Optimization of Rare Ginsenosides and Antioxidant Activity Quality of Ginseng Jiaosu Based on Probiotic Strains and Fermentation Technology
Abstract
The primary active components of plant ginseng are saponins, and rare saponins play a specific antitumor action. In this study, Lactobacillus that can efficiently transform rare saponins were screened as fermentation strains of ginseng enzymes. The fermentation process was optimized to investigate the changes in various biochemical indicators and antioxidant activities of the enzymes before and after fermentation. The results showed that Lactiplantibacillus plantarum had the highest conversion efficiency for diol saponins, among which the content of rare ginsenoside-CK increased by 256%. The optimum optimizing method was a 1 : 1 : 10 ratio of ginseng extract/concentrated apple juice/water, fermentation time of 16 days, initial pH of 6.0, fermentation temperature of 37°C, and sterilization amount of 1.0%. The contents of rare ginsenoside-Rh1, ginsenoside-F2, ginsenoside-Rg3, and ginsenoside-CK increased by 18.48%, 135.73%, 343.03%, and 441.80%, respectively, and the process was stable and controllable. After fermentation, the scavenging rates for hydroxyl radical, DPPH radical, and superoxide anion radical were increased by more than 9%, and new organic acids were produced. In general, this study established an efficient, stable, and controllable ginseng fermentation process to improve the rare saponin content and biological activity of ginseng enzymes, which can provide new ideas for traditional Chinese medicine and medicinal health food in China.
1. Introduction
A healthy diet has drawn more attention as the economy has grown, and pharmaceutical and food-homologous products have gotten much attention and have enormous market potential. The jiaosu is one or more different fruits, vegetables, mushrooms, Chinese herbal remedies, etc., that have been fermented by adding probiotics—natural or synthetic—and contain microorganisms that produce enzymes as well as their interaction metabolites and mutual regulators [1, 2]. The food was full of vitamins, enzymes, minerals, and secondary metabolites, among other nutrients [3]. It not only keeps the natural nutrients in the fermented raw materials but also significantly increases the amount of numerous active substances and creates new bioactive ingredients and biological enzymes that improve digestion and absorption, promote metabolism, and circulate blood. Intestinal and other processes provide an innovative nutritional strategy for creating functional foods [4, 5].
Ginseng (Panax ginseng C.A. Meyer) is one of the world’s most famous medicinal herbs, having thousands of years of application history in traditional Chinese medicine [6]. Ginsenosides are the main active components of ginseng and have irreplaceable pharmaceutical significance in treating human diseases. However, natural saponins do not necessarily have the best physiologically active molecular structure. Studies have shown that ginsenosides are composed of the basic skeleton tetracyclic steroid and linked glycan groups [7, 8]. Hence, ginsenosides can be hydrolyzed to remove part or all the glycans to form rare saponins and thus improve the biological activity of saponins. Ginsenoside-Rh1, ginsenoside-Rg3, ginsenoside-F2, ginsenoside-CK, and other rare saponins have higher medicinal value than ordinary saponins [9].
Several academics have conducted an extensive study on the ginsenosides’ change in recent years. Chemical acid hydrolysis and microbial conversion are two of the most often employed saponin conversion techniques [10]. The advantage of the acid hydrolysis method is that the reaction conditions are simple, the ginsenosides are unstable in low pH acidic environments, and the acid will hydrolyze the connected sugar groups [11]. However, there is also the dehydration and cyclization of the saponin itself under acidic conditions or the change of the conformation of the saponin, which will eventually lead to more by-products in the final product, thereby increasing the separation cost, and only saponin monomers can be obtained, which is unsuitable for researching saponin conversion in the food field [12]. The microbial conversion method is an enzymatic reaction between microorganisms and exogenous substrates, and the substrates are converted into target products by enzymes [13]. During the growth and metabolism of microorganisms, one or more enzymes will be produced to hydrolyze the glycosides connected to the C3, C6, and C20 positions of ginsenosides, thereby transforming the saponins that are more abundant in ginseng, such as Rb1 and Rb2 [14, 15]. Yan et al. studied the biotransformation ability of different fungus on ginsenoside-Rg1 and used LC-MS technology to identify the structure of the transformation product. The results showed that the strains EST-I and EST-II directed the conversion of ginsenoside-Rg1 to ginsenoside-F1 during the fermentation process [16]. Therefore, in the development process of ginseng jiaosu products, increasing the content of ginseng rare saponins through probiotics will greatly improve the application value of ginseng jiaosu products [17].
The enzymatic activity of β-glucosidase produced by bacteria during the metabolic process is the main reason for determining the content of saponins converted into rare saponins [15]. Lactobacillus are a kind of probiotic that produces a variety of natural active substances such as diacetyl, hydrogen peroxide, lactic acid, and bacteriocin [17, 18]. After fermentation by Lactobacillus, in addition to lactic acid production, some macromolecular compounds are converted into small-molecular compounds with high biological activity [18]. According to earlier research, fermentation dramatically boosted the total polyphenol content and antioxidant activity of veggie fruit beverages like pear juice [19]. In addition, Lactobacillus can effectively degrade oxalate by transporting oxalate into cells through isozyme, where oxalate undergoes conversion to oxaloco A by formyl-CoA transferase and further to formate and carbon dioxide by oxalocoenzyme A decarboxylase, ultimately enhancing the functional properties of enzyme products [16, 20, 21]. However, the probiotic fermentation preparation is not ideal for creating raw materials due to the strain specificity, the research on the transformation of ginsenosides by using Lactobacillus is still in the development stage, and the transformation is mainly aimed at a single saponin, and few reports exist on the transformation and application of multiple rare saponins.
Therefore, to obtain fermentation probiotics with higher content of rare saponins (ginsenoside-F2, ginsenoside-Rg3, ginsenoside-CK, ginsenoside-Rh1), more complete types, and more potent drug efficacy after fermentation, four Lactobacillus that can produce glucosidase were first screened in this study. Then, the ginseng enzyme fermentation process was optimized (fermentation time, temperature, inoculation amount, initial pH, etc.) new ideas for Chinese traditional medicine, and medical health products are offered by the changes in indicators and antioxidant activity quality.
