Impact of mass vaccination on the spatiotemporal dynamics of FMD outbreaks in India, 2008–2016
Umanga Gunasekera and Jitendra Kumar Biswal are the co-first authors.
Abstract
Foot-and-mouth disease (FMD) is endemic in India, where circulation of serotypes O, A and Asia1 is frequent. Here, we provide an epidemiological assessment of the ongoing mass vaccination programs in regard to post-vaccination monitoring and outbreak occurrence. The objective of this study was assessing the contribution of mass vaccination campaigns in reducing the risk of FMD in India from 2008 to 2016 by evaluating sero-monitoring data and modelling the spatiotemporal dynamics of reported outbreaks. Through analyzing antibody titre data from >1 million animals sampled as part of pre- and post-vaccination monitoring, we show that the percent of animals with inferred immunological protection (based on ELISA) was highly variable across states but generally increased through time. In addition, the number of outbreaks in a state was negatively correlated with the percent of animals with inferred protection. We then analyzed the distribution of reported FMD outbreaks across states using a Bayesian space–time model. This approach provides better acuity to disentangle the effect of mass vaccination programs on outbreak occurrence, while accounting for other factors that contribute to spatiotemporal variability in outbreak counts, notably proximity to international borders and inherent spatiotemporal correlations in incidence. This model demonstrated a ∼50% reduction in the risk of outbreaks in states that were part of the vaccination program. In addition, after controlling for spatial autocorrelation in the data, states that had international borders experienced heightened risk of FMD outbreaks. These findings help inform risk-based control strategies for India as the country progresses towards reducing reported clinical disease.
1 INTRODUCTION
Foot-and-mouth disease (FMD) is caused by an Aphthovirus in the Picornaviridae family that affects cattle, buffalo, pigs and other domestic and wild ungulates. Classical infection produces clinical signs of fever and vesicles in the mouth, tongue, hoof and udder, affecting production and leading to economic losses (Arzt et al., 2011). However, FMD virus (FMDV) is also known to cause various forms of subclinical infection (Stenfeldt & Arzt, 2020) and has been associated with abortion in cattle in India (Ranjan et al., 2016). FMD is endemic across much of Asia, South America and Africa, where estimated economic losses range from $6.5 to 21 billion USD annually (Knight-Jones & Rushton, 2013). Preventing the transboundary spread of FMD into disease-free countries, including many countries in Europe and North America, plays a major role in shaping international trade policies (Shanafelt & Perrings, 2017). In the past two decades, several widespread viral lineages of serotypes O, A and Asia1 have emerged from South Asia, suggesting that this region is a hotspot for viral evolution and subsequent transboundary spread (Brito et al., 2017b), including the O/ME-SA/Ind2001d lineage that subsequently spread to the Middle East, North Africa and Southeast Asia (Bachanek-Bankowska et al., 2018; Dahiya et al., 2020; Knowles et al., 2016; Subramaniam et al., 2015;Vu et al., 2017). Some of these emerging lineages were first reported from India, possibly originating in India's large susceptible livestock population, or reflecting external introduction and early recognition in India due to its relatively robust surveillance system. Therefore, understanding the epidemiology of FMD in India is critical for supporting regional FMD control initiatives and controlling the disease globally.
India has a population of >300 million cattle and buffalo (20th livestock census, 2019), with some states and administrative units having bovine population sizes on par with individual countries in Africa, Asia and Europe (http://www.fao.org/faostat/en/#data/EK/visualize). In addition, the country is the world's third largest beef exporter and largest producer and consumer of dairy, with dairy products contributing ∼70% of total livestock income of India (Hemme et al., 2003; Kumar et al., 2012). Infection by FMDV can cause significant reductions in milk production, manifesting in losses of up to $200 per animal in India, but economic losses borne by farmers also include treatment, loss in draught power, opportunity costs related to labour and distress sales (GG et al., 2021).
A trivalent FMD vaccine against serotypes O, A and Asia1 has been used as part of India's FMD control strategy, with states enrolled in either of two regular vaccination programs, namely, the central government vaccination program (FMD Control Program, FMDCP) or the Assistance to States to Control Animal Disease (ASCAD) program. FMDCP is a biannual vaccination program in which the vaccine is administered to cattle and buffaloes, whereas ASCAD is an annual vaccination program (Hegde et al., 2014). FMDCP was first implemented in 54 selected districts in 2003–04, and subsequently expanded from southern and central India to the northern part of the country in a phased manner, such that different states were initiated to the program in different years. For FMDCP, monitoring and surveillance are conducted by the Indian government. Pre- and post-vaccination monitoring includes the determination of antibody titres by ELISA before and after each round of vaccination (Pattnaik et al., 2012). At the population level, it is desirable for >80% of animals 12 months and older to have adequate protection (inferred through antibody titres) to minimize the risk of widespread outbreaks within a population (Ferrari et al., 2016).
The World Animal Health Organization (OIE) and the World Food and Agriculture Organization (FAO) have developed a set of outcome-oriented guidelines for FMD-endemic countries to reach FMD-free status, which is known as the progressive control pathway (PCP). India is in the stage 3 of the PCP, and most other countries in Asia are in stage 1, 2 or 3. According to OIE/FAO recommendations, during stage 1, risk from FMD and available control options are identified. By stage 2, a country is expected to have a risk-based strategic control plan with an FMD monitoring and evaluation system in place. In stage 3, a country should continue to monitor disease risk, analyze passive and active surveillance data to show progressive reductions in FMDV occurrence and implement its strategic control plan, which may include pursuing FMD-free zones with vaccination within the country. Vaccination plays a major role in achieving this task.
