Brucellosis in Ethiopia: A comprehensive review of literature from the year 2000–2020 and the way forward
Funding information:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abstract
Brucellosis is a zoonotic disease of considerable economic and public health significance globally. Despite the limited bacteriological evidence, a large number of serological works revealed that it is prevalent both in livestock and humans in Ethiopia. The current comprehensive review was carried out to provide apparent pooled seroprevalence (APS) estimates at individual animal and herd levels in livestock, and identify factors causing variability between studies conducted over the last two decades, show the spatial distribution, as well as summarizes Brucella species reported from livestock. It also provides APS of brucellosis in humans and evaluates the public health awareness of zoonotic brucellosis. In this review, systematic and synthetic review approaches were followed to summarize the available information. For the systematic review and meta-analysis, articles were selected based on predefined criteria. Data extracted from these articles were analysed using meta-analytical approaches to provide APS estimates and in-between study variations for humans and all livestock species considered. Sensitivity analyses and bias assessments were conducted using influence plot analysis and, Egger's and Begg's statistics along with funnel plots, respectively. Synthetic review approaches were used to summarize data on isolates and public health awareness. Pooled seroprevalence estimate of brucellosis at national level was 2.6% (95% CI: 2.2–3.0) in cattle, 4% (95% CI: 3.1–5.1) in goats, 3% (95% CI: 2.3–3.9) in sheep and 3% (95% CI: 2.4–3.7) in camels. At a herd level, 16.3% (95% CI: 12.9–20.5) of cattle, 12.1% (7.1–19.9) of goat, 13.3% (7.6–22.1) of sheep and 19.7% (13.8–27.4) of camel herds in the country had at least one seropositive animal. Cattle in the pastoral/agropastoral production systems had significantly higher (p < .05) APS compared to mixed crop-livestock and urban/peri-urban dairy production systems. Pooled seroprevalence of brucellosis in small ruminants (8.3%, 95% CI: 6.3–10.8) and camels (4.4%, 95% CI: 3.5–5.6) in Afar were significantly higher (p < .05) than in other regions. Reports conducted using ELISA and serial Rose Bengal plate test (RBPT)-ELISA had higher (p < .05) APS estimates than serial RBPT and complement fixation test. Brucella melitensis and B. abortus were reported from goats and cattle, respectively, from three available reports. The APS of brucellosis in humans was 5% (95% CI: 3.3–7.3). Public awareness of brucellosis was low (18.4%), while, practices that expose humans to Brucella infection were high. Scenario-based control interventions on regions and production systems using one health approach are suggested.
1 INTRODUCTION
Brucellosis is one of the economically important diseases of livestock caused by members of the genus Brucella (OIE, 2020a). The disease is characterized by reproductive disorders such as abortion, stillbirth and birth of weak offspring in females and orchitis and epididymitis in male animals causing transient or permanent infertility (Constable et al., 2017). The genus Brucella currently comprises six classical species primarily affecting domestic animals and rodents including B. melitensis of small ruminants, B. abortus of cattle, B. suis of pigs and hares, B. ovis of small ruminants, B. canis of dogs and B. neotomae of desert wood rats; and six novel species identified from marine mammals (B. penippedialis and B. ceti), red foxes (B. vulpes), baboons (B. papionis), a human breast implant (B. inopinata) and rodents (B. microti) (Scholz et al., 2018). While brucellae are host-adapted to preferred hosts, they are capable of infecting other species (Pappas, 2010). For instance, B. abortus, which is host-adapted to cattle, can infect small ruminants (Ocholi et al., 2005) and wildlife (Godfroid, 2018), complicating the epidemiology of the disease and its control measures. Camels are known to be infected by both B. melitensis and B. abortus when they are reared in close contact with small ruminants and cattle, respectively (Gwida et al., 2012; OIE, 2018). The direct economic impact of the disease is associated with loss of replacement stock, reduction in milk production and culling of valuable reproductive age animals further constraining herd expansion. In countries like Ethiopia, where the export of live animals is one of the sources of foreign exchange earnings, brucellosis hinders access to lucrative international markets. Where market accesses are permitted, the requirements by importing countries of testing every individual animal at export quarantines and rejection of those testing positive, further adds up to the economic loss (Franc et al., 2018).