2. Materials and Methods
2.1. Chemicals and Solvents
Ginseng extract and concentrated apple juice were purchased from Infinitus (China) Co., Ltd (Guangzhou City, Guangdong Province, China). The experimental strains were from the Chinese Center for the Preservation and Management of Microbial Cultures. They included Lactobacillus acidophilus (No. GIM1.731), Lactobacillus casei (No. GIM1.204), Lactiplantibacillus plantarum (GIM1.648), and Lactobacillus fermentum (No. GIM1.985). Ginsenoside-Rg1 (Rg1), ginsenoside-Re (Re), ginsenoside-Rb1 (Rb1), ginsenoside-Rb2 (Rb2), ginsenoside-Rd (Rd), ginsenoside-Rg3 (Rg3), ginsenoside-F2 (F2), ginsenoside-CK (CK), and ginsenoside-Rh1 (Rh1) (HPLC, ≥98%) standard products were purchased from Shanghai Yuanye Biotechnology Co., Ltd (Shanghai, China). Standards of p-nitrophenol, glucose, fructose, lactic acid sand organic acid mixture (HPLC, ≥98%) were purchased from Beijing Chemical Co., Ltd. (Beijing, China). The main reagents included peptone, yeast paste, beef paste, ammonium citrate, potassium dihydrogen phosphate, manganese sulfate, and disodium hydrogen phosphate were purchased from TCI Co. Ltd. (Shanghai, China).
2.2. Selection of Transformed Ginsenoside Lactobacillus
2.2.1. Activation and Culture of Lactobacillus Strains
A sterile pipette is used to draw 1.0 mL of the sterilized MRS liquid medium at room temperature, transfer it to a culture tube, shake thoroughly, place 0.5 mL of Lactobacillus liquid on the MRS solid medium, and incubate at 37°C for 36 hours. Streak culture was carried out after three generations of continuous culture. Vigorously growing single colonies were inoculated into a sterilized MRS basal medium and cultured for 36 h.
2.2.2. Determination of β-Glucosidase Enzyme Activity
(1) Extraction of Enzyme Solution. Four Lactobacillus cultures were cultured according to the method described in Section 2.2.1 for further research. 1.0% of the four strains were incubated in MRS medium containing ginseng extract at 37°C for 36 h and centrifuge at 10,000 rpm (11,100 g force equivalent) for 20 minutes at 4°C to collect the supernatant. Ammonium sulfate powder was added at a temperature of 25°C and combined with slow, continuous stirring. The mixture was centrifuged at 13,000 rpm (18,759 g force equivalent) for 20 minutes at 4°C and left to stand at 4°C for 12 hours before the precipitate was collected. The pellet was dialyzed in 10.0 mL of sodium acetate buffer for 24 hours, and the supernatant was crude enzyme solution after centrifugation (18,759 g force equivalent for 10 min, 4°C).
(2) Standard Curve Drawing. We accurately weighed p-nitrophenol (PNP), dissolved it in sodium acetate buffer solution (pH 5.5), and configured it into standard solutions with concentrations of 10, 20, 30, 40, and 50 μmol/mL. We then took 1 mL of the standard solution of different concentrations, added 2.0 mL of 1.0 mmol/mL sodium carbonate solution, and mixed well. A UV-visible spectrophotometer (Shimadzu UV-2450, Japan) is used to measure the UV absorption at a wavelength of 405 nm and then constructed a standard curve with PNP concentration as the X axis and absorbance as the Y axis. Y = 0.0181x + 0.0043, R2 = 0.998, within the concentration range of 10∼50 μmol/mL.
2.2.3. Extraction, Purification, and Detection Methods for Saponins
We added 30.00 g of ginseng extract to medium and mixed well, evenly distributing it into five Erlenmeyer flasks. Each Erlenmeyer flask was sterilized at numbered 1–5 contained 50 mL, 121°C for 20 min, and cooled to room temperature. Subsequently, 0.5 mL of cultured four Lactobacillus fermented liquid medium were added to (NO. 1–4) bottle, and 0.5 mL of medium was added as a blank control to the NO.5. We mixed them well and incubated them at 37°C in an incubator for 3 days for later use.
Referring to the method of Bortolomedi et al. [22], we shook the fermented liquid well, took 10 mL of fermentation broth, and put it in a separating funnel. We degreased it with 90 mL of ether three times and then extracted the degreased fermentation broth with 90 mL of water-saturated n-butanol times. Finally, the water-saturated n-butanol after three extractions was collected, evaporated to dryness under reduced pressure at 45°C, and dissolved in 5 mL of methanol for later use. The saponin content of the sample was detected by high-performance liquid chromatography (HPLC1200, Agilent Technologies, Santa Clara, CA, USA) under the following conditions: mobile phase, A (acetonitrile) and B (water); column, C18 4.6 × 250 mm, 5 μm; column temperature, 30°C; gradient elution flow rate, 1.0 mL/min.
2.2.4. Preparation of Saponin Standards
We accurately weighed 1.0 mg each of the ginsenosides Rg1, Re, Rb1, Rb2, Rd, Rg3, F2, CK, and Rh1 standards in nine 100 mL beakers, added appropriate amount of methanol to dissolve, and ultrasonicated. The ginsenoside chromatogram was plotted from the chromatographic results in Section 2.2.3, and the results are detailed in Appendix S1-1.
For the standard-curve determination of nine saponins, 20.0 mg of the different ginsenosides were accurately weighed, and methanol was added to 20.0 mL. We transferred 1, 2, 3, and 4 mL of saponin standards in four 5 mL volumetric flasks to obtain the standard solution of ginsenosides with different concentrations. The standard solution of ginsenosides with different concentration gradients was determined by the above HPLC method, and the corresponding peak area at different concentrations of each saponin was recorded, with the peak area as the ordinate. The standard curve of ginsenosides was plotted using the saponin concentration (mg/mL) as the abscissa, and the results are detailed in the Appendix S1-2.
2.3. Test Method for Optimization of Ginseng Jiaosu Production Process
2.3.1. Process Flow
The main steps of this experiment is shown in Figure 1. Since glycogen addition, fermentation time, fermentation temperature, initial pH, and strain inoculation are the key technical parameters affecting jiaosu quality, this study was experimentally explored Figure 1.
