29 m6A-RNA Methylation (Epitranscriptomic) Regulators Are Regulated in 41 Diseases including Atherosclerosis and Tumors Potentially via ROS Regulation – 102 Transcriptomic Dataset Analyses
Abstract
We performed a database mining on 102 transcriptomic datasets for the expressions of 29 m6A-RNA methylation (epitranscriptomic) regulators (m6A-RMRs) in 41 diseases and cancers and made significant findings: (1) a few m6A-RMRs were upregulated; and most m6A-RMRs were downregulated in sepsis, acute respiratory distress syndrome, shock, and trauma; (2) half of 29 m6A-RMRs were downregulated in atherosclerosis; (3) inflammatory bowel disease and rheumatoid arthritis modulated m6A-RMRs more than lupus and psoriasis; (4) some organ failures shared eight upregulated m6A-RMRs; end-stage renal failure (ESRF) downregulated 85% of m6A-RMRs; (5) Middle-East respiratory syndrome coronavirus infections modulated m6A-RMRs the most among viral infections; (6) proinflammatory oxPAPC modulated m6A-RMRs more than DAMP stimulation including LPS and oxLDL; (7) upregulated m6A-RMRs were more than downregulated m6A-RMRs in cancer types; five types of cancers upregulated ≥10 m6A-RMRs; (8) proinflammatory M1 macrophages upregulated seven m6A-RMRs; (9) 86% of m6A-RMRs were differentially expressed in the six clusters of CD4+Foxp3+ immunosuppressive Treg, and 8 out of 12 Treg signatures regulated m6A-RMRs; (10) immune checkpoint receptors TIM3, TIGIT, PD-L2, and CTLA4 modulated m6A-RMRs, and inhibition of CD40 upregulated m6A-RMRs; (11) cytokines and interferons modulated m6A-RMRs; (12) NF-κB and JAK/STAT pathways upregulated more than downregulated m6A-RMRs whereas TP53, PTEN, and APC did the opposite; (13) methionine-homocysteine-methyl cycle enzyme Mthfd1 downregulated more than upregulated m6A-RMRs; (14) m6A writer RBM15 and one m6A eraser FTO, H3K4 methyltransferase MLL1, and DNA methyltransferase, DNMT1, regulated m6A-RMRs; and (15) 40 out of 165 ROS regulators were modulated by m6A eraser FTO and two m6A writers METTL3 and WTAP. Our findings shed new light on the functions of upregulated m6A-RMRs in 41 diseases and cancers, nine cellular and molecular mechanisms, novel therapeutic targets for inflammatory disorders, metabolic cardiovascular diseases, autoimmune diseases, organ failures, and cancers.
1. Introduction
Cardiovascular diseases (CVDs) including coronary heart disease, cerebrovascular disease, and peripheral artery disease have risen to the top of the worldwide death toll [1, 2]. Previous studies showed that different risk factors including elevated plasma lipids [3, 4], elevated blood sugar [5], hyperhomocysteinemia [6, 7], and chronic kidney disease [8–10] promote vascular inflammation and atherosclerotic CVDs by different mechanisms including DNA methylation [11], microRNA regulation of mRNA stabilities [12], histone modifications including histone methylations [13–15], immune metabolic programming and trained immunity [16], innate immune activation [17] of endothelial cells (EC) [18–21], EC injury [22], Ly6C high mouse monocyte and CD40+ human monocyte differentiation [23, 24], decreased regulatory T cells (Treg) [25–27], and impaired bone marrow-derived progenitor cells’ vascular repairability [28, 29]. Furthermore, we recently proposed new models that include intracellular organelle dangers [30] and reactive oxygen species (ROS) as an integrated sensing system for metabolic homeostasis and alarming, which showed that metabolic remodeling and dysfunction trigger mitochondrial ROS [31–34], caspase-1/inflammasome activation [8, 10], histone modification enzyme downregulation [14], and increased expressions of trained immunity pathway enzymes. These reports have demonstrated that epigenomic mechanisms play significant roles in connecting metabolic reprogramming and dysfunction to inflammation initiation and gene transcription. However, the mechanism by which epitranscriptomic-RNA methylation controls the progression of many diseases is still unknown.
RNA carries a spectrum of more than 100 chemical modifications including RNA methylation that play significant roles in the regulation of gene expression [35, 36]. As the most dominant mRNA modification, N6-methyladenosine (m6A), installed onto mRNA by the methyltransferase-like 3 (METTL3)/methyltransferase-like 14 (METTL14) methyltransferase complex, is at a frequency of 0.15-0.6% of all adenosines in polyadenylated RNA [37]. In addition, six other RNA methylations have also been reported such as pseudouridine (Ψ), 5-methylcytidine (m5C), N1-methyladenosine (m1A), N4-acetylcytidine (ac4C), ribose methylations (Nm), and N7-methylguanosine (m7G) [38]. At 25-60% of transcriptome, m6A methylation regulates gene expression by influencing numerous aspects of mRNA processes of RNA polymerase II-transcribed mRNAs such as pre-mRNA processing, nuclear export, decay, and translation as well as long noncoding RNAs (lncRNAs) [39]. In addition, m6A plays an important role on noncoding chromosome-associated regulatory RNAs (carRNAs) for gene expression, which includes enhancer RNAs (eRNAs), promoter-associated RNAs (paRNAs), and transposable element transcribed RNA (repeat RNAs). Similar to DNA methylation as a mode of epigenomic regulation, the m6A methylation that occurred in RNA as a mode of epitranscriptomic regulation becomes important in the crucial roles of m6A-mediated gene regulation in many physiological and disease processes [37]. Another important feature of m6A methylation is the reversibility, allowing for the regulation of m6A levels after initial deposition. Fat mass and obesity-associated protein (FTO) and AlkB Homolog 5 (ALKBH5) have been discovered as m6A demethylases (erasers).
The m6A methylation also plays a significant role in human disease pathology. Loss of METTL14 has been shown to increase endometrial cancer cells’ proliferation and tumorigenicity. In contrast, METTL3/METTL14 have been found to play significant roles in acting as oncogenes in acute myeloid leukemia and glioblastoma and promoting or inhibiting roles in hepatocellular carcinoma [40]. Since METTL3/METTL14 have been indicated to be promising drug targets, phase 1 trials of small-molecule inhibitors for METTL3/METTL14 have been planned for 2021-2022 [37]. In addition, METTL3/METTL14 and m6A methylation have been reported to play roles in other diseases such as heart failure, viral infection, type 2 diabetes [37], cardiac remodeling, atherosclerosis, congenital heart disease, inflammation, obesity, insulin resistance, adipogenesis, and hypertension [41]. Moreover, decreased expressions of fat mass and obesity-associated protein (FTO) [42] have been related to heart failure, and overexpression of FTO in failing heart results in decrease of ischemia-triggered loss of cardiac function. Finally, YT521-B homology (YTH, m6A-dependent RNA binding) domain family (YTHDF) [43] reader proteins are also involved in pathogenic processes since YTHDF2 is essential for acute myeloid leukemia initiation and leukemia stem cell development [37].
Despite major advancements in the discipline, the following questions remain unanswered: (1) whether the expressions of 29 m6A-RMRs are modulated in 41 diseases and cancers including acute inflammation, sepsis, acute respiratory distress syndrome, shock, trauma, cardiovascular diseases (CVDs), autoimmune diseases, and organ failures; (2) whether macrophages and CD4+Foxp3+ regulatory T cells (Treg) serve as the key cellular mechanisms underlying the roles of m6A-RMRs in various diseases and cancers; and (3) whether nine types of molecular mechanisms including danger-associated molecular pattern receptors (DAMP receptors); proinflammatory cytokines; immune checkpoint and costimulation receptors; methionine-homocysteine-methyl donation cycle enzymes; m6A-RMRs; proinflammatory transcription factors NF-κB and JAK/STAT; tumor suppressors TP53, PTEN, and APC; histone methyltransferases; and DNA methyltransferases play significant roles in modulating m6A-RMRs. After analyzing 102 transcriptomic datasets according to our flow chart (Figure 1), we made significant findings as summarized in Abstract. Our findings reveal new information about the roles of elevated m6A-RMRs in the pathogenesis of 41 illnesses and tumors, as well as new therapeutic targets for inflammation, sepsis, trauma, organ failures, autoimmunity, metabolic cardiovascular disorders, and cancers.
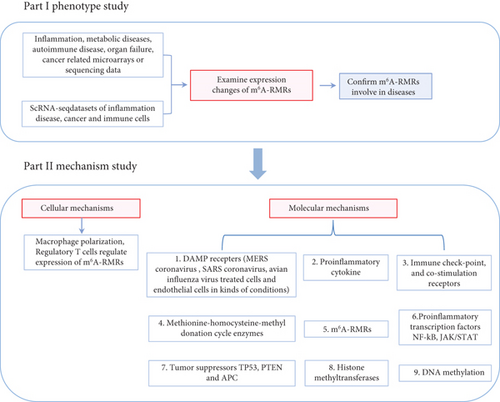
2. Materials and Methods
2.1. Expression Levels of m6A-RMRs in Patients with Various Inflammatory Disorders and Tumors
We collected 23 microarray datasets of acute inflammation, metabolic and cardiovascular diseases, autoimmune diseases, and organ failures (Table 1); one microarray of Middle-East respiratory syndrome (MERS) coronavirus-infected human microvascular endothelial cells; one microarray dataset (nine comparisons) of subacute respiratory syndrome coronavirus- (SARS-CoV-) infected human airway epithelial cells; one microarray dataset (23 comparisons) of influenza virus-infected lung epithelial cells (Table 2); and eight microarray datasets of endothelial cells (Table 3) from National Institutes of Health- (NIH-) National Center for Biotechnology Information- (NCBI-) Gene Expression Omnibus (GEO) databases (https://www.ncbi.nlm.nih.gov/gds/). These datasets were analyzed with GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r/). Some datasets were overlapped with our previous studies [44]. In addition, the Oncomine database (https://www.oncomine.org) was used to analyze the gene expression profile from 19 tumors [45], with threshold parameters of fold change > 2, p < 0.05, and gene rank in the top 10%. Because these microarray studies employed diverse cell types, we were unable to compare the effects of illness circumstances on m6A-RMR regulation in the same cell types. It is worth noting that our strategy was well justified. For example, we and others frequently investigated gene expression in nonideal heterogeneous peripheral blood mononuclear cell populations (PBMCs) in pathophysiological conditions, which are made up of a variety of cell types (also see Discussion).
| GEO ID | Disease/phenotype | Tissue | Comparison | No. of samples | PMID |
|---|---|---|---|---|---|
| Acute inflammation | |||||
| GSE32707 | Lung injury | Whole blood | Sepsis day 0 vs. no sepsis | 30/34 | 22461369 |
| Lung injury | Whole blood | Sepsis day 7 vs. no sepsis | 28/34 | 22461369 | |
| Lung injury | Whole blood | Sepsis/ARDS day 0 vs. no sepsis ARDS | 18/34 | 22461369 | |
| Lung injury | Whole blood | Sepsis/ARDS day 7 vs. no sepsis ARDS | 13/34 | 22461369 | |
| GSE13904 | Septic shock | Whole blood | Sepsis day 1 vs. normal | 32/18 | 19325468 |
| Septic shock | Whole blood | Sepsis day 3 vs. normal | 20/18 | 19325468 | |
| Septic shock | Whole blood | Sepsis/shock day 1 vs. normal | 67/18 | 19325468 | |
| Septic shock | Whole blood | Sepsis/shock day 3 vs. normal | 39/18 | 19325468 | |
| GSE5580 | Severe trauma | Monocytes | Severe trauma vs. health | 7/7 | 17032758 |
| Severe trauma | Leukocytes | Severe trauma vs. health | 7/7 | 17032758 | |
| Severe trauma | T cells | Severe trauma vs. health | 7/7 | 17032758 | |
| Metabolic disease | |||||
| GSE48964 | Obese | Adipose stem cells | Morbidly obese vs. nonobese | 3/3 | 24040759 |
| GSE55200 | MHO | Subcutaneous adipose | MHO vs. LH | 8/7 | 24933025 |
| MUO | Subcutaneous adipose | MUO vs. LH | 8/7 | 24933025 | |
| GSE94752 | Obese IR | Adipocytes | Obese IR vs. lean | 18/9 | 28570579 |
| Obese IS | Adipocytes | Obese IS vs. lean | 21/9 | 28570579 | |
| GSE23343 | T2D | Liver | T2D vs. normal glucose tolerance | 10/7 | 21035759 |
| GSE29221 | T2D | Skeletal muscle | T2D vs. nondiabetes | 12/12 | 23308243 |
| GSE29226 | T2D | Subcutaneous adipose | T2D vs. nondiabetes | 12/12 | 23308243 |
| GSE29231 | T2D | Visceral adipose | T2D vs. nondiabetes | 12/12 | 23308243 |
| GSE28829 | Atherosclerosis | Carotid artery | Advanced plaque vs. early plaque | 16/13 | 22388324 |
| GSE41571 | Atherosclerosis | Plaque macrophages | Ruptured plaques vs. stable plaque | 5/6 | 23122912 |
| GSE1010 | FCH | Lymphoblastic cells | FCH vs. normal | 12/12 | 15388524 |
| GSE6054 | FHC and atherosclerosis | Monocytes | FHC homozygote vs. control | 4/13 | 19040724 |
| GSE6088 | FHC and atherosclerosis | T cell | FHC homozygote vs. control | 3/13 | 19040724 |
| Autoimmune disease | |||||
| GSE97779 | RA | Macrophages ∗ | RA vs. normal | 9/5 | 28813657 |
| GSE109248 | ACLE | Skin | ACL vs. normal | 7/14 | 29889098 |
| CCLE | Skin | CCL vs. normal | 6/14 | 29889098 | |
| Psoriasis | Skin | Psoriasis vs. normal | 17/14 | 29889098 | |
| SCLE | Skin | SCL vs. normal | 12/14 | 29889098 | |
| GSE38713 | UC | Sigmoid colon or rectum | UC active involved vs. normal | 22/13 | 23135761 |
| GSE3365 | UC | PBMC | UC vs. normal | 26/42 | 16436634 |
| CD | PBMC | CD vs. normal | 59/42 | 16436634 | |
| Organ failure | |||||
| GSE76701 | Heart failure | Left ventricle | Failing heart vs. nonfailing heart | 4/4 | 26756417 |
| GSE38941 | HBV-ALF | Liver | HBV-ALF vs. normal | 17/10 | 23185381 |
| GSE37171 | ESRF | Whole blood | Chronic renal failure vs. healthy controls | 75/40 | 23809614 |
| GSE15072 | CKD hemodialysis | PBMC | Hemodialysis vs. healthy controls | 17/8 | 19698090 |
- Abbreviations: No. of samples: number of samples = (number of diseases/number of controls); ARDS: acute respiratory distress syndrome; ALI: acute liver injury; ALF: acute liver failure; PBMC: peripheral blood mononuclear cell; IS: insulin sensitive; IR: insulin resistance; MHO: metabolically healthy obese; MUO: metabolically unhealthy obese; LH: lean health; T2D: type 2 diabetes; FCH: familial combined hyperlipidemia; FHC: familial hypercholesterolemia; RA: rheumatoid arthritis; ACLE: acute cutaneous lupus; CCLE: chronic cutaneous lupus; SCLE: subacute cutaneous lupus; UC: ulcerative colitis; CD: Crohn’s disease; HBV-ALF: hepatitis B virus-associated acute liver failure; ESRF: end-stage renal failure; CKD: chronic kidney disease; PBMC: peripheral blood mononuclear cell, macrophages; ∗macrophages from synovial fluids for RA and blood-derived macrophages for control. All the studies and samples are from human.