Brucellosis is also one of the significant zoonotic diseases affecting 0.83 million individuals worldwide, annually (WHO, 2015). Human brucellosis is most commonly caused by B. melitensis, B. abortus and B. suis (Godfroid et al., 2005). In humans, brucellosis is mostly a chronic debilitating disease manifested by non-specific clinical syndromes such as fever or chills, arthralgia/arthritis, myalgia, malaise, sweating, weight loss and several other symptoms depending on the organ system involved as a result of localization of the organism following bacteremia (Dean et al., 2012; Franco et al., 2007b). The disease is mainly transmitted to humans through direct contact with infected animals and consumption of contaminated raw milk and dairy products made of unpasteurized milk (Pappas et al., 2005). The burden of the disease is believed to be the highest in low and middle-income countries where the disease is endemic in livestock (McDermott et al., 2013) including countries bordering the Mediterranean Sea, the Middle East, central Asian and Latin America. In most countries of Africa, the disease is believed to be endemic. However, only a few official figures are available (Dean et al., 2012a; Pappas et al., 2006).
In Ethiopia, a country with an estimated population of 112,078,730 humans (The World Bank, 2020), 59.5 million heads of cattle, 30.7 million sheep, 30.2 million goats, 1.2 million camels and 11.1 million equines (CSA, 2017), serological studies conducted so far demonstrated that the disease is endemic across greater areas of the country (Asmare et al., 2013; Regassa et al., 2009; Sintayehu et al., 2015; Teshome et al., 2003). However, no official figures are available both for livestock and human brucellosis. Only the presence, absence or suspected statuses of the disease during the various years between 1996 and 2019 in livestock and humans had been reported to the World Organization for Animal Health (OIE) (OIE, 2020b). No control strategy including vaccination is so far been implemented against brucellosis in any of the livestock species in Ethiopia. Studies conducted to estimate the prevalence of brucellosis in the country were conducted by individual researchers in research organizations or higher education institutions. Therefore, they were fragmented and are limited in space, time and scope; as a result, there is a need for summarizing such data to make them useful in understanding the disease burden and its distribution at a national level to devise appropriate intervention strategies. Several narrative reviews (Yilma et al., 2016; Yohannes et al., ) and a few systematic reviews and meta-analyses are available on brucellosis in Ethiopia (Asmare et al., 2014; Tadesse, 2016). While the latter were able to provide pooled prevalence estimates at national and regional levels, they failed to demonstrate the patchy occurrence of the disease. None of the reviews attempted to demonstrate the herd-level pooled prevalence of the disease, an epidemiological feature that is more important in understanding the extent of its distribution across the national herds/flocks. Besides, a large body of literature was made available on the subject area since the publication of the last systematic review. In addition, summarized information on public health awareness of the disease and the circulating Brucella species are lacking. This comprehensive review is, therefore, conducted with the objectives of updating the current epidemiology of brucellosis in both livestock and humans in Ethiopia. The livestock aspect deals with individual- and herd-level brucellosis status, potential predictors and provides a summary of the reported isolates. The appraisal further attempted to illustrate the disease's spatial distribution pattern along the species and describe the public health awareness levels. Finally, it suggests the way forward with a contextual intervention strategy to reduce the economic and public health impact of brucellosis in Ethiopia.
2 MATERIAL AND METHODS
2.1 Literature search strategy
Published articles on livestock and human brucellosis in Ethiopia were searched using CAB direct, African journals online (AJOL), Medline and Web of Science databases. Google Scholar, Yahoo and Bing were used as search engines. Articles written in English were searched online from June 1, 2020 to July 30, 2020. The key strings used in electronic searches were, ‘brucellosis’, ‘livestock’ ‘cattle’, ‘bovine’, ‘camel’, ‘dromedary camel’, ‘goat’, ‘caprine’, ‘sheep’, ‘ovine’, ‘small ruminant’, ‘swine’, ‘pig’, ‘human’, ‘zoonoses’, ‘public health’, ‘professional hazard’, ‘reproductive disorders’, ‘reproductive problems’, ‘abortion’, ‘Brucella’ and ‘Ethiopia’. However, these keywords were rearranged to phrase as close as ‘Livestock brucellosis’, ‘Zoonotic risk of brucellosis’, Brucellosis in cattle’, ‘Bovine brucellosis’ etc. in Ethiopia, in many combinations to match words in the title or the keywords of published documents. Furthermore, references of published review papers were scanned to increase the chance of finding published papers that were not found during keyword searches.