To date, very few studies have been carried out to quantify the spatial distribution of FMD risk in India (Hegde et al., 2014;Sharma et al., 2014), and an evaluation of the success of vaccination programs in reducing outbreaks is key to understanding the role of such programs in controlling FMD and achieving FMD zonal freedom with vaccination. Several studies have recognized the importance of optimizing the vaccination program, controlling animal movements and conducting effective surveillance for FMD control in India (Biswal et al., 2019; Pattnaik et al., 2012). However, rigorous spatial epidemiological methods have yet to be applied to understand how vaccination and other factors relate to the spatiotemporal pattern of outbreaks.
The objective of this study was to assess the contribution of mass vaccination campaigns in reducing the risk of FMD in India by evaluating sero-monitoring data and modelling the spatiotemporal dynamics of reported outbreaks. We first assessed vaccination outputs through an evaluation of antibody titre data collected as part of pre- and post-vaccination monitoring. Using a Bayesian space–time model that accounts for underlying spatial dependencies often present in disease data (Branscum et al., 2008;Chhetri et al., 2010; Machado et al., 2019), we then investigated the impact of mass vaccination programs on the occurrence of reported FMD outbreaks overtime while controlling for other factors that contribute to spatiotemporal heterogeneities. Results presented here will ultimately contribute to evaluation of progress of India's mass vaccination campaigns and support country's progress in the context of the PCP.
2 MATERIALS AND METHODS
2.1 Study area and data sources
There are 28 states and eight union territories in India, and each state is further subdivided into administrative districts. Union territories are a type of administrative division in India governed by central government, in contrast to states which have their own state-level governments. The first phase (phase I) of the FMDCP began in 2003 as a pilot study (Subramaniam et al., 2013). At the beginning of the FMDCP phase I, nine states and one union territory were part of the mass vaccination program, and not all districts within each state were included. In the second phase of FMDCP (starting between 2010 and 2011, depending on the state), all districts within the participating states were part of the vaccination program except for Uttar Pradesh (an administrative unit in northern India with 50.2 million bovine population). In Uttar Pradesh, only 16 out of 75 districts participated in the vaccination program as of 2018. By 2018, all seven union territories and 11 out of 29 states were covered by FMDCP without exceptions. A map of states participating in FMDCP by 2016 is shown in the supplementary materials. During phase II of the FMDCP vaccination program, 38% of the total cattle and buffalo population of India were estimated to have been vaccinated (Mahapatra et al., 2015). In this FMD vaccination program, all cattle and buffalo >6 months of age are eligible for vaccination, with the intent to cover the entire eligible population. However, animals in the last trimester of pregnancy are not vaccinated. Prior to 2020, there has been no mandated two-dose primary vaccination regimen for animals that are vaccinated for the first time. The 3PD50 potency trivalent (serotypes O, A and Asia1) vaccine used in India contains three times the protective dose required to protect 50% of the animal population (Pattnaik et al., 2012). Currently, there are three vaccine manufacturers in India. Since the vaccines have been procured centrally through a techno-commercial tender-bid system (the lowest bid selected during each round of vaccination), there has been no uniformity with respect to the vaccine manufacturer supplying vaccine to a particular state across years.
In India, disease control and reporting rely primarily on passive surveillance conducted by different veterinary authorities including veterinarians and veterinary paraprofessionals, adding up to nearly 65,000 at different administrative levels. Disease reporting is paper based. Outbreaks are directly communicated by mobile phones to district offices, and a monthly report is submitted to the Department of Animal Husbandry. Laboratory diagnosis and confirmation of outbreaks is performed by one of 27 FMD network laboratories and two national laboratories located across the country. FMD outbreak response includes ring vaccination, isolation of animals and treatment against secondary infection and wounds.
Data on pre- and post-vaccination FMDV antibody titres (see below) and annual reported number of outbreaks from each state were obtained from the annual reports of the Directorate of Foot and Mouth Disease of the Indian Council of Agricultural Research (ICAR-DFMD, Ministry of Agriculture), which is the national referral centre for FMD diagnosis. Outbreak data were generated through passive surveillance, where an outbreak was defined as a report of clinically FMDV-infected animals from the same village/district (OIE) which was further confirmed by laboratory tests conducted on referred clinical samples. The number of infected animals was not available for a given outbreak. Outbreak data were reported at the state and not the district level.
2.2 Serological data
As outlined above, mass vaccination of cattle and buffalo was carried out by the Indian government once every 6 months in the selected states and districts that were part of FMDCP. To determine antibody titres pre-vaccination, serum samples were collected at the time of vaccination for each biannual round of vaccination. Samples were collected from animals selected from 10 villages in each district on day of vaccination (pre-vac) and 21–30 days post-vaccination. Sampled animals were selected at random and the pre- and post-vaccination samples may or may not come from the same animal. Minimal meta-data was available at the animal-level, and age was not recorded, though the FMDCP guidelines target animals approximately >2 years of age. On average, the number of animals per state from which samples were collected ranged from 100 to 1000 animals per sampling round.