| GEO ID | Comparison | Cell/tissue | No. of samples | PMID |
|---|---|---|---|---|
| GSE79218 | icMERS-inoculated vs. mock-inoculated (0 hour) | HMEC | 5/5 | 28830941 |
| icMERS-inoculated vs. mock-inoculated (12 hours) | HMEC | 5/5 | 28830941 | |
| icMERS-inoculated vs. mock-inoculated (24 hours) | HMEC | 5/5 | 28830941 | |
| icMERS-inoculated vs. mock-inoculated (36 hours) | HMEC | 5/5 | 28830941 | |
| icMERS-inoculated vs. mock-inoculated (48 hours) | HMEC | 5/4 | 28830941 | |
| GSE47960 | SARS-CoV-infected vs. mock-infected (0 hour) | HAEC | 4/3 | 23935999 |
| SARS-CoV-infected vs. mock-infected (12 hours) | HAEC | 4/3 | 23935999 | |
| SARS-CoV-infected vs. mock-infected (24 hours) | HAEC | 4/3 | 23935999 | |
| SARS-CoV-infected vs. mock-infected (36 hours) | HAEC | 2/3 | 23935999 | |
| SARS-CoV-infected vs. mock-infected (48 hours) | HAEC | 4/3 | 23935999 | |
| SARS-CoV-infected vs. mock-infected (60 hours) | HAEC | 4/4 | 23935999 | |
| SARS-CoV-infected vs. mock-infected (72 hours) | HAEC | 4/4 | 23935999 | |
| SARS-CoV-infected vs. mock-infected (84 hours) | HAEC | 4/4 | 23935999 | |
| SARS-CoV-infected vs. mock-infected (96 hours) | HAEC | 4/4 | 23935999 | |
| H1N1-infected vs. mock-infected (0 hour) | HAEC | 3/3 | 23935999 | |
| H1N1-infected vs. mock-infected (6 hours) | HAEC | 3/3 | 23935999 | |
| H1N1-infected vs. mock-infected (12 hours) | HAEC | 3/3 | 23935999 | |
| H1N1-infected vs. mock-infected (18 hours) | HAEC | 3/3 | 23935999 | |
| H1N1-infected vs. mock-infected (24 hours) | HAEC | 3/3 | 23935999 | |
| H1N1-infected vs. mock-infected (36 hours) | HAEC | 3/3 | 23935999 | |
| H1N1-infected vs. mock-infected (48 hours) | HAEC | 4/3 | 23935999 | |
| GSE49840 | H7N9-infected vs. mock-infected (3 hours) | Calu-3 cells | 4/4 | 24496798 |
| H7N9-infected vs. mock-infected (7 hours) | Calu-3 cells | 4/4 | 24496798 | |
| H7N9-infected vs. mock-infected (12 hours) | Calu-3 cells | 4/4 | 24496798 | |
| H7N9-infected vs. mock-infected (24 hours) | Calu-3 cells | 4/4 | 24496798 | |
| H7N7-infected vs. mock-infected (3 hours) | Calu-3 cells | 4/4 | 24496798 | |
| H7N7-infected vs. mock-infected (7 hours) | Calu-3 cells | 4/4 | 24496798 | |
| H7N7-infected vs. mock-infected (12 hours) | Calu-3 cells | 4/4 | 24496798 | |
| H7N7-infected vs. mock-infected (24 hours) | Calu-3 cells | 4/4 | 24496798 | |
| H5N1-infected vs. mock-infected (3 hours) | Calu-3 cells | 3/4 | 24496798 | |
| H5N1-infected vs. mock-infected (7 hours) | Calu-3 cells | 4/4 | 24496798 | |
| H5N1-infected vs. mock-infected (12 hours) | Calu-3 cells | 4/4 | 24496798 | |
| H5N1-infected vs. mock-infected (24 hours) | Calu-3 cells | 4/4 | 24496798 | |
| H3N2-infected vs. mock-infected (3 hours) | Calu-3 cells | 34 | 24496798 | |
| H3N2-infected vs. mock-infected (7 hours) | Calu-3 cells | 4/4 | 24496798 | |
| H3N2-infected vs. mock-infected (12 hours) | Calu-3 cells | 4/4 | 24496798 | |
| H3N2-infected vs. mock-infected (24 hours) | Calu-3 cells | 4/4 | 24496798 | |
- Abbreviations: No. of samples: number of samples = (number of diseases/number of controls); MERS: Middle East respiratory syndrome; SARS: severe acute respiratory syndrome; H1N1: influenza A, one type of avian influenza virus.
| GEO ID | Comparison | Cell/tissue | No. of samples | PMID |
|---|---|---|---|---|
| GSE59226 | Influenza virus-infected vs. inactivate virus-infected | Human umbilical vein ECs | 2/2 | 25863179 |
| GSE1377 | Kaposi sarcoma-associated herpes virus-infected for 7 days vs. uninfected control | Primary human dermal ECs | 2/2 | 15220917 |
| GSE5883 | 10 ng LPS stimulation for 4 hours vs. no LPS stimulation | Human lung microvascular ECs | 4/4 | NA |
| 10 ng LPS stimulation for 8 hours vs. no LPS stimulation | Human lung microvascular ECs | 4/4 | NA | |
| 10 ng LPS stimulation for 24 hours vs. no LPS stimulation | Human lung microvascular ECs | 4/4 | NA | |
| GSE3920 | 1000 IU IFNα treated for 5 hours vs. untreated control | Human umbilical vein ECs | 5/5 | 17202376, 19553003 |
| 1000 IU IFNβ treated for 5 hours vs. untreated control | Human umbilical vein ECs | 2/5 | 17202376, 19553003 | |
| 1000 IU IFNγ treated for 5 hours vs. untreated control | Human umbilical vein ECs | 3/5 | 17202376, 19553003 | |
| GSE85987 | NOTCH1 siRNA vs. scrambled control siRNA | Human umbilical vein ECs | 3/3 | 29449332 |
| NOTCH1 siRNA+IL-1β treated 24 hours vs. scrambled siRNA | Human umbilical vein ECs | 3/3 | 29449332 | |
| GSE72633 | NOTCH1 siRNA vs. scrambled control siRNA | Human aortic ECs | 3/3 | 26552708 |
| oxPAPC treated vs. scrambled control siRNA | Human aortic ECs | 3/3 | 26552708 | |
| GSE26953 | Oscillatory shear vs. laminar shear (fibrosa) | Human aortic valvular ECs | 6/6 | 21705672 |
| Oscillatory shear vs. laminar shear (ventricularis) | Human aortic valvular ECs | 6/6 | 21705672 | |
| GSE39264 | apoE KO vs. WT | Mouse aortic ECs | 4/6 | 23990205 |
| LPS treated for 4 hours vs. DMEM treated for 4 hours | Mouse aortic ECs | 3/3 | 23990205 | |
| oxLDL treated for 4 hours vs. DMEM treated for 4 hours | Mouse aortic ECs | 3/3 | 23990205 | |
| oxPAPC treated for 4 hours vs. DMEM treated for 4 hours | Mouse aortic ECs | 3/3 | 23990205 | |
- Abbreviations: LPS: lipopolysaccharide; IFN: interferon; NOTCH1: notch receptor 1; ox-PAPC: oxidized-1-palmitoyl-2-arachidonyl-sn-glycerol-3-phosphocholine; siRNA: small interfering RNA; apoE: apolipoprotein E; KO: knockout; WT: wild type; ox-LDL: oxidized low-density lipoprotein; DMEM: Dulbecco’s modified eagle medium; No. of samples: number of samples = (number of diseases/number of controls).
2.2. Expression Profile of m6A-RMRs in Single-Cell RNA Sequencing (scRNA-Seq) Datasets from Studies of Sepsis, Atherosclerosis, Tumors, and Endothelial Cell
Five scRNA-Seq datasets were collected from the Single Cell Portal database (https://singlecell.broadinstitute.org/single_cell), including one study about sepsis, one study about atherosclerosis, two studies about tumors (astrocytoma and melanoma), and one study about endothelial cell populations (Supplementary Table 1). The expressions of 29 m6A-RMRs were online analyzed.
2.3. Expression Regulation Analysis of m6A-RMRs from Deficiency of Folate Cycle and Metabolism-Related Enzymes, m6A-RMRs, H3K4 Methylase, DNA Methyltransferase, Regulatory T Cells’ Signature Genes, Proinflammatory Cytokines, Oncogene, Tumor Suppressors, and Immune Checkpoint Receptors
The 68 microarray datasets in the NIH-NCBI-GeoDataset database (https://www.ncbi.nlm.nih.gov/gds/) were collected in analyzing the regulatory mechanisms of m6A-RMRs (Supplementary Table 2). There are six microarrays about deficiencies of the folate cycle and metabolism-related enzymes and m6A-RMRs, four datasets of deficiencies of H3K4 methylase, six datasets of deficiencies of DNA methyltransferase, three datasets of Treg versus conventional T cells, 11 datasets of deficiencies of Treg cells’ signature genes, one dataset of macrophage polarization, eight datasets of deficiencies of proinflammatory cytokines, nine datasets of deficiencies of oncogene, ten datasets of deficiencies of tumor suppressors, and ten datasets of deficiencies of immune checkpoint receptors.
2.4. Statistical Analysis of Microarray Data
The expression changes of m6A-RMRs are not big enough in diseases; we may miss some important information if the expression change fold thresholds are set high. Thus, the expression changes were listed in the results with p < 0.05 (statistical significance), m6A-RMRs with expression changes more than 1-fold (red) were defined as the upregulated genes, while genes with expression decreased more than 1-fold (blue) were defined as downregulated genes. The missing value was marked with “NA,” and “NA” was excluded in the total count number.
3. Results
3.1. A Few m6A-RNA Methylation Regulators (m6A-RMRs) Are Upregulated, and Most m6A-RMRs Are Downregulated in Sepsis, Sepsis plus Acute Respiratory Distress Syndrome, Sepsis plus Shock, and Trauma, and Upregulated Two RNA Methyltransferases WTAP and PCIF1 and Three RNA Binding Proteins YTHDF3, IGF2BP2, and IGF2BP3 May Promote Acute Inflammations
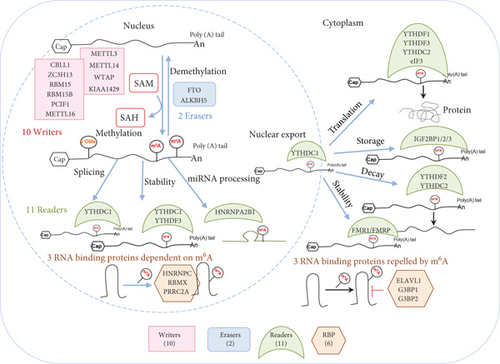
We hypothesized that pathological conditions significantly modulate the expressions of m6A-RMRs in cell type-specific and disease-specific manners. To test this hypothesis, we collected 29 m6A-RMRs in five groups from PubMed database (https://pubmed.ncbi.nlm.nih.gov/) (Figure 2 and Supplementary Table 3). The 29 m6A-RMRs included (1) 10 m6A-RNA methylation writers (methyltransferases) including METTL14, METTL3, WTAP, KIAA1429, ZC3H13,CBLL1, RBM15, METTL16,RBM15B, and PCIF1; (2) two m6A-RNA demethylases, FTO and ALKBH5; (3) 11 RNA binding proteins (readers) including YTHDC1, YTHDF1, YTHDF2, YTHDF3, YTHDC2, HNRNPA2B1, EIF3A, IGF2BP1, IGF2BP2, IGF2BP3, and FMR1; (4) three m6A-dependent RNA binding proteins including HNRNPC, RMMX, and PRRC2A; and (5) three m6A-repelled RNA binding proteins including ELAVL1, G3BP1, and G3BP2. As shown in Figure 3(a), one to four out of 28 m6A-RMRs were upregulated; and 14 to 15 out of 28 m6A-RMRs were downregulated in 0 day and 7 days in patients with sepsis and sepsis plus acute respiratory distress syndrome (ARDS) [46], respectively. In the second datasets with sepsis and sepsis plus shock, two to four out of 26 m6A-RMRs were upregulated; and 10 to 17 out of 26 m6A-RMRs were downregulated in 1 day and 3 days in patients with sepsis and sepsis plus shock [47, 48], respectively. In the third datasets with leukocytes, monocytes, and T cells from trauma, zero to one out of 22 m6A-RMRs was upregulated; and 5 to 11 out of 22 m6A-RMRs were downregulated in leukocytes, monocytes, and T cells from trauma [49], respectively.