2.2 Inclusion and exclusion criteria
This review article considered data from original research articles published in peer-reviewed journals and made available online. Initial screening of the manuscripts was made based on titles and abstracts. Articles that contained information on brucellosis in livestock and humans were passed for full-text evaluation based on the following criteria: (i) brucellosis in cattle, sheep, goats, camels, pigs and humans (yes/no); (ii) clear description of the study objective(s) (prevalence estimation or other objectives); (iii) appropriateness of the study methodology in attaining the set objectives including the study design, representativeness of the sampling, traceability of the sampled animals to actual source population and diagnostic tests employed; (iv) clear description and presentation of results along risk factors and (v) the study period.
In prevalence estimate, the study designs deemed appropriate for the current review were cross-sectional and cohort studies as both designs help in estimating disease burden in populations. The accepted diagnostic tests were those considered to be comparable to or better than complement fixation test (CFT) that included serial Rose Bengal plate test-complement fixation test (RBPT-CFT), serial RBPT-ELISAs and ELISAs alone (OIE, 2018). Rose Bengal plate test alone was excluded as it has low specificity. Random sampling strategies were accepted to select representative samples from the study population, while non-random methods were left out intentionally. Studies conducted on animals in known geographic locations were considered as eligible, while those transported and tested at slaughterhouses/abattoirs and export quarantines were excluded from analysis due to lack of data on the actual origin. In Ethiopia, livestock may be transported from the primary market of origin and traded in several intermediate markets before they are transported to these facilities, therefore, in the absence of animal identification and traceability systems, the terminal markets cannot be considered as sources of the animals (Ayele et al., 2003). In line with the inclusion criteria, manuscripts that fulfilled all the above criteria were rated to be of high quality, those fulfilling criteria for sampling design, representative sampling methods and appropriate laboratory tests were considered of moderate quality even though they may not describe how sample sizes were determined. Articles that did not fulfil any one of the following requirements including study design, representative sampling methods, substandard laboratory assay or unclear result presentations were considered of low quality. Articles rated to be of moderate and above quality were included in the review. Abstracts of inaccessible articles, proceedings, review articles, case reports, outbreak reports, articles published before the year 2000 and those rated as low quality were excluded. In human studies, the natures of questionnaire used were critically assessed from organization perspective for capturing knowledge, attitude and practices of the respondents.
Articles that had data on public health awareness, but not included in the systematic review and meta-analysis for the various reasons given above were used to prepare synthetic reviews to describe the knowledge, attitude and practices of the human populations included in the studies. Similarly, studies published based on microbiological and molecular methods were used to summarize Brucella species affecting livestock and humans in Ethiopia regardless of whether they were included in the systematic review or not.
2.3 Data extraction
Data extraction template was developed by the authors based on the most commonly reported and biologically plausible predictors used in the studies. The template was piloted on selected articles to check for unforeseen shortcomings. Following a discussion on the results of piloted template, ambiguities were removed and the amended one was used for data extraction. Two authors were involved in literature search and data extraction, independently. Once the extraction was completed, the data were cross-checked for inconsistencies between the two extracts. Points of difference were discussed and amendments were made accordingly. In cases where the differences persisted, a third author was invited as a referee. Finally, a unified dataset was produced for subsequent data analyses. Both individual animal- and herd/flock-level data were separately extracted. The dataset contained the following information on livestock: author(s) name, publication year, year of study, manuscript title, journal information (name, volume, issue, and pages), web-link to the article (alternatively, DOI, where available), study area (region, zone, district), species of animal(s), breed, production system, type of animal management, agro-ecology, study design, sampling strategy, diagnostic test, sample size, number of test positives, number of test negatives, prevalence and associated 95% confidence intervals for each of the hypothesized predictors. Similarly, the human data, in addition to all the applicable basic article information and predictors, contained information on the health status of sampled individuals and means of livelihood (where possible) were captured. Zero prevalence reports were increased to 0.2%, to calculate the SE. This is because estimates in the STATA program (STATA Inc, College Station, TX, USA) are based on SE and thus the principle of binomial estimate at 95% confidence interval (CI) was applied during data analysis (Dohoo et al., 2009).
