Anti-Inflammatory and Microbiota-Regulating Property of Deficiency Tonic Medicines in Edible Traditional Chinese Medicine: A Promising Therapy for Depressive Disorder
Abstract
Depression has become the leading cause of disability worldwide. Conventional serotonergic antidepressants fail to meet anticipated outcomes and increase the risk of drug dependency and side effects. Consequently, the significance of diet and nutrition in the prevention and management of depression and anxiety has increasingly received attention. Many years of clinical practice have shown that edible traditional Chinese medicines can relieve depression through their anti-inflammatory properties, potentially acting as a nutritional remedy for depression with a higher acceptance rate and safety. In this review, we elucidated how deficiency tonic medicines in edible traditional Chinese medicines and their ingredients modulate the immune response and gut microbiota to alleviate depression. This article can offer new insights into the antidepressant effect of daily dietary treatments.
1. Introduction
Depression is an average mental disease marked by high morbidity, recurrence, and suicidal tendencies. As stated by the WHO, depression will be the predominant causation of disease burden worldwide by 2030 [1]. Disappointingly, the clinical efficacy of classical monoaminergic antidepressants remains suboptimal, characterized by issues such as delayed response, low cure rate, easy recurrence after drug withdrawal, discontinuation symptoms [2], and inevitable side effects. Therefore, more efficacious and safer antidepressants are needed.
Scholars ascribe the pathogenesis of depression to many hypotheses, including monoamine, inflammation, neurotrophic factors, neurogenesis, and gut microbiota. Among them, inflammation is mainly manifested as immune system disorders, systemic inflammatory cascade reactions, especially the influence of cytokines on the central nervous system (CNS), and the emergence of neuroinflammatory activation. Besides, as a hot spot in the current research on depression, gut microbiota homeostasis has been found to lead to depression, which may be related to immunosuppression and reduced levels of anti-inflammatory substances.
The application of traditional Chinese medicine (TCM) benefits from an extensive historical background in the treatment of mental disorders such as depression, yielding more effective and stable outcomes. A growing body of research has proved that antidepressant mechanism of TCM involve a variety of pathogenic mechanisms of depression mentioned above, and anti-inflammatory, as the most widely recognized biological activity of TCM, has naturally been mentioned more in the research of antidepressant TCM.
The concept of “medicine and food homology” (also known as “edible traditional Chinese medicine”) has existed in Asia since ancient times. This theory suggests that many TCMs can serve as both medicine and food, protecting against diseases. Some edible TCMs have also been included in the pharmacopeia and used as dietary supplements in Europe and America. For example, cinnamon, which has been used as a spice in several cultures for centuries, has also been employed as a stomachic with hypoglycemic properties in clinical practice [3]. Since edible TCMs are of natural origin, they exert therapeutic effects on the entire body based on the holistic concept of Chinese medicine. They are rich in natural active substances that synergistically act on multiple targets. Their unique advantages, such as low toxicity, high efficiency, and good tolerability, make them ideal therapeutic options [4].
Deficiency tonic medicine, a category of Chinese medicine, constitutes the largest portion of edible TCMs (Figure 1(a)). Studies from modern pharmacology suggest that tonic medicine possesses vigorous anti-inflammatory properties and modulates gut microbiota homeostasis [5]. Thus, deficiency tonic medicines can be considered as a repository for developing future antidepressants. For example, saponins and polysaccharides in TCMs possess potent anti-inflammatory effects that may prevent and treat depression by controlling the immune and inflammatory responses. Particularly, they inhibit the harmful effects of cytokines on the CNS and prevent the neuroinflammatory response.
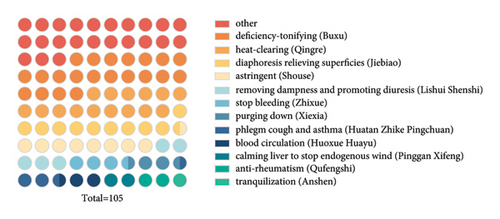
To summarize, it is necessary to explore the potential of edible TCMs as complementary antidepressants. In this review, we discussed how the deficiency tonic medicines affect inflammation and gut dysbiosis associated with depression and recapitulated their effective components. These findings may provide a new strategy for treating depression.
2. Deficiency Tonic Medicine in Edible TCM
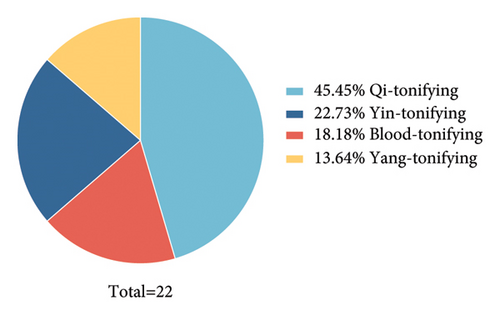
The list of “substances that are both food and Chinese medicine according to tradition” issued by the National Health Commission of China and the State Administration of Market Management and Supervision contains 110 medicinal materials, including those widely used by the public, such as cloves, cinnamon, and black sesame seeds. There are many categories of TCM, such as tonifying deficiency, clearing heat, and stopping bleeding. Deficiency tonic medicines account for the largest proportion of edible TCMs (22 in total, representing 21%) (Table 1).
| Efficacy | Medicine | Pin Yin | Latin name | Medicament portions |
|---|---|---|---|---|
| Qi | Chinese yam | Shan Yao | Dioscorea opposita Thunb. | Rhizome |
| Licorice | Gan Cao | Glycyrrhiza uralensis Fisch. | Root and rhizome | |
| Glycyrrhiza inflata Bat. | ||||
| Glycyrrhiza glabra L. | ||||
| White dolichos | Bai Bian Dou | Dolichos lablab L. | Seed | |
| Longan | Long Yan | Dimocarpus longan Lour. | Aril | |
| Jujube | Zao | Ziziphus jujuba Mill. | Fruit | |
| Ginseng | Ren Shen | Panax ginseng C.A.Mey | Root and rhizome | |
| Codonopsis | Dang Shen | Codonopsis pilosula (Franch.) Nannf. | Root | |
| Codonopsis pilosula Nannf. var. modesta (Nannf.) L.T.Shen | ||||
| Codonopsis tangshen Oliv | ||||
| Astragalus | Huang Qi | Astragalus membranaceus (Fisch.) Bge.var.mongholicus (Bge.) Hsiao | Root | |
| Astragalus membranaceus (Fisch.) Bge. | ||||
| Ganoderma | Ling Zhi | Ganoderma lucidum (leyss. ex Fr.) Karst. | Fruiting body | |
| Ganoderma sinense Zhao, Xu et Zhang | ||||
| North American ginseng | Xi Yang Shen | Panax quinquefolium L. | Root | |
| Blood | Mulberry | Sang Shen | Morus alba L. | Fruit cluster |
| Black sesame seed | Hei Zhi Ma | Sesamum indicum L. | Seed | |
| Donkey-hide gelatin | E Jiao | Equus asinus L. | Hide | |
| Chinese angelica | Dang Gui | Angelica sinensis (Oliv.) Diels. | Root | |
| Yin | P. odoratum | Yu Zhu | Polygonatum odoratum (Mill.) Druce | Rhizome |
| Lily | Bai He | Lilium lancifolium Thunb. | Bulb | |
| Lilium brownie F.E.Brown var.viridulum Baker | ||||
| Lilium pumilum DC. | ||||
| Goji | Gou Qi | Lycium barbarum L. | Fruit | |
| Polygonatum | Huang Jing | Polygonatum kingianum Coll.et Hemsl. | Rhizome | |
| Polygonatum sibiricum Red. | ||||
| Polygonatum cyrtonema Hua | ||||
| Officinale | Tie Pi Shi Hu | Dendrobium officinale Kimura et Migo | Stem | |
| Yang | Cinnamon | Rou Gui | Cinnamomum cassia Presl | Bark |
| Cistanche | Rou Cong Rong | Cistanche deserticola Y.C.Ma | Succulent stem | |
| Eucommiae | Du Zhong Ye | Eucommia ulmoides Oliv. | Leaf | |
They are categorized into four groups (Figure 1(b)). Qi tonic reverses the pathological deviation of internal organs and treats energy deficiency. Blood tonic treats blood and heart insufficiency and improves insomnia and dreams. Yin tonic is often used to nourish Yin, replenish fluids, and improve irritability and impatience. Yang tonic warms the kidneys and improves the loss of appetite and cold in tolerance. A literature search showed that all 22 deficiency tonic medicines possess anti-inflammatory and immunomodulatory properties. Detailed information is explained below.
