Proteomics and immunoblotting analyses reveal antigens that optimize the immunodiagnosis of the infection by Toxocara spp
Abstract
Toxocariasis is an infection caused by the round worms Toxocara canis and Toxocara cati. It occurs worldwide though it is more prevalent in developing countries. For the diagnosis of toxocariasis, the most used method is the indirect enzyme-linked immunosorbent assay (indirect ELISA), based on the detection of specific antibodies using the excreted/secreted products from T. canis larvae (TES) as antigens, but it cross-reacts with several helminth infections. For this reason, there is a need to investigate species-specific immunoreactive proteins, which can be used for the development of a more sensitive and specific diagnosis. This study aims to investigate immunoreactive protein candidates to be used for the development of a more sensitive and specific diagnosis of Toxocara spp. infection in humans. We have used immunoblotting and mass spectrometry to select four Toxocara canis immunoreactive proteins that were recombinantly expressed in bacteria and evaluated as potential new diagnostic antigens (rMUC3, rTES 26, rTES32 and rCTL4). The recognition of these recombinant proteins by total serum IgG and IgG4 was assayed using the purified proteins in an isolated manner or in combination. The IgG ELISAs performed with individual recombinant antigens reached values of sensitivity and specificity that ranged from 91.7% to 97.3% and 94.0% to 97.9%, respectively. Among the analyses, the IgG4 immunoassay was proven to be more effective, revealing a sensitivity that ranged from 88.8% to 98.3% and a specificity of 97.8%–97.9%. The IgG4 ELISA was shown to be more effective and presented no cross-reactivity when using combinations of the rTES 26 and rCTL4 recombinant proteins. The combination of these two molecules achieved 100% sensitivity and specificity. The use of only two recombinant proteins can contribute to improve the current panorama of toxocariasis immunodiagnosis for, with a better optimization and reduced cost.
1 INTRODUCTION
According to the US Centers for Disease Control and Prevention (CDC) (http://www.cdc.gov), human toxocariasis is a zoonotic infection spread worldwide that ranks among the top five most important neglected infectious diseases. It is mostly found in developing countries, where reported prevalence rates reach up to 68.0% (Mendonça et al., 2012). The infection is predominantly caused by the helminths Toxocara canis and Toxocara cati, which have dogs and cats as definitive hosts, respectively (Lee et al., 2010). Humans are considered as paratenic hosts since they are infected by the accidental ingestion of embryonated eggs. In the small intestine, Toxocara spp. infective L3 larvae penetrate the intestinal mucous membrane, accessing blood, lymph vessels and some organs, causing visceral larva migrans, ocular larva migrans, neurotoxocariasis and an asymptomatic disease known as covert toxocariasis (Moreira et al., 2014).
The misdiagnosis of the infection by these helminths leads to a poor management of patients with toxocariasis. In fact, imprecisions related to the diagnosis of the disease and the high prevalence of asymptomatic cases remain as important contributors for the neglected aspect of this infection (Moreira et al., 2014). Importantly, asymptomatic infections can immunomodulate their hosts, leading to an increased production of regulatory cytokines and a low immunological response to vaccines and other infections (Alcântara-Neves et al., 2014).
The diagnosis of toxocariasis is currently based on the analysis of clinical symptoms, serological tests and, more rarely, tissue biopsy. The most commonly used serological method is the indirect enzyme immunoassay (ELISA), based on the detection of specific antibodies against the excretory–secretory Toxocara spp. larval antigens (TES) (Magnaval et al., 2001). However, the production of TES is laborious and involves the obtaining of infected puppies, deworming, separation of eggs from the dissected uteri and in vitro culture of egg-derived larvae, a process that takes approximately 3 months. Moreover, this procedure requires technical and scientific training, has a very low yield and the assay based on the TES antigen has low specificity (de Savigny & Tizard, 1977). Recent studies have shown that the effectiveness of this ELISA test is compromised due to a high level of cross-reactivity with other helminth antigens and that TES proteins also share similar amino acid sequences with common environmental allergens (Watthanakulpanich et al., 2008). Indeed, this extensive cross-reactivity occurs with aeroallergens, causing serological misdiagnosis of helminthic and allergic illnesses (Ponte et al., 2011). To partially overcome this disadvantage, the patients’ sera are usually pre-adsorbed with Ascaris lumbricoides antigens before performing the assay, leading to another laborious and time-demanding procedure (Mendonca et al., 2013).
In this scenario, the search for immunoreactive and specific T. canis antigens is necessary. Some efforts were taken to identify Toxocara spp. immunoreactive antigens through Western blot analysis, but the diagnostic performance of this method is still variable (Despreaux et al., 2016; Ma et al., 2018) and the assay is difficult to be carried out in a large scale. In this work, we have used proteomics associated to immunoblotting analyses to characterize specific TES and T. canis larvae somatic proteins. Four proteins were then expressed in a heterologous system and tested in an immunoenzymatic assay, with the objective to evaluate their potential as specific and sensitive antigens for the development of a toxocariasis immunodiagnosis method.
