Age and episode-associated occurrence of Cryptosporidium species and subtypes in a birth-cohort of dairy calves
Abstract
The role of species-specific immunity in infection patterns of Cryptosporidium spp. in humans and farm animals is not well understood. In the present study, the dynamics of Cryptosporidium infections in a natural cryptosporidiosis model was examined using genotyping, subtyping and whole genome sequencing tools. In a cross-sectional survey of Cryptosporidium spp. in 934 dairy cattle on one farm, marked age-associated differences in the distribution of Cryptosporidium species and C. bovis subtypes were observed. In a closely followed longitudinal birth cohort study of 81 calves over a 9-month period, shedding of C. parvum oocysts by the IIdA19G1 subtype started at 4 days, peaked at 2 weeks and ended mostly by 4 weeks. In contrast, the shedding of C. bovis oocysts started at 2 weeks, peaked initially at 6 weeks and had a second wave during 15th to 23rd weeks. For C. ryanae, calves had mostly only one episode of infection by one subtype, with accumulative infection increasing much slower than C. parvum and C. bovis. Overall, the accumulative infection rates and mean duration of oocyst shedding for calves in the cohort were 97.4% (76/78) and 2.3 weeks, 100.0% (80/80) and 3.9 weeks, and 78.7% (63/80) and 3.2 weeks for C. parvum, C. bovis and C. ryanae, respectively. The oocyst shedding intensity was much lower in C. bovis and C. ryanae infections compared with C. parvum infection, and in the second episode of C. bovis infection compared with the first episode. The two episodes of C. bovis infections were caused by different genome types that differed mostly in nine genes. Cryptosporidium parvum infection was associated with the occurrence of watery diarrhoea. Data from the natural history study of cryptosporidiosis indicate that despite the existence of acquired immunity against homologous pathogens, neonatal animals experience waves of Cryptosporidium infections by different species and genome types.
1 INTRODUCTION
Cryptosporidiosis is a highly prevalent disease associated with significant morbidity and mortality in humans and farm animals, especially in immunocompromised patients and children and pre-weaned domestic ruminants (Ryan et al., 2014). Diarrhoea is the most common clinical symptom, but abdominal pain, low-grade fever, nausea, vomiting and weight loss are also frequently seen (Checkley et al., 2015; Santin, 2020).
There are currently over 40 recognized species of Cryptosporidium, with nearly 20 being identified in humans (Ryan et al., 2021). Among them, C. parvum and C. hominis are the dominant species, while C. meleagridis, C. felis, C. canis, C. ubiquitum, C. cuniculus and C. viatorum are often seen in humans in specific areas (Feng et al., 2018). A few studies have identified age-related distribution of Cryptosporidium species in humans. For example, in children in England, C. hominis is more prevalent in infants under 1 year while C. parvum is more common in toddlers of 1–4 years (Chalmers et al., 2009). In Sweden and the Netherlands, C. parvum is more common in adults, while C. hominis is the predominant species in 0–9 years (Insulander et al., 2013; Wielinga et al., 2008).
Bovine cryptosporidiosis is a commonly used natural model of Cryptosporidium infections (Riggs & Schaefer, 2020). Cattle are commonly infected with four Cryptosporidium spp. including C. parvum, C. bovis, C. ryanae and C. andersoni with age-related occurrence of them (Santin, 2020). In industrialized nations, C. parvum is the dominant species in pre-weaned calves, C. bovis and C. ryanae are common in post-weaned calves and yearlings, whereas C. andersoni is mostly seen in adult animals (Fayer et al., 2006; Santı́n et al., 2004; Santín et al., 2008). In China, pre-weaned calves are mainly infected with C. bovis rather than C. parvum, although the concurrence of C. parvum is increasing (Feng & Xiao, 2017; Guo et al., 2022). Most studies on Cryptosporidium species in cattle are cross-sectional surveys of pre-weaned calves. Thus far, only a few longitudinal cohort studies of Cryptosporidium spp. have been done in dairy cattle in the United State and Sweden (Åberg et al., 2020; Santín et al., 2008; Xiao & Herd, 1994). In most of these areas, however, C. parvum is the only species found in pre-weaned dairy calves. The assumption is that the occurrence of C. bovis and C. ryanae in pre-weaned calves is affected by the presence of C. parvum (Åberg et al., 2020).
In the present study, the natural history of Cryptosporidium infections was examined using the bovine cryptosporidiosis model in an area with common occurrence of C. parvum, C. bovis and C. ryanae in pre-weaned calves. Cross-sectional and longitudinal birth cohort studies, advanced molecular typing and quantitative assessment of oocyst shedding were used in the identification of infection patterns, differentiation of infection episodes and assessment of species- and subtype-specific immunity. The data generated should be useful in understanding the infection dynamics and immunity development in human cryptosporidiosis.
2 MATERIAL AND METHODS
2.1 Study farm
The study was conducted on a commercial Holstein dairy farm in Guangdong, China. About 1200 non-milking dairy cattle were kept on this farm, including calves of 0–6 months (n ≈ 600) and juveniles and heifers of 6–24 months (n ≈ 600). On this farm, calves of less than one month were kept in individual cages, which were cleaned twice daily and reused for different batches of animals. They were fed mainly with milk, with the gradual use of hay and concentrate. Calves were weaned at ∼60 days and transferred to the free stall barns, with 15–20 calves per barn at less than 3 months of age and 50–60 calves per barn at 3–6 months of age. Once the cows become pregnant, they were moved to a nearby farm about 1-km away. Silage and grain supplement are the only feed to post-weaned animals. Faeces and other waste were treated by the solid-liquid separation technique, with the solid faecal material being sold after fermentation and liquid waste being sent to a biochemical treatment system for additional treatment.