Serum samples were tested for reactivity against FMDV using Liquid Phase Blocking (LPB)-ELISA, which was used to infer protective antibody titres against FMDV structural proteins at an inferred protection level of log10 titre of 1.8 (Sharma et al., 2017). Change of titre values pre- and post-vaccination was qualitatively similar for all three serotypes O, A and Asia 1 as vaccination was conducted with a trivalent vaccine. Since serotype O accounts for more than 80% of the outbreaks (Brito et al., 2017b), only antibody titre change for serotype O is shown in the main text. For this study, antibody titre data were only available from states that were part of FMDCP phase I (see Figure S1 for a map of states included in phase I). Because LPB-ELISA cannot discriminate between antibody responses induced by vaccination versus natural infection, we could not determine whether inferred protection was the sole result of vaccination and not from previous natural exposure to FMDV. Data regarding the percent of animals with inferred protection pre- and post-vaccination were summarized for each 6-month round of vaccination.
In contrast to LPB-ELISA, non-structural protein (NSP)-based ELISA differentiates vaccinated animals from naturally infected animals based on elicitation of a response to NSPs that should be absent in vaccine preparations. There is evidence that vaccination can elicit a transient NSP response in vaccinated animals in India (Hayer et al., 2018; Mohapatra et al., 2011). However, the majority of positive samples by NSP-ELISA are expected to be from previously infected individuals. Annual information on sero-positivity in NSP-ELISA was available only for certain states/administrative units for some years. Further, the animals sampled for NSP-ELISA once in a year are not necessarily the same ones as sampled for LPB-ELISA.
2.3 Descriptive analysis
Data from pre- and post-vaccination monitoring obtained through LPB-ELISA from the phase I were analyzed to identify whether there was an increase in percent of animals with inferred protection before and after each individual round of vaccination, as well as to identify trends in inferred protection over time across multiple successive rounds of vaccination.
To evaluate whether there was a correlation between population immunity and FMDV circulation, the association between the percent of animals in each state over a period of 8 years (2008–2016) with inferred protection via LPB-ELISA (an indicator of population immunity) and percent of NSP-ELISA-positive animals (an indicator of previous natural exposure) was evaluated using a Spearman's correlation test.
2.4 Conceptual framework of outbreak risk
A conceptual diagram was created to represent pathways by which hypothesized factors beyond vaccination could influence pathogen spread and the reported number of outbreaks per state (Figure S2), and accounting for these additional sources of variability may allow to better determine the effect of vaccination on outbreak counts at the state level. Details on risk factor variables included in the model are shown in Table S1. Disease spread is expected to be influenced by organized farming practices, and other community activities like animal fairs. Briefly, livestock population data of goat density and pig density were included in our model as categorical variables (high/low, split at the mean). In addition, higher outbreak numbers have been reported in dryer agroclimatic zones in some parts of India (Hegde et al., 2014), potentially due to husbandry practices that are typical of different climatic conditions or due to environmental conditions within those regions that promote survivability of the virus outside the host. Therefore, land cover and annual averages for wind speed, rainfall and temperature were included to capture environmental factors related to outbreak risk (Abatzoglou et al., 2018). Evaluation of intra-annual and seasonal variation was not possible since outbreak numbers were reported as annual values. However, to quantify areas with more extreme seasonality, annual variance (calculated across 12 months) of each environmental variable was also included. All environmental variables were centred at the mean and standardized.
Animal transport within India or across international borders for trading and slaughter may promote disease spread and the occurrence of outbreaks. Because no digitized animal movement databases are maintained for India, road density was utilized as a proxy measure. India is bordered by Pakistan, China, Nepal, Bhutan, Myanmar and Bangladesh. FAO/OIE has categorized India and the surrounding endemic countries into three different ‘pools’ of FMD based on the predominant circulating serotypes and topotypes in each area (Paton et al., 2018). Pool 1 includes Myanmar and China; pool 2 includes India, Sri Lanka, Nepal, Bangladesh and Bhutan; and pool 3 includes Pakistan. Dummy variables were introduced to the analysis indicating whether each state was bordered by a pool 1, pool 2 or pool 3 country, or if the state did not have any international land borders (Table S1).
In addition, the presence and efficiency of veterinary services within a state may influence both vaccination as well as outbreak reporting. The coverage of veterinary services in each state was calculated based on the percentage of veterinarians available relative to the number of veterinarians estimated to be required by that state. This value was obtained from the OIE Performance of Veterinary Services analysis for India.
For each year, states were categorized into two groups based on whether they were part of the FMDCP. All states that had at least one district participating in the vaccination program at some point of time are shown in the Figure S3, and district-level participation expanded through time. During the study period, the majority of districts within a state were enrolled in the program. Detailed information regarding the participation in vaccination program at the district level is available at https://dadfonline.gov.in/FMDCP/Index.aspx. There was no substantial correlation between any variables (Figures S4 and S5).
2.5 Bayesian space–time hierarchical model
2.5.1 Spatial-only model
Because data for many of the risk factors were only available for a single point in time, a spatial-only model was initially built to screen important risk factors among potential predictors. Although we do not expect substantial annual variation in such variables (i.e., cattle population sizes are not expected to change rapidly), the spatial-only model was done so that significance of variables was not inflated due to replication of predictor data across years.
