Efficacy of imidacloprid/flumethrin collar in preventing canine leishmaniosis in Brazil
Abstract
The Leishmania infantum (synonym, Leishmania chagasi) causes life-threatening infection, namely canine leishmaniosis (CanL), which is a chronic zoonosis prevalent in various countries and spread by the bite of the infected Lutzomyia female sandfly in South America. The objective of the study was to assess the effectiveness of a polymer matrix collar containing made up of 10% imidacloprid and 4.5% flumethrin for the prevention of canine leishmaniosis from the hyperendemic region falling under Araçatuba municipality (Brazil). The research included a total of 146 dogs chosen from 75 households. Test were initiated via physical examination; weighing and biological sample collection (blood, popliteal lymph node and conjunctival swab) of these dogs were done in March 2018 (Day 0; GA, control = 69, GB, treated = 77) to initiate laboratory tests. Post-inclusion, the animals were monitored on the 120th, 240th, 360th and 480th days, respectively. The usage of collars continued between 0 and 480 days before being substituted in second (D240) and fourth (D480) follow-up visits. On the whole, 25 dogs in GA (36.2%) and three in GB (3.9%) were found positive for L. infantum infection in a minimum of one diagnostic test used in the research. Therefore, the average collar effectiveness for protection from L. infantum infection was 89.2% (p < .01). In the last follow-up, the average incidence density rate for GA was 30.7%, whereas for GB, it was 2.9%. The imidacloprid/flumethrin collars evaluated in the research were found to be safe and extremely efficient for the prevention of L. infantum infection through Lutzomyia species among the large population of dogs in highly prone endemic regions. This is a dependable and efficient technique aimed at reducing the occurrence and propagation of this illness among the population of canines, which would eventually reduce the human-health-related hazards. In Brazil, Lutzomyia spp. is a leading vector of the infection; thus, the collar can be used to limit infection in dogs and humans.
1 INTRODUCTION
The infectious disease, canine leishmaniosis (CanL), caused by Leishmania infantum, or L. chagasi, that may be transmitted by an infected female Lutzomyia sandfly (Akhoundi et al., 2016; Montalvo et al., 2020; Schwabl et al., 2021), leads to lethal zoonotic disease endemic illness in numerous nations of Central and South America, Mediterranean regions and the Middle East and Asian countries (Maia et al., 2020). Dogs are the main hosts and considered its main domestic reservoir (Dantas-Torres et al., 2019). In addition, a high incidence of canine infection poses a high risk to human well-being (Baneth et al., 2008; Martin-Sanchez et al., 2020).
The progressive spread of visceral leishmaniasis (VL) and overall poor living standards resulted in 40,326 verified human cases and 2794 fatalities in Brazil (Brazil, 2021) from 2009 to 2019, with gradual spreading across all states and a general association with poor living conditions (Marcondes & Day, 2019; Seva et al., 2016). VL infections are significantly less common elsewhere on the continent compared with Brazil (Bern et al., 2008).
Prior experiments (Brianti et al., 2014; Brianti et al., 2016; Otranto et al., 2013; Yimam & Mohebali, 2020) depicting the controlled release of the collar have proven greater safety and efficiency levels in the prevention of L. infantum infection among a large pool of dogs. A single collar is capable of providing protection during a complete season of sand fly (up to 8 months), even in hyper-endemic Southern Italian regions. Constant usage of collars, especially during the season of the sand fly, can be a viable and sustainable technique for deterring leishmaniosis infection in dogs residing in or visiting the endemic zones.
The dearth of awareness in Brazil regarding the efficiency of a polymer matrix collar containing 10% imidacloprid and 4.5% flumethrin (Seresto®, Bayer Animal Health) prompted us to conduct a survey for assessing its efficacy in preventing CanL among privately owned dogs of varying ages found in endemics regions of São Paulo state.