2.2 Animals and sampling for cross-sectional survey
A cross-sectional survey was conducted initially to assess the prevalence of Cryptosporidium spp. on the farm. Altogether, 934 faecal samples were collected from animals of 0–2 (n = 323), 3–6 (n = 305), 7–12 (n = 87), 13–18 (n = 116) and 19–24 months (n = 103). Among them, 40 animals of 0–2 months had diarrhoea at the time of sampling. The data generated were used to guide the design of the longitudinal study of the natural history of cryptosporidiosis in these animals.
2.3 Animals and sampling for longitudinal study
Eighty-one calves born within a 3-week period were selected for the longitudinal birth cohort study of cryptosporidiosis, including 44 calves from birth, 24 calves of 1–2 weeks and 13 calves of 2–3 weeks of age. Among them, one calf died at 4 weeks. Faeces were collected weekly from birth to 3 months of age, biweekly from calves of 3–6 months of age, and monthly from calves more than 6 months of age. From May 2019 to January 2020, a total of 1686 samples were collected from these animals. The consistency of faecal samples was divided into three categories: formed faeces with no diarrhoea (n = 1452), loose faeces with moderate diarrhoea (n = 62), and liquid faeces with watery diarrhoea (n = 172). All faecal samples were collected directly from the rectum of cattle into 50-ml centrifuge tubes by using disposable gloves and stored in 2.5% potassium dichromate at 4°C before DNA extraction.
2.4 DNA extraction
About 200 mg of faecal material from each sample was washed three times with distilled water by centrifugation at 2000 × g for 10 min. Genomic DNA was isolated from the pellet by using a Fast DNA SPIN Kit for Soil (MP Biomedicals, Santa Ana, CA, USA) and stored at −20°C before PCR analysis within 1 year.
2.5 Detection and typing of Cryptosporidium spp.
Cryptosporidium spp. in faecal samples were detected by nested PCR analysis of a ∼830-bp fragment of the SSU rRNA gene in the extracted DNA (Jiang et al., 2005). The species present was determined by sequence analysis of the PCR products. The C. parvum, C. bovis and C. ryanae were subtyped by PCR analysis of ∼830-bp, ∼1300-bp and ∼1000-bp fragments of the gp60 gene, respectively (Feng et al., 2009; Yang et al., 2020; Wang et al., 2021). Each DNA preparation was analysed by PCR at each genetic locus at least twice.
All secondary PCR products of the SSU rRNA and gp60 genes were bidirectionally sequenced using the secondary PCR primers on an ABI 3730 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). The nucleotide sequences generated were assembled using ChromasPro v.1.32 (http://technelysi um.com.au/ChromasPro.html) and aligned with reference sequences from GenBank using ClustalX v.2.1 (http://clust al.org) for the determination of Cryptosporidium species and subtypes.
The genotyping and subtyping results were used in helping to define and differentiate episodes of cryptosporidiosis. Detection of Cryptosporidium spp. after three consecutive negative PCR results and detection of a new species or subtype were considered the beginning of a new episode of cryptosporidiosis.
2.6 Measurement of oocyst shedding intensity
A quantitative PCR (qPCR), the SYBR Green-based 18S-LC2 targeting the SSU rRNA gene, was used to estimate the intensity of oocyst shedding in Cryptosporidium-positive samples (Li et al., 2015). The 20-μl qPCR mix consisted of 10 μl 2 × SYBR Green Real-time PCR Master Mix (ToyoboCo., Ltd., Osaka, Japan), 400 ng/μl bovine serum albumin, 0.5 μM primers (each) and 1 μl DNA. The qPCR was conducted on a LightCycler 480 II (Roche, Indianapolis, IN, USA) using 1 cycle at 95°C for 3 min; 50 cycles at 95°C for 5 s, 55°C for 10 s and 72°C for 40 s; 1 cycle at 95°C for 10 s and 50°C for 30 s and 0.1°C melt steps from 50 to 80°C. The Ct values obtained from the qPCR were used in estimating the number of oocysts per gram of faeces (OPG) in Cryptosporidium-positive samples, using a standard curve generated with negative faecal samples spiked with 101, 102, 103, 104, 105 and 106 oocysts of the C. parvum IOWA isolate.
2.7 Comparative genomic analyses of C. bovis
For whole-genome sequencing, 150-bp or 250-bp paired-end sequence reads were generated from the DNA extracted from immunomagnetic separation-purified oocysts (Guo et al., 2015) using Illumina HiSeq 2500 analysis of an Illumina TruSEquation (v3) library. The completeness of C. bovis genomes in present study was assessed using BUSCO 4.0.6 (Simão et al., 2015). Mauve 2.3.1 (Darling et al., 2010) and GeMoMa 1.7.1 (http://www.jstacs.de/index.php/GeMoMa) were used to align with the published genomes and predict protein-encoding genes, respectively. Burrows Wheeler Alignment (http://bio-bwa.sourceforge.net/), SAMtools (http://www.htslib.org/) and BCFtools (http://www.htslib.org/) were used for single nucleotide polymorphism (SNPs) calling. Maximum-likelihood phylogenies of SNPs across the genomes were constructed using RAxML (http://evomics.org/learning/phylogenetics/raxml/) and substitution rates calculated with the General Time Reversible (GTR) model. In addition, the SNPs were used in principal component analysis (PCA) with SNPrelate (https://www.biostars.org/p/103911/).