3. Inflammation and Gut Microbiota in Depression
3.1. Depression and Inflammation
Immune dysregulation can lead to several diseases, and accumulating evidence suggests the involvement of immune dysregulation in the pathogenesis of psychiatric disorders, such as depression. For example, patients suffering from a cytokine storm are more susceptible to depression [6]. Approximately one third of the patients experienced depression following treatment with proinflammatory cytokines [7]. Particularly, tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6) were upregulated in the cerebrospinal fluid and peripheral blood of patients with depression. In addition, patients with major depression had higher peripheral blood concentrations of acute phase proteins, such as C-reactive protein, chemokines, and adhesion molecules [8]. Multiple meta-analyses have confirmed these findings [9, 10]. Interestingly, the polymorphism of these inflammatory cytokines and mediators is allied with the severity of depression [11]. Besides, patients with depression have increased levels of anti-inflammatory cytokines, such as transforming growth factor-β (TGF-β) and IL-10. Moreover, the abundance of immune cells, such as leukocytes and monocytes, is increased in depressed patients [12]. Nuclear factor kappa-B (NF-κB), an inflammatory signaling molecule, transmits immune signals from the periphery to the brain, partly mediating the effects of cytokines on neurogenesis [13].
Microglia, originating from fetal macrophages, are the major innate immune cells in the CNS accountable for regulating the immune microenvironment in both healthy and disordered brains [14]. Abnormal microglial homeostasis can lead to neuropsychiatric diseases, such as depression. Therefore, depression is a microglial disease [15]. In addition, the absence of other glial cells, such as oligodendrocytes and astrocytes, in various mood-related brain regions, is a morphological anomaly in depression [16, 17]. Indoleamine 2,3 dioxygenase (IDO) can partly explain neuroinflammation-associated depression. IDO is an enzyme mainly synthesized by myeloid cells, such as microglia, after being stimulated by TNF-α and interferon-γ (INF-γ). IDO activation redirects tryptophan metabolism from serotonin synthesis to the production of neuroactive tryptophan metabolites, inducing depressive behavior by modifying glutamatergic neurotransmission [18]. It may be a crucial transmitter in the gut-brain axis linking depression to inflammatory bowel disease [19].
3.2. Depression and Gut Microbiota
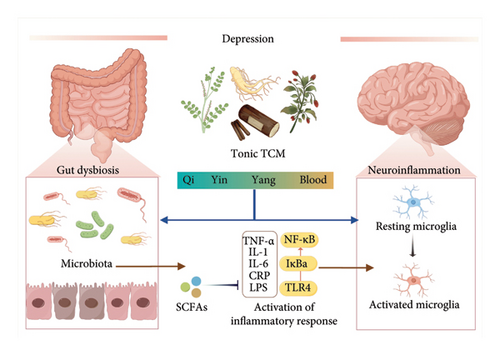
Gut microbiota is another crucial factor in depression. The microbiome refers to a group of microorganisms with an intricate link to the nervous system. Gut dysbiosis can morphologically damage the brain regions associated with mood disorders [20]. Patients with depression have gut dysbios is characterized by altered microbial diversity and changes in the structure of bacterial taxa [21]. An increasing number of microbial-targeted therapeutics have been investigated in depression, such as fecal microbiotatransplantation, probiotics, and synbiotics. Their antidepressant effects may be related to immune suppression, suggesting that microbial dysregulation may lead to depression by inducing inflammatory signals [22]. Mechanistically, changes in microorganisms may impair gut barrier completeness, thereby baring the immune system to inflammatory stimuli. In addition, decreased levels of microbiome-derived anti-inflammatory substances, such as short-chain fatty acids (SCFAs), may induce a depressive phenotype [23] (Figure 2).
4. Deficiency Tonic Medicine in Edible TCM, Inflammation, and Depression
According to modern pharmacology research, tonic medicine can enhance immune capacity tranquilize the mind of patients with impaired disease. In the list of edible TCMs, tonifying herbs have all been reported to have immunomodulatory effects, and some of them have been shown to exert antidepressant efficacy through anti-inflammatory or regulating gut dysbiosis in animal experiments (Table 2). In the following, we will review the anti-inflammatory functions of 22 medicines and their potentials in the treatment of depressive evidence, classified according to the following four types of tonic medicines: Qi, Blood, Yin, and Yang.