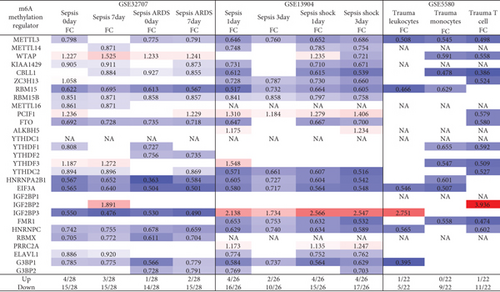
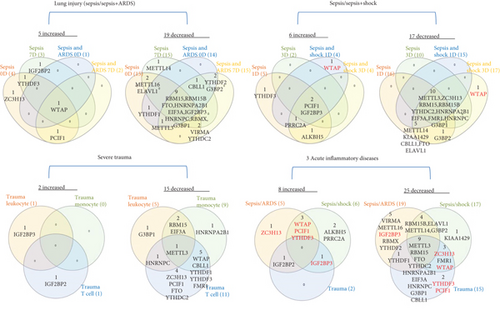
We then analyzed the shared and disease-specific m6A-RMRs using Venn diagram analysis. As shown in Figure 3(b), four diseases including sepsis at 0 day and 7 days and sepsis plus ARDS at 0 day and 7 days shared one RNA methyltransferase WTAP. In the second sepsis datasets, four diseases including sepsis at 1 day and 3 days and sepsis plus shock at 0 day and 7 days shared one RNA methyltransferase PCIF1 and one RNA methylation reader IGF2BP3. In the trauma datasets, three immune cell types, leukocytes, monocytes, and T cells did not share any upregulated m6A-RMRs. When comparing three groups of acute inflammations, sepsis plus ARDS shared three upregulated m6A-RMRs such as writers WTAP, PCIF1, and reader YTHDF3 with sepsis plus shock; sepsis plus ARDS shared upregulation of one RNA binding protein IGF2BP2 with trauma, and sepsis plus shock shared one RNA binding protein IGF2BP3 with trauma.
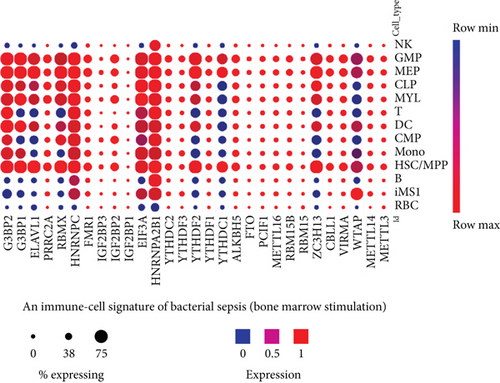
We noticed that in the trauma datasets (Figure 3(b)), three immune cell types, leukocytes, monocytes, and T cells did not share any upregulated RNA methylation regulators, suggesting that upregulation of m6A-RMRs is in a cell-specific manner. To further examine this issue, we collected single-cell RNA-sequencing (scRNA-Seq) datasets in sepsis [57] in a comprehensive single-cell sequencing database (https://singlecell.broadinstitute.org/single_cell). As shown in Figure 3(c), the 11 out of 29 m6A-RMRs (38%) including WTAP, ZC3H13, YTHDC1, YTHDF2, HNRNPA2B1, EIF3A, HNRNPC, RBMX, ELAVL1, G3BP1, and G3BP2 were expressed differentially in 13 different immune cell types (natural killer cell (NK), granulomyeloid progenitor (GMP), megakaryocyte-erythrocyte progenitor (MEP), common lymphoid progenitor (CLP), myeloblasts (MYL), T cells, dendritic cell (DC), common myeloid progenitor (CMP), monocyte (mono), hematopoietic stem cell/multipotent progenitor (HSC/MPP), B cells, CD14+RETN+ALOX5AP+IL1R2+ monocyte (iMS1), and red blood cells (RBC)) in bacterial sepsis. The expressions of most m6A-RMRs except HNRNPA2B1 and HNRNPC were lower in monocyte subsets than those in other peripheral mononuclear cell (PBMC) types (Figure 3(d)).
These results have demonstrated that first, a few m6A-RMRs are upregulated and most m6A-RMRs are downregulated in sepsis, sepsis plus acute respiratory distress syndrome, sepsis plus shock, and trauma; second, two RNA methyltransferases WTAP and PCIF1 and three RNA binding proteins such as YTHDF3, IGF2BP2, and IGF2BP3 are commonly upregulated in sepsis, sepsis plus ARDS, sepsis plus shock, and trauma, suggesting that these five m6A-RMRs are the emergency m6A-RMRs for promoting acute inflammatory diseases; third, nine m6A-RMRs including METTL3, RBM15, FTO, YTHDC2, HNRNPA2B1, EIF3A, HNRNPC, G3BP1, and CBLL1 are commonly downregulated in sepsis, sepsis plus ARDS, sepsis plus shock, and trauma, suggesting that those m6A-RMRs play more important roles in maintaining homeostasis and suppressing inflammation than emergency roles for acute inflammations; and fourth, the expressions of 38% m6A-RMRs in immune cell types in response to sepsis stimulation are different.
3.2. Type 2 Diabetes Has More Modulation of m6A-RMR Expressions Than Atherogenic Diseases and Obesity; Nearly Half of 29 m6A-RMRs Are Downregulated as Atherosclerosis Progression Compared with That of Atherosclerosis Regression
We hypothesized that major metabolic cardiovascular disease groups such as obesity [58, 59], type 2 diabetes, and atherogenic diseases differentially modulate the expressions of m6A-RMRs. To test this hypothesis, we collected five datasets of obesity including obese, metabolically unhealthy obesity (MUO), metabolically healthy obesity (MHO), obese with insulin resistance (ob IR), and obese with insulin sensitivity (ob IS); four datasets of type 2 diabetes; and five datasets of atherosclerosis, familial hypercholesterolemia (FHC) plus atherosclerosis, and familial combined hyperlipidemia (FCH) from the NCBI-GeoDataset (https://www.ncbi.nlm.nih.gov/gds/). As shown in Figure 4(a), in five diseases in the obesity group, zero to three m6A-RMRs were upregulated and zero to 10 m6A-RMRs were downregulated. However, in the four type 2 diabetes datasets, zero to 11 m6A-RMRs were upregulated and one to seven m6A-RMRs were downregulated. The five datasets of atherosclerotic diseases had modulations of m6A-RMRs higher than those in the obesity group but lower than those of type 2 diabetes group: zero to seven m6A-RMRs were upregulated, and zero to seven m6A-RMRs were downregulated.
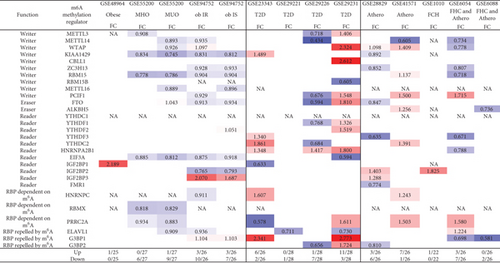
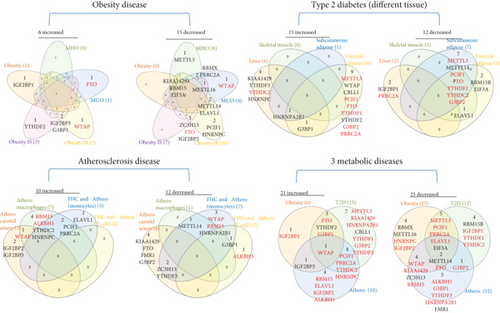
We also analyzed the shared and disease-specific m6A-RMRs using Venn diagram analysis (Figure 4(b)). In six m6A-RMRs upregulated in the obesity group, two regulators IGF2BP3 (RNA methyltransferase) and G3BP1 (m6A repelled RNA binding protein) were shared by obesity IS and obesity IR. Among the 15 m6A-RMRs upregulated in four different tissues in type 2 diabetes, one regulator HNRNPA2B1 (reader) was shared by liver, subcutaneous adipose, and visceral adipose, and one regulator G3BP1 was shared by liver and visceral adipose. In 10 m6A-RMRs upregulated in atherosclerotic diseases, one regulator WTAP (RNA methyltransferase) was shared by atherosclerosis (athero) carotid artery and atheromacrophages, and two regulators PCIF1 (RNA methyltransferase) and PRRC2A (m6A dependent RNA binding protein) were shared by atheromacrophages and FHC and atheromonocytes. Among 21 m6A-RMRs upregulated in three major metabolic cardiovascular disease groups, one regulator WTAP (RNA methyltransferase) was shared by three major groups; one regulator IGF2BP3 was shared by obesity and athero; three regulators FTO (demethylase), YTHDF2 (RNA binding protein), and G3BP1 were shared by obesity and type 2 diabetes; and four regulators such as PCIF1 (RNA methyltransferase), PRRC2A (m6A-dependent RNA binding protein), YTHDC2 (RNA binding protein), and HNRNPC (m6A-dependent RNA binding protein) were shared by type 2 diabetes and atherosclerotic diseases.
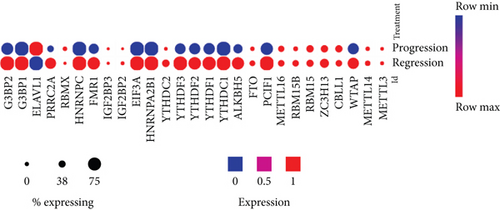
We then examined a hypothesis that atherosclerosis progression and regression differentially modulate the expressions of m6A-RMRs. We collected a dataset of scRNA-Seq in the scRNA-Seq database in the Broad Institute at MIT. As shown in Figure 4(c), the expressions of 14 out of 27 m6A-RMRs including Wtap, Pcif1, Alkbh5, Ythdc1, Ythdf1, Ythdf2, Ythdf3, Hnrnpa2b1, Eif3a, Fmr1, Hnrnpc, Prrc2a, G3bp1, and G3bp2 were decreased in the progressive atherosclerosis compared with those in the regressive atherosclerosis. The expression of Elavl1 was increased in the progressive atherosclerosis compared with that in the regressive atherosclerosis. Of note, the expressions of Virma and Igf2bp1 were not found. These results have illustrated that (1) atherosclerotic macrophages have higher upregulation of m6A-RMRs than atherosclerotic carotid artery, and FHC and atherosclerotic monocytes have more modulation of m6A-RMRs than FHC and atherosclerotic T cells; (2) type 2 diabetes adipose tissues have the highest modulation of m6A-RMRs (11 upregulated and three downregulated) among the 14 metabolic disease datasets; (3) obesity diseases have more downregulation than upregulation of m6A-RMRs, and (4) nearly half of 29 m6A-RMRs are downregulated as atherosclerosis progression compared with that of atherosclerosis regression.
Additionally, for further understanding the m6A-RMRs’s modulation in cardiovascular diseases, our study of m6A-RMRs in atherosclerosis and other three studies of m6A-RMRs in cardiovascular disease (CVDs) [60–62] were compared. From Table 4, METTL14 is upregulated in our study and other two studies; FTO is downregulated in our study and other two studies. At least, these data revealed that in atherosclerosis and other CVDs, m6A writer METTL14 is upregulated and eraser FTO is downregulated.
| m6A-RMRs | Results in our study (Figure 4(a)) | Table 1 in PMID: 34489709 | Table 1 in PMID:34221238 | Table 1 in PMID: 32910911 |
|---|---|---|---|---|
| M6A-RMRs in atherosclerosis | RNA methylation-related enzymes in CVDs | Roles and mechanisms of RNA m6A effectors in CVDs | The association between m6A methylation and CVDs | |
| Upregulated genes | PCIF1, YTHDC2, IGF2BP2, IGF2BP3, HNRNPC, PRRC2A, ELAVL1 | METTL3, YTHDF2, METTL14, KIAA1429, | N/A | METTL14 |
| Downregulated genes | METTL14, KIAA1429,ZC3H13, FTO, YTHDF3, HNRNPA2B1, FMR1, G3BP1, G3BP2 | FTO | ALKBH5, YTHDF2 | FTO |
| Genes without expression changes | METTL3, CBLL1, RBM15B, YTHDF1, YTHDF2, EIF3A, IGF2BP1 | N/A | N/A | N/A |
| Expression of genes not known | METTL16, YTHDC1, RBMX | N/A | N/A | N/A |
| Uncertain (paradox) | WTAP, RBM15, ALKBH5 | YTHDF1 | METTL3, FTO, WTAP | METTL3 |
- Abbreviations: CVDs: cardiovascular diseases; N/A: not available.
3.3. Inflammatory Bowel Diseases and Rheumatoid Arthritis Modulate m6A-RMRs More Than Autoimmune Lupus and Psoriasis; and Some Autoimmune Diseases Share Five Upregulated m6A-RMRs such as PCIF1, G3BP2, G3BP1, WTAP, and FTO
We hypothesized that major autoimmune diseases including rheumatoid arthritis (RA), psoriasis (PS), acute cutaneous lupus (ACLE), chronic cutaneous lupus (CCLE), subacute cutaneous lupus (SCLE), Crohn’s disease (CD), and ulcerative colitis (UC) differentially modulate the expressions of m6A-RMRs. To examine this hypothesis, we collected four microarray datasets (eight comparisons) from the NCBI-GeoDatasets (https://www.ncbi.nlm.nih.gov/gds/). In a RA dataset, four out of 26 m6A-RMRs were upregulated; and 10 out of 26 m6A-RMRs were downregulated. In four lupus datasets, two to six out of 28 m6A-RMRs were upregulated; and one to four out of 28 m6A-RMRs were downregulated. In three colitis datasets, five to eight m6A-RMRs were upregulated; and eight to nine m6A-RMRs were downregulated (Figure 5).