Data on knowledge, attitude and practices concerning brucellosis obtained through questionnaire administrations, were also separately extracted. These include information on the number of individuals interviewed and the number and proportion of individuals who had the characteristics of interest such as knowledge of brucellosis as a zoonosis, habits of raw milk consumption, habits of raw meat consumption, contact with livestock, assistance during livestock delivery and contact with birth fluids and materials (newborns, placenta, dead/aborted fetus). Brucella isolates circulating in livestock in Ethiopia were also compiled from studies conducted using microbiological and molecular techniques separately.
2.4 Data analysis
2.4.1 Meta-analysis
Data retrieved from the available literature that fulfilled the criteria for systematic review and meta-analysis were cleaned and coded for subsequent data analyses. The coded data were imported into STATA 14 (Stata Corp, College Station, Texas). Both individual animal- and herd/flock-level apparent prevalence estimates were log-transformed using the formula, lp = ln[p/(1 – p)], where lp is the logit event estimate; ln is the natural logarithm and p is a study-level estimate. The variance of the logit estimate was calculated as, v (lp) = 1/(np) + 1/[n (1 – p)], where v is variance and n is sample size. The standard error of logit-prevalence (SE) was generated using the formula ln_p = Sqrt(1/n × p × (1 − p)). Cochran's Q statistical test was used to observe the level of heterogeneity between the studies (Cochran, 1954). The true proportion of variability that existed between studies because of heterogeneity was computed using Higgins's (I2) test statistic (Higgins et al., 2003). Besides Galbraith plots were used to visualize the heterogeneity between studies (Anzures-Cabrera & Higgins, 2010). Given the variable context under which the studies were conducted, DerSimonian and Laird (1986) random effect model was used to calculate the pooled prevalence estimate (effect size) of brucellosis using the formula, p = 1/(1 + e–lp) × 100, where ‘e’ is the base of a natural logarithm.
2.4.2 Meta-regression
Following the APS estimate, univariable meta-regression analysis was conducted on predictors to explore factors that might explain the observed heterogeneity. All predictors with generous p-value (p < .25), were checked for the presence of multicollinearity using Kruskal gamma statistic in a cross-tabulation (Dohoo et al., 2009). Non-collinear predictors with gamma values between −0.6 and +0.6 were subjected to multivariable meta-regression to quantify the explainable proportion of the heterogeneity (R2) observed between studies. When the calculated R2 values observed passed beyond the limit of proportion (0, 1), then the outlier values were adjusted to zero when they are negative, while those exceeding one were adjusted to one (Borenstein et al., 2009). For a given predictor, the category-level analysis, however, was dictated by the presence or absence of a sufficient number of observations in respective categories to be compared. In a situation where comparison could not be made, a pooled estimate was computed for the presumed predictor with a minimum of three observations.
2.4.3 Literature bias and sensitivity assessments
For literature bias analysis, both graphical and statistic-based computations were made. Graphical visualizations were made by producing funnel plots for literature bias and influential plots for sensitivity analysis. An influential plot was constructed by removing an individual study report from the analysis every time the estimation was run and observing the effect it had on the stability of the estimate one report at a time for each of the studies. Egger's weighted regression and Begg's rank correlation statistics were the statistical tools used to verify the significance of the observed literature bias (Begg & Mazumdar, 1994; Egger et al., 1997).
2.4.4 Descriptive statistics
A descriptive summary was made to demonstrate knowledge, attitude and practices of studied human populations regarding brucellosis and its risk factors for human infections. A similar approach was used to summarize Brucella species reported from livestock and humans in Ethiopia.