| Efficacy | Edible TCM | Effective component | Depression animal model | Involved mechanisms | References |
|---|---|---|---|---|---|
| Qi | Ginseng | Extract | Chronic restraint stress | Reduce neuroinflammation in amygdala and increase COX-2 and iNOS | [24] |
| Total ginsenosides | Intraperitoneal LPS-induced | Ameliorate neurogenic damage in the hippocampus, decline of hippocampal proinflammatory factors | [25] | ||
| Chronic unpredictable mild stress | Inhibit hippocampal P38 and JNK protein phosphorylation | [26] | |||
| Rg1 | Intracerebral LPS-induced | Decrease circulating IL-6 | [27] | ||
| Chronic dexamethasone-induced | Inhibit NOD-like receptor family domain protein-1 inflammasome | [28] | |||
| Rg3 | Intraperitoneal LPS-induced | Decrease the expression of proinflammatory cytokines and IDO, inhibit microglial activation and regulate the NF-κB pathway | [29] | ||
| Rb1 | Chronic mild stress | Triggers preneurogenic phenotypes of microglia through the activation of peroxisome PPARγ | [30] | ||
| North American ginseng | Extract | Olfactory bulbectomy | Decrease TNF-α, caspase-3, and BDNF and exert a neuroprotective role by modulating the NO pathway | [31] | |
| Astragalus | Astragaloside IV | Repeated restraint stress | Upregulate PPARγ expression, downregulate TNF-α and IL-1β, decline NF-κB phosphorylation, and reduce NLRP3 | [32] | |
| Cycloastragenol | Forced swimming | Stimulate telomerase activity and induce CREB activation followed by tert and bcl2 expression. | [33] | ||
| Ganoderma | Polysaccharides | Chronic social defeat stress | Downregulate IL-1β and TNF-α, inhibit microglia activation and astrocyte proliferation | [34] | |
| Triterpenoids | Maternal Separation | Balance the expression of proinflammatory and anti-inflammatory factors and inhibit microglia activation | [35] | ||
| Ganoderic acid A | Chronic unpredictable mild stress | Restore the inactivation of the ERK/CREB pathway, moderate M1/M2 microglial polarization | [36] | ||
| Licorice | Extract | Reserpine-induced | Increase of brain norepinephrine and dopamine | [37] | |
| Liquiritin | Chronic variable stress | Increase SOD activity, inhibit lipid peroxidation, and lessen production of MDA | [38] | ||
| Licorisoflavan A | Chronic mild stress | Attenuate the BDNF-TrkB, the phosphorylations of ERK1/2/CREB/mTOR pathway, reverse the decreases in synaptic proteins PSD-95 and AMPA receptor subunit GluR1 | [39] | ||
| Blood | Black sesame seed | Sesamol | Chronic unpredictable mild stress | Decrease lipid peroxidation and nitrite levels; increase glutathione levels and superoxide dismutase and catalase activities, and inhibit inflammation surge (serum TNF-α) | [40] |
| Dextran sulfate sodium-induced | Suppress the TLR-4/NF-κB pathway, protect against oxidative stress and upregulate the Nrf2 antioxidant signaling pathway, upregulate the BDNF/TrkB/CREB signaling pathway, restore synaptic impairments, and enhance NE and 5-HT levels | [41] | |||
| Chinese angelica | Extract | Chronic unpredictable mild stress | Increase BDNF and the phosphorylation of CREB/ERK 1/2 | [42, 43] | |
| Z-ligustilide | Chronic unpredictable mild stress | Increase progesterone and allopregnanolone | [44] | ||
| Yin | Lily | Extract | ovariectomized mice | Increase neurotrophic factors (nerve growth factor, BDNF) | [45] |
| Extract | Chronic unpredictable mild stress | Inhibit the activation of microglia and the release of inflammatory mediators by downregulating MYC | [46] | ||
| Lily and Rehmannia decoction | Intraperitoneal LPS-induced | Decrease IL-1β, IL-6, and TNF-α | [47] | ||
| Baihe Zhimu decoction | Chronic unpredictable mild stress | Reverse the suppression of the PI3K/Akt/GSK-3β pathway and the activation of the MAPK pathway | [48] | ||
| Goji | Polysaccharide | Long-term aversive stimuli exposure | Decline the number, diameter, and activation of Iba-1+ LHb neurons | [49] | |
| 2,4-dichlorophenoxyacetic acid-induced | Upregulate the autophagy level by inhibiting the activation of the NLRP3 inflammasome | [50] | |||
| Prenatal chronic stress | Increase the diversity of gut microbiota and reduce corticosterone | [51] | |||
| Polygonatum | Polysaccharides | LPS-induced/chronic unpredictable mild stress | Regulate of the oxidative stress-calpain-1-NLRP3 signaling axis | [52] | |
| LPS-induced | Reverse increase in GFAP+ and NF-κB+ counts and decrease ERK phosphorylation | [53] | |||
| Yang | Cinnamon | Extract | Elevated plus maze | Activation of 5-HT1A autoreceptors | [54, 55] |
| Cinnamaldehyde | Chronic unpredictable mild stress | Decrease COX-2 and PGE2 | [56] | ||
| Cinnamon and ginger | Streptozotocin induced | Elevate total antioxidant capacity, BDNF mRNA and expression of ki67, diminish IL-1β, and GFAP and Caspase-3 | [57] | ||
| Cistanche | Extract | Tail suspension test | Downregulate MAO activity, upregulate DA improve the nerve excitability | [58, 59] | |
| Echinacoside | Forced swimming test | Activate AMPAR-Akt/ERK-mTOR pathway | [60] | ||
| Polysaccharides | Chronic unpredictable mild stress | Keep Th17/treg balance, moderate gut immunity, increase beneficial bacteria and SCFA | [61] | ||
4.1. Qi-Tonifying
4.1.1. Ginseng
The root of Panax ginseng C.A. Meyer has been reputed as the “King of all kinds of herbs” in China since antiquity. It has various neurotrophic and neuroprotective effects in several diseases, such as ischemia/reperfusion injury and depression. Ginsenosides are the premier active component of Panax ginseng C.A. Meyer. Researchers have uncovered that total ginsenosides (GTS) and ginsenosides Rg1, Rg3, Rb1, etc., have certain antidepressant effects. It regulates peripheral and central inflammation, increases the levels of monoamine neurotransmitters, stabilizes the function of the hypothalamic-pituitary-adrenal (HPA) axis, and upregulates brain-derived neurotrophic factor (BDNF). This section mainly focuses on the anti-inflammatory roles of Panax ginseng C.A. Meyer on depression. Ginseng extract has been shown to effectively reduce behavioral despair, evidenced by reduced neuroinflammation in the amygdala and increased mRNA and levels of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) [24]. The results of bioinformatics analysis and experimental verification of GTS displayed that the antidepressant effects of ginseng are connected to its restrictive effects on inflammation, amelioration of neurogenic damage in the hippocampus, decline of hippocampal proinflammatory factors [25], and inhibition of hippocampal P38 and c-Jun N-terminal kinase (JNK) protein phosphorylation. Fractalkine (CX3CL1)/fractalkine receptor (CX3CR1) axis may be a major mediator of the neuroprotective effects of ginseng in depression [26]. Rg1 is a well-known neuroprotective compound that decreases the elevated amount of circulating IL-6 [27] and inhibits the expression of nucleotide-binding oligomerization domain (NOD)-like receptor family domain protein-1 inflammasome [28]. The main component of red ginseng, Rg3, possesses strong immunomodulatory properties. Previous studies have revealed that pretreatment with Rg3 can enhance the duration of immovability in both the tail suspension test (TST) and forced swimming test (FST) and decrease the expression of proinflammatory cytokines and IDO. Microglial activation and regulation of the NF-κB pathway play a pivotal role in these aspects [29]. Rb1 inhibits the inflammatory cascade and alleviates depression-like behavior. Zhang et al. found that Rb1 triggers preneurogenic phenotypes of microglia through the activation of peroxisome proliferators-activated receptor γ (PPARγ) both in vivo and in vitro. Furthermore, microglia exposed to Rb1 augmented the proliferation and differentiation of neural precursor cells [30]. Although the mechanisms underlying the inhibitory effects of Rh3, Rh1, and other components of ginseng on neuroinflammation have been investigated, it is yet unclear whether they possess antidepressant properties. However, it is suggested that they may improve microglia-mediated depression. In terms of gut microbiota, ginseng extract plays a prebiotic role by increasing Lactobacillus and Bifidobacterium in rats [62]. Han et al. explored how red ginseng mitigates anxiety/depression by interacting with microbiota. Oral treatment with red ginseng extract was discovered to reduce depressive/anxiety-like behavior, decrease IL-6 in the hippocampus and hypothalamus, and partly restore gut microbiota abundance after fecal transplantation in humans with ulcerative colitis and depression and colitis induced by E. coli. The number of Bacteroidetes increased while the amount of Proteobacteria declined after treatment with red ginseng [63, 64], indicating that ginseng may alleviate depression-like behavior in mice by modulating the microbiota-gut-brain axis.