The best assembled genome of C. bovis isolates obtained in this study was used as the reference genome. The SNPs were compared and annotated using SnpEff (http://snpeff.sourceforge.net/). The number of SNPs per 1000 bp was calculated for the predicted CDS. In addition, the nucleotide diversity (π) was calculated using DnaSP6 (http://www.ub.edu/dnasp/), the identity of amino acid sequences calculated using ClustalX and the nonsynonymous to synonymous substitution (dN/dS) ratios of genes between C. bovis subtypes using KaKs Calculator 2.0 (Wang et al., 2010).
The highly polymorphic genes identified among C. bovis genomes were annotated by homology analysis of the C. parvum IOWA genome. Sequences of these genes were concatenated and aligned with each other using Muscle (http://www.drive5.com/muscle/). MEGAX (http://www. megasoftware.net/) was used to reconstruct the maximum likelihood trees with the GTR model and 1000 replicates of bootstraps.
2.8 Multilocus sequence typing of C. bovis
Eight polymorphic loci (cgd1_3610, cgd2_2530, cgd3_3410, cgd5_10, cgd5_20, cgd6_390, cgd6_1000 and cgd6_4230) identified in comparisons of C. bovis genomes generated in previous and the present studies were used for multilocus sequence typing (MLST) of C. bovis isolates. Among them, five polymorphic loci (cgd2_2530, cgd3_3410, cgd5_10, cgd5_20 and cgd6_1000) were identified by comparative analyses of two published and two in-house C. bovis genomes (Xu et al., 2020). The remaining three (cgd1_3610, cgd6_390 and cgd6_4230) were identified from comparisons of the XXVIb and XXVIc genomes obtained in this study. Maximum likelihood trees of the concatenated MLST sequences were generated using MEGAX and genetic distances calculated with the GTR model.
2.9 Statistical analysis
Data were analysed using the R package version 3.5.0 (https://www.r-project.org/). The χ2 test was used to compare differences in Cryptosporidium infection rates between age groups or animals with or without diarrhoea. Logistic regression was used to assess the association between putative risk factors (Cryptosporidium species and age of cattle) and the occurrence of diarrhoea. Initially, the strength of association was assessed using a univariate model. Variables with p < .2 were thereafter used to build a multivariate model. Odds ratios (OR) with 95% confidence intervals (CI) and p < .05 were used to identify risk factors for diarrhoea occurrence in calves.
3 RESULTS
3.1 Cryptosporidium occurrence identified by cross-sectional survey
Cryptosporidium spp. were identified in 136 of the 934 (14.6%) samples collected in the cross-sectional survey. The highest infection rate was 29.4% (95/323) in cattle of 0–2 months (Figure 1a). Cryptosporidium infection was significantly correlated with young age (χ2 = 89.398, p < .001). Animals with diarrhoea (11/40; 27.5%) had a higher infection rate than those (125/903; 13.8%) without diarrhoea (χ2 = 5.789, p = .016, OR = 2.361, CI = 1.150-4.847).
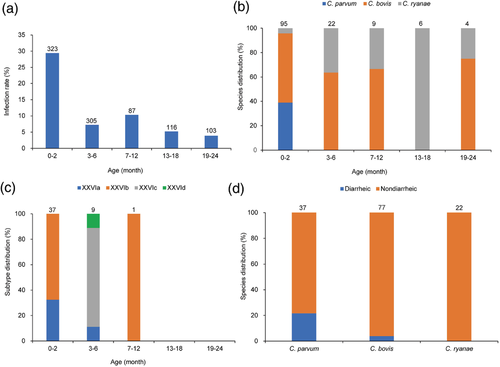
Three Cryptosporidium species were detected, including C. parvum (n = 37), C. bovis (n = 77) and C. ryanae (n = 22). Cryptosporidium parvum was only identified in calves of 0–2 months, while C. bovis and C. ryanae were detected in animals of a broad range of age (Figure 1b). Among the three species, C. bovis was the dominant species even for animals of 0–2 months of age. The gp60 sequence analysis showed that all C. parvum isolates (n = 37) belonged to the IIdA19G1 subtype, while the C. bovis isolates (n = 47) belonged to four subtypes, including XXVIa (n = 13), XXVIb (n = 26), XXVIc (n = 7) and XXVId (n = 1). Among the latter, XXVIa and XXVIb were found mainly in animals of 0–2 months, while XXVIc and XXVId were found only in animals of 3–6 months (Figure 1c). In addition, the occurrence of C. parvum infection was significantly associated with diarrhoea (χ2 = 28.255, p < .001, OR = 7.455, CI = 3.160-17.597). Infections with C. ryanae and C. bovis were not associated with the occurrence of diarrhoea (Figure 1d).