2.4.5 Proportional symbol map
Thematic maps with circles of variable sizes were used to represent the quantitative aspect of occurrence and distribution. To this end, proportional dot maps were developed using ArcGIS version 10.4.1 software (ESRI, Redlands, California, USA). Before map generation, data for cattle, camels and small ruminants were generated at a district level together with the frequency of the reports. Mean apparent prevalence and 95% confidence intervals were generated based on a binomial distribution for each of the districts. In a situation where there was only one report, the available report was considered to represent the district in question. The data were then used to generate the proportional dot maps. The systematic review and meta-analysis part was finally compiled based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (Liberati et al., 2009; Moher et al., 2009) (Supplementary Table 1).
3 RESULTS
3.1 Literature search result
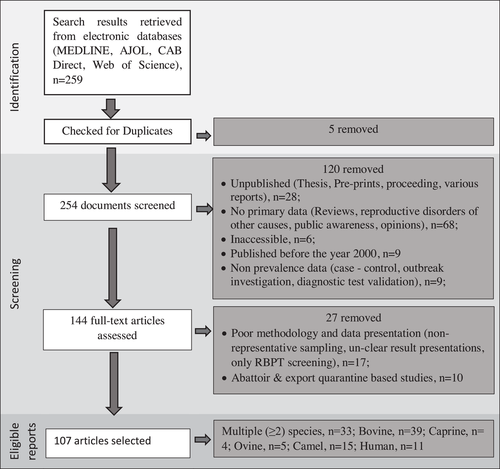
Online databases search queries identified 259 documents containing the search phrases. From these, 152 were rejected for various reasons stipulated in the inclusion and exclusion criteria. Accordingly, five of the articles were rejected for being duplicates, and 120 of the articles for lack of prevalence or incidence data, inaccessible articles, unpublished, or published before the year 2000. The rest 27 articles were rejected for methodologies that were not sufficient to provide representative population estimates including descriptive designs, purposive individual animal sampling, lack of traceability of study subjects and inconclusive laboratory procedures. Eventually, 114 group-level and 306 individual-level observations were extracted along consistently reported variables at a district level for livestock and 31 observations for humans, while the public health awareness aspect of this review was organized based on 52 individual-level observations. Data on brucellosis prevalence were altogether acquired from 107 published articles (Figure 1, Supplementary Tables S2 and S3). Additionally, nine articles that do not provide disease burden were included for synthetic review of bacterial isolation (n = 2) and public awareness (n = 7) (Supplementary Table 2).
3.2 Study appraisal and data extraction
In the course of valid literature selection, preliminary screening was initially made on scanning of the title and abstract. When an article passes the preliminary assessment, it was subjected to full-length appraisal. The appraisal was done using the predefined quality assessment criteria given above in the material and method section. From a design perspective, only cross-sectional studies were available for pooled prevalence estimates. The screening tests used were serological including RBPT, serial RBPT-CFT, serial RBPT-ELISA, ELISA, lateral flow assay (LFA), and slide agglutination test.
Specific livestock species addressed in this review were cattle, sheep, goats and camels. Small ruminants and camels were all of the local breeds, but in cattle, exotic breeds including Friesian, Jersey, their crosses and local zebu were part of the data. The production systems under investigation were categorized into crop-livestock, urban/peri-urban and pastoral/agropastoral systems, while agroecology was classified into highland, midland and lowland. The management aspect was considered from intensive/semi-intensive and extensive perspectives. Diagnostic tests considered for comparison were RBPT-CFT (serial testing), RBPT-ELISA (serial testing) and ELISAs alone. The rest were excluded due to the lack of a sufficient number of observations (less than three per category). Administrative regions-wise, all the 9 regions and the two city administrations were considered, but respective comparisons were done based on the number of observations per category. In human data, the professional background and clinical status of the people tested were considered. Clinical status was grouped into apparently healthy and sick individuals. Occupationally, the list included pastoralists, farmers, animal health workers, artificial insemination technicians, butchers and abattoir workers. There were also volunteers and apparently healthy blood donors who were tested for brucellosis. For sake of investigating exposure risk levels, farmers in the mixed crop-livestock production system and apparently healthy blood donors from urban setups were put under low level of exposure risk while, pastoralists and all animal health professionals, artificial inseminators, butchers and abattoir workers were labelled as groups with high exposure risk. The public perception aspect was characterized in line with the level of awareness on brucellosis (yes/no), raw milk consumption (yes /no), raw beef consumption habit (yes/no) and exposure to aborted material (yes/no) for synthetic review. Ultimately, this comprehensive review captured original study reports conducted based on 44,750 cattle, 16,510 camels, 20,164 goats and 13,278 sheep. Group-level data were collected from 3532 cattle herds, 666 camel herds and 1257 small ruminant flocks. The reports on human brucellosis captured data from 4472 individuals of different backgrounds.