4.1.2. North American Ginseng
Panax quinquefolium L., also named American ginseng, is an herb originally found in North America and Asia. It is known for its capacity to manage the immune system and protect the CNS. The results of an in vitro experiment revealed that a neutral polysaccharide separated from American ginseng can effectively minimize the production of IL-1β, IL-6, and nitric oxide (NO) by macrophages, indicating that this polysaccharide can treat inflammation and inflammatory diseases [65]. In rats with gastric mucosal damage, American ginseng treatments declined the proinflammatory levels of IL-1β and COX-2 [66]. As for gut immunity, administration of American ginseng mitigated chemically-induced colitis and downregulated inflammatory cytokines, such as IL-1α. Lowered growth of Bacteroidetes and Verrucomicrobia, coupled with increased growth of Firmicutes, implies that North American ginseng may ameliorate gut dysbiosis [67]. Besides, American ginseng has been found to protect against nerve damage. Extract of American ginseng recovered the cognitive function [68]. American ginseng saponins also relieved the symptoms of anxiety [69] and significantly inhibited microglia cells in the brains of rats with acute ischemic stroke [70]. A study unveiled the potential of American ginseng in alleviating depression. Olfactory bulbectomy-induced depression increased corticosterone levels and induced oxidative-nitrosative damage in rats, which was associated with increased levels of TNF-α, caspase-3, and BDNF in both cerebral cortex and hippocampus. American ginseng reversed all these alterations and exerted a neuroprotective role by modulating the NO pathway [31].
4.1.3. Astragalus
Astragalus is the root of Astragalus membranaceus, a Qi tonifying adaptogenic herb in TCM. It vigorously protects the nervous system, immune system, and cardiovascular system. The use of Astragalus polysaccharides (ASP) has been shown to prevent metabolic stress-mediated astroglios and activate microglia near senile plaques in the brain, thus improving sporadic Alzheimer’s disease [71]. As a type of astragaloside, isoastragaloside I decreased the synthesis of iNOS and COX-2 and alleviated lipopolysaccharides (LPS)-induced expression of IL-1β, TNF-α, and iNOS in BV2 cells. NF-κB phosphorylation was reduced, and its transactivation function was inhibited through phosphoinositide 3-kinase (PI3K)/Akt and MAPK signaling pathways, suggesting its potential to treat neurological diseases in connection to neuroinflammation [72]. Astragaloside IV (AS-IV) can hinder microglia activation by promoting the glucocorticoid pathway [73] and avert neuronal ferroptosis via the erythroid 2-related (Nrf2)/heme oxygenase-1 (HO-1) signaling pathway, thereby enhancing delayed ischemic neurological deficits and reducing neuronal death [74]. Besides, AS-IV, in combination with calycosin, an isoflavone phytoestrogen isolated from Astragalus, mitigated the symptoms of Parkinson’s disease (PD) by downregulating inflammatory cytokines [75–77]. Notably, AS-IV significantly decreased LPS-induced and repeated restraint depressive-like behaviors by blocking neuroinflammation and upregulating PPARγ expression. It significantly downregulated TNF-α and IL-1β, declined NF-κB phosphorylation, and reduced NLRP3 inflammasome in the hippocampus of mice [32]. Cycloastragenol (CAG), an aglycone of astragaloside IV, has distinct telomerase-activating properties. It can induce telomerase activity and activate cyclic adenosine monophosphate response element-binding protein (CREB) in PC12 cells and primary neurons. Oral administration of CAG lessened depression-like behavior in experimental mice [33]. Thus, AS-IV has the potential to be an antidepressant. A study shed light on the antifatigue properties of ASP and measured its efficacy and mechanism in treating chronic fatigue syndrome (CFS), particularly through gut-brain axis. Specifically, ASP restored the structure of gut microbiota and enhanced the production of SCFAs and anti-inflammatory bacteria, which increased SCFA levels in the cecal content after inducing CFS. At the same time, ASP reversed the unconventional production of Nrf2, NF-κB, and their downstream factors in the brain-gut axis [78].
4.1.4. Ganoderma
Lingzhi is the Chinese name of Ganoderma lucidum, which was considered to be the most precious medicine in ancient China. As edible mushrooms exist in our daily diet, the effects of Lingzhi on inflammatory reactions are of potential interest to optimize the function of our immune system. Ganoderma-rich active ingredients such as polysaccharides and triterpenes are utilized as anti-inflammatory and immunomodulatory agents, protecting the CNS. Numerous stress-modeling animal experiments have confirmed their effectiveness in reducing depression. For example, Ganoderma lucidum polysaccharides (GLPs) downregulated IL-1β and TNF-α and inhibited microglia activation and astrocyte proliferation in the hippocampus of mice subjected to chronic social defeat stress [34]. GLP can also help reduce inflammation-related microglial migration, morphological alterations, and phagocytosis [79]. Furthermore, GLP mitigated cognitive impairment in mice with chronic cerebral hypoperfusion and D-galactose rats. GLP increased the abundance of Foxp3+ Treg cells to increase the concentrations of IL-10 and regulate aberrant energy metabolism in the brain of mice with chronic cerebral hypoperfusion [80]. GLP decreased TNF-α, IL-6, and phospho-p53 levels and incremented anti-inflammatory cytokines levels in D-galactose rats [81]. In addition to GLP, Ganoderma lucidum triterpenoids (GLTs) can deploy anti-inflammatory effects in the peripheral and central nervous system to reduce depression-like behaviors. GLT treatment can normalize the expression of inflammation-related factors in the prefrontal cortex, dorsal hippocampus, and ventral hippocampus [35], which are three crucial regions of the brain connected with anxiety and depression. In addition, Ganoderic acid A, one of the chief bioactive triterpenoids, attenuated neuronal damage and depressive-like behaviors in the rat model of poststroke depression, restored the inactivation of the extracellular-signal regulated kinase (ERK)/CREB pathway, and moderated M1/M2 microglial polarization, evidenced by shrank iNOS and CD86 expression and boosted Arg-1 and CD206 expression [36]. In terms of intestinal immunity and microbiota ecology, Ganoderma lucidum extract significantly maintained intestinal barrier integrity, thereby reducing metabolic endotoxemia [82], inhibiting macrophage infiltration, and downregulating inflammatory markers [83]. Ganoderma lucidum extract also aggrandized the number of SCFA-producing bacteria and decreased the relative abundance of Proteobacteria, a marker of intestinal dysbiosis, and Escherichia-Shigella [84]. However, these effects were observed in high-fat diet and colitis disease models, not in the rat model of mental diseases and depression.
4.1.5. Licorice
Licorice, alias Gan-cao in Chinese, extensively exists in food worldwide due to its potent sweetness. The Chinese pharmacopoeia officially included Glycyrrhiza uralensis Fisch., Glycyrrhiza inflata Bat., and Glycyrrhiza glabra L. as licorice. Licorice has been shown to possess antidepressant properties that effectively decrease the duration of immobility in FST and TST [37], with flavonoid glycyrrhizin upgrading superoxide dismutase (SOD) activity, inhibiting lipid peroxidation, and cutting down malondialdehyde production [38]. In addition, licorice functions as a monoamine oxidase inhibitor, modulating dopamine D1, D3, and antidiuretic hormone V1A receptors [85]. Licorisoflavan A also reversed chronic mild stress-induced differences in FST and TST without modifying open field test movements. The BDNF/tyrosine kinase B (TrkB) pathway and α-amino-3-hydroxy-5-methyl-4-isoxazole-propionicacid (AMPA) receptors were shown to be involved in such effects [39]. On the other hand, the flavonoids of licorice exerted neuroprotective effects. In vitro experiments demonstrated that licorice hindered the generation of NO, IL-1, prostaglandin E2 (PGE2), and Iba1 in LPS-induced BV2 cells [86, 87] through the mitogen-activated protein kinases (MAPKs) pathway [88]. In vivo experiments found that licorice improved cognitive impairment by ameliorating LPS-induced neuroinflammation in mice, evidenced by inhibition of toll-like receptor 4 (TLR4) and NF-κB pathways and downregulation of proinflammatory factors [89]. Of interest, a triterpenoid saponin called glycyrrhizic acid is a hindrance of high-mobility group box 1 (HMGB1), which can exhibit proinflammatory activities. Upon extracellular release, HMGB1 predominantly triggers intracellular signaling by combineing with the receptor for advanced glycosylation endproducts and TLR4. Glycyrrhizic acid has therapeutic effects on several nervous diseases, including neuroinflammatory diseases, seizure, Alzheimer’s disease, and PD [90]. The aforementioned preclinical studies support the notion that licorice can potentially serve as a therapeutic alternative for neuroinflammatory depression.