2 MATERIALS AND METHODS
2.1 Ethical statement
A prospective study based on the standards of Good Clinical Practise (VICH GL9 GCP, 2000. https://www.ema.europa.eu/en/vich-gl9-good-clinical-practices) and guidelines of the Statistical principles for veterinary clinical studies (VICH CVMP/EWP/81976/2010, 2012. https://www.ema.europa.eu/documents/scientific-guideline/guideline-statistical-principles-clinical-trials-veterinary-medicinal-products-pharmaceuticals_en.pdf) have been followed. The entire range of experimental processes involving animals adhered to the ethical principles specified for research on animals by the College of Animal Experimentation and were sanctioned by the Ethics Committee on Animal Use (CEUA) of the Faculdade de Odontologia de Araçatuba and of the Faculdade de Medicina Veterinária de Araçatuba (FMVA) Unesp, Campus de Araçatuba, process FOA n° 00870–2016 and by the Research Ethics Committee (CEP) of the Faculdade de Odontologia de Araçatuba, Unesp, Campus de Araçatuba, process FOA n° 61241216.4.0000.5420.
2.2 Study design and experimental procedures
Randomized controlled clinical trials (RCT) were held in the form of a survey in the CanL prone regions of Araçatuba municipality (21°12′32″S, 50°25′58″W, altitude 390 m above sea level) of Sao Paulo state in Brazil.
The area under examination is characterized by a high frequency of CanL, human cases and vector L. longipalpis (Courtenay, Dilger, et al., 2019). Additionally, the targeted area (Figure 1) has been identified by the Brazilian National Information System for Notifiable Diseases as an intensively transmission-prone zone (average of human cases of 2015–2019: 10.4 cases per year) (Brazil, 2021).
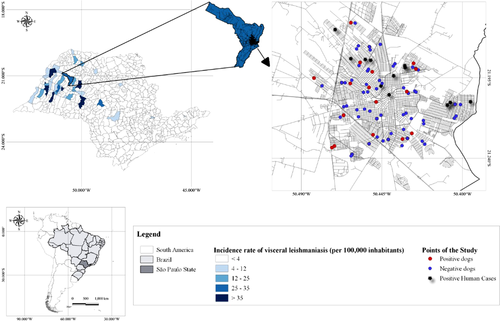
A software named WinEpi (http://www.winepi.net/f108.php) was employed for determining the minimal sample size for every group (69 animals) that helped in identifying the differences between the two segments in any direction of the two populations. The following hypothesis were taken in account: expected incidence rate in group A = 4% and expected incidence rate in group B = 15% (power = 95% and confidence level = 95%). Enrolment of 188 dogs instead of 138 individuals (69 per groups) was done to compensate for likely losses in the duration of the study.
Information regarding the sex, age, weight and the existence of clinical symptoms was recorded in epidemiological data. Well-trained veterinarians formed the investigation team for the collection of biological samples from the canines.
The research included 188 canines that were examined in 96 houses via door-to-door visits organized in February 2018. The study included only those canines that were tested negative in the screening, after which 146 animals from 75 households were chosen for the research. After a physical examination and weighing of these animals in March 2018 (Day 0), biological samples including blood, popliteal lymph node aspiration and conjunctival swab were taken from them for laboratory testing. Figure 2 displays a schematic diagram for briefing the empirical design employed in this investigation. The study was carried out on dogs that meet inclusion criteria (standard general health, the longevity of ≥24 weeks, unexposed to endectocides products or immunosuppressive drugs in the past 6 months and tested negative in L. infantum serology test (DPP and ELISA) along with conjunctive and lymph node PCR, and lymphatic node cytology. A table of randomly arranged digits and a random treatment allocation model were engaged for the random selection of the canines in this study. In order to minimize direct touch between animals of both groups in a single house and to avoid possible transmission of active material from treated canines to the untreated ones, randomization was carried out via households. Therefore, dogs of the collar and control groups were chosen from separate households to prevent contamination of the control animals by the collars' repellent.
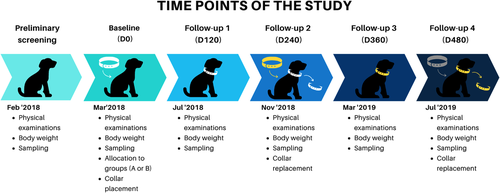
The day of inclusion (Baseline—D0) was marked by the fitting of imidacloprid 10% + flumethrin 4.5% collars to dogs in GB depending on the weight of their body (small collar: <8 kg/large collar: >8 kg), whereas dogs in GA were not given any treatment to act as negative subjects.