2 METHODS
2.1 Sample collection and ethical aspects
Serum samples were obtained from 280 children, 4–11 years old, originally recruited in a cross-sectional study in Northeastern Brazil that evaluated the prevalence of allergy and helminth infections (Silva et al., 2017). This study was approved by the Committee of Ethics in Research of the Maternidade Climério de Oliveira, Federal University of Bahia (UFBA), Salvador, Brazil, under the registration number CEP.004/2010. An informed consent form for participation in all stages of the research was signed by all the parents or guardians of the children.
2.2 Worm collection and antigen obtaining
Worms were collected from the faeces of piperazine-treated puppies. The TES antigen was obtained from the supernatant of T. canis larvae cultures, as described by de Savigny (de Savigny & Tizard, 1977) and modified by Alcantara-Neves and collaborators (Alcantara-Neves et al., 2008; da Silva et al., 2018). The larvae somatic extract was obtained by washing them with PBS three times by centrifugation, followed by grinding in a tissue macerator in the presence of zirconium/silica beads (BioSpec Products Inc., Bartlesville, OK, USA) and further delipidification with several centrifugation steps in the presence of ethyl ether. Following, the extract was centrifuged at 8000 × g for 10 min at 4°C and a solution of protease inhibitors (Sigma-Aldrich, St. Louis, MO, USA) was added to the supernatant, which was sterilized by filtration using a 0.22 μM membrane. Samples were aliquoted and stored at −70°C until use.
The adult A. lumbricoides extract was obtained using 20 adult worms obtained through deworming a child with piperazine and mineral oil. The worms were washed in PBS, cut and subjected to thermal shock (alternate cycles of treatments with liquid nitrogen and heating at 37°C). The worms were then macerated as described for T. canis larvae; delipidification steps were performed with ethyl ether in a volume equal to that of the macerate, incubated for 30 min under agitation and centrifuged for 15 min. The supernatant containing the soluble extract was separated from the debris. A protease cocktail (Sigma-Aldrich, St. Louis, MO, USA) was added to the supernatant. The protein concentration was determined using the Bradford method and the extract was aliquoted and stored at −70°C until use.
To obtain the Blomia tropicalis extract, this acarid was isolated, cultivated and purified as previously described (Baqueiro et al., 2010). Briefly, mites isolated from domestic dust samples and grown at 25°C and 75% relative humidity were purified by flotation in 5 M NaCl. The fraction containing the mites was collected until it was free of impurities (>97% purity) and washed with distilled water. The mites were crushed in a tissue grinder in the presence of zirconium/silica beads (BioSpec Products, Inc., Bartlesville, USA) along with alternating cycles of thermal shock. Following, the suspension was centrifuged and the supernatant was delipidated by means of successive washes in ethyl ether, followed by centrifugation. The procedure was repeated until the sample was lipid-free and stored at −20°C.
Dermatophagoides pteronyssinus was obtained from a commercial laboratory (Greer Laboratories, Cambridge, MA, USA) as dehydrated mites and the extracts were prepared as described for Blomia tropicalis. Periplaneta americana and Blatella germanica were grown in a fish aquarium in the presence of watered cotton and fish food. Their extracts were obtained as previously described for A. lumbricoides.
2.3 Cross-reactivity analysis
In order to analyse the cross-reaction of antibodies between Toxocara spp. and A. lumbricoides antigens, and environmental allergens, 40 serum samples from children seropositive for Toxocara spp.-specific IgG (detected through TES-based ELISA), 40 serum samples from children presenting positive results in a dermic test for environmental allergens sensitization (ImmunoCap and Phadiatop, Phadia/Thermo, Upsala, Sweden) and 40 sera from individuals with negative results for atopy, antibodies against Toxocara spp., asthma and with negative stool examination for A. lumbricoides were selected. Atopy was defined by the detection of IgE specific for B. tropicalis-Dermatophagoides pteronyssinus, pollen extracts, fungi extract, and dog and cat epithelia antigens (Phadiatop; Phadia/Thermo).
Serum samples from Toxocara spp. seropositive individuals were pre-adsorbed with extracts of A. lumbricoides (8 mg/ml), B. tropicalis (4 mg/ml), D. pteronyssinus (6 mg/ml), P. americana and/or B. germanica (5 mg/ml). Sera were pre-adsorbed with the extracts, separately or combined, in the presence of polyethylene glycol (3%) (PEG 15,000; Sigma Chemical Co. San Louis, MO, USA) and sodium azide 0.1%. After homogenization and incubation for 30 min at room temperature (RT), the suspensions were centrifuged at 8000 × g and 4°C. Supernatants were removed and kept at −70°C for further analysis.
2.4 Protein analysis with one-dimensional gel electrophoresis and immunoblotting
The TES and larval extract proteins were separated on 12% polyacrylamide gel in the presence of sodium dodecyl sulphate (SDS-PAGE). Samples were added in the SDS-PAGE in duplicate (two gels). The first gel was used for band visualization only; the second gel was used for transferring and immunoblotting, with the objective of checking which bands would be immunogenic and specific only for Toxocara spp. The gels were run for 2 h at 20 mA (120 V); for band visualization, the gels were stained with Coomassie Brilliant G250 (Neuhoff et al., 1988). The TES and larval extract samples were transferred to two nitrocellulose membranes and blocked for 2 h with PBS/T/5% milk to prevent unspecific bonds. Then, the blots were cut into strips and a pool of Toxocara spp. seropositive sera was added (diluted 1:100), pre-adsorbed or not with A. lumbricoides antigens and extracts of environmental allergenic organisms. Following, anti-human IgG antibody conjugated to alkaline phosphatase (Sigma, USA) diluted 1:10,000 was added and incubated for 1 h at RT. The bands were visualized with nitro blue tetrazolic chloride (NBT-BCIP solution, Sigma, USA). The reaction was stopped by washing the strips with distilled water. The immunoblot strips were divided into three groups: without pre-absorption, pre-adsorbed with A. lumbricoides antigens and pre-adsorbed with A. lumbricoides antigens combined with environmental allergenic organism extracts.