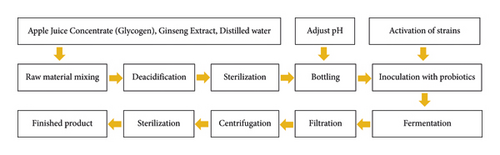
2.3.2. Single-Factor Test of the Fermentation Process
The rare saponin content was used as the investigation index to optimize the process parameters glycogen addition amount, fermentation time, fermentation temperature, initial pH, and strain inoculation amount.
(1) Fixed Ginseng Extract. 1 : 10 addition ratio of distilled water, initial pH of 6, 1% strain inoculation rate, 37°C fermentation for 16 days, optimized glycogen addition amount, and 0.0, 5.0, 10.0, 15.0, and 20.0 g of concentrated apple juice.
Fixed ginseng extract/concentrated apple juice/distilled water addition ratio of 1 : 1 : 10, initial pH of 6, strain inoculation rate of 1.0%, 37°C optimized fermentation time level, and 0, 1, 4, 8, 12, 16, 20, 24, and 28 days for time node sampling.
Fixed ginseng extract/concentrated apple juice/distilled water addition ratio of 1 : 1 : 10, strain inoculation rate of 1.0%, fermentation for 16 days, optimized fermentation temperature levels, and 39, 37, 35, 33, and 31°C.
Fixed ginseng extract/concentrated apple juice/distilled water addition ratio of 1 : 1 : 10, initial pH of 6, strain inoculation rate of 1.0%, fermentation at 37°C for 16 days, optimization of initial pH values of 4.0, 5.0, 6.0, 7.0, and 8.0; fixed ginseng extract/concentrated apple juice/distilled water addition ratio of 1 : 1 : 10, initial pH of 6, 37°C fermentation for 16 days, and optimal inoculum addition amounts of 0, 0.1%, 0.5%, 1.0%, 2.0%, and 5.0% (bacteria concentration of 6.5109 CFU/mL).
2.3.3. Verification of Optimal Fermentation Processes
According to the results of process optimization, the fermentation broth was prepared according to the ginseng extract/concentrated apple juice/water ratio of 1 : 1 : 10. It was then mixed and pasteurized at 60°C for 30 min. We adjusted the initial pH 6 of the fermentation broth in an ultraclean table after cooling and then added the cultured L. plantarum to the Erlenmeyer flask with an inoculation amount of 1.0% and mixed evenly and sealed. The blank group without bacteria was set, and all were placed in a 37°C incubator for fermentation for 16 days. Three batches of samples were continuously fermented and then extracted and determined for saponins according to Section 2.2.3 methods.
2.4. Analysis of Biochemical Indexes of the Fermentation Process of the Jiaosu Optimal Process
To reduce the experimental error caused by external addition, this experiment mainly used dipotassium hydrogen phosphate and potassium dihydrogen phosphate to adjust the pH of the initial fermentation. pH measurements were determined with an PHS-3C meter (Bellingham and Stanley Ltd, UK). Detailed information on methods for determining the antioxidant properties of glucose, fructose, lactic acids, organic acids, and flavonoids is provided in Appendix S2 of this manuscript.
2.5. Statistical Analysis
Results were expressed as the mean value ± standard deviation (mean ± SD), and all experiments were performed in triplicate. Analyses of the significance of differences were performed by Tukey’s HSD test using SPSS.20 software (SPSS Inc., Chicago, IL).
3. Results and Discussion
3.1. Transformation of Ginsenoside Strain Screening
The enzymatic activity size of β-glucosidase produced by bacteria during metabolism is the main factor determining the conversion of saponins into rare saponins [23]. Figure 2 shows the enzyme activity of β-glucosidase induced by four Lactobacillus. The figure shows that the order of β-glucosidase enzyme activity from largest to smallest was L. plantarum > L. fermentium > L. casei > L. acidophilus. Therefore, L. plantarum was tentatively defined as a fermented and transformed ginsenoside strain.
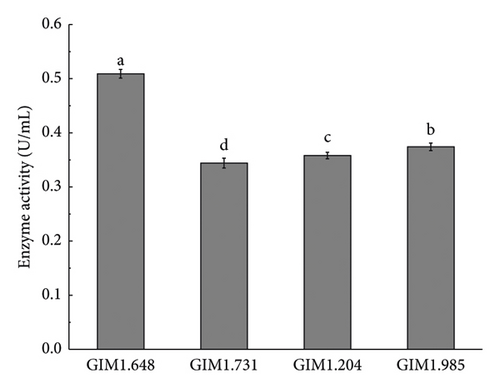
Figure 3 shows the content of various saponins in four Lactobacillus fermentation broths and unsterilized fermentation broth. As shown in the figure, the concentration of saponins of the four Lactobacillus changed greatly after fermentation. Compared with unsterilized fermentation broth, the contents of ginsenosides Rb1, Rb2, Rd, Re, and Rg1 were reduced, while the contents of rare saponins F2, Rg3, CK, and Rh1 were significantly increased. Studies have shown that the conversion pathways of diol saponins by bacteria include Rb1/Rb2 ⟶ Rd ⟶ F2 ⟶ CK and Rb1 ⟶ Rd ⟶ Rg3. Therefore, the content of ginsenoglycol saponins Rb1, Rb2, and Rd decreased because the enzymes produced by the strain partially converted them into Rg3, F2, and CK. Ginsenoside-Rh1 is one of the secondary metabolites of triol saponin Re and Rg1, which is why the concentration of saponins Re and Rg1 decreases and the concentration of Rh1 increases.