The follow-up checking of the animals was done on 120, 240, 360 and 480th days of inclusion, respectively. Physical examination of dogs was ensured at every follow-up session, which included the collection of blood, conjunctival swab and popliteal lymph node aspiration samples, as illustrated in Figure 2.
The collars were required to be put on from day 0 to 480 until being substituted at follow-up 2 (D240) and 4 (D480) or in case of being lost or increased weight of the animal exceeding the threshold value of 8 kg.
The collars were discarded and substituted from the dogs in the GB group on days 240 and 480, respectively. All dogs enrolled in the study were monitored by their owners, and researchers (veterinarians) were informed of all the changes observed in them. During the entire duration of the trial, additional ectoparasiticides were neither used in dogs nor in the environment. Due to the nature of the veterinary product (collar) used in the research, the employees performing lab analysis were advised to apply blinding.
2.3 Laboratory procedures
A peripheral blood sample (5 ml) was taken via brachiocephalic vein puncture in the vacuum tubes for centrifugation at 1000×g for 10 min. The serum was segregated to be stored at −20°C for future tests.
Serologic examination of CanL has been conducted in compliance with the latest Visceral Leishmaniasis Surveillance and Control Program (VLSCP) convention, including immunochromatographic evaluation (dual-path platform, TR-DPP®-LVC; Bio-Manguinhos, Rio de Janeiro) before performing the enzyme-based immuno-sorbent test (ELISA).
DPP issued by the Brazilian Ministry of Health was conducted in alignment with the producers’ guidelines. This is a subjective assessment for testing the anti-Leishmania antibody in L. donovani complex through the antigen-like recombinant protein K28 (components K26, K39 and K9) (Rocha et al., 2020).
ELISA kits were employed for sample analysis (Canine Leishmaniasis EIE kit; Bio-Manguinhos, Fiocruz, Rio de Janeiro, Brazil), in line with the manufacturer's guidelines. A soluble antigen is used in this test in the form of promastigote of parasites such as Leishmania major-like (ELISA-L. major-like).
Fine Needle Aspiration Biopsy (FNAB) was employed to perform cytology via popliteal lymph node smears that were stained with Diff-Quick and tested for Leishmania amastigotes through direct microscopic analysis (100×). A favourable outcome involves the finding of typical amastigotes with kinetoplasts, and nucleus coupled with lymphoblastic amplification, macrophages, plasmocytes, eosinophils and lymphoglandular hyperplasia (Santos et al., 2014).
For diagnosing infection caused by L. infantum, collection of conjunctival swabs was done with the help of sterile cotton swabs, which are specially produced for bacterial separation. A sample from each eye was taken for gathering the exfoliation cells by swiping the swab across the surface of the lower eyelids of both eyes. Sterile tubes were used to store the conjunctival swabs with no additional solution, and swabs were frozen (−20°C) till the tests were performed.
PCR for enhancement of Leishmania DNA was done on all conjunctival swabs and lymph node popliteal samples. In accordance with the producer's recommendations, DNA extraction was done with the help of a PureLink® Genomic DNA Kit (Invitrogen, USA).
Nested PCR was employed to diagnose the existence of Leishmania DNA via a variable portion of the tiny sub-unit rRNA gene (nested SSU rRNA-PCR). Twin pairs of primers, namely outer (P221: 5′-GGTTCCTTTCCTGATTTACG-3′ and P332: 5′-GGCCGGTAAAGGCCGAATAG-3′) and inner (P223: 5′-TCCCATCGCAACCTCGGTT-3′ and P333: 5′-AAGCGGGCGCGGTGCTG-3′) primers were used in this study (Kothalawala & Karunaweera, 2016). Amplification of kDNA of Leishmania was enabled by newly designed P221 and P332 primers (1st PCR). Despite the potential amplification of additional kinetoplastids like Leptomonas and Crithidia by P221 and P332 primers (2nd PCR), designing internal primers (P223 and P333) was specific to the Leishmania genus (Cruz et al., 2002). The reaction mixture of PCR was made by combining outer primers of the nested PCR (forward primer (10 μM), P221: 5′-GGTTCCTTTCCTGATTTACG-3′ and reverse primer (10 μM) P332: 5′-GCCGGTAAAGGCCGAATAG-3′) in the proportion of 10:1 (inner primers: outer primers). PCR traits (final concentration) included 10 μl DNA, 2 mM MgCl2, 0.2 mM dNTPs, 15 pmol primers, 1.4U Taq, 1× buffer (Promega) and 5 μl 1st PCR (PCR product was previously diluted 1:200 in ultra-pure water), 2 mM MgCl2, 0.2 mM dNTPs, 1.5 pmol primers, 0.7U Taq, 1× buffer (Promega), for both 1st and 2nd PCR, respectively.