3 TRYPSINIZATION OF IN-GEL PROTEINS AND ANALYSIS WITH LC/MS/MS OF THE TES AND THE SOMATIC EXTRACT OF T. CANIS LARVAE
The gel bands corresponding to the proteins that reacted only with the pre-adsorbed pools of sera (considered as Toxocara spp. specific) were digested using the ProteoExtract trypsin digestion kit, according to the manufacturer's instructions (Calbiochem, San, Diego, CA, USA). Peptides generated by proteolysis were analysed by reverse-phase liquid chromatography mass spectrometry (LC-MS/MS), performed as previously described (da Silva et al., 2018).
3.1 Data analysis and identification of Toxocara canis proteins
The PEAKS Studio 7 software was used to identify the proteins analysed with mass spectrometry using the following parameters: maximum of two lost trypsin cleavages, cysteine carboxymethylation and M oxidation as fixed modifications, acetylation of any N-terminal amino acid, tolerance to precursor ions of 10 ppm and fragmented ion tolerance of 0.2 Da. The protein sequences were then submitted to the Blast server (http://www.ncbi.nlm.nih.gov/BLAST) for homology analysis and annotation of the ontology (GO) gene. The interpretation of results at protein level was performed using the blast2GO software (www.blast2go.de/b2ghome).
3.2 Gene synthesis, expression and purification of selected recombinant proteins
The coding sequences of MUC3 (UNIPROT code Q9U9J2) and TES 26 (UNIPROT code A0A0B2UWT5) were synthesized and cloned into the pD444-CH vector by the company DNA 2.0 (Newark, USA). CTL4 (UNIPROT code Q9XYV5) and TES 32 (UNIPROT code O44927) coding sequences were synthesized and cloned into the vector pET–28 a (+) (GenScript USA Inc., Piscataway, NJ, USA). All codons were harmonized for expression in Escherichia coli (DE3) based on its codon usage.
The purification of the proteins was performed by affinity chromatography using the nickel column HisTrap HP (GE Healthcare, Chicago, IL, USA). The elution of the recombinant proteins was carried out with the 20-mM Tris buffer plus 300–400 mM imidazole. The fractions containing the purified recombinant proteins were combined and dialysed with PBS. Then, the protein concentrations were determined with the Bradford method. The purification process was then assessed by Western blotting.
Although the patented recombinant TES 26 molecule (PCT/MY2009/000044) (Genbank A0A0B2UWT5) has the same molecule name, the rTES-26 used in this study is different from that. In fact, not only due to the different access code (A0A0B2UWT5), but because they have low sequence similarity (58%), indicating that they are different sequences. Therefore, this error should be corrected in the future with different names for each sequence. Figure S1 shows the alignment of the two sequences.
4 DETECTION OF ANTI-TOXOCARA SPP. TOTAL IgG AND IgG4 WITH INDIRECT ELISA
The TES antigen and the four recombinant proteins diluted in carbonate/bicarbonate buffer, pH 9.6 were coated on polystyrene high-binding 96-well ELISA plates, and incubated overnight at 4°C. The plates were blocked with 100 μl of PBS containing 0.05% of Tween and 10% (v/v) of bovine foetal serum (BFS, Gibco/Invitrogen; São Paulo-SP, Brazil) (PBS/T/BFS) and then incubated for 1 h at RT. Pre-adsorbed sera (100 ml) were incubated for 1 h at RT. 100 μl of secondary antibody was added (biotinylated anti-human IgG or anti-human IgG4; BD Pharmingen, San Jose, CA, USA), diluted in PBS/T/BSF 2.5% for 1 h at RT. After washing, 100 μl of streptavidin-peroxidase (BD Pharmingen) diluted 1:500 in PBS/T/BSF 2.5% were added to each well and the plates were incubated for 30 min at RT. Following, 100 μl of a solution containing the ortho phenylenediamine chromogen (OPD – Sigma Chemical Co. San Louis, MO, USA) and H2O2 was added to each well. The enzymatic reaction was stopped by adding 25 μl of 2N sulphuric acid and the optical densities were read using a spectrophotometer (Biotek EL-800, CA, USA) at 490 nm. The detection limit was determined by the cut-off point of the assay, calculated from the average of optical densities (OD) plus three times the standard deviation of the results of 10 serum samples from healthy individuals (negative for intestinal helminths and seronegative for Toxocara spp. infection).