For model selection purposes, univariable analyses were first performed separately for each variable in Table S1. Backward selection was then performed from a full multivariable model by removing the variables with the widest confidence interval that overlapped zero. From among those different models, the simplest model that was <2 ΔDIC from the model with the lowest DIC value was considered the best-fit spatial-only model (Spiegelhalter et al., 2002). Risk factors in the best-fit spatial-only model were considered as candidate variables in the space–time model alongside temporally variable risk factors (in which yearly data were available from 2008 to 2016).
2.5.2 Space–time model
To incorporate temporal effects into the model, a BYM2 model was used (Riebler et al., 2016). Several possible model structures exist to incorporate the temporal effect (summarized in Table 1): time (year) can be considered as a random effect (ωt, Equation 1 in Table 1), a structured effect (γt), in which a random walk is used to account for between-year dependencies (ωt + γt, Equation 2) and/or as a random, structured and space–time interaction (ωt + γt + δit, Equation 3). The best model structure was selected from amongst these models using DIC. This structure was then used to evaluate the impact of vaccination programs on relative risk while accounting for other sources of variability (hypothesized risk factors) as well as spatial and temporal correlations in outbreak incidence.
| Model | Specification | DIC | Posterior predictive-values (lower, upper) |
|---|---|---|---|
| Equation 1. Spatial only model | log(θit) = α + vi + υt | 224.99a | (15.2, 0) |
| Equation 2. Space–time model (time as an unstructured effect) | log(θit) = α + vi + υt + ωt | 2765.52 | (63.7, 26.1) |
| Equation 3. Space–time model (time as a structured effect) | log(θit) = α + vi + υt + ωt + ýt | 2768.51 | (63.7, 26.1) |
| Equation 4. Space–time model (space–time interaction) | log(θit) = α + vi + ut + ωt + γt + δit | 1222.6 | (33, .7) |
- Each model adds additional components to the previous model.
- aDIC for the spatial-only model is not comparable to space–time model.
We also calculated how much variability is explained by each component that made up the final model structure. Once the best model structure was selected, variable selection was performed as described for the spatial-only model, including temporally variable risk factors and spatial-only factors from the spatial-only model as candidate fixed effects. Excess risk (ER) for a given state was calculated as the proportion of the posterior for each fitted θit that exceeded .8.
2.5.3 Prior sensitivity analysis
Since prior distributions can influence model results, we conducted a sensitivity analysis on the priors. The model was refitted with different penalized complexity priors and non-informative priors to evaluate the extent to which our results were sensitive to different prior assumptions (Figure S6). We ultimately used non-informative penalized complexity priors, which are applicable for a large class of hierarchical models (Simpson et al., 2014). Penalized priors consider that there is a base model and that the complex model that we obtain is a result of deviation from the base model. For Gaussian Random Field distributions, the base model can be given as π(x/ε) where ε = 0. The objective of using the penalized priors is to make the model similar as possible to the base model. Penalized priors can also account for model overdispersion (Simpson et al., 2014).
2.5.4 Model diagnostics
The fit of the final model (selected based on DIC, as described above) was evaluated using posterior predictive p values, defined as p(yi* ≤ yi|y), where yi* is the posterior of the predicted distribution from the model. Posterior predictive p values can be interpreted as an approximation of the proportion of the predicted distribution for yi that is more extreme than the observed value, and values of p(yi* ≤ yi|y) near 0 and 1 indicate poor model fit. If the model is performing well, then a greater portion of the posterior of the predicted values should be >.1 and <.9 (Blangiardo & Cameletti, 2015). In addition, the proportion of marginal variance for random effects and each model component was checked in the final model. The explained variability from the covariates was obtained as a percentage of change of standard deviation from the null model to the model with all the selected covariates.
2.6 Software
All analyses were performed in R statistical software. Different packages such as tidyverse 1.2.123 (Wickham et al., 2019), spdep 0.7−425 (Bivand and Wong, 2018), dplyr, stringr and ggplot2 were used. For the Bayesian models, INLA 19.09.03 (Rue et al., 2009) were used, and model results were processed with INLAOutputs 19.09.03 (Baquero, 2018).