4.1.6. White dolichos
White dolichos, the mature seed of Dolichos lablab L., is traditionally applied to treat gastrointestinal diseases in China. Soluble dietary fibers in white dolichos significantly promoted the release of RAW 264.7 macrophage-mediated secretory molecules, including NO, IL-6, and TNF-α [91]. Along with polysaccharides, it can act as a potential prebiotic for Bifidobacterium and Lactobacillus, notably increasing SCFA production [91, 92]. White dolichos extract inhibited gastrointestinal inflammation by downregulating inflammatory factors like IL-8, iNOS, and TNF-α, thus alleviating gastrointestinal diseases [93, 94]. Meanwhile, white dolichos extract alleviated anxiety-like behavior in mice with irritable bowel syndrome through the gut-brain axis. White dolichos extract notably decreased c-Fos expression in the prefrontal cortex, indicating its effect on the brain’s neural response to detrimental intestinal stimuli [94].
4.1.7. Codonopis
Codonopsis Radix, known as “Dangshen” in China, originates from the root of Codonopsis pilosula. Several research studies have discovered that Codonopis is abundant in nutritional elements with anti-inflammatory characteristics. Among them, polysaccharide obtained from Dangshen (DSP) can preserve the equilibrium of Th1/Th2 cells and Tregs/Th17 cells in vivo [95] and augment CD4+ to CD8+ T ratio in vitro [96], thus stimulating the proliferation of lymphocytes [97]. DSP has been shown to reduce TNF-α and IL-1β secretion, regulate the actuation of MAPK and NF-κB signaling pathways, and enhance the ability of macrophages for bacterial phagocytosis in the mice model of pulmonary disease [98, 99]. Besides, DSP and two other bioactive ingredients, oligosaccharides and inulin, can improve gut dysbiosis, thus improving macrophage infiltration and strengthening mucosal immunity [100, 101]. Of greater significance, the previous one can boost anti-inflammatory activity by suppressing inflammatory factors within the brain and mitigating the spatial learning and memory deficits generated by scopolamine [102].
4.1.8. Chinese Yam
The dried root of Dioscorea opposite Thunb, known as Chinese Yam, strengthens the immune system, regulates the gastrointestinal system, and provides neuroprotective benefits [103]. Galacturonan [104], galactan [105], and water-soluble polysaccharides [106] were the specific components of Dioscorea opposite Thunb involved in such effects. The extract reduced intestinal inflammation, inhibited the growth of pathogenic bacteria, and increased SCFA levels to alleviate diarrhea [107]. In terms of immunomodulatory function, polysaccharides and glycoproteins are the key active components enhancing macrophage activation and cytokine secretion [108]. The enhancement of lymphocyte proliferation and differentiation in immune-suppressed mice (with a notable increase in CD4+/CD8+ ratio) is connected with the actuation of TLR4/NF-κB [109] and MAPK/NF-κB signaling pathways [110]. In addition, sesquiterpene reversed LPS-induced increment in IFN-γ and IL-17 concentrations. The proportion of Th cells, DC cells, and NK cells increased, while the proportion of myeloid-derived suppressor cells and Tregs decreased [111]. The inhibitory effect of polysaccharides on inflammation helped recover from cancer-related fatigue [112], prevented neuronal injury and cell necrosis, and retained the integrity of the blood-brain barrier. The protective effect relies on Ca2+/Calmodul independent protein kinase (CAMmβ)-mediated adaptation of the Nrf2/HO-1 signaling pathway [113].
4.1.9. Longan
Longan is the pulp of Dimocarpus longan Lour fruit, a traditional medicinal and edible material in Asian countries. Longan extracts can serve as anti-inflammatory agents through the NF-κB signaling pathways [114]. Polysaccharides (LP), the main constituents of longan, promote concanavalin A-induced splenocyte proliferation, macrophage phagocytosis, and serum INF-γ and IL-2 secretion, demonstrating its value as a supplement to immunotherapy [115]. In vitro experiments indicated that the immunomodulatory effects of LP relied on the actuation of the PI3K/AKT and myeloid differentiation factor 88 (MyD88)/TNF receptor associated factor 6 (TRAF6) pathways by TLR2 and TLR4 [116]. A review of LP highlighted its essential role in intestinal immune response and the soundness of the intestinal barrier [117]. Consuming LP can maintain the integrity of mucosal barrier through the secretion of intestinal immunoglobulin A, consequently upgrading both systemic and intestinal mucosal immunity [118].
4.1.10. Jujube
Jujube, alternatively referred to as the fruit of Ziziphus jujuba Mill., possesses an exceptional nutritional value. The list of edible TCM explicitly mentions that jujube belongs to dates or black dates. Studies have unveiled that jujube water extracts induce the transcription of IL-1β, IL-6, and TNF-α in RAW 264.7. Pretreatment with extracts suppressed these inflammatory factors in LPS-stimulated macrophages [119], which may be related to the rich structurally diverse triterpenes [120, 121]. The extract, along with jujuboside B can reduce lung inflammation in mice suffering from respiratory diseases. Moreover, the former successfully decreased the abundance of neutrophils and macrophages in alveolar lavage fluid, reducing interstitial inflammatory cell infiltration and TNF-α levels in the lung [122, 123]. The extract downgraded the increased levels of proinflammatory indicators in the hippocampus and prefrontal cortex, indicating its potential neuroprotective effect on scopolamine-induced cognitive dysfunction in rats. This implies that jujube extract possesses antiamnesia properties, partly owing to its anti-inflammatory activity [124].
4.2. Blood Tonifying
4.2.1. Black Sesame Seed
Black sesame seed is the mature seed of Sesamum indicum L., which is one of the first documented plants used for its seeds. Sesaminol triglucoside, the main sesame lignan, can be converted to a catechol moiety by intestinal microbiota [125] and downregulate LPS-induced inflammatory markers in monocyte-derived macrophages [126]. In addition, both sesamol and sesamin, two types of phytoestrogen isolated from sesame, inhibited inflammatory signaling pathways like MAPK-p38 and NF-κB-p65 pathways in vitro and in vivo [127–130]. Besides, there have been numerous studies indicating that sesamol or sesame oil can mitigate cognitive impairment by inhibiting NF-κB-mediated inflammatory response and potentiating Nrf-2-mediated antioxidant response [131–134]. Many studies indicated their efficacy in alleviating depression. Sesamol has therapeutic benefits for depression and cognitive impairment associated with diabetes [135]. Chronic treatment with sesamol notably veered chronic stress-induced behavior, attenuated inflammation, and decreased serum TNF-α levels in stressed mice [40]. Remarkably, Xia et al. illuminated that sesamol alleviated the symptoms of dextran sulfate sodium (DSS)-induced colitis in mice, depression-like behaviors, and neuroinflammatory responses by suppressing the TLR-4/NF-κB pathway via the gut-brain axis [41].