4.1 Standardization of indirect ELISAs based on T. canis recombinant proteins
The immunoreactivity, sensibility and specificity of the assays using the selected recombinant proteins were evaluated through an indirect ELISA. For this, a total of 280 sera from children living in a toxocariasis endemic area were used. The total IgG and IgG4 responses to the recombinant proteins TES 26, MUC3, TES 32 and CTL4 were evaluated. A 182 positive control serum samples (presenting antibodies against Toxocara spp.) and 100 negative control serum samples (with negative results for the presence antibodies against Toxocara spp.) were assayed. The methodology used was the same as described above, adding TES, rTES 26, rCTL4, rTES32 and rMUC3 as coating antigens, isolated or in a 1:1 association. A checkerboard titration method was used to determine the best antigen concentration, serum sample dilution and conjugated antibody dilution. To evaluate cross-reactivity, serum samples from patients presenting A. lumbricoides (n = 45), hookworms (Necator americanus and Ancylostoma duodenale) (n = 10), Trichuris trichiura (n = 27), Schistosoma mansoni (n = 10) eggs in stool samples and sera from individuals sensitized by environmental allergens (N = 100) were used.
4.2 Statistical analysis
The Kolmogorav–Smivov test was used to analyse data normality. Positive/negative (P/N) values were used for data normalization of different batches of ELISA results. Receiver operating characteristics (ROC) were analysed using the software GraphPad Prism version 7. ROC curves were generated by plotting sensitivity versus specificity, and the area under the ROC curve (AUC) was used to conduct pairwise comparison of the diagnostic performance. Cut-off values were determined using ROC analysis and defined as the OD values that yielded the highest sum of sensitivity and specificity, and positive and negative predictive values were calculated as previously described (Barral et al., 2019). Absorbance readings and arithmetical means of the number of EPGs were analysed by the Pearson's correlation coefficient (PCC). All data are presented as mean ± standard deviation (SD) of at least three independent assays. Student's t-test and one-way ANOVA were used for comparison between groups and significance was set at p < .05.
5 RESULTS
5.1 Cross-reactivity analysis shows higher reactivity in non-absorbed sera
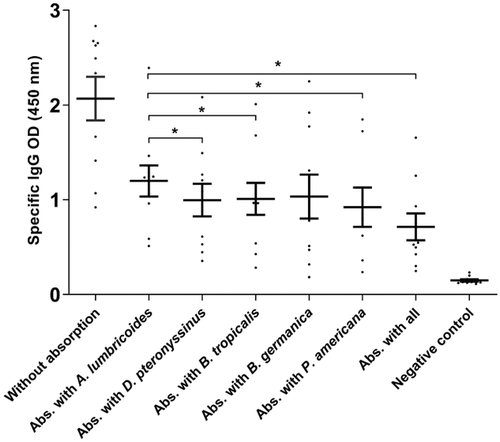
The indirect ELISA assay showed considerably decreased OD levels when results from sera pre-adsorbed with environmental allergens were compared to sera pre-adsorbed only with A. lumbricoides antigens. There were statistically significant differences between results from non-absorbed and absorbed serum samples, when sera were absorbed with extracts of D. pteronyssimus (p < .017), B. tropicalis (p < .019), P. americana (p < .037), B. germanica (p < .01) and especially with the pool containing all allergens (p < .04), which were compared to the Toxocara spp. positive serum samples. However, there was no statistically significant difference when non-absorbed Toxocara spp. positive serum samples were compared with samples pre-adsorbed with B. germanica extract (Figure 1).
5.2 Immunoblotting revealed protein bands that reacted exclusively with T. canis antigens
Figure S2a and c shows the band profiles of TES and larval antigen extracts in the immunoblotting assay. It was observed that 127 protein bands reacted with anti-Toxocara spp. antibodies. The molecular weights of these proteins varied between 23 and 100 kDa in TES (Figure S2b) and 30 and 190 kDa in the larval extract (Figure S2d). The bands of the 1D gel that correspond to the immunoreactive fractions are shown in Figure S2b and d. In total, there were 33 bands (12 in TES and 21 in larval extract) showing reactivity only with anti-Toxocara spp. These bands were selected for trypsin digestion and subsequent analysis using LC-MS/MS.
5.3 Composition of immunoreactive fractions, determined by means of LC-MS/MS
Tables S1 and S2 show the composition of immunoreactive fractions, as determined by LC-MS/MS. Nine specific immunoreactive bands were found on T. canis larval TES, and all identified proteins were classified according to their functionality, based on the Gene Ontology (GO) annotations of Blast2GO. The most relevant proteins were identified as having molecular weights of 23–28 kDa, represented herein by the names TES-32, MUC-3 CTL4 and TES-26. In addition, muc-2, muc-4 and muc-120 were also identified, as well as members of the 30 kDa band called CTL-4 and collectin-12.
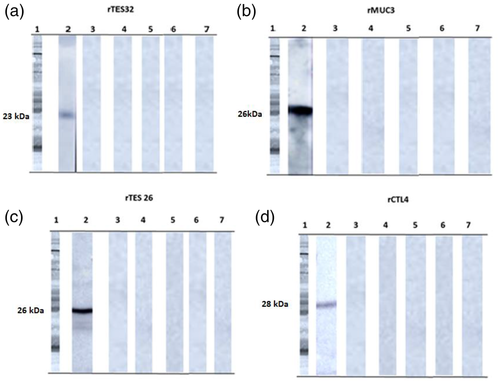
Western blot analysis of the recombinant protein expressions confirmed the expression of the bands of interest for immunodiagnosis (Figure S3e). Three recombinant proteins (rTES 26, rTES 32 and rCTL4) were successfully expressed as soluble proteins, with MW of 26, 23 and 28 kDa, respectively; rMUC3 needed to be re-solubilized and presented a MW of 26 kDa (Figure S3a–d).