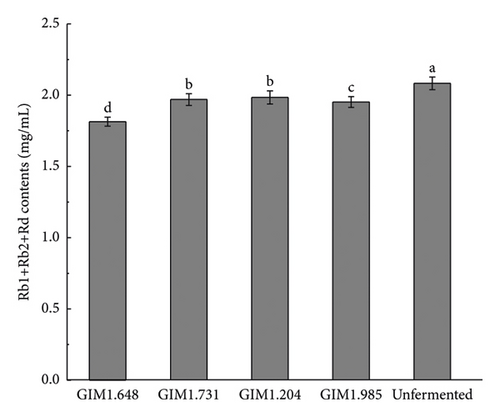
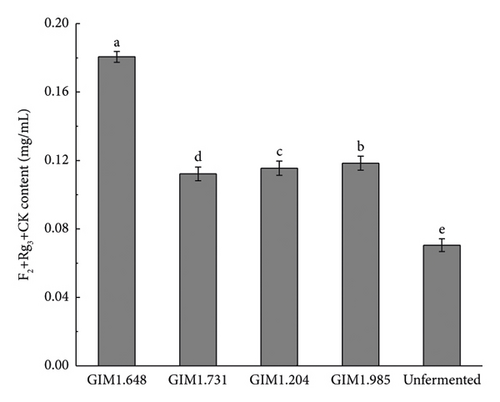
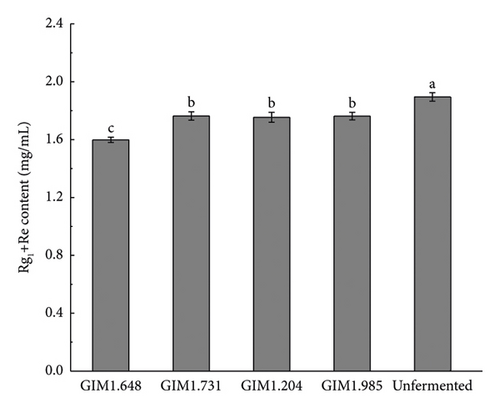
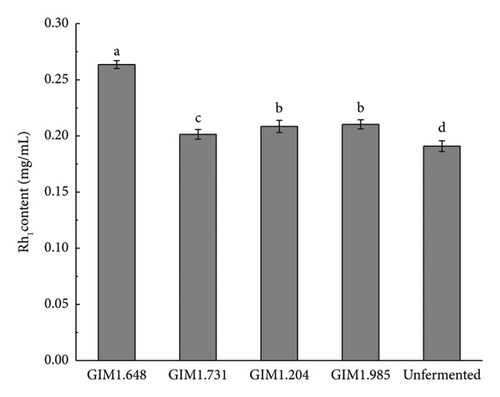
Compared with the other three Lactobacillus, after fermentation by L. plantarum, the concentrations of ginsenoglycol saponins Rb1, Rb2, and Rd in the fermentation broth decreased significantly, and the total content before and after fermentation decreased by 0.2687 mg/mL. Meanwhile, the full content of the transformation products Rg3, F2, and CK also increased the most, from 0.0705 ± 0.0011 mg/mL to 0.1806 ± 0.0024 mg/mL, of which the CK content increased the most, increasing by 256%. The concentration of the original ginsenotriol type ginsenoside-Re and ginsenoside-Rg1 decreased more after fermentation by four bacteria, but the concentration of Rh1, one of the transformation products, was not too obvious. The main reason may be that it was converted into another ginsenotriol saponin conversion product. In summary, L. plantarum produced the highest activity of β-glucosidase, and the total content of rare saponins F2, Rg3, and CK converted after fermentation was the highest. Therefore, in this experiment, L. plantarum was selected as the fermentation strain for the next step of the research.
3.2. Determination of Lactobacillus Fermentation Process
3.2.1. Experiment for the Determination of Glycogen Addition Amount
The initial glycogen amount will affect the concentration of bacteria in the fermentation broth during the stable period of the strain, thereby affecting the enzyme-producing activity of the strain, which in turn will affect the conversion rate of saponins [24]. Determining the proportion of glycogen can not only maximize the conversion rate of saponins after fermentation but also reduce the sugar content in ginseng jiaosu after fermentation, thereby increasing the categories of ginseng jiaosu beneficiaries. It can be seen from Table 1 that when the amount of apple juice is less than 10.0 g, the content of each rare saponin increases with the increase in apple juice content. When the amount of apple juice added exceeded 10.0 g, the upward trend of rare saponins tended to flatten. Therefore, ginseng jiaosu can be prepared according to the ratios of ginseng powder (10.0 g), apple juice (10.0 g), and water (100 mL) as fermentation raw materials; that is, ginseng jiaosu can be prepared according to the ginseng powder/concentrated apple juice/water ratio of 1 : 1 : 10.