The PCR process continued for 15 cycles in the first step (about 45 min) and 35 cycles in the second step (about 1.5 h) (Deepachandi et al., 2019). The PCR conditions in the first step exhibited 95°C for 3 min, succeeded by 15 cycles of 94°C for 30 s, 53°C for 30 s and 72°C for 30 s, before the final step depicting 95°C for 1 min. After being incubated at 95°C for 1 min, the tubes were inverted many times for dissolving the intrinsic primers and were then spun and sent back to the thermocycler for the next round of amplification. In the second step, the PCR programme continued at 95°C for 3 min, followed by 35 cycles of 94, 65 and 72°C for 30 s each before the final step of 5 min duration at 95°C. Double distilled water fluids were used for detection in all PCR cycles. 2.0% agarose gel made of bromide ethidium was used to test 10 μl of PCR primers against the DNA ladder of 100 bp. The visibility of DNA bands was enabled by an ultraviolet light transilluminator.
2.4 Entomological survey
A couple of trapping cages were kept 50 cm raised from the ground level in every house from March 2018 to July 2019 that were exposed for a duration of 12 h from 7 pm till 7 am and placed in-situ for a period of 72 h. Additionally, vector surveys were carried out with the aid of seven standard CDC light traps whose bulbs were removed before placing them at 2 positions: one within the building, ideally in the master bedroom, and another in the dog's sleeping area or at the kennel's entrance. Therefore, each trap was connected to both hosts, including dogs and people. Captured sand flies were sexed and counted under a stereomicroscope. According to Gaglio et al. (2014) sandfly specimens were set ready to be examined under a microscope for their identification via morphological criteria at the level of species. As mentioned earlier, the females of Lu. longipalpis were submitted to the PCR, as described above, procedure after sexualization and distinctive identification.
2.5 Statistical analysis
The homogeneity of the groups was assessed retrospectively on the basis of baseline characteristics (age, weight and gender) of day 0 with the help of a Chi-square test or Fisher's exact test for qualitative data (weight and gender) and analysis of variance for quantitative data (age).
Any dog can be deemed afflicted with L. infantum only upon exhibiting positive results in either of the diagnostic analyses (DPP, ELISA, PCR on lymph node and on conjunctival swabs, or cytology). The assessment of effectiveness was done after comparing the number of dogs infected by L. infantum in the entire study group.
Leishmania incidence was computed as year-crude incidence (YCI) (considering CI for the first year and for the second year) through the following method: YCI = number of dogs newly infected with L. infantum/(number of negative dogs initially enrolled − number of lost or dead dogs) × 100.
The incidence rate of infection has also been computed by means of incidence density rate (IDR) (Moreira et al., 2004), which is monitored under a regular 30 days/month cycle, in order to mitigate the issue of dogs missed during follow-up. The number of newly infected dogs in either of the diagnostic tests divided by the number of follow-up months yielded the IDR of each follow-up session (the number of months between the prior and latest evaluation for each dog at risk of being infected by L. infantum). The incidence calculation did not consider the dogs that were examined only a single time due to their death or loss. Final IDRs were later presented on a yearly basis.
where A reflects the percentile of positively diagnosed dogs in the control group and B implies the percentile of positive canines in the treatment-administered groups. The programmes like STATA/SE, Version 16.1, Software (Stata Corp LLC, College Station, Texas, USA) and Microsoft® Excel® - Microsoft 365 assisted in performing randomization and statistical evaluations whose significance level was fixed at the p value of <.05.