5.4 ELISA standardization of total IGG and IGG4 to T. canis selected recombinant antigens for serodiagnosis compared to T. canis larvae excreted–secreted products (TES) and non-selected recombinant T. canis antigens
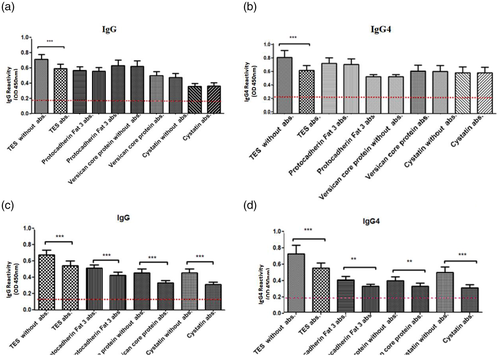
The serum reactivity of the total IgG and IgG4 antibodies to the recombinant antigens TES-26, MUC-3, TES-32 and CTL4 of T. canis was evaluated using as a gold standard the humoral response to TES. There was no statistically significant difference between absorbed and non-absorbed sera when using the selected recombinant proteins, either for total IgG or for IgG4 (Figure 2a and b). Meanwhile, both TES and other T. canis immunoreactive recombinant molecules presented a significant reduction in antibody titres when tested with sera absorbed with helminths and environmental allergen's extracts, showing that these antigens are not specific as those selected for the immunodiagnosis assay (Figure 2c and d).
5.5 Selection by indirect immunoassay of recombinant proteins with the best profile for immunodiagnosis
A panel with validation parameters was built, and the results of the validation of the study of these ELISAs are shown in Tables 1–3. In the IgG ELISA using the rMU3, rTES26, rCTL4 and TES32 antigens, sensitivities of 91.7%, 92%, 93.5% and 94.55, respectively, were observed, as well as specificities of 95.7%, 94%, 96.6% and 94.9%, respectively. The IgG4 ELISA using the same antigens showed sensitivities of 92.3%, 97.3%, 94.5% and 96.7%, respectively, with specificities of 97.9%, 97.8%, 97.8% and 97.9%, respectively.
| Recombinant proteins | ||||||||
|---|---|---|---|---|---|---|---|---|
| rTES 26 | rMUC3 | rCTL4 | rTES32 | |||||
| Parameters | Total IgG | Ig4 | Total IgG | Ig4 | Total IgG | Ig4 | Total IgG | Ig4 |
| Total of tested samples | 280 | |||||||
| Positive controls | 182 | |||||||
| Negative controls | 98 | |||||||
| Truly positive | 177 | 168 | 174 | 179 | 174 | 173 | 173 | 177 |
| Truly negative | 90 | 96 | 87 | 97 | 87 | 93 | 93 | 95 |
| False negatives | 16 | 14 | 8 | 5 | 12 | 10 | 10 | 6 |
| False positives | 4 | 2 | 15 | 1 | 3 | 2 | 5 | 2 |
| Cut-off | 0.287 | 0.241 | 0.257 | 0.243 | 0.227 | 0.252 | 0.228 | 0.233 |
| Sensitivity (%) | 91.7 | 92.3 | 92.0 | 97.3 | 93.5 | 94.5 | 94.5 | 96.7 |
| Specificity (%) | 95.7 | 97.9 | 94.0 | 97.8 | 96.6 | 97.8 | 94.9 | 97.9 |
| Accuracy (%)* | 95.3 | 94.2 | 93.2 | 97.6 | 93.2 | 95.0 | 95.0 | 97.1 |
| PPV (%) | 97.7 | 98.8 | 94.0 | 99.4 | 94.0 | 98.81 | 97.2 | 98.8 |
| NPV (%) | 84.9 | 87.2 | 9.6 | 95.0 | 87.8 | 90.2 | 90.2 | 94.0 |
| Kappa (K)** | 0.94 | 0.96 | 0.94 | 0.98 | 0.94 | 0.97 | 0.97 | 0.96 |
| Repeatability (%)*** | 99.2 | 98.2 | 98.8 | 98.5 | 99.7 | 98.9 | 99.6 | 95.7 |
| Reproducibility (%) | 97.9 | 99.5 | 97.9 | 98.1 | 97.9 | 95.3 | 99.3 | 99.3 |
- * Accuracy was calculated from the proportion of correct results, i.e., the total of truly positive plus truly negative results.
- ** The level of agreement between the results of each ELISA and the TES ELISA was determined using the Kappa index (K).