| Different fermentation conditions | Rb1 | Rb2 | Rd | F2 | Rg3 | CK | Rg1 | Re | Rh1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Saponin content (mg/100 mL) | ||||||||||
| Apple juice added | 0 g | 67.01 ± 2.12a | 81.48 ± 3.18a | 50.62 ± 1.86a | 4.04 ± 0.14c | 1.91 ± 0.09c | 3.94 ± 0.35c | 45.04 ± 1.52a | 138.68 ± 4.39a | 20.31 ± 1.12b |
| 5 g | 52.47 ± 4.58a | 75.58 ± 3.21ab | 47.82 ± 1.66ab | 5.68 ± 0.11b | 2.41 ± 0.07b | 5.84 ± 0.34b | 40.41 ± 2.31ab | 123.12 ± 4.92a | 21.93 ± 1.04ab | |
| 10 g | 56.35 ± 5.19b | 67.66 ± 4.51b | 44.33 ± 2.18ab | 7.99 ± 0.27a | 6.52 ± 0.22a | 12.94 ± 0.65a | 36.07 ± 3.25b | 102.25 ± 7.61b | 24.07 ± 1.25ab | |
| 15 g | 55.68 ± 3.59b | 67.01 ± 3.24b | 43.83 ± 1.64ab | 8.04 ± 0.21a | 6.63 ± 0.10a | 13.51 ± 0.32a | 35.81 ± 1.45b | 100.18 ± 3.47b | 24.35 ± 1.14ab | |
| 20 g | 54.92 ± 3.85b | 66.34 ± 3.85b | 43.12 ± 2.13b | 8.11 ± 0.19a | 6.81 ± 0.13a | 13.92 ± 0.18a | 35.11 ± 1.64b | 99.45 ± 2.18b | 24.62 ± 0.97a | |
| Fermentation temperature | 33°C | 63.94 ± 2.98a | 75.56 ± 3.89ab | 48.67 ± 1.88b | 5.86 ± 0.12c | 4.37 ± 0.06d | 9.94 ± 0.35b | 44.14 ± 1.67ab | 132.68 ± 4.57ab | 21.91 ± 1.03b |
| 35°C | 59.91 ± 2.69ab | 72.52 ± 3.36b | 46.41 ± 1.76bc | 6.64 ± 0.16b | 5.33 ± 0.08b | 11.84 ± 0.45a | 38.34 ± 2.26ab | 119.38 ± 5.28bc | 22.73 ± 1.12b | |
| 37°C | 56.35 ± 5.19b | 67.66 ± 4.5c | 44.33 ± 2.18c | 7.996 ± 0.27a | 6.52 ± 0.22a | 12.94 ± 0.65a | 36.07 ± 3.25b | 102.25 ± 7.61c | 24.07 ± 1.25a | |
| 39°C | 59.98 ± 3.05ab | 73.01 ± 3.10b | 45.83 ± 1.59c | 6.58 ± 0.14b | 4.83 ± 0.10c | 12.18 ± 0.42a | 38.85 ± 1.72ab | 121.37 ± 4.49abc | 22.15 ± 1.09b | |
| 41°C | 65.71 ± 2.94a | 79.54 ± 3.15a | 50.86 ± 2.03a | 4.91 ± 0.09d | 3.87 ± 0.07e | 8.29 ± 0.29c | 46.08 ± 1.85a | 139.43 ± 5.08a | 20.54 ± 1.06b | |
| Initial pH | pH 4 | 65.64 ± 4.86a | 73.68 ± 3.68a | 51.85 ± 1.94a | 6.67 ± 0.12c | 5.45 ± 0.18b | 10.33 ± 0.49b | 47.35 ± 2.28a | 135.21 ± 6.63a | 18.82 ± 1.08b |
| pH 5 | 61.53 ± 5.01b | 69.29 ± 4.12b | 48.26 ± 2.56b | 7.27 ± 0.21abc | 5.98 ± 0.19ab | 11.45 ± 0.58ab | 42.66 ± 3.19ab | 126.84 ± 6.81ab | 20.112 ± 1.13ab | |
| pH 6 | 56.35 ± 5.19c | 67.66 ± 4.51c | 44.33 ± 2.18c | 7.996 ± 0.27a | 6.52 ± 0.22a | 12.94 ± 0.65a | 36.07 ± 3.25c | 102.25 ± 7.61b | 24.07 ± 1.25a | |
| pH 7 | 62.97 ± 4.82ab | 69.15 ± 5.01b | 45.49 ± 2.03c | 7.89 ± 0.22ab | 6.36 ± 2.80a | 12.64 ± 0.54a | 37.1 ± 2.71b | 119.69 ± 7.12ab | 21.74 ± 1.18ab | |
| pH 8 | 67.24 ± 4.59a | 76.61 ± 4.28a | 50.24 ± 2.24a | 7.21 ± 0.18bc | 5.85 ± 0.15ab | 11.94 ± 0.60ab | 44.2 ± 3.34a | 127.95 ± 6.92ab | 19.83 ± 1.32ab | |
| Inoculation amount | 0.10% | 65.62 ± 4.46a | 79.14 ± 4.18a | 50.91 ± 2.58a | 4.43 ± 0.21c | 3.36 ± 0.25c | 3.94 ± 0.57c | 44.61 ± 4.11a | 139.68 ± 8.84a | 20.30 ± 1.04b |
| 0.50% | 60.08 ± 4.83b | 73.22 ± 5.03b | 47.21 ± 2.24a | 6.71 ± 0.31b | 4.86 ± 0.34b | 8.37 ± 0.63b | 40.29 ± 3.58b | 120.54 ± 6.91ab | 21.26 ± 1.17b | |
| 1.0% | 56.35 ± 5.19c | 67.66 ± 4.51c | 44.33 ± 2.18b | 7.996 ± 0.27a | 6.52 ± 0.22a | 12.94 ± 0.65a | 36.07 ± 3.25c | 102.25 ± 7.61b | 24.07 ± 1.25a | |
| 1.50% | 56.94 ± 5.03c | 66.94 ± 4.76c | 45.40 ± 2.95b | 8.01 ± 0.18a | 6.58 ± 0.28a | 13.26 ± 0.66a | 35.43 ± 3.39c | 116.09 ± 8.35ab | 24.16 ± 1.36a | |
| 2.00% | 56.03 ± 4.75c | 65.71 ± 4.69c | 44.08 ± 2.71b | 8.13 ± 0.24a | 6.64 ± 0.19a | 13.51 ± 0.71a | 35.03 ± 4.05c | 115.84 ± 7.15ab | 24.74 ± 1.13a | |
- Results are expressed as mean ± standard deviation (n = 3). Different letters in same column indicate significant differences at the 5% level.
3.2.2. Determination of Fermentation Temperature of Lactobacillus
Too high or too low temperature will affect microbial metabolism of enzyme production and enzyme activity, thereby affecting the fermentation process. In the industrial fermentation process, the fermentation temperature will also be controlled to increase the accumulation rate of fermentation products [25]. Therefore, the conversion results of nine saponins were obtained by single-factor experiments on temperature, as shown in Table 1. It can be seen from Table 2 that when fermentation is for 16 days in the range of 31–37°C, the concentration of saponins Rg1, Re, Rb2, Rd, and Rb1 in the fermentation broth gradually decreases with the increase in temperature. When the temperature increases, the growth and metabolism of bacteria in the fermentation broth is vigorous, the activity of enzymes gradually increases, and the efficiency of converting saponins becomes higher. When the temperature exceeds 37°C, the concentration of saponins Rg1, Re, Rb2, Rd, and Rb1 in the fermentation broth is higher than that at 37°C. This is mainly because when the temperature reaches a certain extent, the enzyme will be inactivated when the temperature is increased. The changes in rare saponins Rh1, F2, Rg3, and CK are just the opposite; hence, the fermentation temperature is set at 37°C.