3.2 Natural history of cryptosporidiosis in cattle in longitudinal study
3.2.1 Infection dynamic of Cryptosporidium spp.
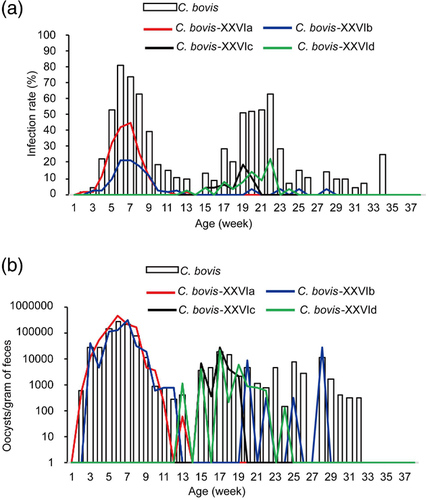
Based on the outcome of the cross-sectional survey, the natural history of Cryptosporidium infections was longitudinally followed in a birth cohort of 81 calves for 38 weeks of age. Calves started to shed Cryptosporidium spp. in faeces on day 4 of life. The infection rate increased rapidly during the first 2 weeks, with almost all calves infected by 3 weeks. Overall, calves in the study experienced three waves of cryptosporidiosis, with peak infection rates at 2–3 weeks, 6–8 weeks and 19–22 weeks (82.7-92.7%, 83.8-86.3% and 59.5-66.7%, respectively). Starting from 24 weeks, the infection rates remained low (Figure 2a).

The pattern for oocyst shedding intensity mirrored the one for infection rates. High oocyst shedding was seen during the first 3 weeks of life, reaching the mean level of 1.18 × 106 ± 2.15 × 106 OPG at the peak. The oocyst shedding decreased briefly afterwards, but increased again at 5 weeks, reaching a second peak at 6 and 7 weeks. The OPG values decreased gradually to low levels at 12 and 13 weeks, and increased again at 14 weeks and maintained at the level until 31st week. Among the three peaks in oocyst shedding, the second and third peaks were much broader than the first one; the average OPG value during the first shedding period was approximately one log higher than that of the second shedding period, while the one for the second shedding period was approximately one log higher than that of the third shedding period (Figure 2b).
Of the 1686 faecal samples collected from the longitudinally followed calves, 747 were positive for Cryptosporidium spp. (Table S1) Among them, three Cryptosporidium species were identified, including C. parvum (n = 147), C. bovis (n = 449) and C. ryanae (n = 151). In addition, there were ten co-infections of C. parvum and C. bovis at 2–4 weeks of age, and three co-infections of C. bovis and C. ryanae at 6–10 weeks of age (Figure 2a, Table S2).
3.2.2 Infection dynamic of C. parvum
Cryptosporidium parvum was the dominant species in the first 3 weeks of life. It first appeared in calves on day 4. The highest infection rate was 92.7% (63/68) at 2 weeks, and the accumulative infection rate before weaning was 97.4% (76/78). Afterwards, infections due to C. parvum decreased rapidly. After 5 weeks of age, there was nearly no more detection of the species (Figure 2a). The intensity of oocyst shedding followed the infection pattern, with peak OPG of C. parvum reaching an average of 1.20 × 106 ± 2.16 × 106 at 2 weeks (Figure 2b).
Of the 147 C. parvum-positive samples, IIdA19G1 was the only subtype identified. Therefore, calves on the farm inevitably experienced one episode of C. parvum infection, which occurred shortly after the birth of the animals. The mean length of C. parvum infection was 2.3 ± 0.5 weeks.
3.2.3 Infection dynamic of C. bovis
Cryptosporidium bovis started to appear in calves on day 9. Infection with the species, however, increased mostly after the waning of C. parvum infection (Figure 2a). The accumulative infection rate of C. bovis during the study period was 100.0% (80/80) by 8 weeks. Compared with one peak for C. parvum, there were two infection peaks for C. bovis at 6–8 weeks and 19–22 weeks of age. The average infection rate during the first peak was higher than during the second peak. The mean length of C. bovis infection was 3.9 ± 1.2 weeks for the first infection episode and 4.0 ± 2.4 weeks for the second infection episode (Figure 4a).
The shedding of C. bovis oocysts followed the infection pattern, but with slower decreases in OPG values (Figure 2b). The average OPG value (2.81 × 105 ± 8.03 × 105) at the first C. bovis infection peak was lower than that of C. parvum (1.20 × 106 ± 2.16 × 106), but much higher than at the second C. bovis infection peak (8.04 × 102 ± 1.14 × 103).
Of the 449 C. bovis-positive samples identified in the longitudinal study of Cryptosporidium infections, four subtypes were detected, including XXVIa, XXVIb, XXVIc and XXVId. Before 12 weeks of age, only XXVIa and XXVIb subtypes were identified. At 14 weeks and thereafter, XXVIc and XXVId were the dominant C. bovis subtypes, with occasional detection of XXVIb at 20 weeks and thereafter (Figure 3a and Table S2). The oocyst shedding intensity for the four C. bovis subtype families followed the pattern of infection rates (Figure 3b). Among the 80 surviving calves, 35 had infections by two subtype families and 11 had infections by three. In addition, due mostly to subtyping failure, 33 calves had infection by one subtype family and one calf had C. bovis infection with unknown subtype family (Figure 4b).