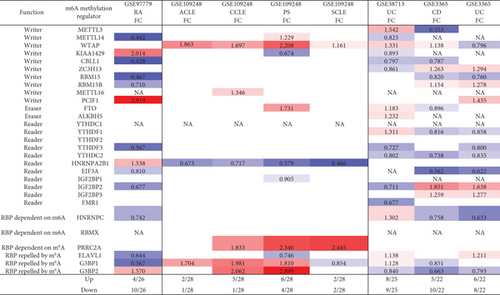
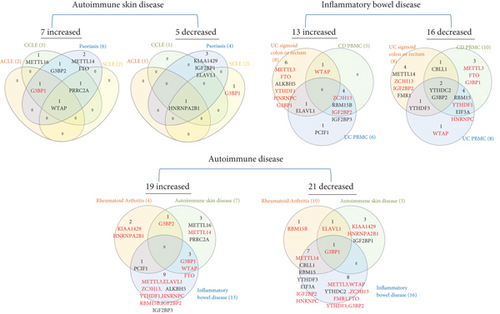
We also analyzed the shared and disease-specific m6A-RMRs using Venn diagram analysis. In seven m6A-RMRs upregulated in the autoimmune skin disease group, one m6A-RMR WTAP was shared by four autoimmune skin diseases; one m6A-RMR G3BP1 was shared by three autoimmune skin diseases including ACLE, CCLE, and psoriasis; one m6A-RMR PRRC2A was shared by three autoimmune skin diseases including CCLE, psoriasis, and SCLE; and one m6A-RMR G3BP2 was shared by two autoimmune skin diseases including CCLE and psoriasis. In 13 m6A-RMRs upregulated in the inflammatory bowel disease group, one m6A-RMR ELAVL1 was shared by UC sigmoid colon or rectum and UC PBMCs; one m6A-RMR WTAP was shared by UC sigmoid colon or rectum and CD PBMCs; and four m6A-RMRs including ZC3H13, RBM15B, IGF2BP2, and IGF2BP3 were shared by CD PBMCs and UC PBMCs. In 19 m6A-RMRs upregulated in all eight autoimmune diseases, one m6A-RMR PCIF1 was shared by RA and inflammatory bowel disease; one m6A-RMR G3BP2 was shared by RA and autoimmune skin diseases; and three m6A-RMRs including G3BP1, WTAP, and FTO were shared by autoimmune skin diseases and inflammatory bowel diseases. Taken together, our results have shown that first, inflammatory bowel diseases and RA modulate m6A-RMRs more than autoimmune lupus and psoriasis; second, autoimmune skin diseases share four upregulated m6A-RMRs including G3BP1, G3BP2, PRRC2A, and WTAP; third, inflammatory bowel diseases share six upregulated m6A-RMRs including ELAVL1, WTAP, ZC3H13, RBM15B, IGF2BP2, and IGF2BP3; and fourth, autoimmune diseases have no any commonly shared m6A-RMRs but share five upregulated m6A-RMRs between groups including PCIF1, G3BP2, G3BP1, WTAP, and FTO.
3.4. Some Organ Failures Share Eight Upregulated m6A-RMRs between Groups including Four Writers WTAP, PCIF1, RBM15, and RBM15B; Two m6A-Dependent RNA Binding Proteins PRRC2A and HNRNPC; and Two m6A-Repelled RNA Binding Proteins IGF2BP2 and IGF2BP3; End-Stage Renal Failure (ESRF) Downregulates 85% of 26 m6A-RMRs, and Upregulation of 45% m6A-RMRs in Hemodialysis from ESRF (15%) May Be Associated with Clinical Benefits
We hypothesized that major organ failures including heart failure, hepatitis B virus-associated acute liver failure (HBV-ALF), end-stage renal failure (ESRF), and hemodialysis modulate the expressions of m6A-RMRs. We collected four microarray datasets from the NCBI-GeoDatasets (https://www.ncbi.nlm.nih.gov/gds/) to examine this hypothesis. As shown in Figure 6, in the heart failure dataset, six out of 26 m6A-RMRs were upregulated; and seven out of 26 m6A-RMRs were downregulated. In the HBV-ALF dataset, eight out of 26 m6A-RMRs were upregulated; and 17 out of 26 m6A-RMRs were downregulated. In the ESRF dataset, four out of 26 m6A-RMRs were upregulated; and 22 out of 26 m6A-RMRs were downregulated. In the hemodialysis dataset, 10 out of 22 m6A-RMRs were upregulated; and five out of 22 m6A-RMRs were downregulated. We also analyzed the shared and disease-specific m6A-RMRs using Venn diagram analysis. In 20 m6A-RMRs upregulated in four organ failure groups, one upregulated m6A-RMR (RBM15B, RNA methyltransferase) was shared by HF and HBV-ALF; one upregulated m6A-RMR (PRRC2A) was shared by HF and ESRF; two upregulated m6A-RMRs (IGF2BP2 and IGF2BP3, RNA binding proteins) were shared by HBV-ALF and ESRF; and four m6A-RMRs including WTAP (RNA methyltransferase), RBM15 (RNA methyltransferase), PCIF1 (RNA methyltransferase), and HNRNPC (m6A-dependent RNA binding protein) were shared by HBV-ALF and hemodialysis.
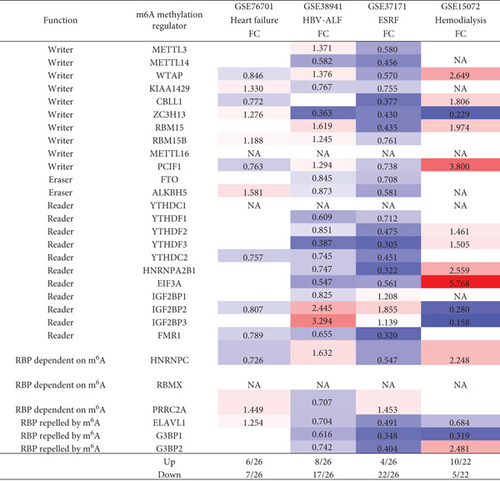
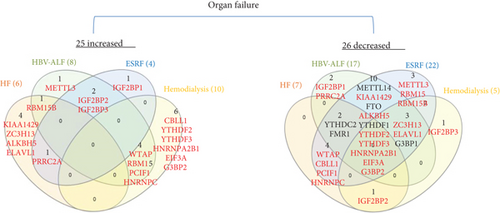
These results demonstrated that first, four major organ failures have no commonly shared upregulated m6A-RMRs but share eight m6A-RMRs between groups including RBM15B, IGF2BP2, IGF2BP3, PRRC2A, WTAP, RBM15, PCIF1, and HNRNPC; second, hemodialysis is the only organ failure that upregulates more m6A-RMRs than downregulates m6A-RMRs; other organ failures have less upregulation of m6A-RMRs than downregulation of m6A-RMRs; third, surprisingly, ESRF and hemodialysis have no any shared upregulated m6A-RMRs, suggesting that the clinical improvements of hemodialysis from ESRF are benefited from upregulation of m6A-RMRs by hemodialysis; and fourth, ESRF modulates 100% of 26 m6A-RMRs, which is the highest modulation rate found among all the diseases examined.
3.5. MERS-CoV Infections at 36 Hours in Human Microvascular Endothelial Cells Modulate the Highest Numbers of m6A-RMRs (Upregulated 20 and Downregulated Four) among Three Types of Viral Infections (MERS-CoV, SARS-CoV, and Influenza Virus); and the Differences in Modulating the Expressions of m6A-RMRs May Be Related to the Virulence of Virus and Virus Replication
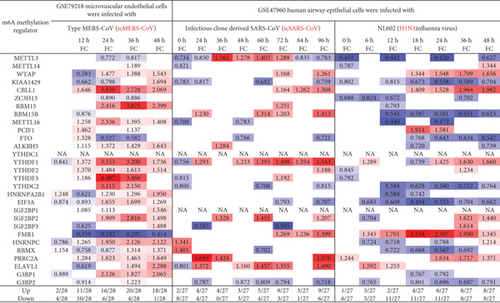
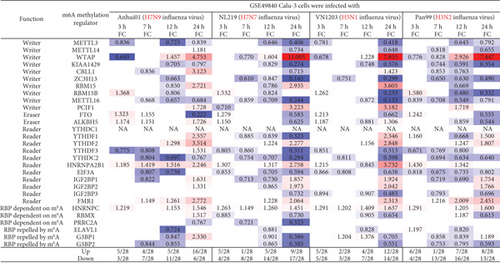
We hypothesized that viral infections with Middle East respiratory syndrome (MERS) coronavirus infection [63], severe acute respiratory syndrome (SARS) coronavirus (SARS-CoV) [64], and influenza virus infections [65] modulate the expressions of m6A-RMRs. To test this hypothesis, we collected three groups of microarray datasets from the NCBI-GeoDatasets (https://www.ncbi.nlm.nih.gov/gds/). We found that MERS-CoV infection of human microvascular endothelial cells at the time course of 0, 12, 24, 36, and 48 hours (h) [63] after infection resulted in upregulation of two to 20 out of 28 m6A-RMRs and downregulation of one to 10 out of 28 m6A-RMRs (Figure 7). In addition, SARS-CoV infection of human airway epithelial cells at the time course of 0, 24, 36, 48, 60, 72, 84, and 96 hours (h) [64] after infection resulted in upregulation of two to nine out of 27 m6A-RMRs and downregulation of zero to eight out of 27 m6A-RMRs. H1N1 influenza virus infection of human airway epithelial cells at the time course of 0, 6, 12, 18, 24, 36, and 48 hours after infection resulted in upregulation of one to eight out of 27 m6A-RMRs and downregulation of five to 11 out of 27 m6A-RMRs. Moreover, infections of Calu-3 cells (a non-small-cell lung cancer cell line) with four different strains of influenza viruses (H7N9, H7N7, H5N1, and H3N2) [65] resulted in upregulation of four to 16, one to nine, two to 12, and one to eight out of 28 m6A-RMRs, respectively, and downregulation of three to 11, four to 17, three to 14, and four to 16 out of 28 m6A-RMRs, respectively. Taken together, these results have demonstrated that (1) MERS-CoV infections in human microvascular endothelial cells modulate the highest numbers of m6A-RMRs (upregulated 20 and downregulated four at the time course of 36 hours) among the three types of viral infections examined; (2) similar to MERS-CoV infection, SARS-CoV infections in human airway epithelial cells upregulated more m6A-RMRs than downregulated but significantly less than that in MERS-CoV infections; (3) influenza virus (H1N1) infection in human airway epithelial cells downregulated more m6A-RMRs than upregulated; and (4) novel avian-origin influenza virus (IAV) H7N9 infections at 24-hour postinfection upregulated 16 and downregulated six out of 28 m6A-RMRs, which are significantly different from that of upregulation of nine, 12, and eight and downregulation of 17, 14, and 13 out of 28 m6A-RMRs by highly pathogenic avian-origin influenza viruses H7N7, H5N1, and human seasonal H3N2 IAV, respectively. The differences in modulating the expressions of m6A-RMRs may be related to the virulence of the virus, virus replication, and other aspects [65].
3.6. Proinflammatory Lipid oxPAPC and NOTCH1 Knockdown Modulates m6A-RMRs More Than Other DAMP Stimulation of ECs including LPS, oxLDL, and IFNs; and Two m6A-RMRs Such as Hnrnpa2b1 and Eif3a Were Differentially Expressed in Three Aortic EC Clusters
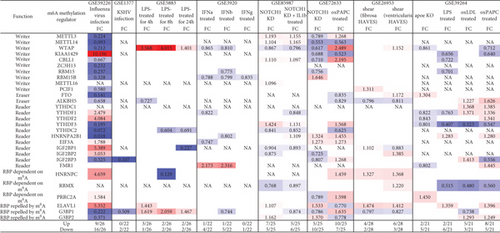
We hypothesized that pathogen-associated molecular patterns (PAMPs)/danger-associated molecular patterns (DAMPs) modulate the expressions of m6A-RMRs in various endothelial cells. To examine this hypothesis, we collected eight microarray datasets from various endothelial cells stimulated by PAMPs/DAMPs. As shown in Figure 8(a), influenza virus infection of human umbilical vein endothelial cells (HUVEC) resulted in upregulation of nine out of 26 m6A-RMRs (with the highest upregulation of writer KIAA1429 for 10 folds) and downregulation of 16 out of 26 m6A-RMRs. By comparison, Kaposi’s sarcoma-associated herpesvirus (KSHV) infection of human dermal endothelial cells modulated m6A-RMR expressions significantly less than that of influenza virus infection, upregulating zero and downregulating two out of 22 m6A-RMRs. Toll-like receptor 4 (TLR4) ligand lipopolysaccharide (LPS) stimulation of human lung microvascular endothelial cells for 4, 8, and 24 hours resulted in upregulation of two to three m6A-RMRs (with the high upregulation of writer WTAP) and downregulation of one to two m6A-RMRs, respectively. In addition, cytokine interferon-α (IFNα), IFNβ, and IFNγ treatments of HUVEC led to upregulation of zero to one and downregulation of one to five out of 22 m6A-RMRs, respectively. The knockdown (KD) of proinflammatory NOTCH1 signaling and NOTCH1 KD plus proinflammatory cytokine interleukin-1β (IL-1β) in HUVEC led to upregulation of seven and five out of 25 m6A-RMRs and downregulation of five and six out of 25 m6A-RMRs. The differences of m6A-RMR expression modulation between NOTCH1 KD and NOTCH1 KD plus IL-1β may contribute to the inflammatory status of treated HUVEC [66]. In contrast, another report found that NOTCH1 is antiatherogenic; and proinflammatory lipids oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (Ox-PAPC) decrease NOTCH1 expression in human aortic endothelial cells (HAEC) [67]. In this experimental setting, NOTCH1 KD in HAEC resulted in upregulation of five and downregulation of 10 out of 25 m6A-RMRs; proinflammatory lipid oxPAPC stimulation in HAEC led to upregulation of 10 and downregulation of seven out of 25 m6A-RMRs. It was reported that oscillatory shear (OS) present on the fibrosa stimulates fibrosa human aortic valve endothelial cells (HAVEC), which may contribute to aortic valve disease [68]. In this experimental setting, oscillatory shear on fibrosa HAVEC and ventricularis HAVEC resulted in upregulation of four and six and downregulation of two and three out of 28 m6A-RMRs, respectively. Moreover, mouse aortic endothelial cells (MAEC) isolated from atherogenic apolipoprotein E-deficient (ApoE-/-) mice [3], TLR4 ligand LPS-treated MAEC, another TLR4 ligand oxidized low-density lipoprotein- (oxLDL-) stimulated MAEC [69, 70], and oxPAPC-stimulated MAEC [71] upregulated two, two, five, and eight out of 21 m6A-RMRs and downregulated five, six, three, and five out of 21 m6A-RMRs, respectively.