4.2.2. Chinese Angelica
Chinese angelica, the root of Angelica sinensis (Oliv.) Diels (AS), is widely used in TCM for treating neurodegeneration, aging, depression, and inflammation. Ligustilide, the primary constituent of AS, can ameliorate aging-related neurodegeneration or neuroinflammation by downregulating the expression of chemokines and inflammatory factors (NLRP3, IL-1β, and NF-κB) and upregulating neurotrophic factors (postsynaptic density protein (PSD) 95, PSD93, and BDNF) [136–138]. An optimal formula modulates neuroinflammation and oxidative stress by inhibiting the expression of IL-1b, myeloperoxidase, protein kinase C gamma type, amyloid-β (Aβ) precursor protein, and Aβ in the hippocampus, thereby preventing cognitive dysfunction and Aβ deposition in rats treated with Aβ25-35 [139]. The combination of Chinese angelica and Paeonia lactiflora in the Chinese herbal compound formula had an anti-inflammatory role on LPS-induced BV-2 cells by impeding the TLRs/NF-κB signaling pathway and attenuating the release of inflammatory factors, such as iNOS and TNF-α [140]. Both AS and Chinese herbal formula possess antidepressant properties by amplifying BDNF in the hippocampus [42, 43]. It has been suggested that ligustilide can act as an antidepressant through the biosynthesis of progesterone and allopregnanolone in the brain [44].
4.2.3. Mulberry
The fruit cluster of Morus alba L., so-called Sangshen or mulberry fruit, possesses medicinal properties such as neuroprotective and immunomodulatory effects. Polysaccharides of both pyrrole alkaloids and mulberry fruits can enhance phagocytosis, macrophage activation, and TNF-α and NO secretion inmacrophages [141, 142]. The compound Cyclo (L-Pro-L-Val) notably inhibited the phosphorylation of IκBα and NF-κB and activation of iNOS and COX-2 [143]. Mulberry fruit extract can activate TLR4 through the downstream MAPK and NF-κB signaling pathways and finally induce Th1 response [144, 145]. The ethanol extract of mulberry fruits and its fractions included sufficient total phenolic and flavonoid content to restore stimulation-induced increase in the NO level by downregulating iNOS expression [146]. As a result, they can downregulate reactive oxygen species production, NO generation, and apoptosis-associated proteins, thereby protecting against PD-induced neurotoxicity [147]. Besides, Bang et al. verified that mulberry vinegar mainly alleviates neuroinflammation by modulating the NF-κB signaling pathway and glial activation [148].
4.2.4. EJiao
EJiao is derived from the dermis of the Equus asinus L. donkey, well-known for its tonic properties that enhance blood circulation and nourish yin. Ejiao drastically declined the amount of inflammatory cytokines such as IL-6, TNF-α [149], and IL-1β and inhibited NF-κB and pyrin domain-containing protein 3 (NLRP3) inflammasome, thus improving the NF-κB signaling pathway and decreasing the expression of downstream proteins associated with pyroptosis of inflammatory cells [150]. EJiao may prevent and treat Alzheimer’s disease, as it increases cell viability in neuronal-like PC12 cells treated with H2O2 and reduces Aβ accumulation [151]. The combination of Korean Red Ginseng and EJiao enhanced thymic regulatory T cells, restored CD3+ T cells and circulation, and recovered proteins of the JAK/STAT pathway in the spleen of immune-compromised mice [152].
4.3. Yin Tonifying
4.3.1. Lily
The bulb of Lilium lancifolium Thunb, Lilium brownie F.E.Brown var. viridulum Baker, and Lilium pumilum DC, as a medicinal lily named “Bai-he,” is renowned for its tranquility. Even the “Synopsis of the Golden Chamber,” an ancient Chinese medical book written 1,800 years ago, refers to an emotional disorder known as “lily disease.” Similar to estradiol, the aqueous extracts of Liliumaqueous alleviated anxiety, depression, and cognitive dysfunction in ovariectomized mice and effectively increased neurotrophic factors (nerve growth factor, BDNF), indicating its potential as a novel therapy for mood abnormalities after menopause [45]. Further studies uncovered that lilium saponins mediate the antidepressant properties of Liliumaqueous. Pharmacological experiments have shown that they can enhance brain-gut synergism by hindering the hyperactivity of the HPA axis and modulating the secretion and release of various brain-gut peptides. Despite the extensive anti-inflammatory properties of the lilium extract, only a single study conducted solely on lilium alone and exhibited it exerts antidepressant effects by inhibiting MYC, activating microglia, and releasing inflammatory mediators [46]. In particular, the combination of lilium with other medications, such as two classical prescriptions named Lily Rehmannia decoction and Lily Anemarrhenae decoction, is often employed to improve mood. Clinical trials have found significant improvements in anxiety/somatization and despair scores with fewer side effects after combining Lily Rehmannia with chemical drugs [153]. Animal experiments verified the efficacy of Lily Anemarrhenae in alleviating depressive symptoms. It was discovered that both can reduce IL-1β, IL-6, and TNF-α levels in peripheral blood [47] and hippocampal tissue. PI3K/Akt and MAPK were identified as potential signaling cascades based on network pharmacology [48]. The neuroprotective effects of Lilium Rehmannia decoction were explored in an in vitro experiment model of corticosterone-induced neurocytotoxicity. Specifically, we confirmed the interaction between the medicated serum and the microbiota, which produces various active substances, decreases intestinal permeability, and improves neurotransmitter deficits and inflammatory imbalances [154]. This proves that lilium can regulate the inflammatory response, thereby exerting antidepressant effects.
4.3.2. Goji
Lycium barbarum, alternatively referred to as Goji berry, is a native natural nourishment of Asian countries mainly used in soups, herbal teas, tinctures, wines, and juices for its beneficial properties. Behavioral and biochemical evidence suggests that polysaccharides (LBP), the major component of Lycium barbarum, have neuroprotective effects against depression-like behavioral and cognitive dysfunction. LBP lessen oxidative stress and upregulate the expression of protective neurotrophic factors, which can effectively promote hippocampal neurogenesis [155, 156] and reduce neuronal apoptosis [157]. Lin et al. found that the antidepressant property of LBP antidepressant was connected with a marked decline in the number, diameter, and activation of Iba-1+ LHb neurons [49]. Zhou et al. discovered that LBP can inhibit the NLRP3 inflammasome [50]. In vitro experimental studies displayed that Goji extracts shrink M1 proinflammatory factors (iNOS, IL-1β, et al.) and elevate M2 anti-inflammatory factors (arginase-1 and IL-4) [158]. So et al. conducted a clinical trial in a group of adolescents with mild depression. They indicated that the LBP group displayed greater alterations in Hamilton Depression Scale (HAMD-24) scores compared to the baseline at 6 weeks, higher remission rates (HAMD-24 total score ≤7), and no intervention-related side effects compared to the placebo group [159]. Of interest is the possible involvement of gut microbiota in the anti-inflammatory effects of Goji. LBP may modulate the intestinal microbiota of maternal rats, thus decreasing the effect of stressors on the emotional damage of offspring. The LBP group may increase the relative abundance of the Firmicutes and lower the one of Bacteroidetes [51]. Lycium anthocyanins (ACN) meliorated neuroinflammation by inhibiting the TLR4 signaling pathway, which was blocked upon depletion of gut microbiota. ACN-induced increment in the abundance of Lactobacillus spp. may alleviate neuroinflammation [160]. The aqueous extract of Goji ameliorated bile acid-mediated activation of microglia and neuroinflammation in the gut-brain axis pathway induced by a high-fat and high-fructose diet, significantly increased probiotic Lactococcus, and alleviated cognitive impairment [161]. To summarize, these findings suggest a holistic interplay between Goji and neuroinflammatory depression, partly mediated by the alleviation of gut microbiota dysbiosis.