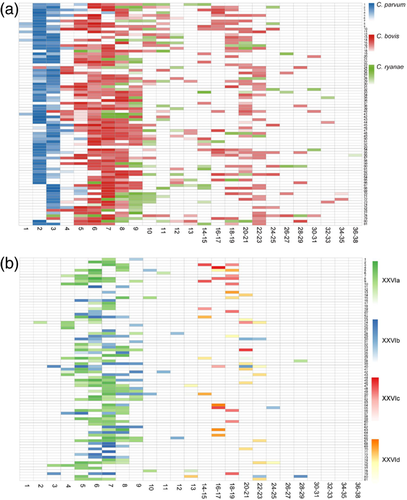
3.2.4 Infection dynamic of C. ryanae
For C. ryanae, the earliest detection of the pathogen was the 20th day of life. The infection rate of the species increased slowly afterwards, reaching a peak of 31.7% (25/79) at 9 weeks of age. Overall, the infection pattern of C. ryanae followed the one for C. bovis, but with much lower frequency (Figure 2a). The accumulative infection rate of C. ryanae in the study period was 80.0% (64/80). Among the 151 C. ryanae-positive samples collected during the longitudinal study, only one subtype was identified. Therefore, most calves had only one episode of C. ryanae infection spreading over an extended period (Figure 4a). The mean length of C. ryanae infection was 3.2 ± 0.9 weeks.
3.2.5 Correlation between Cryptosporidium spp. and diarrhoea and age
In χ2 analysis of data from the longitudinal study, only C. parvum infection was associated with the occurrence of moderate diarrhoea (χ2 = 8.457, p = .004, OR = 4.810, CI = 1.503-15.389) and watery diarrhoea (χ2 = 38.182, p < .001, OR = 9.409, CI = 1.503-15.389) in pre-weaned calves. In contrast, infections with C. ryanae and C. bovis were not associated with the occurrence of any diarrhoea (Table S3).
Results of logistic regression analysis confirmed that C. parvum infection was a risk factor for the occurrence of watery diarrhoea (p < .001, OR = 8.49, CI = 3.66-21.53). Unlike the result of χ2 analysis, C. ryanae infection was marginally associated with the occurrence of watery diarrhoea in both univariate (p = .028, OR = 1.75, CI = 1.04-2.81) and multivariate analyses (p = .041, OR = 1.72, CI = 1.00-2.83) of the data. However, this could be due to the fact that diarrhoea was more common in post-weaned calves during the study (p < .001, OR = 4.29, CI = 3.13-6.00; p = .000, OR = 5.83, CI = 3.36-10.99), as C. ryanae infection occurred mostly after the weaning (Table 1, Figure 2a).
| Diarrhoea status | Factor | Univariate model | Multivariate model | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | ||
| Watery diarrhoea | Any Cryptosporidium | 0.88 | 0.63-1.21 | .420 | NA | NA | NA |
| C. parvum | 1.43 | 0.84-2.30 | .164 | 8.49 | 3.66-21.53 | <.001 | |
| C. bovis | 0.50 | 0.32-0.75 | .001 | 0.87 | 0.55-1.35 | .558 | |
| C. ryanae | 1.75 | 1.04-2.81 | .028 | 1.72 | 1.00-2.83 | .041 | |
| No Cryptosporidium | 1.00 | Reference | 1.00 | Reference | |||
| Pre-weaned (<2 months) | 0.31 | 0.20-0.47 | <.001 | 0.95 | 0.29-3.28 | .929 | |
| Post-weaned (2-6 months) | 5.17 | 3.52-7.81 | <.001 | 10.69 | 4.77-30.53 | <.001 | |
| Juveniles (>6 months) | 0.15 | 0.05-0.33 | <.001 | NA | NA | NA | |
| Moderate diarrhoea | Any Cryptosporidium | 0.45 | 0.24-0.78 | .007 | 0.50 | 0.25-0.98 | .320 |
| C. parvum | 1.37 | 0.56-2.89 | .439 | NA | NA | NA | |
| C. bovis | 0.24 | 0.08-0.54 | .002 | 0.28 | 0.10-0.64 | .007 | |
| C. ryanae | 0.82 | 0.25-2.04 | .707 | NA | NA | NA | |
| No Cryptosporidium | 1.00 | Reference | 1.00 | Reference | |||
| Pre-weaned (<2 months) | 0.43 | 0.22-0.79 | .010 | 0.89 | 0.34-2.44 | .822 | |
| Post-weaned (2-6 months) | 2.21 | 1.3-3.87 | .004 | 1.96 | 0.95-4.59 | .088 | |
| Juveniles (>6 months) | 0.81 | 0.35-1.63 | .585 | NA | NA | NA | |
| Any diarrhoea | Any Cryptosporidium | 0.72 | 0.54-0.96 | .027 | 1.01 | 0.71-1.43 | .050 |
| C. parvum | 1.45 | 0.91-2.22 | .101 | 6.97 | 3.41-15.03 | <.001 | |
| C. bovis | 0.41 | 0.27-0.59 | <.001 | 0.68 | 0.45-1.01 | .065 | |
| C. ryanae | 1.50 | 0.93-2.34 | .082 | 1.40 | 0.86-2.24 | .164 | |
| No Cryptosporidium | 1.00 | Reference | 1.00 | Reference | |||
| Pre-weaned (<2 months) | 0.32 | 0.22-0.46 | <.001 | 0.68 | 0.3-1.54 | .353 | |
| Post-weaned (2-6 months) | 4.29 | 3.13-6.00 | <.001 | 5.83 | 3.36-10.99 | <.001 | |
| Juveniles (>6 months) | 0.29 | 0.15-0.49 | .367 | NA | NA | NA | |
- CI, confidence interval; NA, not available; OD, odds ratio.