- *** Repeatability was calculated by testing the positive and negative pools 10 times on the same day under the same conditions. Reproducibility was obtained by testing the positive and negative pools at different times by five different laboratory technicians. The results are expressed as percentage (%) of correct results.
| Combinations of recombinant antigens | ||||||
|---|---|---|---|---|---|---|
| rTES 26 and rMUC3 | rTES 26 and rTES 32 | rTES 26 and rCTL4 | ||||
| Parameters | Total IgG | IgG4 | Total IgG | IgG4 | Total IgG | IgG4 |
| Total of tested samples | 280 | |||||
| Positive controls | 182 | |||||
| Negative controls | 98 | |||||
| Truly positive | 179 | 179 | 179 | 177 | 166 | 182 |
| Truly negative | 95 | 97 | 97 | 95 | 83 | 98 |
| False negatives | 7 | 4 | 3 | 5 | 16 | 0 |
| False positives | 3 | 1 | 1 | 1 | 4 | 0 |
| Cut-off | 0.294 | 0.233 | 0.234 | 0.271 | 0.257 | 0.266 |
| Sensitivity (%) | 96.2 | 97.8 | 98.3 | 97.2 | 91.2 | 100.0 |
| Specificity (%) | 96.9 | 98.9 | 98.9 | 98.9 | 95.4 | 100.0 |
| Accuracy (%)* | 97.8 | 97.6 | 97.6 | 97.1 | 94.3 | 100.0 |
| PPV (%) | 98.3 | 99.4 | 99.4 | 99.4 | 97.6 | 100.0 |
| NPV (%) | 93.1 | 96.0 | 97.0 | 95.0 | 83.8 | 100.0 |
| Kappa (K)** | 0.91 | 0.98 | 0.95 | 0.96 | 0.93 | 0.99 |
| Repeatability (%)*** | 99.3 | 99.3 | 97.3 | 97.9 | 98.5 | 98.1 |
| Reproducibility (%) | 97.5 | 96.1 | 98.2 | 97.3 | 98.6 | 99.4 |
- * Accuracy was calculated by the proportion of correct results, i.e., the total of truly positive plus truly negative results.
- ** The level of agreement between the results of each ELISA and the TES ELISA was determined using the Kappa index (K).
- *** Repeatability was calculated by testing the positive and negative pools ten times on the same day under the same conditions. Reproducibility was obtained by testing the positive and negative pools at different times by five different laboratory technicians. The results are expressed as percentage (%) of correct results.
| Combinations of recombinant antigens | ||||||
|---|---|---|---|---|---|---|
| rMUC3 and rCTL4 | rCTL4 and rTES 32 | rTES32 and rMUC3 | ||||
| Total IgG | IgG4 | Total IgG | IgG4 | Total IgG | IgG4 | |
| Total of tested samples | 280 | |||||
| Positive controls | 182 | |||||
| Negative controls | 98 | |||||
| Truly positive | 170 | 179 | 177 | 182 | 173 | 179 |
| Truly negative | 96 | 97 | 90 | 97 | 93 | 97 |
| False negatives | 12 | 3 | 16 | 4 | 10 | 8 |
| False positives | 2 | 1 | 4 | 1 | 4 | 1 |
| Cut-off | 0.259 | 0.211 | 0.287 | 0.241 | 0.252 | 0.243 |
| Sensitivity (%) | 93.4 | 98.3 | 91.7 | 97.8 | 94.4 | 95.7 |
| Specificity (%) | 97.9 | 99.0 | 95.7 | 98.9 | 95.8 | 98.9 |
| Accuracy (%)* | 95.0 | 98.5 | 95.3 | 99.2 | 95.0 | 97.6 |
| PPV (%) | 98.8 | 99.4 | 97.7 | 99.4 | 97.8 | 99.4 |
| NPV (%) | 88.9 | 97.1 | 84.9 | 96.0 | 90.2 | 92.4 |
| Kappa (K)** | 1.00 | 0.99 | 0.94 | 0.98 | 0.97 | 0.98 |
| Repeatability (%)*** | 98 | 98 | 98 | 98 | 88.7 | 98.5 |
| Reproducibility (%) | 99.3 | 95 | 97.9 | 96.3 | 96.7 | 98.1 |
- * Accuracy was calculated by the proportion of correct results, i.e., the total of truly positive plus truly negative results.
- ** The level of agreement between the results of each ELISA and the TES ELISA was determined using the Kappa index (K).
- *** Repeatability was calculated by testing the positive and negative pools ten times on the same day under the same conditions. Reproducibility was obtained by testing the positive and negative pools at different times by five different laboratory technicians. The results are expressed as percentage (%) of correct results.
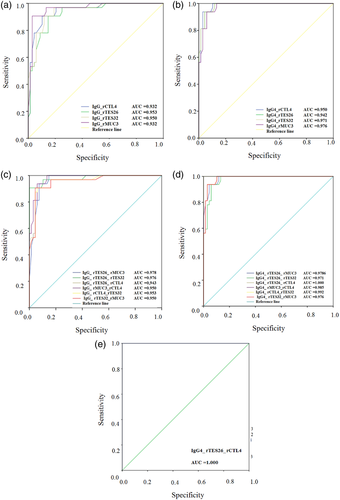
When using combinations of molecules in the immunoassays, responses to IgG and IgG4 antibodies were even better; the combination of rTES 26 with rCTL4 in IgG4 ELISA reached 100% sensitivity and specificity. The positive predictive values (PPV) and negative predictive values ranged from 84.9% to 100%. In some IgG4 immunoassays, the levels of agreement were 100% (k = 1.0, p < .01). The test also provided high reproducibility and repeatability and a 1.00 Kappa index. In order to assess the diagnostic accuracy of recombinant proteins, ROC curves of individual antigens were generated, and the corresponding AUC was determined (Figure 3a–e). The assay using rTES26 combined with rCTL4 showed the best combination of sensitivity and specificity, 100% for both, respectively, with 100% precision, as shown by the area under the curve (AUC) of the receiver's operating characteristic curve ROC (Figure 3e).