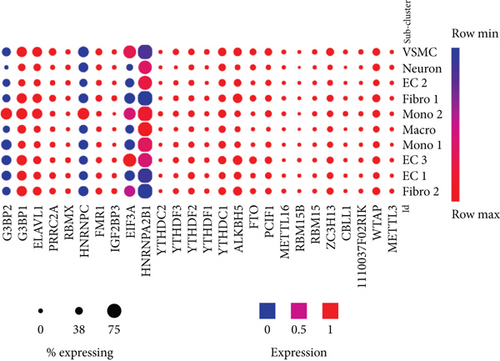
We then examined the expressions of m6A-RMRs in three mouse aortic endothelial cell (EC) clusters identified recently with single-cell RNA sequencing (scRNA-Seq) [72]. As shown in Figure 8(b), 12 out of 26 m6A-RMRs (46%) including Wtap, Zc3h13, Pcif1, FTO, Alkbh5, Ythdc1, YthDf2, Ythdf3, Fmr1, Prrc2a, Elavl1, and G3bp1 were in medium expression levels in almost all aortic cell populations of normal mouse aortas; and two m6A-RMRs such as Hnrnpa2b1 and Eif3a were differentially expressed in three aortic EC clusters, which were Cytl1+Gkn3+ endothelial cell (EC) cluster 1, Fabp4+Gphbp1+Rgcc+ EC cluster 2, and Ccl21a+Lrg1+ EC cluster 3 [72]. Of note, the expressions of Mettl14, Igf2bp1, and Igf2bp2 were not found.
These results conclude that (1) influenza virus infection of HUVEC results in expression modulation of most m6A-RMRs (25 out of 26), which were similar to that found in influenza virus infections of human airway epithelial cells and Calu-3 lung cells for 24 hours in Figure 7; (2) proinflammatory lipids oxPAPC and NOTCH1 knockdown modulate m6A-RMRs more than other DAMP stimulation of ECs including LPS, oxLDL, and IFNs; (3) ten m6A methyltransferases were not significantly modulated in MAEC from ApoE KO aorta with or without further stimulations of LPS, oxLDL, and oxPAPC, respectively (Figure 8, GSE39264); and (4) 12 out of 26 m6A-RMRs (46%) were in medium expression levels in almost all aortic cell populations of normal mouse aortas; and two m6A-RMRs including Hnrnpa2b1 and Eif3a were differentially expressed in three aortic EC clusters.
3.7. Upregulated m6A-RMRs Were More Than Downregulated m6A-RMRs in Various Cancer Types; Head and Neck Cancer, Cervical Cancer, Brain and CNS Cancer, Other Cancer, and Kidney Cancer Upregulated ≥10 m6A-RMRs; and IGF2BP3, G3BP1, IGF2BP2, and HNRNPC Were Upregulated in ≥10 Cancer Types
We hypothesized that the expressions of m6A-RMRs are modulated in various cancer cell populations. To test this hypothesis, we collected 19 cancer datasets in a comprehensive cancer gene expression database Oncomine (https://www.oncomine.org) [45] including brain and CNS cancer, head and neck cancer, esophageal cancer, gastric cancer, bladder cancer, breast cancer, cervical cancer, ovarian cancer, colorectal cancer, kidney cancer, liver cancer, lung cancer, pancreatic cancer, prostate cancer, leukemia, lymphoma, melanoma, myeloma, sarcoma, and other cancer (Figure 9(a)). As shown in Figure 9, the numbers in red indicated the numbers of studies with upregulated m6A-RMRs; and the numbers in blue indicated the numbers of studies with downregulated m6A-RMRs. The results showed that upregulated m6A-RMRs were more than downregulated m6A-RMRs in various cancer types (number of red cells is more than that of blue cells). Of note, the “Significant Unique Analyses” indicated the numbers of studies in which the analyzed genes were significantly upregulated (red) or downregulated (blue). The “Total Unique Analysis” indicated the numbers of studies in which the analyzed genes were included (p < 0.05, fold change > 2). Three m6A-RMRs such as METTL16, YTHDC1, and PRRC2A were not found.
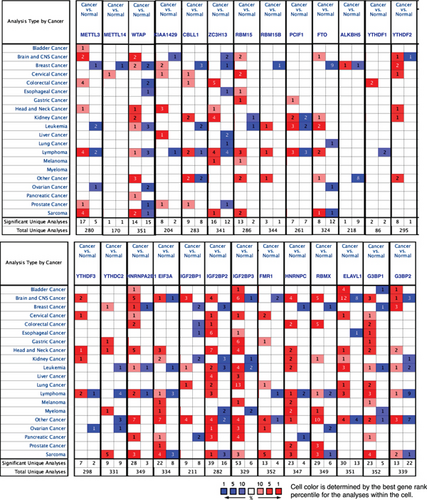
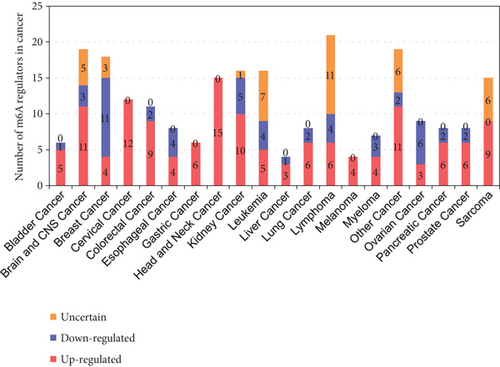
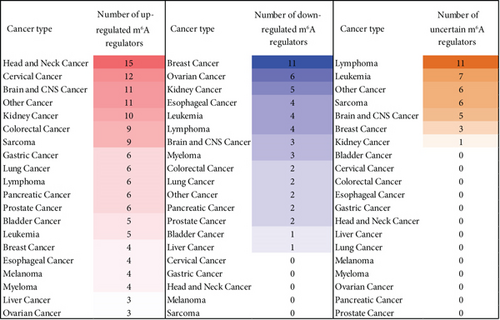
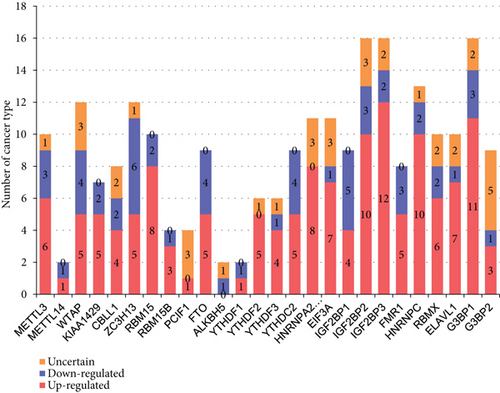
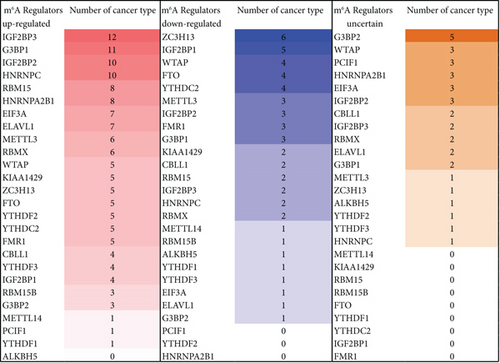
We then determined the top cancer types that upregulated or downregulated m6A-RMRs the most. As shown in Figures 9(b) and 9(c), head and neck cancer, cervical cancer, brain and CNS cancer, other cancer, and kidney cancer upregulated ≥10 m6A-RMRs. In contrast, breast cancer, ovarian cancer, kidney cancer, esophageal cancer, leukemia, and lymphoma downregulated ≥four m6A-RMRs. In addition, lymphoma, leukemia, other cancer, sarcoma, and brain and CNS cancer modulated ≥five m6A-RMRs in uncertain manners. We also determine the top m6A-RMRs that upregulated or downregulated in cancer types the most. As shown in Figures 9(d) and 9(e), IGF2BP3, G3BP1, IGF2BP2, and HNRNPC were upregulated in ≥10 cancer types. In contrast, ZC3H13, IGF2BP1, WTAP, FTO, and YTHDC2 were downregulated in ≥four cancer types. In addition, G3BP2, WTAP, PCIF1, HNRNPA2B1, EIF3A, and IGF2BP2 were modulated in ≥three cancer types in uncertain manners.
The results indicated that most m6A-RMRs were downregulated in acute inflammatory diseases, while in cancer, the upregulated m6A-RMRs were more than downregulated m6A-RMRs. This situation is because acute inflammation is a part of an innate immune system reaction that can be produced by various factors, such as signaling pathways of the receptors for danger-associated molecule patterns (DAMPs/PAMPs) derived from pathogens, viruses, bacteria, and toxic substances as well as cytokine signals and stress hormone signal. These factors may activate the interactions and cross-talks among the receptors of cytokines, viruses, DAMPs, or PAMPs and promote the migration of macrophages or neutrophils to the area of inflammation [73]. However, during cancer occurs, tumor cells will generate tumor antigens that activate adaptive immune responses and induce T cells and B cells. T cell activation could cause a variety of immune signaling, including T cell antigen receptor signaling, costimulation signaling, immune checkpoint coinhibition signaling, and cytokine signaling. The composition of signaling receptors and pathways is different between acute inflammation and cancer. Therefore, RNA methylation and its transcription, splicing, and mRNA stability are also different under these two situations.
We further hypothesized that m6A-RMRs were differentially expressed as different cancer progression. To examine this hypothesis, the expression matrix GSE114783 was downloaded from the NIH-NCBI-GeoDataset database (https://www.ncbi.nlm.nih.gov/gds); and the expressions of m6A-RMRs were screened. The heat map was generated from Cluster web tool [45]. As shown in Supplementary figure 1A, the results showed that the expressions of m6A-RMRs were different among healthy controls, hepatitis B virus carriers, and the patients with liver cirrhosis and hepatocellular carcinoma, especially genes in the red box; compared with healthy controls, there were more upregulated m6A-RMRs in patients with chronic hepatitis B (CHB) group than those in healthy controls. Potentially, due to the tumor heterogeneity or the small sample sizes, there were no differentially expressed m6A-RMRs in the hepatocellular carcinoma group compared with healthy controls. In addition, as shown in Supplementary figure 1B, using the same method as Supplementary figure 1A, the expressions of m6A-RMRs were analyzed in human preinvasive and invasive cervical squamous cell carcinomas and normal cervical epithelia. The differential expressions of m6A-RMRs were shown in the red box. The 22 m6A-RMRs were examined in these three groups. The results showed that the expressions of WTAP were downregulated; and the expressions of four m6A-RMRs such as HNRNPA2B1, IGF2BP2, FMR1, and HNRNPC were upregulated in patients with preinvasive and invasive cervical squamous cell carcinomas compared with normal controls. Moreover, as shown in Supplementary figure 1C, the expressions of m6A-RMRs were analyzed in prostate cancer from benign prostatic hyperplasia to metastatic prostate cancer. The expressions of RBMX were gradually increased from prostatic intraepithelial neoplasia to hormone-naïve prostate cancer compared with normal samples. There were more obvious differential expressions of m6A-RMRs between adjacent prostate cancer samples and prostate carcinoma samples. The expressions of two m6A-RMRs such as EIF3A and RBMX were upregulated in prostate carcinoma samples compared with normal prostate epithelium-adjacent samples.
Finally, we examined the expressions of 29 m6A-RMRs [74] in single-cell RNA-Seq data. As shown in Supplementary figure 2A, in a single-cell RNA-seq analysis of astrocytoma dataset [75], 23 out of 28 m6A-RMRs (82%) (except ALKBH5, IGF2BP1, IGF2BP2, IGF2BP3, and G3BP2) were in the medium to high expression levels in malignant cells of astrocytoma. In addition, as shown in Supplementary figure 2B, in a melanoma intratumor heterogeneity dataset [76], 24 out of 29 m6A-RMRs (83%) (except RBM15B, IGF2BP1, IGF2BP3, HNRNPA2B1, and HNRNPC) were in the medium to high expression levels in cancer-associated fibroblasts (CAF) of melanoma.
Taken together, these results have demonstrated that first, upregulated m6A-RMRs were more than downregulated m6A-RMRs in various cancer types; second, head and neck cancer, cervical cancer, brain and CNS cancer, other cancer, and kidney cancer upregulated ≥10 m6A-RMRs; third, IGF2BP3, G3BP1, IGF2BP2, and HNRNPC were upregulated in ≥10 cancer types; fourth, four m6A-RMRs such as HNRNPA2B1, IGF2BP2, FMR1, and HNRNPC were upregulated in patients with preinvasive and invasive cervical squamous cell carcinomas; and the expressions of two m6A-RMRs such as EIF3A and RBMX were upregulated in prostate carcinoma samples; and fifth, 82-83% of 29 m6A-RMRs were in the medium to high expression levels in astrocytoma and melanoma.