| Time (d) | Rb1 | Rb2 | Rd | F2 | Rg3 | CK | Rg1 | Re | Rh1 |
|---|---|---|---|---|---|---|---|---|---|
| Saponin content (mg/100 mL) | |||||||||
| 0 | 69.82 ± 6.14a | 83.84 ± 4.26a | 53.43 ± 1.46a | 3.72 ± 0.25c | 1.52 ± 0.11c | 2.41 ± 0.18f | 47.23 ± 2.11a | 142.33 ± 9.25a | 19.15 ± 1.13a |
| 1 | 69.07 ± 7.23a | 81.89 ± 4.61ab | 52.05 ± 1.85a | 3.91 ± 1.91c | 1.94 ± 0.09c | 4.82 ± 0.14e | 46.25 ± 3.52a | 135.17 ± 8.68ab | 19.52 ± 1.87a |
| 4 | 66.08 ± 4.41a | 78.57 ± 2.83abc | 50.81 ± 3.27a | 4.74 ± 0.13bc | 2.52 ± 0.24bc | 6.82 ± 0.23d | 43.04 ± 2.55ab | 124.65 ± 7.62abc | 20.54 ± 2.12ba |
| 8 | 62.24 ± 5.86a | 75.50 ± 1.71abc | 47.64 ± 2.32ab | 5.84 ± 0.31abc | 4.06 ± 2.60abc | 9.57 ± 0.30c | 40.67 ± 3.98ab | 116.85 ± 8.30abc | 21.92 ± 3.50ba |
| 12 | 58.56 ± 6.96ab | 72.04 ± 5.52abc | 46.45 ± 5.39b | 6.62 ± 0.35ab | 5.37 ± 0.18ab | 11.25 ± 0.74b | 38.32 ± 2.31ab | 109.51 ± 7.43bc | 22.94 ± 1.89b |
| 16 | 56.35 ± 5.19b | 67.66 ± 4.51c | 44.33 ± 2.18b | 7.96 ± 0.27a | 6.52 ± 0.22a | 12.94 ± 0.41a | 36.07 ± 3.25b | 102.25 ± 7.61c | 24.07 ± 1.25ab |
| 20 | 55.95 ± 6.79b | 68.67 ± 3.99bc | 44.04 ± 7.36b | 8.06 ± 0.21a | 6.61 ± 0.30a | 12.80 ± 0.45a | 35.84 ± 2.68b | 101.68 ± 4.97c | 23.93 ± 2.41a |
| 24 | 55.49 ± 5.40b | 67.91 ± 3.48c | 43.88 ± 6.51b | 8.01 ± 0.26a | 6.72 ± 0.16a | 12.76 ± 0.61a | 35.94 ± 3.31b | 101.31 ± 6.42c | 23.84 ± 2.95a |
| 28 | 55.67 ± 6.17b | 68.55 ± 2.63bc | 43.94 ± 3.93b | 7.91 ± 0.33a | 6.76 ± 7.36a | 12.83 ± 0.56a | 35.39 ± 1.90b | 102.05 ± 8.14c | 23.78 ± 1.75a |
- Results are expressed as mean ± standard deviation (n = 3). Different letters in same column indicate significant differences at the 5% level.
3.2.3. Determination of the Initial pH of the Fermentation
Adjusting the initial pH of the fermentation broth facilitates the growth of microorganisms in the fermentation broth, which affects the activity of the enzymes produced by metabolism, thus affecting the product’s conversion rate. It can be seen from Table 1 that the initial pH of the fermentation broth has a significant effect on the conversion of L. plantarum into saponins. When the initial pH of the fermentation broth was 4–6, the concentrations of saponins Rg1, Re, Rb2, Rd, and Rb1 gradually decreased with the increase in pH, while the contents of rare saponins Rh1, F2, Rg3, and CK gradually increased. When the fermentation broth pH is 6–8 and fermentation is for 16 days, the content of saponins Rg1, Re, Rb2, Rd, and Rb1 also increases with the increase in pH, while the content of rare saponins Rh1, F2, Rg3, and CK gradually decreases. Therefore, based on the conversion rate of rare saponins, the initial optimal pH of fermentation is set to 6.
3.2.4. Determination of the Inoculation Amount of Strains
It can be seen from Table 1 that the inoculation number of bacteria is also an essential factor affecting the saponin transformation in the fermentation process. With the increase in the inoculation amount of L. plantarum, the concentrations of saponins Rg1, Re, Rb2, Rd, and Rb1 gradually decreased, while the contents of rare saponins Rh1, F2, Rg3, and CK gradually increased, and the content of saponins tended to balance when the inoculation amount was greater than 1.0%. The explanation is that when the inoculation amount is too low, the strain will reach the maximum growth concentration after a long time, and so it will prolong the growth time and reduce the transformation efficiency. When the inoculation amount reaches a certain value, the excessive inoculation amount will bring waste of resources to production. Hence, the inoculation amount of the final selected strain is 1.0%.
3.2.5. Experiment for Determining Fermentation Time
The growth of microorganisms is cyclical. The length of fermentation time not only determines the growth state of microorganisms but also determines the activity of metabolite enzymes. Fermentation products will be with the consumption of nutrients in the fermentation broth from production to increase, maintain, or decrease, and so determining the end point of fermentation is conducive to the improvement of industrial production capacity and economic benefits [26, 27]. It can be seen from Table 2 that the content of protodiol ginsenosides Rb1, Rb2, and Rd gradually decreased during fermentation, and the concentrations of three saponins in the fermentation broth decreased to the minimum at 16 days, while the concentrations of Rg3, F2, and CK gradually increased with time and reached their peak at 16 days and then maintained a balanced state. The content of Re and Rg1 of the original ginsenotriol-type ginsenoside also gradually decreased, and it reached the lowest concentration at 16 days. The concentration of the transformation product Rh1 was not obvious, as can be seen from the conversion mechanism of the protriol type ginsenoside. It may be that Rh1 undergoes hydrolysis into F1 by the enzyme, resulting in the concentration of Rh1 in the fermentation broth to not significantly increase. In summary, the concentration of rare saponins Rg3, F2, CK, and Rh1 increased during the fermentation process and reached the maximum at 16 days; thus, 16 days could be set as the end point of fermentation of L. plantarum into ginsenosides.