3 RESULTS
At the preliminary screening 146 (77.7%) among the 188 canines were tested negative for anti-L. infantum antibody circulation at initial screening (Table 1). One hundred forty-six (GA = 69, GB = 77) dogs were enrolled on Day 0 for being studied and involved in the efficacy calculation. These canines were dispersed homogeneously (p > .05) on the basis of sex, weight and age. No loss of dogs was witnessed in this research due to daily interaction with dog owners. Finally, outcomes of the variable diagnostic tests revealed that 25 dogs (36.2%) in GA and three (3.9%) in GB showed positive test results for L. infantum, in at least one of the diagnostic tests employed.
| Baseline (D0)–March 2018 | |||||
|---|---|---|---|---|---|
| Variable | Preliminary screening (February 2018) | p Value | Untreated (Group A) | Collared (Group B) | p Value |
| Number of screened animals | 188 | NC | 69 (47.3%) | 77 (52.7%) | .56 |
| Mean age (years)/range | 4.26/0–17 | NC | 4.27 | 4.92 | |
| Sex | |||||
| Female | 95 (50.5%) | .94 | 37 (25.0%) | 39 (27.0%) | .85 |
| Male | 93 (49.8%) | 32 (22.0%) | 38 (26.0%) | ||
| Weight | |||||
| Small (0.1–2 kg) | 65 (34.6%) | .83 | 34 | 31 | .39 |
| Medium (2.1–7.9 kg) | 55 (29.3%) | 23 | 26 | ||
| Large (>8 kg) | 68 (36.2%) | 20 | 12 | ||
| ELISA positive | 32 (17.0%) | 0 | 0 | ||
| DPP positive | 21 (11.2%) | NC | 0 | 0 | NC |
| ELISA and/or DPP positive | 42 (22.3%) | 0 | 0 | ||
During the investigation, the number of seropositive dogs in GA rose from 7 (1st follow-up) to 20 (4th follow-up), and positive cases (n = 24) were also reported in PCR and/or cytology (Table 3). Until the completion of the research, all dogs were positive. The number of seropositive dogs in GA grew from 7 (first follow-up) to 20 (fourth follow-up) during the exploration, with 24 dogs being positive in PCR on samples of conjunctival swabs and popliteal lymph node and/or in cytology on lymph node aspirate (Table 2). One positive dog in GB (DPP and cytology) was positive even after the second follow-up and remained so in all methods until the final follow-up, whereas the remaining two dogs turned positive in the last follow-up and they were both PCR positive.
| Group | ID | Follow-up 1 (D120)–July 2018a | Follow-up 2 (D240)–November 2018a | Follow-up 3 (D360)–March 2019a | Follow-up 4 (D480)–July 2019a | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DPPb | ELISAb | PCRb | Cytology | DPPb | ELISAb | PCRb | Cytology | DPPb | ELISAb | PCRb | Cytology | DPPb | ELISAb | PCRb | Cytology | ||
| GA | A3/S3/37 | pos | neg | pos | pos | neg | neg | pos | neg | pos | neg | neg | pos | pos | neg | pos | pos |
| GA | A3/S5/43 | pos | neg | pos | neg | neg | neg | pos | neg | neg | neg | pos | pos | neg | neg | pos | pos |
| GA | A5/S5/74 | pos | neg | neg | pos | pos | neg | pos | pos | neg | neg | pos | pos | pos | neg | pos | neg |
| GA | A6/S5/97 | pos | pos | neg | neg | pos | neg | neg | neg | pos | pos | neg | neg | pos | pos | pos | pos |
| GA | A7/S4/115 | pos | neg | neg | pos | neg | neg | neg | pos | pos | neg | pos | pos | neg | pos | pos | neg |
| GA | A1/S6/123 | pos | pos | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | pos |