5.6 Recognition of recombinant antigens by sera pools of individuals seropositive to T. Canis TES, recombinant antigens and other helminth extracts
In order to evaluate the performance of the recombinant molecules, IgG and IgG4 ELISAs were conducted. We observed the presence of low cross-reactivity, both in the assays made with isolated molecules and in those where we used combinations of recombinant proteins (Figure S4a–m). The IgG4 ELISA was shown to be more effective and lacked cross-reactivity when using combinations between the rTES 26 and rCTL4 (Figure 4d), rMUC3 and rCTL4 (Figure S4j) and rCTL4 and rTES 32 (Figure S4l). It is worth mentioning that the low cross-reactions occurred in sera from individuals infected with A. lumbricoides (Figure S4c, f, g and i), T. trichiura (Figure S4b), Ancilostoma spp., S. mansoni (Figure S4m) and environmental allergens (Figure S4f).
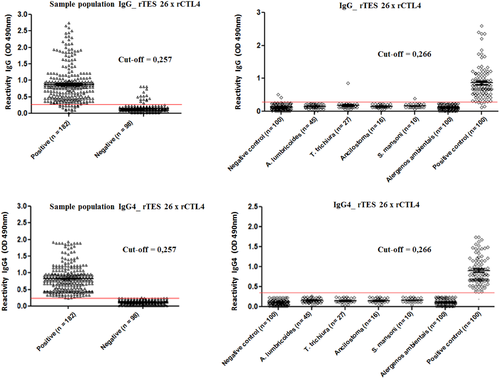
The immunorecognition of TES and the recombinant molecules in the sera from individuals infected by Toxocara spp. and other helminths and sensitized with allergens was also evaluated. The results showed that the serum pool of children infected with Toxocara spp. not only strongly recognized the TES antigens, but also r26, rMUC3, rTES 32 and rCTL4. The recombinant molecules were not recognized by antibodies from the sera of individuals infected with helminths or environmental allergens (Figure 5).
6 DISCUSSION
The indirect ELISA using TES as antigen, currently used as the gold standard method for detecting human toxocariasis, uses T. canis larvae excreted–secreted proteins as antigens (Silva et al., 2017). For the obtaining of this antigen, it is needed to deworm infected pups and cultivate the larvae of the parasite, which will produce the TES. Thus, the obtaining of this antigenic solution is time-demanding and requires specialized personnel. In addition, helminths and allergenic arthropod molecules, such as those from mites and cockroaches, may cross-react with antibodies against Toxocara spp. (Santiago Hda et al., 2015). These cross-reactions interfere with the effectiveness of the immunodiagnostic assay for both groups of diseases (helminthiasis and allergies) and are a major problem for the accurate diagnosis of this helminth infection and of allergies in toxocariasis endemic areas, as well as in areas with high prevalence of allergic diseases (Mueller et al., 2015). The current indirect ELISA commercial kit used for toxocariasis diagnostic presents sensitivity and specificity of 91% and 86%, respectively (NovaTec Immunodiagnostica GmbH, Dietzenbach, Germany). That led us to infer that the diagnostic method using TES, without the necessary precautions (pre-absorption of cross-reactive molecules), is useful only for disease screening, because positive results may be cross-reactions. Therefore, we seek to develop a highly specific, sensitive and reliable method for the detection of anti-Toxocara spp. antibodies (Alcântara-Neves et al., 2014).
The results showed a statistically significant difference between the reactivity of T. canis antigens when sera pre-adsorbed only with A. lumbricoides were compared to those that were absorbed with A. lumbricoides and environmental allergenic organism extracts (Figure 1). This finding indicates that the practice of pre-absorbing sera only with the A. lumbricoides antigenic extract (Mendonca et al., 2013) is not enough to eliminate cross-reactivities. These cross-reactions can impair the specificity of the diagnosis of toxocariasis, since it may lead to false-positive results when IgGs against several aeroallergens are present. This cross-reaction can also interfere in the diagnosis of atopy, since it is well known that helminths induce the production of a large amount of IgE (Alcântara-Neves et al., 2014).
The results of the proteomic analyses, when categorized according to the genetic ontology, revealed proteins with a potential interest for immunodiagnosis that are involved in different processes. These proteins present 60% similarity with molecules from the other species, lower percentage of identity and homology with other aminoacidic sequences. These are important characteristics to be considered for the enhancement of specificity of an immunodiagnostic assay (Zheng et al., 2020).
The recombinant molecules of T. canis selected for the toxocariasis immunodiagnosis herein were rMUC3, rTES 26, rTES 32 and rCTL4, all of them present in TES. It is already shown that these molecules can induce the activation of the host humoral immune response. It is worth mentioning that other Toxocara species infect humans, especially Toxocara cati. They share many molecules with T. canis and only the molecular diagnosis through PCR can distinguish among these infections (Özbakış & Doğanay, 2019). Because of the difficulty in differentiating the two species of Toxocara in the sera of infected individuals, we could not perform a cross-reactivity assay using sera with antibodies against T. canis and T. cati. Therefore, we prefer to state that our assay can detect antibodies against Toxocara spp., since they differentiated infection by Toxocara spp. of infections with other species of helminths.