3.2.6. Verification of Experimental Results by the Optimal Fermentation Process
As shown in Table 3, according to the optimized process for three batches of continuous fermentation, the RSD of the ginsenoside content in the fermentation broth after fermentation is <2%, indicating that the reproducibility of this fermentation process is good. The contents of ginsenosides Rg1, Re, Rb2, Rd, and Rb1 in the fermentation broth after fermentation decreased by 0.1121, 0.2767, 0.181, 0.0985, and 0.159 mg/mL, respectively. The concentration of rare saponin Rh1 increased by 18.48% before and after fermentation. The concentration of saponin F2 increased by 135.73%, and the concentration of Rg3 increased by 343.03%. The concentration of saponin CK increased by 441.80%. Therefore, after fermentation by L. plantarum, the content of rare saponins in the fermentation broth is greatly increased, and other kinds of saponins may be generated in the process. Therefore, this fermentation process can effectively increase the types and contents of rare saponins in the ginseng jiaosu.
| Saponin content before fermentation (mg/100 mL) | Saponin content after fermentation (mg/100 mL) | RSD of saponins after fermentation (%) | |
|---|---|---|---|
| Rb1 | 68.77 | 52.81 | 1.10 |
| Rb2 | 82.86 | 67.93 | 1.11 |
| Rd | 54.27 | 44.50 | 1.29 |
| F2 | 03.61 | 08.51 | 1.68 |
| Rg3 | 01.58 | 07.01 | 1.71 |
| CK | 02.44 | 13.22 | 1.53 |
| Rg1 | 46.53 | 34.77 | 1.63 |
| Re | 43.11 | 15.44 | 0.93 |
| Rh1 | 19.13 | 22.63 | 1.68 |
3.3. Determination of Biochemical Indexes under Optimal Process
3.3.1. Changes in Glucose, Fructose, and Organic Acid during Fermentation
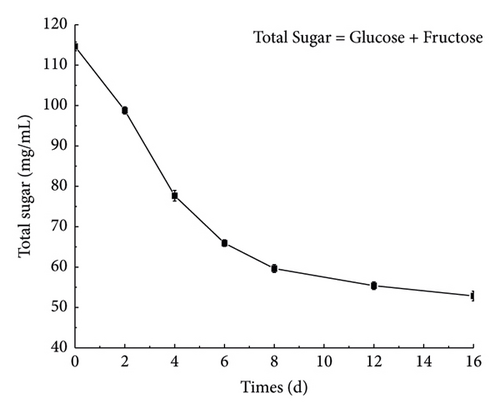
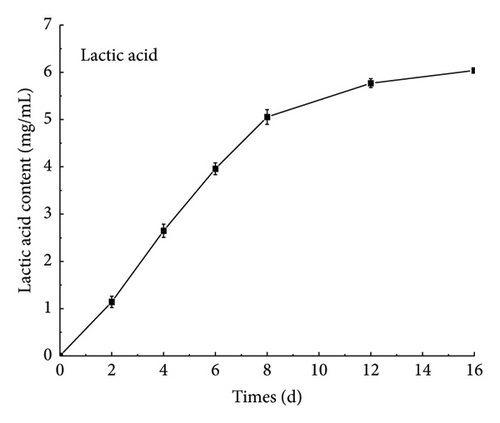
It can be seen from Figure 4(a) that the content of glucose and fructose decreased with the fermentation, and the total amount decreased from 114.63 ± 0.28 mg/mL in the beginning to 52.84 ± 0.31 mg/mL on the 16th day. In the early stage of fermentation, the nutrients in the fermentation broth were sufficient, and L. plantarum used glucose and fructose in large quantities for growth and metabolism. In the middle and late stages of fermentation, because of the lack of growth space and the continuous consumption of nutrients, L. plantarum entered the decline period, and the total number of Lactobacillus colonies in the fermentation broth greatly decreased. Thus, the consumption of the two sugars declined, and the sugar content decreased slowly [28, 29]. Figure 4(b) shows that the content of lactic acid increases with time during the fermentation process, reaching 6.04 ± 0.045 mg/mL after 16 days of fermentation. L. plantarum will produce lactic acid during metabolism. Lactic acid accumulates in the fermentation broth, thereby reducing the pH in the fermentation broth. When the pH is reduced to a certain extent, it will inhibit the growth of Lactobacillus. In the late stage of fermentation, L. plantarum will die, and so the lactic acid content increases slowly in the late fermentation stage. As shown in Appendix S1-3, after ginseng jiaosu fermentation, organic acids are generated, such as tartaric acid, malic acid, and succinic acid, in fermented liquid. Organic acids can not only improve the flavor of ginseng jiaosu but also adjust the pH of the intestinal tract to improve the health value of ginseng jiaosu [30].
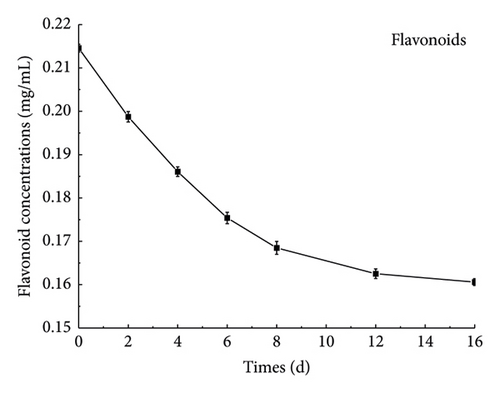
3.3.2. Determination of Flavonoid Content in the Fermentation Process
Figure 4(c) shows that the flavonoids in the fermentation process of ginseng jiaosu were in a downward trend; the content of flavonoids in the fermentation broth is 0.212 ± 0.03 mg/mL when it is not fermented, and the flavonoid content drops to 0.161 ± 0.04 mg/mL after 16 days of fermentation. The content of flavonoids in the prefermentation period mainly has the following two reasons: one is that the enzyme β-glucosidase produced by Lactobacillus can hydrolyze flavonoids. The second may also be that some flavonoids are modified into other substances, such as flavonol glycosomes [31]. In the late stage of fermentation, the content of flavonoids in the fermentation broth tends to remain stable, which may be because in the late stage of fermentation, microbial metabolism stopped, enzyme activity was not high, or the flavonol glycosum was hydrolyzed into flavonoids with the fermentation process. Hence, the total amount of flavonoids in the fermentation broth tends to remain unchanged.