3.2.6 Genome characteristics of C. bovis
Whole genome sequence data were obtained from five isolates of representative C. bovis subtypes with genome assemblies of 9.02- 9.07 Mb in 105–441 contigs (Table S4). BUSCO analysis showed that over 400 of the 446 core protein-encoding genes of apicomplexan parasites were included (Figure S1). Comparative genomic analysis showed the presence of 437–2650 SNPs between subtypes XXVIa, XXVIb and XXVIc (Table S5). In PCA and phylogenetic analyses, genomes of subtype XXVIa and XXVIb clustered together away from those of subtype XXVIc (Figure 5a and b).
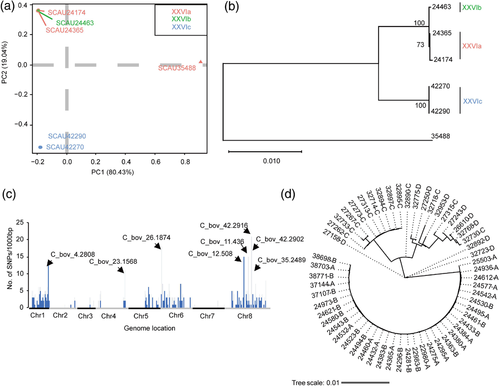
Nine highly polymorphic genes were identified among the sequenced genomes, including C. bovis-specific C_bov_12.508, C_bov_42.2902 and C_bov_42.2916 and orthologs of cgd1_590, cgd1_3610, cgd3_1780, cgd6_390, cgd6_1080 and cgd6_4230 in C. parvum (Figure 5c). They encode the GP60 glycoprotein, a rhoptry protein, a WYLE family protein, and six hypothetical proteins. Two of them are secretory proteins with signal peptides (Table 2). In addition, among the nine genes, four had dN/dS ratios > 1, thus were under positive selection. In contrast, the gp60 gene (C_bov_15.895) had dN/dS ratio < 1, thus was under purifying selection (Figure S2). A phylogenetic tree constructed using concatenated sequences from these highly polymorphic genes showed subtypes XXVIa and XXVIb were divergent from subtype XXVIc (Figure S3).
| Gene in C. bovis | Ortholog in C. parvum | Annotation | Nucleotide diversity (pi) | Signal peptide | TMHMM | Amino acid identity to SCAU42270 (%) |
|---|---|---|---|---|---|---|
| C_bov_18.895 | cgd6_1080 | GP60 | 0.330 | YES | 0 | 47.8 |
| C_bov_35.2489 | cgd3_1780 | Rhoptry protein | 0.045 | YES | 1 | 81.1 |
| C_bov_42.2902 | — | Hypothetical protein | 0.025 | NO | 0 | 89.3 |
| C_bov_11.436 | cgd6_4230 | Signal peptide-containing protein | 0.030 | YES | 0 | 91.3 |
| C_bov_42.2916 | — | Hypothetical protein | 0.022 | YES | 1 | 94.0 |
| C_bov_12.508 | — | WYLE protein family | 0.012 | YES | 2 | 96.8 |
| C_bov_4.2808 | cgd1_3610 | Signal peptide-containing protein | 0.008 | NO | 1 | 98.0 |
| C_bov_23.1568 | cgd6_390 | Hypothetical protein | 0.006 | NO | 0 | 98.4 |
| C_bov_26.1874a | cgd1_590 | Hypothetical protein | — | YES | 0 | — |
- a The gene in the two subtype XXVIc samples was not fully assembled.
To examine the genomic characteristics of C. bovis in the cohort calves, 80 C. bovis-positive samples of the four subtypes, including XXVIa (n = 20), XXVIb (n = 20), XXVIc (n = 17) and XXVId (n = 23), were selected for a MLST analysis at eight genetic loci (cgd1_3610, cgd2_2530, cgd3_3410, cgd5_10, cgd5_20, cgd6_390, cgd6_1000 and cgd6_4230). The amplification efficiency ranged from 86.2% to 97.5%. Among the 80 isolates analysed, XXVIa and XXVIb isolates were identical to each other in sequences at all eight loci, XXVIc had sequence polymorphism at seven loci except cgd2_2530, while XXVId had sequence polymorphism at all eight loci. Altogether, 56 of the 80 isolates were characterized at all eight loci successfully. A phylogenetic tree constructed using concatenated sequences from the eight polymorphic loci showed that XXVIc and XXVId were genetically related to each other, forming a large cluster divergent from the cluster formed by XXVIa and XXVIb. Between XXVIc and XXVId, XXVId had higher genetic diversity (Figure 5d).
4 DISCUSSION
In this study, we have unravelled the infection dynamics of C. parvum, C. bovis and C. ryanae in a natural cryptosporidiosis model. Calves had four episodes of cryptosporidiosis during the longitudinal follow-up period. The two episodes of C. bovis infections were caused by two genome types that did not segregate by subtype at the commonly used subtyping locus gp60. These data suggest that the infection patterns of Cryptosporidium spp. are affected by the species and genome types of the pathogens due to the lack of cross-species and full species-specific immunity after the primary infection.