3.8. M1 Macrophage Polarization Upregulates Seven m6A-RMRs including METTL14, WTAP, PCIF1, HNRNPA2B1, IGF2BP2, FMR1, and G3BP1, among Which WTAP and IGF2BP2 May Not Only Promote M1 Polarization but Also Inhibit M2 Polarization
We hypothesized that macrophage polarization from M0 into type 1 proinflammatory macrophages (M1) or M2 anti-inflammatory macrophages modulate the expressions of m6A-RMRs. To examine this hypothesis, we collected a microarray dataset GSE85346 from the NCBI-GeoDataset database. As shown in Figure 10, M1 macrophage polarization upregulated seven m6A-RMRs including METTL14, WTAP, PCIF1, HNRNPA2B1, IGF2BP2, FMR1, and G3BP1 whereas M2 polarization into M2a, M2b, or M2c downregulated three m6A-RMRs including WTAP, IGF2BP2, and IGF2BP3. These results suggest that these M1 polarization upregulated seven m6A-RMRs may promote proinflammatory M1 polarization. In contrast, two M1 polarization-upregulated and M2 polarization-downregulated m6A-RMRs such as WTAP and IGF2BP2 may not only promote M1 polarization but also inhibit M2 polarization.
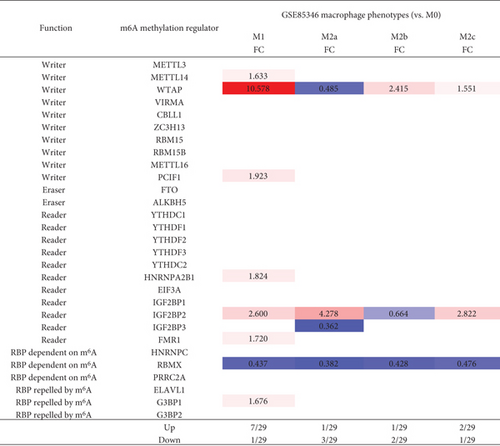
Macrophages are diverse immune cells polarized by numerous stimuli, resulting in a wide range of traits and functions. The polarized process from M0 to M1 is induced by the stimulations of TLR ligands or Th1 cytokines, like TNF-a, IFN-γ, and CSF2 signaling. Under inflammation, these macrophage polarization signals are specific and robust [77], whereas, during tumorigenesis, so many factors can be involved. Moreover, different cancers are related to different cell types. A great amount of different signaling cross-talks may cause chaos signaling [78]. Consequently, the signaling that modulates the upregulation of WTAP could be diffused by the chaos cross-talking signaling.
3.9. The 86% of m6A-RMRs Were Differentially Expressed in the Six Spleen Treg Clusters; and 8 Out of 12 Treg Signature Genes including FOXO1, HDAC9, Dicer, BLIMP1, GATA3, EP300, BCL6, and PPAR Significantly Regulated the Expressions of m6A-RMRs
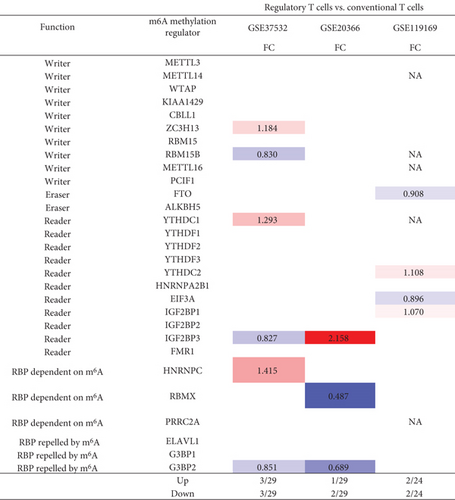
Our and others’ reports demonstrated that CD4+Foxp3+ regulatory T cells play significant roles in suppressing immune responses, inflammations, atherosclerosis [13, 25–27, 70, 79–83], and antitumor immune reactions [84] and promoting tissue regeneration. We hypothesized that m6A-RMRs are modulated in Treg differentiation and Treg responses to tissue microenvironment. As shown in Figure 11(a), Treg upregulated six m6A-RMRs such as ZC3H13, YTHDC1, YTHDC2, IGF2BP1, IGF2BP3, and HNRNPC in three Treg datasets compared to that of CD4+Foxp3- effector T cells.
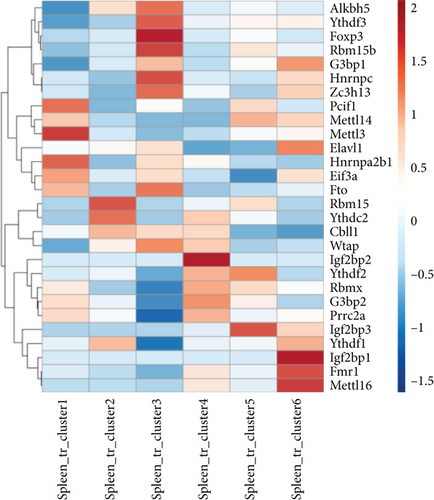
In addition, a recent report with single-cell RNA sequencing (scRNA-Seq) characterizes spleen Treg into six clusters including S100a4highS100a6high cluster 1 (activated), Itgb1high cluster 2 (activated), Dusp2highNr4a1highFoxp3highIL2rahigh cluster 3 (activated), Ikzf2highFoxp3high cluster 4 (resting), Bach2high cluster 5 (resting), and Satb1highSellhigh cluster 6 (resting) [85]. We hypothesized that the m6A-RMRs are differentially expressed in these six Treg clusters. As shown in Figure 11(b), four m6A-RMRs including PCIF1, METTL3, HNRNPA2B1, and EIF3A were upregulated in the cluster 1 Treg; two m6A-RMRs including RBM15 and YTHDC2 were upregulated in the cluster 2 Treg; seven m6A-RMRs including ALKBH5, YTHDF3, RBM15B, HNRNPC, ZC3H13, FTO, Wand TAP were upregulated in cluster 3 Treg; four m6A-RMRs including IGF2BP2, RBMX, G3BP2, and PRRC2A were upregulated in cluster 4 Treg; three m6A-RMRs including METTL14, YTHDF2, and IGF2BP3 were upregulated in cluster 5 Treg; and five m6A-RMRs including G3BP1, ELAVL1, IGF2BP1, FMR1, and METTL16 were upregulated in cluster 6 Treg. Therefore, 25 out of 29 m6A-RMRs (86%) were differentially expressed in the six spleen Treg clusters.
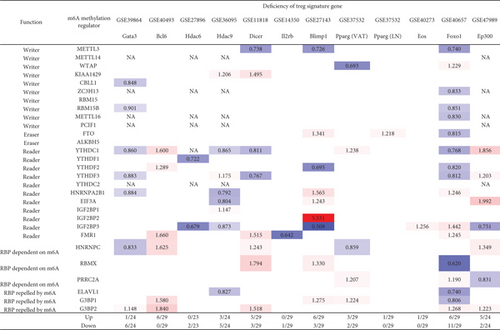
Moreover, we hypothesized that Treg signature genes play significant roles in regulating the expressions of m6A-RMRs. To examine this hypothesis, we collected the microarray datasets of 12 Treg signature gene deficiencies. As shown in Figure 11(c), the deficiencies of type 2 T helper (Th2) transcription factor (TF) GATA binding protein 3 (GATA3), follicular Th cell (Tfh) TF B cell lymphoma 6 protein (BCL6), histone deacetylase 6 (HDAC6), HDAC9, microRNA maturation enzyme DICER, interleukin-2 receptor b chain (IL2rb), B-lymphocyte-induced maturation protein 1 (BLIMP1, PR domain Zinc finger protein 1), peroxisome proliferator activated receptor gamma (PPARg) (visceral adipose tissue, VAT), PPARg (lymph nodes, LN), IKAROS family zinc finger 4 (EOS), Foxhead box 1 (FOXO1), and histone acetyltransferase P300 (EP300) modulated and upregulated 1, 6, 0, 3, 5, 0, 6, 3, 1, 1, 6, and 5 m6A-RMRs, respectively, and downregulated 6, 0, 2, 5, 3, 1, 3, 2, 0, 0, 11, and 2 m6A-RMRs, respectively. The 12 Treg signature genes were ranked based on the total modulation numbers when they were deficient, FOXO1 (17) > HDAC9 (8) = Dicer (8) = BLIMP1 (8) > GATA3 (7) = EP300 (7) > BCL6 (6) > PPARg (VAT) (5) > HDAC6 (2) > IL − 2RB (1) = PPARG (LN) (1) = EOS (1). It has been reported that mechanistic target of rapamycin kinase (mTOR)/interferon regulatory factor 4 (IRF4)/GATA3 promotes IL-4+ Th2-like Treg; phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/AKT serine/threonine kinase 1 (Akt)/Foxo, Th1 TF T-box transcription factor 21 (T-bet), hypoxia inducible factor 1 subunit alpha (HIF-1a), glycolysis promote interferon-gamma (IFNg)+ Th1-like Treg, microbiota/MAF BZIP transcription factor (c-Maf)/signal transducer and activator of transcription 3 (Stat3)/RAR-related orphan receptor C (RORgt), aryl hydrocarbon receptor (AhR) promote IL-17+ Th17 like Treg, and mTOR/Stat3/hepatocyte nuclear factor 1-alpha (TCF1) promote Bcl6+ follicular Treg (Tfr).
Our results have shown that (1) Treg upregulated six m6A-RMRs including ZC3H13, YTHDC1, YTHDC2, IGF2BP1, and HNRNPC; (2) 25 out of 29 m6A-RMRs (86%) were differentially expressed in the six spleen Treg clusters, suggesting that m6A-RMRs play significant roles in reprogramming Treg activation status (clusters 1-3) and resting status (clusters 4-6); and (3) the transcription data from 12 Treg signature gene deficiencies suggest that eight out of 12 Treg signature genes including FOXO1, HDAC9, Dicer, BLIMP1, GATA3, EP300, BCL6, and PPARγ regulate the expressions of m6A-RMRs significantly; and m6A-RMRs play important roles in reshaping Treg stability and plasticity.
3.10. Immune Checkpoint Receptors TIM3, TIGIT, PD-L2, and CTLA4 Play Significant Roles in Modulating the Expressions of m6A-RMRs; and Inhibition of Costimulation Receptor CD40 with Anti-CD40 Antibody Significantly Upregulates the Expressions of m6A-RMRs
We recently reported that cosignaling receptors localized on cell membrane including costimulation receptors and immune checkpoint receptors (coinhibition receptors) serve as a prototypic cell-cell contact interaction and regulate T cell plasticity, immune tolerance, cellular physiology, and sterile inflammatory disorders [86]. We hypothesized that cosignaling receptors regulate the expressions of m6A-RMRs. We collected 10 cosignaling receptor deficiency/suppression microarray datasets from the NIH-NCBI GeoDataset database. As shown in Figure 12, cytotoxic T-lymphocyte-associated protein 4 (CTLA4) KO and anti-CTLA4 upregulated 2-3 m6A-RMRs and downregulated one m6A-RMR. CD80+ programmed cell death 1 ligand 2 (PD-L2)+ (memory B cells) [87] upregulated three and downregulated five m6A-RMRs. PD-L1 and anti-PD-L1 upregulated one m6A-RMRs and downregulated zero m6A-RMRs. T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3) KO, CD3+CD8+TIM-3- cells [88], and CD8+PD1+TIM3- cells [89] upregulated 0-5 m6A-RMRs and downregulated 2-11 m6A-RMRs. T cell immunoreceptor with Ig and ITIM domain- (TIGIT-) Treg upregulated 3 m6A-RMRs and downregulated 13 m6A-RMRs. In addition to the modulation of m6A-RMR expressions by immune checkpoint receptors, the modulation with anti-CD40 (tumor necrosis factor receptor superfamily member 5), a costimulation receptor, resulted in upregulation of 5-12 m6A-RMRs and downregulation of 1-2 m6A-RMRs. Taken together, these results have demonstrated that immune checkpoint receptors TIM3, TIGIT, PD-L2, and CTLA4 play significant roles in modulating the expressions of m6A-RMRs; and inhibition of costimulation receptor CD40 with anti-CD40 antibody significantly upregulates the expressions of m6A-RMRs, which is associated with suppression of autoimmune responses by anti-CD40 antibody therapy [90].
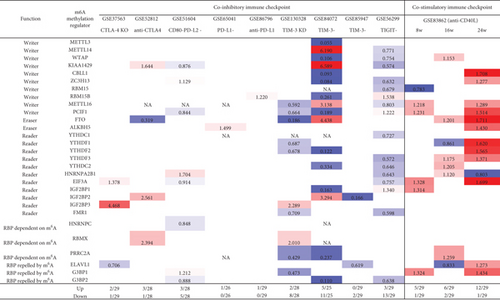
3.11. Proinflammatory Cytokine Signaling Pathways Significantly Modulate the Expressions of m6A-RMRs, and Suppression of Proinflammatory Cytokine Pathways Upregulate More Than Downregulate the Expressions of m6A-RMRs
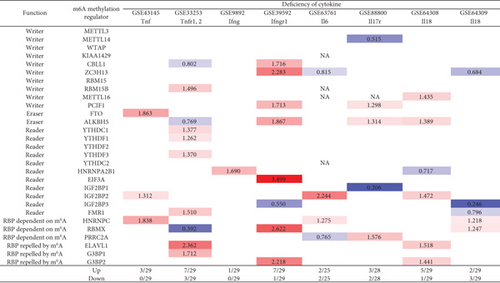
We recently reported that cytokines including IL-17 [91–93], IL-2 [26], and IL-35 [15, 94, 95] play significant roles in inflammation regulation, Treg maintenance, and various pathologies. We hypothesized that signal pathways of cytokines and cytokine receptors regulate the expressions of m6A-RMRs. We collected eight cytokine and cytokine receptor microarray datasets from the NIH-NCBI GeoDataset database. As shown in Figure 13, deficiencies of tumor necrosis factor-α (TNFα, TNF receptor 1-2 (TNFR1-2), interferon-γ (IFNγ), IFNγ receptor-1 (IFNγR1), IL6, IL-17 receptor (IL17R), IL18, and IL18 upregulated 3, 7, 1, 7, 2, 3, 5, and 2 m6A-RMRs, respectively, and downregulated 0, 3, 0, 1, 2, 2, 1, and 3 m6A-RMRs, respectively. These results have demonstrated that (1) proinflammatory cytokine signaling pathways significantly modulate the expressions of m6A-RMRs, and (2) suppression of proinflammatory cytokine pathways upregulates more than downregulates the expressions of m6A-RMRs.