3.4. Determination of Antioxidant Activity under Optimal Processes
Figure 5 shows the changes in hydroxyl radical scavenging rate, DPPH radical scavenging capacity, and superoxide anion radical during broth fermentation. Hydroxyl free radicals have a strong oxidizing ability and damage the cell membrane structure of the human body in body, thereby causing an inflammatory response, tumors, diabetes, aging, and other diseases [32]. DPPH free radical scavenging ability is the reactant’s antioxidant capacity in a short period. The scavenging capacity of DPPH radicals in food reflects its antioxidant capacity. Superoxide anion radicals mainly damage cell membranes, including vascular endothelial cell membranes and substructures, and thus trigger a series of biochemical reactions harmful to the body [33].
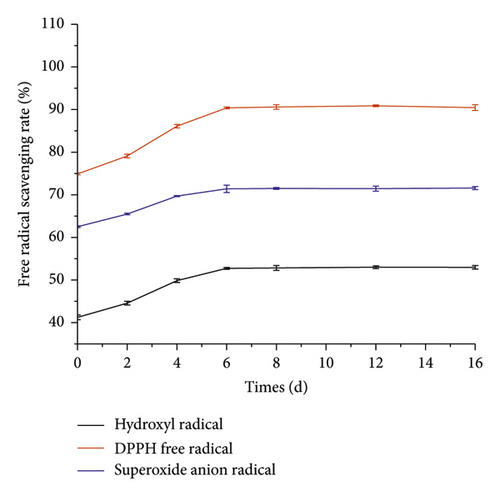
The hydroxyl radical reached 53.01 ± 0.54% after 16 days of fermentation, an increase of 11.75% before fermentation, and the hydroxyl radical scavenging rate of ginseng jiaosu after fermentation was equivalent to about twice the clearance of 0.012 mg/mL of vitamin C. Therefore, fermentation of L. plantarum can improve the hydroxyl free radical scavenging rate of ginseng jiaosu. The scavenging rate of DPPH radicals in the fermentation broth was 74.86 ± 0.45% when it was not fermented, and the scavenging rate of DPPH radicals in the fermentation broth reached 90.45 ± 0.48% after 16 days of fermentation, which was 15.98% higher than that before fermentation. The scavenging rate of DPPH free radicals by ginseng jiaosu after fermentation was equivalent to 1.35 times that of 0.12 mg/mL vitamin C. Compared with hydroxyl radical scavenging ability, the fermentation broth has a more vital ability to scavenge DPPH free radicals. Therefore, L. plantarum fermentation can increase the DPPH free radical scavenging rate of ginseng jiaosu. The superoxide anion radicals in the fermentation broth reached 71.58 ± 0.41% after 16 days of fermentation, an increase of 9.07% compared with before fermentation. After fermentation, the scavenging rate of superoxide anion radicals by ginseng jiaosu was equivalent to 1.55 times the scavenging rate of 0.12 mg/mL for Vc. Therefore, L. plantarum fermentation can improve the antioxidant activity of ginseng jiaosu. Cho et al. found that fermentation can improve the antioxidant activity of ginseng when studying the antioxidant effect of fermentation on ginseng, which is consistent with the results of this experiment [34]. Relevant studies have shown that Lactobacillus can produce some active substances that scavenge free radicals extracellularly during metabolism and at the same time secrete chelates of specific metal ions into the environment, thereby reducing the content of free radicals in the fermentation broth [35, 36], which may be the reason for the increase in antioxidant activity of ginseng jiaosu fermentation.
4. Conclusions
To improve the health value of ginseng jiaosu, this study, screening strains, screens out edible probiotics that can stably convert saponins and explores a stable and controllable ginseng jiaosu fermentation process. The results show that L. plantarum has the highest conversion efficiency for diol saponins, and the content of Rg3, F2, and CK increases after fermentation, among which the content of rare saponin CK is the most obvious, rising by 256%. The optimum optimizing method was a 1 : 1 : 10 ratio of ginseng extract/concentrated apple juice/water, fermentation time of 16 days, initial pH of 6.0, fermentation temperature of 37°C, and sterilization amount of 1.0%. For the best process verification, saponins Rh1, F2, were found. The contents of Rg3 and CK increased by 18.48%, 135.73%, 343.03%, and 441.80%, which could be used for a stable process for jiaosu fermentation. Under the optimal process, the total amount of glucose and fructose in the fermentation process decreased by 61.79 mg/mL, the flavonoid content decreased by 0.051 mg/mL, and the lactic acid content reached 6.04 ± 0.045 mg/mL, the scavenging rates for hydroxyl radical, DPPH radical, and superoxide anion radical were increased by more than 9%, and new organic acids were produced. Overall, this study establishes an efficient, controlled, and stable ginseng enzyme fermentation process to improve the rare saponin content of ginseng enzymes which is intended to provide a theoretical basis for the health value and development and utilization of ginseng jiaosu products. Later, the causes of the ginseng enzyme’s increased antioxidant activity during fermentation can be further researched. The pharmacological properties of the ginseng enzyme can also be examined to emphasize its medicinal benefits.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
Yuqing Xu contributed data curation and wrote the article. Yuqian Tang reviewed the article. Tao Liu contributed data curation and methodology. Haocheng Liu reviewed and edited the article. Jiguo Yang contributed materials and analysis tools. Li Meng developed software and validated the article.
Acknowledgments
The authors would like to thank all those who contributed directly or indirectly to the project. This research was funded by the Heyuan Branch, Guangdong Laboratory for Lingnan Modern Agriculture Project (no. DT20220027) and the Key Research and Development Program of Guangdong Province (no. 2021B0707010002).
Open Research
Data Availability
The data supporting the current study are available from the corresponding author upon request.




