. However, this can lead to a decrease in fluorescence intensity. This process is identified as dynamic quenching or collision quenching [116, 117].
FRET quenching is a process in which the emission spectrum of carbon dots overlaps the absorption spectra of the quencher molecule [118]. The energy transfer happens without emission and results in dipole-dipole interaction [119, 120].
The photoinduced transfer involves the transfer of electrons among the quencher and carbon dots molecules. The electron acceptors form anionic free radicals whereas electron donors form cationic free radicals [121]. PET is mainly categorized into reduced PET (CQDs act as electron acceptors) in oxidized PET (CQDs act as electron donors). Inner filter effects occur mainly due to high concentrations of carbon dots or quencher molecules in solution. It is a process in which the emission or excitation of light from carbon dots is absorbed by quencher molecules or CQDs themselves [122, 123]. Static quenching and FRET quenching are primarily utilized in acrylamide sensing applications whereas dynamic quenching and other quenching mechanisms are relatively less used in the detection of food additives and other pollutants [124, 125]. In another study, John et al. [106] fabricated CQDs from plumbago leaves by adopting the hydrothermal treatment at 200°C for 6 hours for picric acid detection. The CQDs were fabricated using the hydrothermal method from okra at 200°C for 7 hours for the detection of colors in food samples [107]. In another study, Zhao et al. [60] tailored carbon dots from orange peel by the hydrothermal approach for 12 h at 220°C. This study explores the detection of folate, Fe3+, and Ca2+.
5.2.1. Challenges Using CQDs as Fluorescence Probe in Real Food Samples
CQDs have shown great potential as fluorescence probes in food safety analysis, but there are several challenges and limitations associated with their use in real-world food samples. The complex matrix interferences of food samples can significantly affect the performance of CQD-based sensing probes [126–128]. The presence of various organic and inorganic components in food samples can lead to false positive or false negative results, making it challenging to accurately detect and quantify target contaminants. CQDs are known for their excellent photostability and chemical stability, but their stability under different conditions, such as varying pH levels, temperatures, and ionic strengths, can significantly impact their performance as fluorescence probes [129, 130]. The stability of CQDs in food samples with varying pH levels and ionic strengths needs to be thoroughly investigated to ensure accurate and reliable detection of target contaminants. The comparability of CQD-based sensing probes with standard methods for food safety analysis is crucial for their widespread adoption. While CQD-based sensing probes offer several advantages, such as high sensitivity, selectivity, and ease of use, their comparability with established methods, such as chromatography and mass spectrometry, needs to be established to ensure their reliability and accuracy [131, 132].
5.2.2. Determination of Acrylamide via CQDs Fluorescence Spectroscopy
CQDs have emerged as one of the most promising approaches in the determination of acrylamide via fluorescence quenching [133]. Various methods are employed to fabricate the CQDs from different carbon sources, resulting in the formation of functional groups on the surface of the CQD. The hydroxyl, carboxy, and amino groups are primarily present on the surface which leads to the development of different binding sites. The interaction between the surface groups and target analytes resulted in a change in the fluorescence properties of CQDs. The interaction between the acrylamide molecules and functional groups of CQDs mainly leads to the formation of covalent bonds. The interaction between the carbon (CH2) of acrylamide and the amine (NH2) group of CQDs results in the formation of adducts. This reaction facilitates the formation of an amide product via the hydration reaction of the imide compound [134]. Furthermore, covalent conjugation among the carboxylic group of CQD and the amide group of acrylamide undergoes an amide coupling reaction, involving the formation of amide linkages. The interaction among these molecules of acrylamide and carbon dots serves as a decrease in fluorescence intensity (Figure 4).