3 RESULTS
3.1 Descriptive results
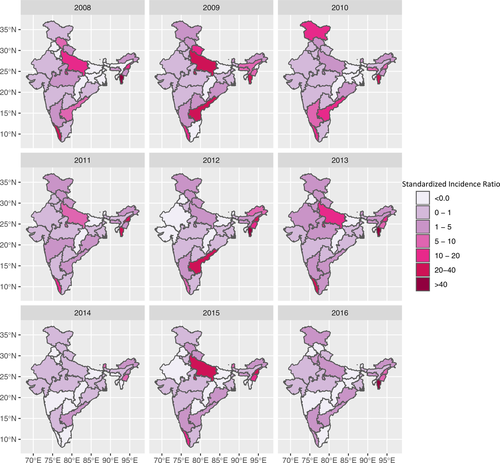
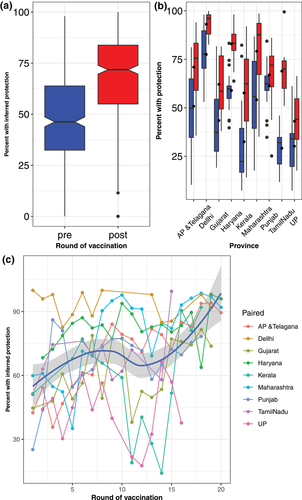
A total of 3282 outbreaks were reported over a period of 9 years from 2008 to 2016, with substantial heterogeneity in the spatial and temporal occurrence of outbreaks (as shown by SIR values) across states and years (Figure 1). During this time period, we summarized the antibody titre data measured in 1,002,437 animals via LBP-ELISA. Antibody titre data were only available for states that were part of FMDCP phase I. This pre- and post-vaccination monitoring demonstrated that the percent of animals with inferred protection for serotype O generally increased after vaccination (Figures 2(a) and 2(b)), but there was high variation between states and across time (Figure 2(c)). Similar trends were observed for serotypes A and Asia1 (Figure S7). For states participating in FMDCP phase 1, Figure S1 shows a choropleth map of the post-vaccination serotype O percent protection titres, averaged across all vaccination rounds
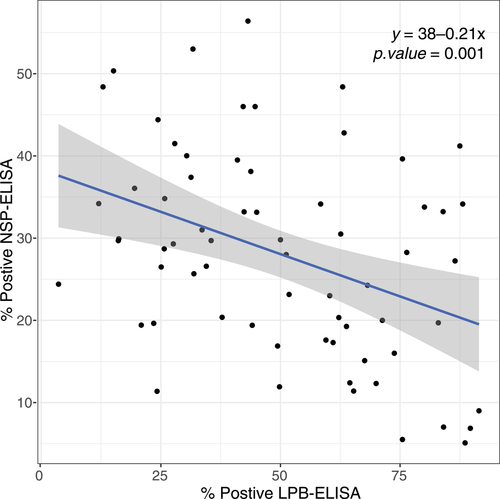
States where NSP-ELISA and the LPB-ELISA were carried are shown in Figures S8 and S9. For years in which NSP-ELISA data were available (2009–2016), there was no significant correlation between NSP sero-prevalence and the number of outbreaks per state per year (σ = .17, p value = .10) or SIR (σ = .11, p value = .32). However, the percent of animals with inferred protection via LBP-ELISA was negatively correlated with raw outbreak numbers (σ = −.29, p < .001) and SIR (σ = −.25, p value = .03). The most striking correlation was between the percent of animals positive to LPB-ELISA and NSP-ELISA (σ = −.39, p < .01; Figure 3). Because it was not possible to incorporate ELISA data into space–time models, we performed a simple ad-hoc linear regression on the percent of animals positive to LPB-ELISA and NSP-ELISA to estimate to relationship between these two measures (Figure 3).
3.2 Bayesian modelling results
3.2.1 Selection of best model structure
Table 1 shows the different space–time models that were tested to select the best fitting model structure based on DIC. For the selected model structure that best fit the data (Equation 4, Table 1), the unstructured spatial effect accounted for 62% of the variability, whereas the structured spatial effect accounted for only 14.6% of the variability (Table S2). This suggests there was relatively little correlation in the occurrence of reported outbreaks across neighbouring states through time. The other factor that accounted for substantial variability was the space–time interaction effect.
3.2.2 Univariable analyses of potential risk factors
Variables for which data were only available for 1 year were first screened in univariable spatial-only models, whereas time-varying variables were screened in univariable spatiotemporal models. Variables that were associated with reported outbreaks (credible interval of odds ratio does not overlap one) are shown in the Table 2. The complete list of variables is included in the supplementary materials (Tables S3 and S4). All univariable models included the underlying terms that accounted for spatially structured and unstructured effects, as well as temporal effects if applicable.
| Univariable model | DIC | Coefficient (credible interval) |
|---|---|---|
| (a) Spatial-only models | ||
| No international border | 228.35 | .52 (.06, .74) |
| Bordered by country of FMD pool 1 | 229.33 | 6.11 (1.72, 21.93) |
| Pig density (reference: low) | 229.81 | 2.23 (1.06, 4.72) |
| Forest coverage density (reference: low) | 230.25 | 3.81 (2.07, 7.06) |
| (b) Space–time model | ||
| Participation in the vaccination program (reference: no) | 1224.01 | .41 (.22, .78) |
3.2.3 Best-fit multivariable model
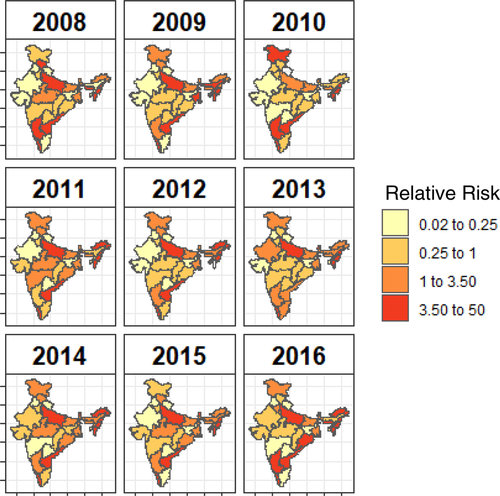
Fixed effects identified from the best-fit multivariable spatial-only model (Table S5) were included as candidates in the multivariable space–time model. Two predictors were retained in the final space–time model: no international border and participation in the vaccination program (Table 3). Fitted relative risk values calculated from the best-fit model for each state are shown in Figure 4. Relative risk is interpreted as how many times more (or less) likely outbreaks are relative to the number of outbreaks that would be expected in a state based on its population size (observed-to-expected ratio). Most border areas show continuous high risk throughout the years. In addition, risk increased in some areas, whereas it decreased in others. ER peaked in many states between 2011 and 2013 (Figure S10).