4.3.3. Polygonatum
Polygonatum genus contains 79 species worldwide. Among these, the rhizomes of P. sibiricum, together with P. cyrtonema and P. kingianum, used as tonic herbs, are named “Huang-jing” in China. Polysaccharide is one of the main bioactive composites possessing various beneficial effects on depression, inflammation, neurotoxicity, gut microbiota, and so on. It can preserve synaptic plasticity and neuronal damage in both D-gal-induced impairment of learning and memory and single prolonged stress (SPS)-induced post-traumatic stress disorder mice model, averting oxidative stress and reducing inflammation [162, 163]. It lowered the levels of inflammation-related markers (hippocampal NLRP3 and apoptosis-associated speck-like protein, serum IL-1β, and TNF-α) and corticosterone and ameliorated the SPS-induced inflammatory response. Besides, numerous studies have investigated its antidepressant effect. Polygonatum polysaccharides cushioned LPS-mediated lessening in Nrf2 expression in the hippocampus but inhibited calpain-mediated inflammatory pathways, thereby alleviating depression-like behavior [52]. Shen et al. found that polysaccharides can remarkably reverse the shrinkage of hippocampal 5-hydroxytryptamine (5-HT) and alleviate depression-like behaviors in both LPS-treated and chronic unpredictable mild stress (CUMS) mice models. Notably, polysaccharides reversed LPS-induced increase in GFAP+ and NF-κB+ counts and decreased ERK phosphorylation, which may intervene the transcriptional activation of NF-κB [53]. In addition to its neuroprotective effects, Polygonatum polysaccharide also improves intestinal functions. It can regulate the structure of the gut microbiome and stimulate the production of SCFAs to preserve the integrity of intestinal morphology and barrier function, thus inhibiting intestinal inflammation in aged mice and obese rats [164, 165]. Meanwhile, it can attenuate DSS-induced colitis in mice by significantly reducing the production of proinflammatory cytokines, increasing the expression of antioxidant genes (SOD1 and Nrf-2), and selectively enhancing the development of probiotics such as Bifidobacterium and Alistipes [166].
4.3.4. P.odoratum
Polygonatum odoratum, a perennial rhizomatous medicinal plant, is utilized for medicinal purposes such as fever and gastritis. The immune response of macrophages treated with hot water-extractable polysaccharides of rhizome is the most powerful among the components of Polygonatum odoratum [167]. In vivo, P. odoratum rhizome extract administration increased the mucous of gastric epithelial tissue, decreased leukocyte infiltration, and regulated inflammatory factors [168]. Notably, both P. odoratum polysaccharides and methanol extract of P. odoratum can modulate gut microbiota, increasing the abundance of SCFA-producing bacteria. P. odoratum polysaccharides reduce the relative abundance of Clostridium, Coprobacillus, and Sutterella. The methanol extract of P. odoratum primarily comprises flavonoids and coumarins, decreasing the abundance of H2S-producing bacteria [169, 170].
4.3.5. Officinale
Officinale is the stem of Dendrobium officinale Kimura et Migo. Phytochemical studies revealed that Dendrobium officinale polysaccharides (DOPSs) are the predominant medicinal compound. It can engender the immune response via the TLR4/NF-кB signaling pathway [171], modulate gut microbiota, decrease the amount of detrimental bacteria, such as Helicobacter, Sutterella, and Escherichia-Shigella, etc., and increase the amount of beneficial bacteria such as SCFA-producing bacteria such as Allobaculum, Bifidobacterium, and Lactobacillus [172–175]. In addition, it enhances the expression of tight junction proteins, protecting intestinal permeability and alleviating the damage to intestinal mucosa [172, 176]. As for its neuroprotective effect, DOPS can reduce neuroinflammation by handicapping the buildup of TNF-α and IL-1β and activating astrocytes and microglia during cognitive decline [177]. Moreover, ultrafine Officinale powder can relieve fatigue and depression by regulating the neuroendocrine-immune network. For instance, it can alleviate hormonal changes in the HPA axis, downregulating 5-HT and dopamine and increasing IgG and IgM levels and CD4+/CD8+ T cell ratio [178].
4.4. Yang Tonifying
4.4.1. Cinnamon
Cinnamon, also called “Gui-pi” in condiments, is obtained from the bark of Cinnamomum cassia Presl young branches, which has numerous pharmacological effects, including antitumor, cytoprotective, neuroprotective, and anti-inflammatory properties. Oral administration of the water extracts markedly repressed the activation of astrocytes and microglia, downregulated IL-1β and TNF-α and reduced neuropathic cold allodynia following injection with oxaliplatin, a chemotherapy drug [179]. By modulating the serotonin-1a and GABAergic systems, the ethanol extracts effectively raised the number of entries and the time spent in the open arms of the elevated plus maze test, acting as a potent anxiolytic agent [54, 55]. Several phytochemical studies have isolated and identified numerous components of Cinnamomum cassia Presl, with cinnamaldehyde being the most prominent. In various models, trans-cinnamaldehyde (TCA) ameliorated memory deficit and enhanced synaptic plasticity. It accelerated the degradation of iNOS mRNA in LPS-stimulated microglia, decreasing NO production. In LPS-challenged mice, TCA reduced morphological changes and IL-1β release from primary microglia [180]. In presenilin knockout mice, TCA disrupted the NF-κB signaling pathway and reduced microglial activation and proinflammatory mediator levels, thereby suppressing neuroinflammation [181]. Similar to TCA, 2′-hydroxycinnamaldehyde downregulated NO and reduced proinflammatory cytokines by inhibiting ERK, JNK, and NF-κB [182]. Yao et al. demonstrated that cinnamaldehyde possesses antidepressant properties. It decreased COX-2 and PGE2 concentration in the frontal cortex and hippocampus of stressed rats, reversing behavioral abnormalities [56]. Furthermore, the combination of Cinnamomum cassia and Zingiber officinale reversed diabetes-induced depressive-like alterations. The combination mitigated the degenerative changes in the hippocampus and elevated serum levels of insulin, total antioxidant capacity, hippocampal BDNF mRNA, and expression of ki67, while diminishing serum glucose, IL-1β, and hippocampal expression of GFAP and Caspase-3 [57].