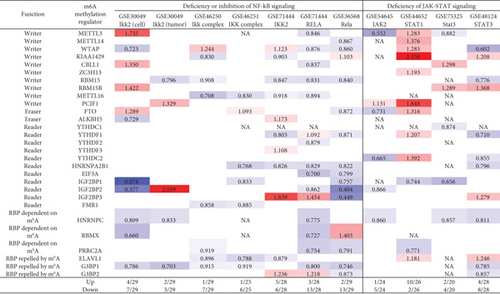
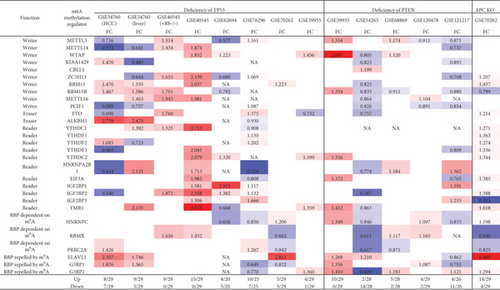
3.12. NF-κB Components and JAK/STAT Signaling (Except STAT1) Play Important Roles in Upregulating More Than Downregulating m6A-RMRs; and Tumor Suppressors TP53, PTEN, and APC Play Significant Roles in Downregulating More Than Upregulating m6A-RMRs
We and others reported that proinflammatory/immune regulatory transcription factors (TFs), Janus kinase (JAK)/signal transducer and activator of transcription (STAT) [94], nuclear factor kappa B (NF-κB) [96], tumor protein P53 (TP53), phosphatase and tensin homolog (PTEN), and adenomatous polyposis coli (APC regulator of WNT signaling pathway, APC) [97] play significant roles in regulating inflammation, Treg maintenance, cytokine responses, tumorigenesis, and development. We hypothesized that proinflammatory and immune regulatory TFs, NF-κB, and JAK/STAT pathways and three tumor suppressors TP53, PTEN, and APC pathways regulate the expressions of m6A-RMRs. We collected five NF-κB subunit deficiency datasets (seven comparisons), one JAK2 deficiency dataset, one STAT1 deficiency dataset, two STAT3 deficiency datasets, six TP53 deficiency datasets (eight comparisons), five PTEN datasets, and one APC dataset from the NIH-NCBI GeoDataset database. As shown in Figure 14, deficiencies of IKK2 (cell line), IKK2 (tumor), IKK complex, IKK complex, IKK2, RELA, and Rela upregulated 4, 2, 1, 1, 5, 3, and 2 m6A-RMRs, respectively, and downregulated 7, 3, 7, 6, 6, 13, and 13, respectively. Of note, deficiencies of NF-κB signaling components resulted in more downregulation than upregulation of m6A-RMRs. Deficiencies of JAK2, STAT1, STAT3, and STA3 resulted in upregulating 1, 10, 2, and 4 m6A-RMR, respectively, and downregulating 5, 2, 4, and 8 m6A-RMRs, respectively. STAT1 deficiency upregulated more than downregulated m6A-RMRs, but deficiencies of JAK2 and STAT3 downregulated more than upregulated m6A-RMRs. Deficiencies of TP53 in eight datasets upregulated 8, 9, 9, 15, 4, 10, 3, and 4 m6A-RMRs, respectively, and downregulated 7, 5, 0, 0, 5, 7, 3, and 1 m6A-RMRs, respectively. Of note, six out of 8 TP53-deficient datasets had more upregulation than downregulation of m6A-RMR, suggesting that tumor progression triggered by TP53 deficiencies may require more upregulation than downregulation of m6A-RMRs. Deficiencies of PTEN in five datasets upregulated 10, 2, 5, 4, and 4 m6A-RMRs, respectively, and downregulated 0, 14, 2, 2, and 11 m6A-RMRs, respectively. Of note, three out of five PTEN deficient datasets had more upregulation than downregulation of m6A-RMR, suggesting that tumor progression triggered by PTEN deficiencies may require more upregulation than downregulation of m6A-RMRs. Deficiencies of APC upregulated 14 and downregulated 4 m6A-RMRs, respectively. These results have exhibited that (1) NF-κB signaling components play important roles in upregulating more than downregulating m6A-RMRs; (2) JAK/STAT signaling pathways play important roles in upregulating more than downregulating m6A-RMRs except STAT1; and (3) tumor suppressors TP53, PTEN, and APC play significant roles in downregulating more than upregulating m6A-RMRs, suggesting that upregulation of m6A-RMRs may favor more than inhibit tumor progression.
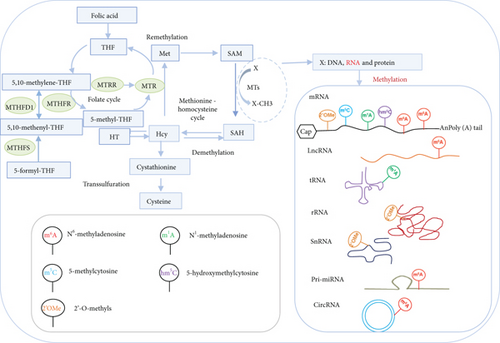
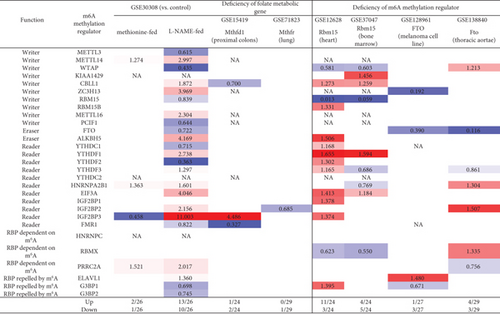
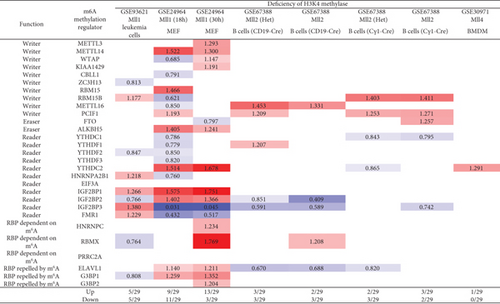
3.13. Methionine-Homocysteine Cycle Enzyme Mthfd1 Downregulates More Than Upregulates m6A-RMRs; One m6A Writer RBM15 and One m6A Eraser FTO Significantly Modulate the Expressions of m6A-RMRs; and H3K4-Specific Methyltransferase MLL1 and DNA Methyltransferase, DNMT1, Significantly Regulate the Expressions of m6A-RMRs
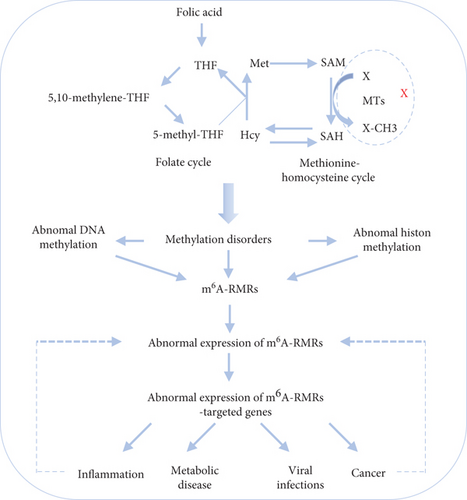
We reported that hyperhomocysteinemia promotes differentiation of the inflammatory Ly6C+ monocyte [5, 98, 99], decreased high-density lipoprotein (HDL) [100, 101], endothelial cell activation, and programmed cell death [6] and atherosclerosis [102]. We hypothesized that m6A-RMRs, histone lysine methylases, DNA methyltransferases, and methyl donating homocysteine-methionine metabolism cycle play significant roles in regulating the expressions of m6A-RMRs as a feedback mechanism. To examine this hypothesis, we collected numerous microarray datasets including a methionine diet-fed model, Mthfd1 KO, Mthfr KO, eight histone 3 lysine 4 (H3K4) methylase KOs, and eight DNA methyltransferase KOs from the NIH-NCBI-GeoDataset database. As shown in Figure 15(a), the methionine diet-fed model upregulated three m6A-RMRs and downregulated one m6A-RMR; deficiencies of methylenetetrahydrofolate dehydrogenase (Mthfd1) and methylenetetrahydrofolate reductase (Mthfr) resulted in upregulation of one and zero m6A-RMR, respectively, and downregulation of two and one m6A-RMRs, respectively. Deficiencies of m6A-RMRs RBM15 and FTO upregulated 1 to 11 m6A-RMRs and downregulated 3-5 m6A-RMRs. As shown in Figure 15(b), deficiencies of histone lysine 4 methylase in eight datasets upregulated one to 13 m6A-RMRs and downregulated zero to 11 m6A-RMRs. Among the eight comparison datasets, three datasets of deficiencies of H3K4-specific methyltransferase MLL1 [103] modulated m6A-RMRs the most, with 10 out of 29, 20 out of 29, and 16 out of 29 m6A-RMRs, respectively. Moreover, deficiencies of DNA methyltransferase 1 (DNMT1) in GSE54841, GSE27434, GSE44277, and GSE86147; DNMT3A at GSE54841 and GSE42304; and DNMT3B at GSE54841 and GSE75401 upregulated 0, 1, 0, 3, 0, 1, 0, and 0 m6A-RMRs, respectively, and downregulated 2, 8, 12, 15, 1, 3, 1, and 1 m6A-RMRs, respectively. Among the eight datasets of DNA methyltransferase deficiencies, DNMT1 deficiencies modulated the most with 9, 12, and 18 m6A-RMRs except GSE54841 (Figure 15(c)). Taken together, these results have demonstrated that (1) methionine diet-fed model and methionine-homocysteine cycle enzyme Mthfr do not significantly regulate the expressions of m6A-RMRs but methionine-homocysteine cycle enzyme Mthfd1 downregulates more than upregulates m6A-RMRs; (2) one m6A writer RBM15 and one eraser FTO significantly modulate the expressions of m6A-RMRs, suggesting a feedback mechanism for regulation of m6A-RMR expressions; (3) H3K4-specific methyltransferase MLL1 significantly regulates the expressions of m6A-RMRs; and (4) DNA methyltransferase, DNMT1, modulates the expressions of m6A-RMRs.

3.14. The 18 Out of 165 ROS Regulators (11%) Were Modulated by Four Human m6A Writers KIAA1429, METTL14, METTL3, and WTAP, and Gene KO Data Showed That 40 Out of 165 ROS Regulators (24%) Were Modulated by m6A Eraser FTO and Two m6A Writers METTL3 and WTAP
Our and others’ reports showed that reactive oxygen species (ROS) play significant roles not only in mediating endothelial cell activation and vascular inflammation [104] but also in sensing metabolic homeostasis and stress in organelle metabolic process as an integrated system. An important question remained whether m6A-RMRs regulate ROS regulators. We hypothesized that m6A-RMRs regulate the expressions of ROS regulators. To examine this hypothesis, the ROS regulators were the targets of RNA methylation regulators in human cell studies as shown in Table 5. The TREW data (Target of m6A Readers, Erasers, and Writers) was download from Met-DB v2.0 (MeT-DB V2.0: the MethylTranscriptome DataBase Version 2.0 http://180.208.58.19/metdb_v2/html/index.php) [105]. The 165 ROS regulators were examined as we reported. The result showed that 18 out of 165 ROS regulators (11%) (F2RL1, PDK4, TIGAR, BCL2, SESN2, GNAI2, DDIT4, SH3PXD2A, FOXM1, AATF, TGFB1, TSPO, G6PD, GNAI3, and CYP1B1) were modulated by four m6A writers KIAA1429, METTL14, METTL3, and WTAP (several ROS regulators were modulated in more than one position in chromosome or by more than one RNA methylation regulators) (Table 5). The deficiencies of four m6A writers (KIAA1429, METTL14, METTL3, and WTAP) downregulated the m6A modification of ROS regulators. As shown in Supplementary Table 4, the expressions of ROS regulators were also the targets of RNA methylation regulators in mouse cell studies. TREW (Target of m6A Readers, Erasers and Writers) was download from Met-DB v2.0 (MeT-DB V2.0: the MethylTranscriptome DataBase Version 2.0 http://180.208.58.19/metdb_v2/html/index.php) [105]. The 165 ROS regulators were examined; and the result showed that 40 out of 165 ROS regulators (24%) were modulated by m6A eraser FTO and two m6A writers METTL3 AND WTAP. The deficiency of m6A eraser FTO upregulated the m6A modification of ROS regulators, and the deficiencies of two m6A writers METTL3 or WTAP downregulated the m6A modification of ROS regulators.