| Fixed effect | Coefficient 95% credible interval |
|---|---|
| Intercept | 1.64 (.72, 3.68) |
| No international border | .27 (.08, .99) |
| Participation in the vaccination program (reference: no) | .45 (.24, .84) |
- Coefficients and credible intervals have been exponentiated to be on the odds scale.
The sensitivity analysis of model priors demonstrated that similar DIC and p values were produced regardless of choice of priors.
4 DISCUSSION
In this study, we first conducted a descriptive analysis of epidemiological outcomes of government-aided FMD vaccination programs in India. For states where the vaccination under FMDCP was implemented, our analysis showed that the percent of animals with inferred protective antibody titres fluctuated across years and states, but there was a general increase in the percent of animals with protective antibody titres after each round of vaccination and across time. We then analyzed the distribution of reported FMD outbreaks by using a Bayesian space–time model to map high-risk areas, identify factors that influence risk in order to inform risk-based control strategies and assess the impact of mass vaccination in reducing outbreaks. This model demonstrated that states that were included in the vaccination program and did not have an international border experienced reduced risk of FMD outbreaks. These results quantitatively demonstrate how effective FMD control in India, and potentially other endemic countries, can be supported by expansion of mass vaccination programs, sero-monitoring, and movement restrictions at international borders.
India has used FMD vaccination as a control measure since the 1980s. The trivalent vaccine produced in India has a protective effect against the circulating outbreak strains of serotype O, A and Asia1, and studies have been carried out on vaccine safety and efficacy (Mahapatra et al., 2015; Mohanty et al., 2015; Subramaniam et al., 2015). Approximately one-third of India's cattle and buffalo population have been vaccinated through the government's FMDCP program (Pattnaik et al., 2012). Following PCP guidelines for stage 3, India has conducted pre- and post-vaccination sero-monitoring according to OIE guidelines to monitor the population-level immunity since 2008. For a given round of vaccination, as expected, antibody titres were higher post-vaccination compared with pre-vaccination (Figures 2(a) and S1). However, there was substantial heterogeneity across states and between years (Figures 2(b) and 2(c)), and the percentage of animals with inferred protection mostly fell below OIE's recommendation of >80% population of animals 12 months and older, though the exact threshold for herd immunity will vary as a result of spatial heterogeneity, age distribution and vaccine effectiveness (Ferrari et al., 2016). Furthermore, the pre-vaccination monitoring data showed that (Figure 2) the percent of animals with inferred protection routinely fell to <50% prior to the next round of vaccination. Unfortunately, limited meta-data were available for the sampled animals that precluded a more detailed age-based or spatial analysis of serology data. It is possible that younger animals that have received fewer vaccines doses compared to the other animals may not sustain durable antibody titres; a two-dose primary course of vaccination would enhance the duration of immunity in younger animals (Knight-Jones et al., 2015). However, even at suboptimal levels, vaccination can still reduce disease incidence (Skowronski et al., 2012;Ohmit et al., 2013; Wan-Ju et al., 2018).
Population demographics, turnover and waning immunity may contribute to periodic dips below the 80% threshold (Knight-Jones et al., 2016). According to a study conducted in Turkey, biannual mass vaccination can leave gaps in population-level immunity. Among other factors, young animals may not have received sufficient vaccine doses to attain long-lasting immunity, and animals in late pregnancy are sometimes not vaccinated, resulting in declines in population-level immunity just prior to the subsequent round of vaccination (Knight-Jones et al., 2016). Due to herd demographics and semi-intensive management practices, it was concluded that vaccination without biosecurity may not be able to control FMD in Turkey (Knight-Jones et al., 2016). Similar dynamics may also occur in India, as shown by the high spatial and temporal variation in the percent of animals with inferred protection, and these spatial and temporal gaps in herd immunity may allow for the persistence and spread of FMDV in the country. As cattle slaughter is prohibited in India, the presence of stray cattle populations may also hinder achieving the targeted vaccine coverage. The proportion of animals with inferred protection could also have been influenced by inconsistent vaccine administration and transport conditions, delay in re-vaccination, lack of booster doses in the primo-vaccinated calves and transboundary introduction of naïve animals, which could have contributed to variable antibody titres.