| GA | A4/S4/131 | pos | neg | pos | pos | pos | neg | neg | pos | neg | neg | pos | pos | pos | neg | pos | pos |
| GA | A1/S2/4 | neg | neg | neg | neg | pos | neg | neg | pos | neg | pos | neg | pos | pos | pos | neg | pos |
| GA | A3/S2/33 | neg | neg | neg | neg | pos | pos | neg | neg | pos | pos | neg | neg | pos | neg | pos | pos |
| GA | A3/S3/37 | neg | neg | neg | neg | neg | neg | pos | pos | neg | neg | pos | pos | pos | neg | pos | pos |
| GA | A5/S2/67 | neg | neg | neg | neg | neg | neg | neg | neg | pos | neg | neg | pos | pos | neg | pos | pos |
| GA | A6/S1/82 | neg | neg | neg | neg | neg | neg | neg | neg | pos | pos | neg | pos | neg | pos | neg | pos |
| GA | A6/S2/92 | neg | neg | neg | neg | neg | neg | neg | neg | neg | pos | neg | neg | pos | pos | neg | pos |
| GA | A6/S5/99 | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | pos | pos | pos | pos | pos | pos |
| GA | A2/S3/15 | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | pos | pos | neg | pos |
| GA | A2/S5/27 | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | pos | neg | pos | pos |
| GA | A3/S4/35 | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | pos | neg |
| GA | A3/S6/44 | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | pos | neg | pos | pos |
| GA | A4/S3/50 | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | pos | pos |
| GA | A4/S7/59 | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | pos | neg | pos | pos |
| GA | A6/S2/87 | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | pos | pos |
| GA | A6/S3/92 | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | pos | pos | neg |
| GA | A7/S3/107 | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | pos | pos | neg | neg |
| GA | A7/S5/116 | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | pos | pos | neg | pos |
| GA | A1/S4/120 | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | pos | neg | pos |
| Total by test | 7 | 5 | 5 | 7 | 8 | 10 | 20 | 24 | |||||||||
| Total (pos any test) | 7 | 9 | 13 | 25 | |||||||||||||
| GB | A7/S6/114 | neg | neg | neg | neg | pos | neg | neg | pos | pos | neg | neg | neg | pos | pos | pos | pos |
| GB | A6/S3/88 | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | pos | neg | pos | pos |
| GB | A6/S5/90 | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | pos | pos | neg |
| Total by test | 0 | 0 | 1 | 1 | 1 | 0 | 3 | 3 | |||||||||
| Total (pos any test) | 0 | 1 | 1 | 3 | |||||||||||||
- apos, positive; neg, negative.
- bDPP, dual-path platform; ELISA, enzyme-linked immunosorbent assay; PCR, polymerase chain reaction.
Twenty-two out of 28 (79%) positive dogs had a clinical symptom indicating CanL during the final follow-up; for instance, lymphadenomegaly (GA: n = 19; GB: n = 1)), cutaneous symptoms like alopecia, dermatitis, ulcers, lesions on the ears, face and limbs (GA: n = 16; GB: n = 1), onychogryphosis (GA: n = 16) conjunctivitis (GA: n = 10) and cachexia (GA: n = 5).
The YCI computed through dogs that continued in the study till the final scheduled follow-up (D480) was recorded as 3.9% (three out of 77) for GA and 36.2% (25 out of 69) for GB, respectively (p < .001). Table 3 reflects every group's IDRs (month and year) during all the follow-up sessions.