A reliable immunodiagnosis of toxocariasis is highly associated with the use of specific molecules as antigens. In the present work, we selected molecules belonging to families that until now have only been described for their ability to elicit an immune response characterized by the production of specific IgG and IgG4 antibodies against the T. canis TES. Regarding IgG isotypes, IgG4 is significantly produced as a consequence of Th2 response activation, as seen on helminthic infection, and IgG4 responses to TES may reflect ongoing infection with migrating larvae (Ma et al., 2018). In addition, IgG4 blocks IgE binding on the surface of mast cells and basophils, modulating the effector immune response against the parasite (Calcagno et al., 2017). A previous study revealed significant increases in the specificity of ELISAs developed to identify Toxocara spp.-specific antibodies, since an IgG4-ELISA made with recombinant proteins (rTES-120 and rTES-30) achieved 100% sensitivity.
After immunoproteomic analysis and subsequent indirect immunoassays, it was observed that the four recombinant molecules showed good performance, mainly when applied in combination, such as CTL4 and TES 26. These proteins belong to the family of lectins and are responsible for mediating the inflammatory immune response, participating in cell adhesion and polarization (Barroso et al., 2014). TES-26, or phosphatidylethanolamine, is a protein anchored in the plasma membrane of the parasite, and involved in lipid transport and cell signalling; therefore, it is a promising candidate for immunodiagnosis because it is able to induce a significant humoral immune response (Figueroa-Santiago & Espino, 2014; Zhan et al., 2015).
The percentage of sensitivities and specificities obtained with these recombinant antigens was shown to be close to that of other T. canis recombinant antigens from (Figueroa-Santiago & Espino, 2014; Zhan et al., 2015). In addition, there use of only two molecules can optimize testing and reduce costs. Therefore, the high specificities obtained herein can be explained by the fact that the recombinant antigen solutions are simple and homogeneous, in contrast to antigenic extracts. In addition, these recombinant antigens are not glycosylated because they were expressed into a prokaryotic expression system, and the lack of post-translational modification reduces cross-reactivity with antibodies that recognize carbohydrates branches (De Andrade Lima Coêlho et al., 2005; Mohamad et al., 2009). The results obtained herein agree with those of Mohamad et al. (2009), who obtained more satisfactory results using IgG4-based immunoassays when evaluating classes and subclasses of anti-Toxocara immunoglobulins.
This study demonstrated that the four molecules generated satisfactory diagnosis results, especially when used in combination. It was observed that the IgG detection-based immunodiagnostic assay showed good specificity and sensitivity levels. However, the use of IgG4 exceeded our expectations, and its effectiveness in the performance of the method is clear, especially when using recombinant molecules combined, in this case rTES 26 and rCTL4, resulting in 100% sensitivity and specificity. Elevated levels of IgG4 have been associated with active infections by other helminths, such as Onchocerca volvulus and Wuchereria bancrofti (Lucius et al., 1992). IgG4 responses to TES may reflect continuous infection with migrating larvae. Longitudinal analyses of antibodies against Toxocara spp. after treatment are rare. In addition, IgG4 can block the binding of IgE to the surface of mast cells and basophils, modulating the effector responses (James & Till, 2016).
7 CONCLUSION
The present study identified immunoreactive and specific T. canis proteins that may constitute promising tools for the detection of anti-Toxocara spp.-specific antibodies. We were able to efficiently select, express and test four molecules that showed an identity percentage above 50%, and 50% homology between species, increasing the probability of being specific antigens. The assays developed herein allowed the serodiagnosis of human toxocariasis with high sensitivity and specificity. These results are encouraging for the replacement of antigenic extracts by recombinant proteins for the toxocariasis diagnosis. The immunoassay developed in this work, with the combination of two molecules, allowed the serodiagnosis of human toxocariasis with 100% sensitivity and specificity, respectively. These results are encouraging or the replacement of antigenic extracts by recombinant proteins in the diagnosis of toxocariasis.
ACKNOWLEDGEMENTS
We thank FAPESB (Fundação de Apoio à Pesquisa do Estado da Bahia), project n. APP0099/2016, CAPES (Coordination for the Improvement of Higher Education Personnel) project n. 077/2012, MCTI/CNPQ/FNDCT Program Contract n. 5737862008, and Transversal Action Regional Research Networks In Ecosystems, Biodiversity and Biotechnology n. 79/2013 (RENORBIO) for supporting this project, and the Department of Molecular Biology at the University of Salzburg, Salzburg, Austria, for supporting the proteomic work. CNPq (Conselho Nacional de Pesquisa e Desenvolvimento) provided a scholarship to LACP and NMAN. RDP is a Technological Development Fellow from CNPq (Proc. 313350/2019-1).
CONFLICT OF INTEREST
The authors declare no conflicts of interest in the completion and reporting of results for this study.
ETHICS STATEMENT
This study was approved by the Committee of Ethics in Research of the Maternidade Climério de Oliveira, Federal University of Bahia (UFBA), Salvador, Brazil, under the registration number CEP.004/2010. An informed consent form for participation in all stages of the research was signed by all the parents or guardians of the children.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