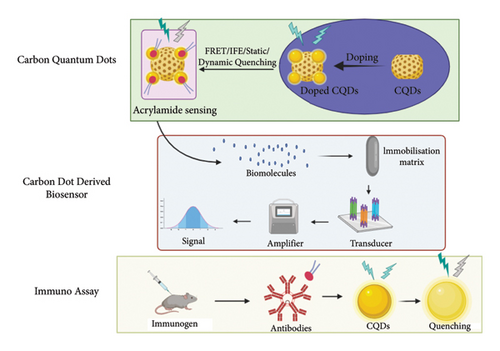
Surface functionalization refers to the chemical alteration of the surface resulting in the enhancement of fluorescence properties of CQDs by providing the active binding sites for the analyte detection. These functional groups act as excitation energy traps that result in the synthesis of a sensitive fluorescence probe. Several agents are employed for the surface modification such as hetero atoms, thiol groups, and N- acryloxy succinimide for advanced detection of acrylamide.
The citric acid-derived CQDs were employed for the detection of acrylamide in white bread crust and water samples. The microwave-assisted approach utilizes 2.0 g citric acid and 0.95 mL ethylenediamine as a carbon source, further incorporating 25 mM of NAS solution in 1 : 10 with CQD solution for the surface treatment to develop C=C bonds. Therefore, these bonds can assist in the polymerization of acrylamide resulting in the change among funcionalized carbon dots. This study employed the excitation and emission wavelengths of 350 nm and 445 nm, respectively. Furthermore, an increase in distance between NAS-modified CQD in the presence of acrylamide leads to a decrease in the fluorescence intensity. However, in the absence of acrylamide, the functional groups of carbon nanoparticles tend to polymerize, resulting in a reduction in distance among CQDs. This cost-effective technique provides a LOD of 8.1 × 10−7 M and 2.6 × 10−7 M in white bread and water samples, respectively [61].
In a recent study, Pattnayak and Mohapatra [135] employed glutathione-capped carbon dots integrated with gold nanoparticles for the determination of acrylamide in potato chips and fried bread sticks. The 100 mg chlorophyll was utilized as a precursor, mixed with 10 mg glutathione and 1 mL of ethylenediamine for the surface treatment. The FTIR analysis displayed the presence of the –SH group on the surface of CQDs, leading to the nucleophilic addition to acrylamide. These glutathione-modified CQDs (GSCQD) were integrated with gold nanoparticles, resulting in the development of a fluorescence nanoprobe (GSCQD-Au) based on the FRET process. The fluorescence intensity of this nanoprobe significantly increased in the presence of acrylamide in solution owing to the disruption in the energy transfer between GSCQD and Au nanoparticles. The interruption in the energy transmission due to the interaction of –SH with C=C of acrylamide, resulting in the thioester formation. This phenomenon leads to fluorescence quenching and, subsequently, quantification of acrylamide in real food samples. This sensitive technique assisted in attaining a LOD of 0.12 pM. The fluorescence quenching method is appropriate for the determination of acrylamide as it donates and accepts in the hydrogen bond with highly electronegative atoms.
In another study, Rong et al. [136] fabricated a glutathione-modified fluorescence sensor mainly based on the FRET for the determination of acrylamide in potato chips. The incorporation of GSH resulted in the fluorescence spectra overlap between lanthanide-doped nanoparticles and GSH-modified rhodamine B absorption. Consequently, the FRET process leads to fluorescence quenching and there was a significant reduction in fluorescence intensity. This method achieved an LOD of 0.68 nM.
Fluorescence spectroscopy with its streamlined sample preparation and its diverse options to compare rapid results is also appealing and economical.
(1) CQD Derived Biosensor. A biosensor is a quantitative analytical device comprised a biological element such as protein, single-stranded DNA, antibodies, and aptamers and a transducer. The transducer converts biological signals into electrical or colorimetric responses. The measurement of these responses is directly associated with the concentration of analyte in the sample. The biosensor can be categorized into electrochemical, fluorescence, and colorimetric. Fluorescence-based biosensors emerge as a potential analytical approach in food safety and quality management.
The integration of biological elements with the CQD provides a distinctive nanoprobe for the detection of acrylamide as the formation of an adduct between acrylamide and biological elements via hydrogen bonding. These noncovalent interactions between nitrogenous bases of DNA and acrylamide molecules resulted in fluorescence quenching. This showcased a significant potential approach for the determination of acrylamide in real food samples.