| Group/follow-up | Cohort dogs (neg at previous sampling) | New cases | Dog-months | IDRa/ month | New cases | Dog-years | IDRa/year | Efficacy (%) |
|---|---|---|---|---|---|---|---|---|
| GA (Control) | ||||||||
| Baseline (D0) | 69 | – | ||||||
| Follow-up 1 (D120) | 62 | 7 | 276 | 2.5 | 7 | 23 | 30.4 | |
| Follow-up 2 (D240) | 57 | 3 | 248 | 1.2 | 3 | 21 | 14.5 | |
| Sub-total | 57 | 10 | 524 | 1.9 | 10 | 44 | 22.9 | |
| Follow-up 3 (D360) | 53 | 4 | 228 | 1.8 | 4 | 19 | 21.1 | |
| Follow-up 4 (D480) | 36 | 11 | 212 | 5.2 | 9 | 18 | 62.3 | |
| Sub-total | 36 | 15 | 440 | 3.4 | 13 | 37 | 40.9 | |
| Total | 36 | 25 | 964 | 2.6 | 23 | 80 | 31.1 | |
| GB (Collar) | ||||||||
| Baseline (D0) | 77 | |||||||
| Follow-up 1 (D120) | 77 | 0 | 308 | 0.0 | 1 | 26 | 0.0 | 100.0 |
| Follow-up 2 (D240) | 76 | 1 | 308 | 0.3 | 1 | 26 | 3.9 | 91.0 |
| Sub-total | 76 | 1 | 616 | 0.2 | 2 | 51 | 1.9 | 91.0 |
| Follow-up 3 (D360) | 76 | 0 | 304 | 0.0 | 0 | 25 | 0.0 | 93.6 |
| Follow-up 4 (D480) | 74 | 2 | 304 | 0.7 | 4 | 25 | 7.9 | 89.2 |
| Sub-total | 74 | 2 | 608 | 0.3 | 4 | 51 | 3.9 | 90.0 |
| Total | 74 | 3 | 1224 | 0.2 | 6 | 102 | 2.9 | 89.2 |
- aIDR, incidence density rate.
The mean IDR of GA and GB was registered at 31.1 and 2.9%, respectively, at the time of the concluding follow-up session. However, the IDR in the GA of the initial two follow-ups was 22.9, whereas the third and fourth follow-ups reflected the IDR of 40.9. The IDR value of GB was 1.9 and 3.9 for the first/second and third/fourth follow-ups, respectively.
The average collar effectiveness in protecting dogs against L. infantum infection was therefore 89.2%. (p < .01). The collar's efficiency in the first evaluation (D0–D240) and the second evaluation (D240-D480) was 91.0 and 90.0%, respectively. There were neither complications nor any side effects of using the collar in the dogs that were given the treatment. Thirteen GA dogs that exhibited tick infections were given individual treatment by manual removal.
Specimens of sand flies (n = 1557) were collected from March 2018 to July 2019. Lu. longipalpis (198 females and 1291 males; 95.63%) was the maximally evident species in both the years, followed by Nyssomyia intermedia (34 females and 20 males; 3.47%), Nyssomyia whitmani (five females and three males; 0.51%), Nyssomyia neivai (0 female and two males; 0.13%), Pintomyia pessoa (one female and one male; 0.13%), Evandromyia edwardsi (one female and 0 male; 0.06%) and Migonemyia migonei (0 female and one male; 0.06%).
1498 (amplitude: 8–338) samples of Lu. longipalpis were taken in the entomological assessment wherein 1291 (amplitude: 5–293) samples were from males and 198 (amplitude: 3–45) from females. The mean temperature and humidity of the evaluation period were 25.12°C and 53.52%, respectively.
Maximum samples of Lu. longipalpis were accumulated during December 2018 (n = 338) and January/February 2019 (n = 222) with peak mean temperature (26.43°C) and a moderate monthly average of relative humidity (55.55%).
Identification of five (2.53%) Lu. longipalpis females (n = 198) that were diagnosed positive for L. infantum were identified with the help of PCR amplification of parasite DNA technique.
4 DISCUSSION
High safety and efficacy standards (average efficacy = 89.2%) of 10% imidacloprid/4.5% flumethrin collar have been successfully proved in the prevention of infection caused by L. infantum among the dogs residing in CanL hyper-endemic regions, as it is extremely crucial arthropod-transmitted zoonotic illnesses of dogs found globally. This research has been performed with a 10% imidacloprid/4.5% flumethrin collar in Brazil for the first time ever.
The current research attempts to verify and expand this understanding via examining the collar in a larger diverse population of dogs residing in a CanL hyper-endemic region. An annual incidence (36.2%) and the median IDR (70.2%) reported in the reference population of dogs were verified to corroborate the hyperendemicity of the research location at the time of the last follow-up.
Identical findings of RCT were presented by Brianti et al. (2014); Brianti et al. (2016); Otranto et al. (2013), depicting the insecticide-impregnated (imidacloprid and flumethrin) efficiency of the collars above 85% (93.4, 88.3 and 100%, respectively) retrieved from dog shelters of Italy.