4.4.2. Cistanche
Rou-cong-rong, the Chinese name for Cistanche deserticola, is commonly referred to as “desert ginseng.” It mitigated Alzheimer’s disease and depression by decreasing monoamine oxidase activity, increasing dopamine concentration, enhancing neural excitability, reducing serum corticosterone concentration, and improving immune dysfunction [58, 59]. For example, the extracts exert antidepressant effects by provoking the AKT/GSK3β signaling pathway, increasing the number of Nissl bodies, and reducing neural apoptosis in the hippocampus of CUMS rats. Echinacoside, a form of phenylethanoid glycoside, has been shown to have neuroprotective effects. It alleviated seizure-like behavior, peripheral neuropathic pain, and PD by decreasing proinflammatory cytokine expression and inhibiting microglia overactivation [183–185]. Importantly, echinacoside executed an antidepressant-like role by triggering the 5-methyl-4-isoxazolepropionic acid receptor (AMPAR)/Akt/ERK/mammalian target of rapamycin (mTOR) pathway in the hippocampus and upregulating the expression of BDNF [60]. Cistanche polysaccharide, another active component, modulates gut microbiota and affects amino acid metabolism imbalance to improve intestinal immunity, indirectly affecting CNS to improve neurological damages [186]. Liu et al. elucidated that Cistanche polysaccharide can significantly improve depressive behaviors and gut dysfunction in rats. It kept Th17/Treg balance, moderated gut immunity, increased the abundance of beneficial bacteria, elevated SCFA levels, and altered amino acid metabolism, consequently improving gut homeostasis [61].
4.4.3. Eucommia Leaf
As the leaves of Eucommia ulmoides Oliv., Eucommia leaf (EUL) was utilized in clinical practice. It can target inflammatory factors and shrink the expression of NO and TLR4 [187, 188]. Chlorogenic acid and aucubin may be their main ingredients inhibiting inflammatory factors [189, 190]. It can also improve intestinal morphology, downregulate leukocyte infiltration [191], and adjust intestinal microbiota structure, decreasing the abundance of Bacteroidaceae and elevating the one of Akkermansiaceae and Ruminococcaceae [192]. In addition, ulmoidol suppressed the TLR4/CD14 signaling pathway, thus inhibiting the NF-κB/MAPK signaling pathway. The mechanism at hand pertains to the prevention of PU box binding-1, alleviating neuroinflammatory responses in microglia [193]. Kim et al. reported that ethyl acetate fraction derived from EUL can downregulate the levels of p-JNK, p-IκBα, and TNF-α in the lung and olfactory bulb due to PM2.5 toxicity, consequently decreasing the expression of inflammatory factors in the hippocampus [194].
5. Discussion and Prospect
5.1. The Multiple Antidepressant Mechanism of Edible TCM Remains to be Explored
Due to the diverse range of bioactive components in edible TCM, the neuroprotective effect is mediated through multiple pathological links through the combination of multifold elements, paths, and targets. Therefore, they may relieve depression via different mechanisms, including regulating peripheral and central inflammation, modulating metabolism, modifying monoamine neurotransmitters, and stabilizing the function of the HPA axis. Some of the abovementioned deficiency tonic medicines in edible TCM have been announced to have antidepressive effects. Their mechanism of action for depression is still being explored based on the traditional hypothesis of depression pathogenesis—regulating neurotransmitters and endocrine homeostasis. The role of inflammation in therapy of depression needs to be further clarified. TCM with anti-inflammatory properties may ameliorate neuroinflammation by modulating the same inflammatory cytokines and pathways, such as White dolichos, Codonopis, and Chinese Yam, although their protective effects on CNS-related diseases, such as mental and neurological disorders, are yet to be totally understood.
5.2. Discovery of Antidepressant Active Ingredient Could Aid Functional Food Development
Similar to functional food, edible TCMs share a common treatment approach, utilizing their bioactive elements, which are identified as ingredients in foods or nutritional supplements, as the basis for pharmacodynamic effects. Understanding the structure and components of these constituents is essential for the new-generation functional food. In this review, we outlined that the primary elements, polysaccharides and saponins, are significantly influential. With the support of genomics, proteomics, metabolomics, high-throughput screening and various other techniques, the extraction and identification of bioactive components of edible TCMs can elucidate the material basis of the antidepressant effects, increase safety, and contribute to the design of multitarget, multilevel mechanistic studies. This lays the groundwork for further development of edible TCMs into new-generation functional foods with therapeutic potential for depression.
5.3. Exploring the Clinical Potential of Combined TCM for Depression Treatment
Although a single edible TCM or its components markedly improved depression, the majority of previous studies were animal based, with no clinical data. However, TCMs are being widely used in daily clinical practice. For instance, the Xiaoyao pill, made of seven Chinese herbs, including Bupleurum chinense DC, Angelica sinensis (Oliv.) Diels, Glycyrrhiza uralensis Fisch et al. is a classic formula for treating anxiety and insomnia. The gut microbiota converts its major components like benzoic acid, liquiritigenin, glycyrrhetinic acid, and saikogenin D into substances with antidepressant properties. They can pass the blood-brain barrier, potentially preventing depression by directly inhibiting the expression and activity of fatty acid amide hydrolase in the brain [138]. Compared to using only a single species, combining these drugs preserves their pharmacological effects and safety, reduces negative reactions, or increases their effectiveness. Furthermore, multiple compounds in edible TCM may act synergistically and exhibit toxic antagonism.
Therefore, more combinations of antidepressant TCM are urgently needed to be experimentally investigated for their efficacy in the clinical setting, randomized controlled trial, for example, to compare their antidepressant efficacy with that of current first-line antidepressant and to investigate the side effects, compliance, and other aspects. The scientific popularization of antidepressive tonic medicines also needs to be mentioned and expanded. Dietary nutrients may affect the gut microbiota and influence the metabolic status of the host [195]; nutritional supplements developed around them, therefore, also hold broad promise because of their food properties.
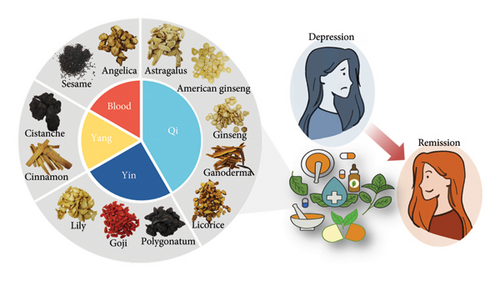
6. Conclusion
This article reviewed recent studies that examined how tonic herbs occupy the largest category of edible TCM and affect inflammation associated with depression. The gut microbiota serves as an important mediator in the regulation of the body’s inflammatory response, and we have also listed the effects of various herbs on the microbiota together. According to current evidence, all 22 deficiency tonic medicines have anti-inflammatory and immunomodulatory functions, and 12 of them have definite antidepressant effects (Figure 3). Most of the active substances that play a major role are polysaccharides. As a novel treatment strategy, edible TCM is a type of food that is always used in our diet. Thus, it has been commonly applied in our clinical practice, as its side effects are less than chemically synthesized drugs. All of these advocate a strategy for optimizing therapeutics for depressive disorders. It should be noted that tonic medicine is merely scratching the surface of the vast reservoir in TCM, and the harmonious fusion of medicine and food has emerged as a prominent trend. It can greatly aid in the exploration and advancement of efficacious antidepressant medicines and food products and help design rational treatment strategies with the aid of contemporary medicine and advanced analytical techniques.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
S.Y Ren conceptualized, investigated, and reviewed and edited the study. P.L Qin wrote the original draft and performed formal analysis and investigation. J Yang contributed to project administration and supervised the study. G Wang supervised the study. All the authors have read and agreed to the published version of the manuscript. Siyu Ren and Peilin Qin contributed equally to this work.
Acknowledgments
We thank Bi-Rui Wen for the support of Chinese medicinal materials photos in producing Figure 3. Figure 2 was drawn by Figdraw. This research was funded by the National Natural Science Foundation of China (No. 82201681 and 82171526), the research projects of Beijing Anding Hospital (YJ2022-03).
Open Research
Data Availability
Data sharing is not applicable to this article as no new data were created or analysed in this study.