Dairy cattle apparently experience waves of Cryptosporidium infection during early life. As shown in the present longitudinal birth cohort study, most calves had three infection peaks during the first six months. Although three Cryptosporidium species were responsible for them, there was no absolute association between individual infection peaks and species. The first infection was clearly due to C. parvum. In contrast, C. bovis was associated with both the second and third infection peaks, while C. ryanae was detected at low frequency over the second and third infection periods, mostly after the first peak by C. bovis. As there was a well-defined separation between the initial C. bovis infection and the appearance of C. ryanae, almost all dairy calves in the longitudinal studies had four episodes of cryptosporidiosis. This is in contrast with the previous reports of two episodes of cryptosporidiosis in longitudinal birth-cohort studies in the United States and Europe (Åberg et al., 2020; Santín et al., 2008; Xiao & Herd, 1994). In two of these studies, however, some oocyst shedding was observed after the initial peak by C. bovis and C. ryanae, but was attributed to sporadic shedding of oocysts from the initial infection (Åberg et al., 2020; Santín et al., 2008). In humans, children also experience multiple episodes of cryptosporidiosis by different Cryptosporidium species before the development of full protective immunity (Cama et al., 2008; Kattula et al., 2017).
Dairy calves in different areas appear to have different infection patterns of Cryptosporidium spp. Among the three major intestinal species, C. parvum has the same infection pattern in dairy calves everywhere regardless the nature of endemicity (highly prevalent versus only seen on some farms) and subtypes (IIa in most areas vs. IId in China and Sweden) involved. In almost all studies conducted thus far on farms with C. parvum, infection with the species starts soon after birth, peaks at 2–3 weeks of age, and recovers mostly by 4 weeks (Cai et al., 2017; Qi et al., 2020; Santín et al., 2008; Soltane et al., 2007; Thomson et al., 2019). In contrast, the occurrence of C. bovis and C. ryanae in dairy calves differs by areas. In most areas, C. bovis and C. ryanae are mainly found in post-weaned calves, with peak occurrence at 16–20 weeks of life (Lichtmannsperger et al., 2020; Santı́n et al., 2004; Santín et al., 2008; Smith et al., 2014; Soltane et al., 2007; Thomson et al., 2019). In China and Sweden where C. parvum occurs only on some farms and are mainly cause by IId rather than IIa subtypes, C. bovis and C. ryanae are a common presence in pre-weaned dairy calves (Åberg et al., 2020; Cai et al., 2017; Guo et al., 2022; Qi et al., 2020; Silverlas & Blanco-Penedo, 2013). Therefore, it is likely that the occurrence of C. parvum affects the occurrence of C. bovis and C. ryanae in pre-weaned dairy calves. In a longitudinal birth cohort study of cryptosporidiosis in dairy calves on a Swedish farm without C. parvum, C. bovis infection occurred earlier, with peak infection rates at 3–5 weeks of life, compared with 6–9 weeks in the present study (Åberg et al., 2020). This is similar to the pattern seen on beef farms without C. parvum (Björkman et al., 2015; Rieux et al., 2014). In a recent short-term birth cohort study of Cryptosporidium spp. in 25 dairy calves on a farm with C. parvum in Xinjiang, China, C. bovis started to appear at 4 weeks soon after the waning of C. parvum infection and reached peak by 9 weeks (Qi et al., 2020). This is in agreement with the pattern of cryptosporidiosis identified in the present study. Therefore, on Chinese dairy farms with IId subtypes of C. parvum, most calves have experienced two episodes of cryptosporidiosis by weaning, compared with the occurrence of only IIa subtypes of C. parvum in other countries. Reasons for the occurrence of C. bovis and C. ryanae in pre-weaned dairy calves in China even in the presence of C. parvum are not yet clear.
In this natural model of cryptosporidiosis, there is no apparent cross-species immunity after an episode of cryptosporidiosis. Calves experienced only one episode of C. parvum infection during the 9-month follow-up period, indicating the existence of species-specific immunity. This was also supported by the occurrence of only one episode of C. ryanae infection in these calves. The immunity against one Cryptosporidium species, however, is not strong enough to prevent infection with other species, as most animals infected with C. parvum had subsequent infections with C. bovis and C. ryanae, which have the same predilection site as C. parvum. In fact, most calves in the study had two episodes of infections with C. bovis. However, they were caused by different subtype families, indicating that there could be protective immunity against Cryptosporidium spp. at the subtype level. Previously, results of longitudinal birth cohort studies of cryptosporidiosis in infants in low- and middle-income countries also suggested that there was no apparent cross-species immunity against Cryptosporidium spp. in humans (Cama et al., 2008; Kattula et al., 2017).
The protective immunity against C. bovis has apparently led to the unique distribution of subtypes between the two episodes of C. bovis infections. In the present study, the first episode of C. bovis infection was caused by subtypes XXVIa and XXVIb, which differ in the whole genome by only 405–437 SNPs, most of which are at the gp60 locus. In contrast, the second episode of C. bovis infection was caused by XXVIc and XXVId. Although we have failed in obtaining the whole genome sequences of XXVId, the MLST data obtained indicated that XXVIc and XXVId are genetically related and there are >2000 SNPs between XXVIc and XXVIa or XXVIb at the genome level. Therefore, while the immunity developed against XXVIa confers cross-protection against infection with XXVIb because of high genome sequence identity, this immunity is not strong enough against subtypes XXVIc and XXVId with divergent genomes. Partial immunity, however, could be operating at the subtype level, as the second C. bovis infection had lower intensity of oocyst shedding than the first infection.