We also investigated the relationship between the occurrence of FMDV within states and vaccination data (i.e., participation in FMDCP or the percent of animals with inferred protection via LPB-ELISA). We used two imperfect measures to quantify the extent of FMD circulation: standardized incidence ratios (SIR, based on reported outbreaks) and NSP-based sero-prevalence. Outbreak reporting can be inconsistent and likely provides an incomplete picture of FMDV incidence. In contrast, the NSP-ELISA data captured the percentage of animals with an anti-NSP response, which is indicative of natural infection. A naturally infected animal is also expected to be positive on LPB-ELISA, thus the percent of animals with inferred protection (based on LPB-ELISA) cannot discriminate between immunity due to vaccination or natural infection. However, during the period under study, the percent of animals positive on LBP-ELISA and NSP-ELISA was negatively correlated. In addition, fewer outbreaks and lower SIRs were reported in states with higher proportion of animals LPB-ELISA positive. These results suggest that (a) LPB-ELISA data can be interpreted as an indicator of vaccine coverage rather than natural virus circulation and (b) areas with higher vaccine coverage experienced reduced circulation of FMDV (as shown by low NSP sero-prevalence and fewer reported outbreaks). Indeed, a simple ad hoc analysis suggests that NSP sero-prevalence declined by ∼2.1% for every 10% increase in vaccine coverage (inferred from LPB-ELISA positivity; Figure 3). These results are in agreement with a cross-sectional study conducted in 2014, which identified that herds in states in the biannual vaccination program reported lower disease incidence (Sharma et al., 2014). Once we accounted for spatiotemporal dynamics in the space–time model, participation in the FMDCP reduced outbreaks by ∼55%.
To better understand heterogeneities in outbreak occurrence within India, we developed a Bayesian space–time model that allowed us to examine risk factors associated with outbreak risk alongside model components that accounted for the spatial interdependency of risk across states. Although reported outbreak numbers are likely an underestimate of the true number of outbreaks, analyzing patterns of reported outbreak occurrence does advance our understanding about spatial factors associated with outbreaks. An examination of the variance explained by each component making up the model's structural backbone (Table S2) revealed initial insights into processes shaping outbreak risk. For example, the structured spatial effect explained relatively little variation, indicating that the outbreak risk in one state was not closely correlated with the occurrence of outbreaks in neighbouring states. This pattern may be because states in India are large, and a smaller spatial scale would better capture the local spatial dynamics of outbreak propagation. Also, this result suggests that outbreaks or control programs in one state would not have large impacts on the adjacent state.
From our model, it is evident that relative risk of outbreaks changes through time and space, though there are some states that were more consistently at higher risk (Figure 4). The two variables retained in the final model were participation in the FMDCP vaccination program and not having an international border. The relative risk of outbreaks in states that were part of the FMDCP during 2008–2016 was about one half that of states that were not part of the program. This is consistent with our descriptive analysis on the importance of vaccination. It is also notable that we observed a benefit of the FMDCP in the number of reported outbreaks state-wide, despite the observed variability in percent of animals with inferred protection and that the FMDCP did not always extend to all districts within the state.
The other important risk factor identified by the model was a ∼70% reduction in the risk of outbreaks in states with no international border. Given that many northern states did not participate in the FMDCP vaccination program until later in the study period, part of this effect may be due to the shorter duration of time that bovine populations in these states received vaccination, which could potentially impact population immunity. Our model included FMDCP participation and international borders as additive effects and did not account for different lengths of time that a state was part of the FMDCP, which could lead to a slight overestimation of the risk associated with international borders. Nonetheless, our results in combination with other published research suggest that international borders increase the risk of outbreaks. In a longitudinal study conducted in 2014 to determine serological herd immunity, border states such as Assam, Rajasthan, Jammu and Kashmir, West Bengal and Uttar Pradesh were at high risk due to low population immunity. There were also instances where high incidence of FMD was observed in border states even where herd immunity was high (Sharma et al., 2014). We observed the same pattern of border states having greater ER (Figure S10), including the states of Meghalaya, Assam, Arunachal Pradesh, Tripura and Jammu and Kashmir.
The potential for transboundary introductions of novel FMDVs into India from neighbouring countries may in part explain the risks associated with international borders. Alternatively, transboundary value chains may result in high risk in certain border states if animals are transported to border states from elsewhere within India prior to exportation. Legal and illegal animal movement occurs between neighbouring countries, but the extent of such transboundary movements depends on the countries involved (Landes et al., 2016). Previous studies have identified that cattle and buffaloes are transported from India to Malaysia through Myanmar and Vietnam (all pool 1 countries), which may lead to the dissemination of FMDV (Rweyemamu et al., 2008; Smith et al., 2016; Bartels et al., 2017). Interestingly, our spatial-only model suggests that bordering a country in pool 1 carried a higher risk, which would be consistent with the idea that transboundary movements with pool 1 countries shapes FMD risk within India, although this variable was not retained in the space–time model.
ER peaked between 2009, 2011 and 2013 in almost all states (Figure S10). During 2013, widespread FMD outbreaks occurred in India, caused by the strain O/ME-SA/Ind2001d. This strain also spread to other countries in the Middle East and Southeast Asia at this time (Brito et al., 2017a; Subramaniam et al., 2015), suggesting that periods of ER in India may also translate to heightened frequencies of transboundary transmission.
India is in the stage 3 of the PCP for FMD. Countries within this stage should engage in ongoing monitoring of risk and implementation of risk-based strategies to define a pathway to obtain freedom from FMD (with vaccination) in at least one geographic zone, including analysis of passive/active surveillance data to document epidemiological evidence of reductions in FMD incidence. Related to this, our results suggest that a feasible strategy may be to continue trying to decrease prevalence in identified high risk areas to mitigate the impact of the disease with special focus on states that possess international borders. Alternatively, low risk areas identified from this spatial analysis could help delineate areas in which zonal freedom may be more readily attainable.