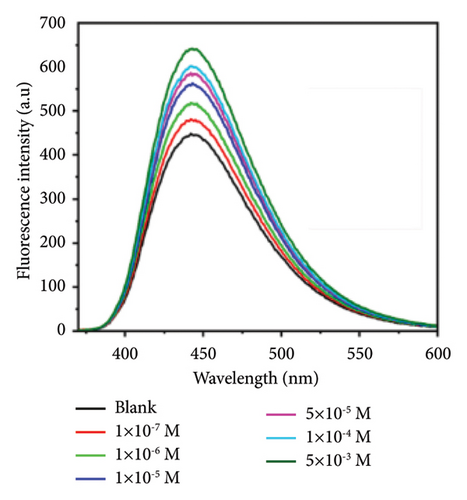
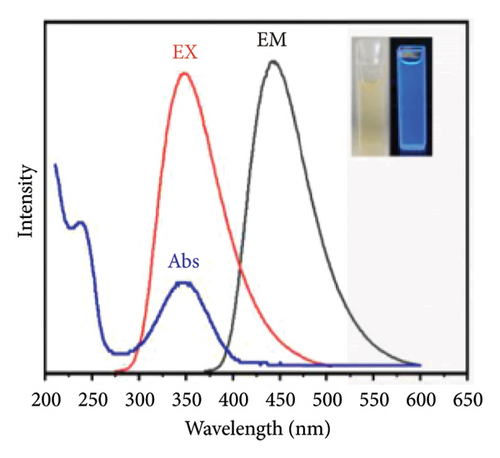
A fluorescence-based biosensor comprised of single-stranded DNA and citric acid-derived CQDs was utilized for the detection of acrylamide in a bread crust. The microwave-assisted technique employed excitation and emission wavelengths of 350 nm and 445 nm respectively. The surfaced functional groups (−COOH and −NH2) of CQD were shielded by the single-stranded DNA through noncovalent bond formation. This study outlines the hydrogen bond formation between acrylamide and nitrogen bases such as pyrimidine and purine of single-stranded DNA, resulting in the formation of a DNA-acrylamide adduct. Acrylamide can disrupt the bond between the guanine and cytosine bases by forming hydrogen bonds with the guanine base, which leads to a reduction in the fluorescence intensity of carbon dots. This fluorescence approach provided an LOD of 2.41 × 10−8 M along with a recovery rate of 91–98% and RSD of 1.15–2.3% [137]. In this context, Figure 5(a) illustrates the fluorescence spectrum of biosensors with different concentrations of acrylamide ranging from 1 × 10−7 to 5 × 10−3 M, revealing an increased intensity relationship. This CQD-derived biosensor provides a simple, rapid, precise, highly selective, sensitive, and easily reproducible approach but the stability of particular biological elements is a primary concern for the implementation of fluorescence-based biosensors in acrylamide detection. Figure 5(b) highlights the shift in the wavelength of the fluorescence spectrum due to the presence of acrylamide.
(2) CQD-Derived Fluorescence Immunoassay. Immunoassay is a biochemical analytical approach in which interaction among the antigen and antibodies results in determining the concentration of analyte in the sample. It is primarily based on the bonding between the antibody and antigen. This technique is commonly utilized along with special enzymes or fluorescence molecules resulting in the development of an economical and sensitive approach. The fluorescence immunoassay based on the inner filter effect was employed for the quantification of acrylamide in drinking water, potato chips, and cookie samples. The antibodies were produced by administering an immuno dose of 0.5 mg/mL to white rabbits. Xanthydrol was utilized for derivatization leading to the conversion of acrylamide into xanthyl acrylamide (XA), which has a high affinity towards polyclonal antibodies. The hydrothermal approach was utilized to fabricate carbon dots from phenylenediamine and glutamic acid at 180°C for 12 h. The carbon dots were used as the fluorescence material, employing an excitation wavelength of 415 nm and an emission wavelength of 512 nm. This fluorescence-based immunoassay approach showcased the inner filter effects among the carbon dots and p-nitrophenol, leading to fluorescence quenching. The proposed method provided the LOD of 0.16 µg/L along with a recovery rate of 82–103%, and these results were validated HPLC-MS/MS [138].