The three major intestinal Cryptosporidium species appear also differ in pathogenicity. The early occurrence of C. bovis and C. ryanae in pre-weaned dairy calves in the study area offers a good opportunity to study their pathogenicity, as this is the period when neonatal animals experience diarrhoea most. As expected, C. parvum infection was strongly associated with the occurrence of watery diarrhoea despite the factor that a IId subtype rather than a IIa subtype was involved. Nevertheless, there was no such an association with C. bovis infection. In fact, C. bovis was mostly detected in animals without diarrhoea during this period. There was a weak association between C. ryanae infection and diarrhoea in some of the statistical analyses, but this could be due to the small numbers of diarrheic animals involved. This is in agreement with the results of other studies elsewhere, which mostly detected no association between C. bovis or C. ryanae infection and the presence of diarrhoea (Rieux et al., 2013; Cai et al., 2017; Thomson et al., 2019; Åberg et al., 2020; Qi et al., 2020; Zhang et al., 2021). This is expected, as the intensity of oocyst shedding by C. bovis and C. ryanae is significantly lower than C. parvum, even in pre-weaned calves as commonly seen in China and Sweden (Åberg et al., 2020).
The use of advanced molecular characterization tools and quantitation of oocyst shedding by qPCR have played a major role in clarifying the distribution of Cryptosporidium species and immunity development against cryptosporidiosis. In the present study, molecular diagnostic tools were used not only in the differentiation of the three morphologically similar Cryptosporidium species but also in subtyping C. bovis identified. This led to the identification of four well-defined episodes of cryptosporidiosis in most calves over the 9-month follow-up period. Data from MLST and comparative genomic analyses of C. bovis have further indicated that the unique occurrence of XXVIa and XXVIb in the first episode and XXVIc and XXVId in the second episode of C. bovis infections was due to incomplete acquired immunity against parasites of divergent genomes. This was confirmed by the lower oocyst shedding intensity in the second episodes of C. bovis infection. Previously, additional spikes in oocyst shedding after the primary C. bovis infection were present in the few longitudinal birth cohort studies of bovine cryptosporidiosis conducted (Åberg et al., 2020). The use of advanced typing tools would have identified the presence of additional episodes of C. bovis infection. The lack of additional episodes of C. parvum and C. ryanae infection was likely due to the presence of only one subtype of each species on the study farm. The presence of four C. bovis subtypes on the study farm also indicates that genetic recombination is likely common in this species, as seen in the lack of segregation of genomes by gp60 subtype in the present study. This will likely result in instable transmission of the species at the gp60 subtype level. High genetic diversity at the subtype family level and differences in subtype occurrence between pre-weaned and post-weaned dairy calves have been recently identified in C. bovis, with apparent genetic recombination among some of the subtype families in gp60 sequences (Wang et al., 2021).
5 CONCLUSION
Using longitudinal follow-up and advance characterization of pathogens in a birth cohort of calves, we have revealed the dynamics of Cryptosporidium infections in a natural cryptosporidiosis model. Data from the study have shown the occurrence of four episodes of cryptosporidiosis over the first nine months of life, with no apparent cross-species immunity among the three intestinal Cryptosporidium species involved. The alternation of infections by different C. bovis subtype families in these animals before and after weaning suggests that protective immunity against cryptosporidiosis is operative at the subtype level. Despite the early appearance of C. bovis and C. ryanae in life, these species remain non-pathogenic. Elucidation of genetic determinants for the unique spectrum of Cryptosporidium species distribution and pathogenicity would be key to the understanding of cryptosporidiosis in humans and development of effective drugs and vaccines.
ACKNOWLEDGEMENTS
This work was supported in part by National Natural Science Foundation of China (U1901208 and 32030109), Guangdong Major Project of Basic and Applied Basic Research (2020B0301030007), the 111 Project (D20008), and Innovation Team Project of Guangdong University (2019KCXTD001).
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ETHICS STATEMENT
This study was approved by the Ethics Committee of the South China Agricultural University. Faecal samples from dairy cattle were collected with the permission of the farm managers and the cattle were handled in accordance with the Animal Ethics Procedures and Guidelines of the People's Republic of China.
AUTHOR CONTRIBUTIONS
Conceptualization: L.X., Y.F. and Y.G. Methodology: L.X. and S.H. Formal analysis: L.X., Y.F., S.H., M.W., W.H., and D.S. Writing - original draft: S.H. Writing - review & editing: Y.F., L.X. and Y.G. Funding acquisition: L.X. and Y.F. Sampling: S.H., W.W. and R.L. Supervision: L.X., Y.F. and Y.G.
Open Research
DATA AVAILABILITY STATEMENT
Age and clinical information and Cryptosporidium detection associated will all faecal samples collected in the longitudinal study are presented in Supplementary Table S1 online at the journal website. Whole genome sequence data from C. bovis, including raw sequence reads and genome assemblies, were deposited in GenBank under BioProject PRJNA794035. Representative sequences of the gp60 gene of C. bovis and C. ryanae were deposited in GenBank under accession numbers OM136967-OM136970, and OM165200.