There are several caveats to the interpretation of the serological data that present limitations to this study. First, NSP-ELISA data were available from only 24 out of 29 states for 6 years. In addition, transient increases of NSP titres can occur within 21 days of vaccination in up to 15% of previously uninfected animals, which complicates the interpretation of NSP-ELISA results particularly if vaccination history is not available (Mohapatra et al., 2011; Hayer et al., 2018). Second, animals can be positive on an LPB-ELISA from either vaccination or natural infection. The negative correlation between NSP-ELISA and LPB-ELISA data suggests that (a) rates of LPB-ELISA positivity likely represented vaccination rather than natural infection, and (b) rates of NSP-ELISA positivity were not coupled with vaccination. However, serial testing and monitoring for clinical signs is necessary to identify the changes in antibody titres in infected and vaccinated animals to determine whether animals have acquired antibodies due to infection or vaccination (Mohanty et al., 2015). Related to this, when pre- and post-vaccination antibody titres were compared at the state level, samples were not coming from the same animal which limits the conclusions we can draw from this comparison. In addition, serum samples were not collected in an age stratified manner, and information on age of animals was not available. Not only is the number vaccine doses received and hence LPB-ELISA positivity potentially tied to age, but there is also an age-related increase in post-vaccinal NSP-responses and the probability of lifetime infection (de Carvalho Ferreira et al., 2017; Stenfeldt & Arzt, 2020). While lack of age information may hinder the interpretation of sero-reactivity, particularly at the individual-level, the robust sample sizes within each state should somewhat mitigate this issue for population-level estimates, as each state's sampled population should contain a relatively representative mixture of animals of various ages. Age-stratified sampling has been implemented as of 2020, but not for the study period investigated here. Finally, data for years beyond 2016 were not included in the present study because the country began using Solid Phase Competitive ELISA assessment of population immunity, and this different diagnostic technique could create inconsistencies and confusion in the interpretation of patterns of protection at the population level.
Another limitation is that the outbreak data came from passive surveillance, and there may be substantial under-reporting. If there are spatial biases in the extent to which outbreaks are under-reported, then this could introduce spatial biases to the SIR data and the data used for the space–time model. These types of potential bias are common in observational epidemiological studies that rely on passive surveillance; however, we believe there is still value in describing large-scale patterns of FMD incidence. In addition, we have no information about the number of animals infected in each outbreak, which means that small and large outbreaks receive equal weight in our analysis. Finally, no environmental or climatic factors were retained in our best-fit model. This may be an artefact and limitation of the state-level spatial and yearly temporal scale of our analysis, which did not allow us to capture finer-scale spatial variation or seasonal effects. Future analyses could overcome the limitations imposed by the spatial and temporal resolution of our outbreak data by tabulating outbreak data on a finer spatiotemporal scale, thus enabling a better evaluation of the importance of environmental risk factors.
5 CONCLUSION
In this study, we show that the standardized incidence of FMD outbreaks have reduced over time with the implementation of mass vaccination, though the percent of animals with inferred protection was highly variable through space and time and often fell below the desired threshold of >80%. Over the same time period, states with a higher percentage of animals with inferred protection had a significantly lower number of reported outbreaks. Through implementing a Bayesian space–time model, we demonstrate that states that were part of the FMDCP experienced a ∼50% reduction in the risk of reported outbreaks. Our results also demonstrate a substantial risk of outbreaks associated with international borders, suggesting a role of transboundary movements of animals or fomites in shaping FMD incidence. This study provides a rigorous spatial analysis of the impact of vaccination programs in FMD circulation, which will contribute towards efforts to reduce disease prevalence. Further studies may be necessary of detailed risk factor identification at a granular level and to assess the impact of other control measures, such as implementing biosecurity measures, movement control and systematic age-stratified sero-surveillance, to control FMD in India.
ACKNOWLEDGEMENTS
Support for this work was provided by the Indian Council of Agricultural Research, New Delhi. This work was funded by the United States Department of Agriculture (USDA) Agricultural Research Service (ARS) Project 1940-32000-061-00D, the United States Department of State, Biosecurity Engagement Program through the USDA-ARS Office of International Research Program and a specific collaborative agreement between USDA-ARS and University of Minnesota. The authors are thankful to DAHD, Government of India for the support and AICRP on FMD Centers for providing the serum samples for analyses.
ETHICAL STATEMENT
Not applicable.
AUTHOR CONTRIBUTION
K. V., U. G. and J. K. B. conceived the project. U. G. analyzed data, performed Bayesian modelling and wrote the manuscript. J. K. B. provided the data, and R. R., S. S., M. R., J. K. M., B. P. and R. P. S. were involved in data collection and interpretation of results. K. V. W. and G. M. participated in the development of the Bayesian model, study design, interpretation of results and helped to draft the manuscript. A. P. and J. A. contributed to the study design, interpretation of the results and helped to draft the manuscript. All authors edited and gave the final approval for publication
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All datasets supporting our findings are available from the ICAR on reasonable request.