| Targeted-ROS regulator | Type | m6A methyl-regulator | p adj | p_treated | p_control | Log2_OR | Log2_RR | q | Experiment (modification-RNA methylation regulators-cell) | PMID |
|---|---|---|---|---|---|---|---|---|---|---|
| F2RL1 | Writer | KIAA1429 | 0.01 | 0.35 | 0.59 | -1.39 | -0.74 | 893.65 | m6A-KIAA1429-si-A549 | 24981863 |
| F2RL1 | Writer | KIAA1429 | 0.02 | 0.46 | 0.75 | -1.86 | -0.72 | 1959.11 | m6A-KIAA1429-si-A549 | 24981863 |
| PDK4 | Writer | KIAA1429 | 0.02 | 0.11 | 0.69 | -4.12 | -2.60 | 91.22 | m6A-KIAA1429-si-A549 | 24981863 |
| TIGAR | Writer | KIAA1429 | 0.04 | 0.19 | 0.73 | -3.56 | -1.95 | 309.32 | m6A-KIAA1429-si-A549 | 24981863 |
| BCL2 | Writer | METTL14 | 0.03 | 0.63 | 0.95 | -3.37 | -0.58 | 130.91 | m6A-METTL14-sh2-A549 | 24981863 |
| SESN2 | Writer | METTL3 | 0.03 | 0.25 | 0.46 | -1.35 | -0.88 | 80.55 | m6A-METTL3-kd-Hela | 24316715 |
| GNAI2 | Writer | METTL3 | ≤0.01 | 0.26 | 0.41 | -0.99 | -0.66 | 474.67 | m6A-METTL3-kd-Hela | 24316715 |
| GNAI2 | Writer | METTL3 | ≤0.01 | 0.26 | 0.41 | -0.99 | -0.66 | 474.67 | m6A-METTL3-kd-Hela | 24316715 |
| DDIT4 | Writer | METTL3 | ≤0.01 | 0.27 | 0.44 | -1.05 | -0.68 | 1549.26 | m6A-METTL3-kd-Hela | 24316715 |
| DDIT4 | Writer | METTL3 | ≤0.01 | 0.27 | 0.44 | -1.05 | -0.68 | 1549.26 | m6A-METTL3-kd-Hela | 24316715 |
| DDIT4 | Writer | METTL3 | ≤0.01 | 0.27 | 0.44 | -1.05 | -0.68 | 1549.26 | m6A-METTL3-kd-Hela | 24316715 |
| SH3PXD2A | Writer | METTL3 | 0.03 | 0.55 | 0.60 | -0.29 | -0.13 | 2889.11 | m6A-METTL3-kd-Hela | 24316715 |
| SH3PXD2A | Writer | METTL3 | 0.03 | 0.55 | 0.60 | -0.29 | -0.13 | 2889.11 | m6A-METTL3-kd-Hela | 24316715 |
| SH3PXD2A | Writer | METTL3 | 0.03 | 0.55 | 0.60 | -0.29 | -0.13 | 2889.11 | m6A-METTL3-kd-Hela | 24316715 |
| SH3PXD2A | Writer | METTL3 | 0.03 | 0.55 | 0.60 | -0.29 | -0.13 | 2889.11 | m6A-METTL3-kd-Hela | 24316715 |
| FOXM1 | Writer | METTL3 | 0.02 | 0.33 | 0.40 | -0.43 | -0.27 | 427.19 | m6A-METTL3-kd-Hela | 24316715 |
| AATF | Writer | METTL3 | ≤0.01 | 0.19 | 0.39 | -1.40 | -1.00 | 132.56 | m6A-METTL3-kd-Hela | 24316715 |
| TGFB1 | Writer | METTL3 | ≤0.01 | 0.46 | 0.60 | -0.79 | -0.37 | 1205.15 | m6A-METTL3-kd-Hela | 24316715 |
| TSPO | Writer | METTL3 | 0.01 | 0.25 | 0.37 | -0.78 | -0.54 | 186.38 | m6A-METTL3-kd-Hela | 24316715 |
| G6PD | Writer | METTL3 | ≤0.01 | 0.36 | 0.40 | -0.28 | -0.17 | 2992.01 | m6A-METTL3-kd-Hela | 24316715 |
| GNAI3 | Writer | WTAP | 0.02 | 0.17 | 0.46 | -2.01 | -1.41 | 154.65 | m6A-WTAP-kd-Hela | 24316715 |
| GNAI3 | Writer | WTAP | 0.02 | 0.17 | 0.46 | -2.01 | -1.41 | 154.65 | m6A-WTAP-kd-Hela | 24316715 |
| TIGAR | Writer | WTAP | 0.01 | 0.19 | 0.56 | -2.42 | -1.54 | 104.56 | m6A-WTAP-kd-Hela | 24316715 |
| CYP1B1 | Writer | WTAP | 0.02 | 0.50 | 0.95 | -4.33 | -0.93 | 583.55 | m6A-WTAP-si-A549 | 24981863 |
| DDIT4 | Writer | WTAP | ≤0.01 | 0.46 | 0.73 | -1.66 | -0.66 | 825.45 | m6A-WTAP-si-Hek293T | 24981863 |
- Met-DB v2.0 contains a significant increase in context-specific m6A peaks and single-base sites predicted from 185 samples from 26 separate studies for 7 species. It has also been updated to include a new database for targets of m6A readers, erasers, and writers, as well as additional functional data gathering. The abbreviation TREW stands for Target of m6A Readers, Erasers, and Writers. To discover their target sits, we collected ParCLIP-seq and MeRIP-seq data for 8 regulator/reader proteins (including FTO, KIAA1429, METTL14, METTL3, WTAP, HNRNPC, YTHDC1, and YTHDF1) from 10 independent studies. Then, the differential m6A peaks that showed significant hypermethylation (hypomethylation) after knocking down of a demethylase (methylase) were determined to the target peaks. p_treat: peak of treated group; p_control: peak of control group; OR: odds ratio; RR: relative risk or risk ratio.
4. Discussion
Recent progress has reported that m6A-RNA methylation [50] plays a significant role in regulating cardiovascular diseases (CVD) and CVD-related diseases and complications such as cardiac remodeling, atherosclerosis, heart failure, inflammation adipogenesis, obesity, insulin resistance, hypertension, and type 2 diabetes mellitus [41]. In addition, m6A-RNA methylation plays critical roles in the pathogenesis of cancers and tumors [106, 107], aging [107], immune responses and autoimmunity, and viral infections [108, 109]. However, two major issues remain unknown: first, transcriptomic regulation of a complete list of m6A-RNA methylation regulators in various diseases and second, cellular mechanisms and molecular mechanisms underlying transcriptomic changes of m6A-RNA methylation regulators in pathophysiological conditions. To solve these problems, we performed a transcriptomic data mining of the expressions of 29 m6A-RNA methylation regulators in diseases and cancers and made significant findings: (1) a few m6A-RMRs were upregulated; and most m6A-RMRs were downregulated in sepsis, acute respiratory distress syndrome, shock, and trauma; (2) half of 29 m6A-RMRs were downregulated in atherosclerosis progression compared with those of regression; (3) IBD and RA modulated m6A-RMRs more than lupus and psoriasis; and some autoimmune diseases share five upregulated m6A-RMRs; (4) some organ failures shared eight upregulated m6A-RMRs; end-stage renal failure (ESRF) downregulated 85% of m6A-RMRs; and upregulation of m6A-RMRs in hemodialysis more than in ESRF may have clinical benefits; (5) MERS-CoV infections modulated m6A-RMRs the most among viral infections; (6) oxPAPC and NOTCH1 knockdown modulated m6A-RMRs more than other DAMs stimulation of endothelial cells including LPS, oxLDL, and IFNs; (7) upregulated m6A-RMRs were more than downregulated m6A-RMRs in cancer types; five types of cancers upregulated ≥10 m6A-RMRs; (8) M1 macrophages upregulated seven m6A-RMRs; WTAP and IGF2BP2 may not only promote M1 but also inhibit M2 polarization; (9) 86% of m6A-RMRs were differentially expressed in the six spleen Treg clusters; and 8 out of 12 Treg signatures significantly regulated m6A-RMRs; (10) immune checkpoint receptors TIM3, TIGIT, PD-L2, and CTLA4 significantly modulated m6A-RMRs; and inhibition of costimulation receptor CD40 with anti-CD40 significantly upregulated m6A-RMRs; (11) proinflammatory cytokines significantly modulated m6A-RMRs; (12) NF-κB and JAK/STAT pathways (except STAT1) upregulated more than downregulated m6A-RMRs; and TP53, PTEN, and APC downregulated more than upregulated m6A-RMRs; (13) methionine-homocysteine cycle enzyme Mthfd1 downregulated more than upregulated m6A-RMRs; m6A writer RBM15 and one m6A eraser FTO significantly modulated m6A-RMRs; and H3K4 methyltransferase MLL1 and DNA methyltransferase, DNMT1, significantly regulated m6A-RMRs; and (14) 40 out of 165 ROS regulators were modulated by m6A eraser FTO and two m6A writers METTL3 and WTAP.
For some diseases, both eraser and writing enzymes of m6A-RMRs have changed or have the same expression trend. In order to explain this phenotype, we checked the m6A-RMRs changes in Met-DB v2.0 and the result supports m6A-RMRs regulate expression of m6A-RMRs themselves (Supplementary Tables 5 and 6). When the writer METTL3 or WTAP was knocked down, the expression of eraser ALKBH5 also was down regulated (Supplementary Table 5). Then, Protein-Protein Interaction (PPI) analysis was performed by using STRING (https://string-db.org/) and the result also suggest the interactions among m6A-RMRs are complex (Supplementary Figure 3). Additionally, the expression of two kinds of m6A-RMRs such as writer WTAP and eraser FTO is positively correlated in some tumors (Supplementary Figure 4). So, in some diseases, writers and erasers both are upregulated or downregulated, which is reasonable and the main function of m6A-RMRs can be confirmed by using some well-designed experiments.
One of the potential issues related to database mining is that we were unable to compare the impact of different regulators in controlling the expressions of m6A-RMRs in the same cell types since the original microarray studies we looked at employed different cells. Although our database mining strategy was not optimal, our approach was justified in filling up a critical knowledge gap. This is, in fact, a common practice that we and others [110] often use in studying gene expression in nonideal, heterogeneous peripheral blood mononuclear cell populations (PBMCs) in disease conditions versus healthy conditions, and PBMCs are actually made up of a variety of cell types, including B cells (~15%), T cells (~70%), monocytes (~5%), and natural killer (NK) cells (~10%) among others [111]. Another limitation of the current study is that, due to the low-throughput nature of verification techniques in every laboratory, including ours, we were unable to confirm every result we uncovered using high-throughput data analyses. We recognize that in the future, carefully designed in vitro and in vivo experimental models will be required to confirm regulator gene deficiency-upregulated m6A-RMRs further and the underlying mechanisms we disclose here.
Based on our findings, we proposed a novel working model in Figure 16. First, we recently proposed a new theory that because of their connections with three metabolic pathways including folate cycle, transsulfuration pathway, glutathione synthesis, polyamine metabolism, and methionine salvage pathway, homocysteine-methionine cycle serves as a sensor-receptor system to sense the intracellular metabolic homeostasis and stresses of four amino acids such as methionine, homocysteine, serine, and arginine as well as vitamin B12 and folate; second, similar to protein phosphorylation/dephosphorylation-based signaling pathways, the metabolic homeostasis and stress signals relay the metabolic reprogramming signals into cellular methylation processes via various methyltransferases to methylate DNAs, proteins, histones, RNAs, and other molecules. The methylations of those important molecules regulate their biological functions; third, m6A-RNA methylation is a dominant RNA methylation for various RNA types including mRNAs, tRNAs, rRNAs, and noncoding RNAs. Throughout transcriptomic data analyses of 102 microarrays, RNA-Seq, and single-cell RNA-Seq related to 41 diseases in six categories organ failures, viral infections, metabolic diseases, acute inflammations, cancers, and autoimmune diseases, our data have demonstrated that several layers of regulatory systems regulate the transcriptomic changes of m6A-RMRs in diseases as well as pathophysiological conditions in various cell types, which include (1) cell surface receptors such as cytokine receptors, viral receptors, danger-associated molecular pattern (DAMP) receptor, pathogen-associated molecular pattern (PAMP) receptors, immune checkpoint receptors, and cosignaling receptors. Of note, immune checkpoint receptors and cosignaling receptors are the prototypic membrane protein interactions between cells; (2) cellular mechanisms such as macrophage polarization, endothelial cell activation, CD4+Foxp3+ regulatory T cell activation, resting status, and pathophysiological changes of other cells; and (3) nuclear transcription factors (TFs) including proinflammatory TFs NF-κB, Jak-STATs, and tumor suppressors TP53, PTEN, and APC. In summary, our results have demonstrated that transcriptional regulations of m6A-RMRs are highly significant mechanisms in regulating m6A-RNA methylations related to various pathophysiological processes and diseases. Our findings provide novel insights on the roles of m6A-RMRs in the development of inflammatory disorders and malignancies as well as novel pathways for future therapeutic strategies for inflammatory diseases, sepsis, trauma, organ failures, autoimmune diseases, metabolic CVDs, and cancers.
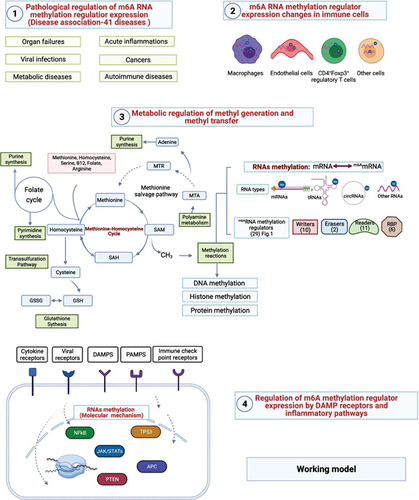
Conflicts of Interest
The authors declare no conflict of interest.
Authors’ Contributions
ML carried out the data gathering and data analysis, and prepared tables and figures. KX, FS, YS, RZ, YL, YS, CDIV, LL, SW, SPK, GJC, JS, HS, XJ, and HW aided with analysis of the data. XFY supervised the experimental design, data analysis, and manuscript writing. All authors read and approved the final manuscript. Ming Liu and Keman Xu shared the first authorship.
Acknowledgments
ML was supported by a fellowship from the School of Basic Medical Science, Shanxi Medical University. This research was funded by NIH grants to HW and XFY.
Open Research
Data Availability
All the datasets used in this study are publicly available. The analyzed results in this study are included within the article and Supplementary Materials.