The fluorescence-based immunoassay provides a potent approach for acrylamide detection with highly sensitive and precise results but this approach involves complex procedures and requirements.
Table 5 shows the applications of CQD derived-fluorescence probes in acrylamide detection by observing the shift in emission peak while applying the excitation peak of wavelength with their LOD. To summarize, CQDs fabricated from food and industrial waste illustrate tremendous potential in diverse applications such as the detection of acrylamide and other pollutants using their strong fluorescent properties.
| Food matrix | CQD derived fluorescence probe | Excitation range (nm) | Emission range (nm) | LOD | References |
|---|---|---|---|---|---|
| White bread crust drinking water | CQDs compared LC-MS | 350 | 445 |
|
[61] |
|
Glutathione CQD Gold nanoprobe | 460 | 520 | 0.12 pM | [135] |
| White bread | Biosensor (single-stranded DNA with carbon quantum dots) | 350 | 445 | 2.41 × 10−8 M | [137] |
| Drinking water |
|
415 | 512 | 0.16 µg/L | [138] |
| Potato fries (water extracts) | Biosensor (double stranded with gold nanoparticles) | 490 | 510–560 | 0.5 × 10−6 M | [139] |
- 1LOD: limit of detection.
5.3. Future Recommendation
Future advancements in fluorescence sensing using CQDs for acrylamide detection in food hold significant promise. Researchers are actively working to enhance the sensitivity, selectivity, and reliability of CQD-based sensors for detecting acrylamide, a harmful compound formed during food processing. Novel strategies such as surface functionalization and molecular imprinting are being explored to improve the specificity of CQDs towards acrylamide detection while minimizing interference from other compounds. In addition, efforts are underway to develop portable and userfriendly sensing devices integrated with CQDs, enabling rapid on-site detection of acrylamide levels in various food products. Furthermore, ongoing research aims to optimize detection protocols and validate CQD-based sensing methods for regulatory compliance and widespread adoption in the food industry, contributing to improved food safety and consumer health.
6. Conclusion
Acrylamide is a heat-induced neurotoxin mainly biosynthesized in plant-derived food. The conversion of acrylamide into reactive metabolites such as epoxide, glycidamide, and mercapturic acid have adverse and toxic effects on human health. These metabolites disrupt hormonal secretion, and neurological functionality resulting in various such as cancer, and neurological syndromes. Therefore, the detection of acrylamide is critical to ensure product quality and human health. Quantification of acrylamide requires extraction and purification of analyte by utilizing techniques such as QuEChERS, DLLM, SPE, and Carrez clarification. Established analytical methods like GC-MS, LC-MS, and other chromatographic techniques reliably detect acrylamide, but there are several drawbacks associated such as technical expertise, lengthy analysis times, high cost, demanding conditions, complex pretreatments, and potential toxic exposure. Therefore, future acrylamide detection should be rapid, simple, affordable, and accurate for ensuring food and product quality and safety. Despite their significant advantages, carbon dots still face hurdles in their structure, luminescence mechanisms, and specification during reaction time and temperature. The posttreatment process is time consuming along with low quantum yield in the long wavelength region. The posttreatment process involves purification, functionalization, and surface passivation steps to enhance carbon dots’ properties. Challenges in achieving high quantum yield, especially in the long wavelength region, arise from incomplete passivation and nonradiative energy loss. These obstacles impact scalability and sensitivity in detection applications. The surface functionalization of CQDs provides a promising approach for the development of highly targeted nanoprobes with specific binding affinities. In-depth studies are required, which are critical for the implementation of sustainable and consumer-friendly approaches that reduce acrylamide formation while sustaining product quality and industrial feasibility.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
We sincerely acknowledge the Department of Food Technology and Nutrition, School of Agriculture, Lovely Professional University, Phagwara, Punjab, India, for granting necessary financial support.
Open Research
Data Availability
The data used to support the findings of this study are from previously reported studies and datasets, which have been cited.