RCTs are prospective studies that set the benchmark for adopting efficient and safe treatment approaches. RCTs make it evident that a novel treatment is better than the corresponding contemporary standard therapy or an antidote. RCTs are used in clinical trials to solve patient issues and serve as the foundation for the approval granting decisions of regulatory agencies during the creation of new medications. In addition to meta-analysis, the greatest standard of proof is served by high-quality RCTs posing a less potential likelihood of systematic bias (Hariton & Locascio, 2018; Kabisch et al., 2011).
The profoundly rampant disease observed in the canines of this region could also be determined by Leishmaniasis-induced symptoms (lymphadenomegaly; cutaneous symptoms like alopecia, dermatitis, ulcers, lesions apparent on ears, face and limbs; onychogryphosis; conjunctivitis and cachexia) visible in 22 out of 28 dogs (78.6%) in groups A and B after being exposed to sandflies.
Three collared dogs seroconverted (DPP or ELISA), became PCR positive or displayed clinical signs indicative of CanL, which can be attributed to the anti-feeding activity of flumethrin. Indeed, this compound is known to reduce the number of insect bites and, in turn, the infection challenge, thereby preventing a shift towards a non-protective immune response and the development of disease (Vlkova et al., 2011).
The conduction of investigations exclusively with the use of collars extracted from deltamethrin-impregnated dogs exhibited differing rates of efficiency in Brazil. The total rate of protection from CanL exceeded the estimated values computed in the research works engaging deltamethrin-bred collars (highest 70.3%) in Brazil, including 49.0% in Bahia state (Leite et al., 2018), 50% in the state of Minas Gerais (Reithinger et al., 2004), 52% in Minas Gerais state et al., 2020), 53–59% in Rio Grande do Norte state (Kazimoto et al., 2018), 57.7% in São Paulo state (Lopes et al., 2018), 63% in Minas Gerais state (Coura-Vital et al., 2018), 65% in São Paulo state et al., 2010) and 70.3% Minas Gerais state (Silva et al., 2019).
The maximal efficacy for deltamethrin-bred collars was 84% in other nations, including 50% in Italy (Maroli et al., 2001), 53.2% in Iran (Gavgani et al., 2002), 45.1% in Italy (Foglia Manzillo et al., 2006), 84% in Italy (Ferroglio et al., 2008), 63% in Italy (Cassini et al., 2013), 61.8% in Italy (Brianti et al., 2016) and 50% in Iran (Courtenay et al., 2019).
A mathematical model contrasting several CanL limiting approaches demonstrated the greater efficacy and successful outcomes of using dog collars rather than subjecting them to vaccination or euthanasia, considering that a high collar rate remains in place (Seva et al., 2016). Moreover, the proof of collars being an effective and ethical technique for limiting CanL is increasingly evident (Dantas-Torres et al., 2019).
The entomological survey indicated the extensive prevalence of Lu. longiplapis, which is considered to be the most significantly competent vector of L. infantum, across all the tracked locations of Brazil. The continual occurrence of sandflies, therefore, marks a serious risk of L. infantum generated infection among dogs and human beings in this region. The results of this study accord with Silva et al. (2021) findings and confirm the rate of 4.2% positivity of Lu. longipalpis in the areas of São Paulo state of Brazil.
The recognition of female Lu. longipalpis across all seasons of both collection durations indicates the potential possibility of infection throughout the entire year, thus substantiating research that was done in different epidemic locations under variable conditions (Mestre et al., 2011; Mota et al., 2019; Resende et al., 2006).
5 CONCLUSION
The collar used in this research is made of 10% imidacloprid/4.5% flumethrin and has proven to be highly secure and efficient in the prevention of L. infantum infection caused by Lutzomyia species largely among the dogs residing in hyper-endemic regions. The threats to human health can be finally reduced through a dependable and successful plan of reducing the occurrence and transmission of this infection among the canine populations. Since Lutzomyia species is the principal vector of the disease in Brazilian regions, the usage of the collar might reduce infection in dogs and humans.
ACKNOWLEDGEMENTS
This research received financial support provided by São Paulo Research Foundation (FAPESP) under project number 2017/24538-0.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author Dr. Santos-Doni ([email protected]), upon reasonable request.