3.3 Meta-analysis
The APS of brucellosis in livestock was estimated using random-effect model as the studies were conducted under different contexts. Accordingly, the highest APS estimate was calculated for goats while the lowest was for cattle. At a herd/flock level, camels had the highest and goats had the lowest APS estimates. The estimates given in Table 1 and Figure 2 revealed the presence of significant heterogeneity among the studies.
| Study level | Species | Number of reports | Pooled estimate (95% CI) | Q (df) | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Individual animal | Cattle | 149 | 2.6 (2.2–3.0) | 1167.9 (148) | 87.3 | 0.7890 |
| Goats | 58 | 4.0 (3.1–5.1) | 807.8 (56) | 92.9 | 0.8690 | |
| Sheep | 55 | 3.0 (2.3–3.9) | 301.5 (54) | 82.1 | 0.6750 | |
| Camel | 44 | 3.0 (2.4–3.7) | 217.1 (43) | 80.2 | 0.3781 | |
| Herd/flock | Cattle | 80 | 16.3 (12.9–20.5) | 4662 (79) | 83.3 | 1.1462 |
| Goats | 14 | 12.1 (7.1–19.9) | 53.9 (13) | 77.8 | 0.7955 | |
| Sheep | 10 | 13.3 (7.6–22.1) | 45.4 (9) | 82.4 | 0.6296 | |
| Camel | 10 | 19.7 (13.8–27.4) | 37.6 (9) | 76.1 | 0.3316 |
- CI: confidence interval, Q: Cochran test statistics, I2: Higgin's statistics/indexes, τ2: Tau squared statistics, df: degrees of freedom.
3.4 Geographic distribution pattern
The spatial distribution pattern of brucellosis in this review was illustrated by summarizing the available apparent prevalence reports at a district level (Supplementary Table 4). Accordingly, 60 non-zero district-based mean prevalence reports were retrieved for cattle (Figure 3), 65 for small ruminants (Figure 4) and 18 for camel (Figure 5) brucellosis. Districts reporting only zero prevalence were not included in the figures. For cattle, these include 11 districts of Oromia, two from Somali (one of them reporting zero twice), one from Afar, one from Amhara (reporting zero twice), one from SNNPR and one from Tigray regional states.
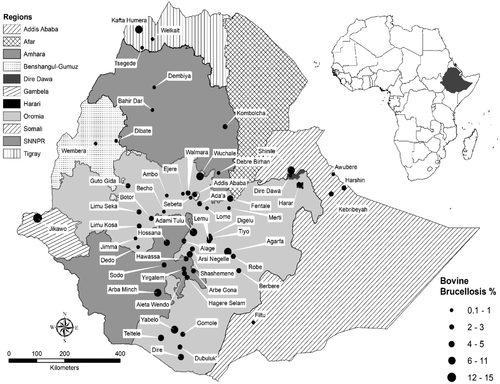
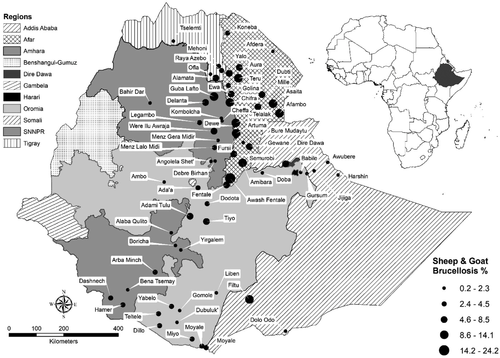
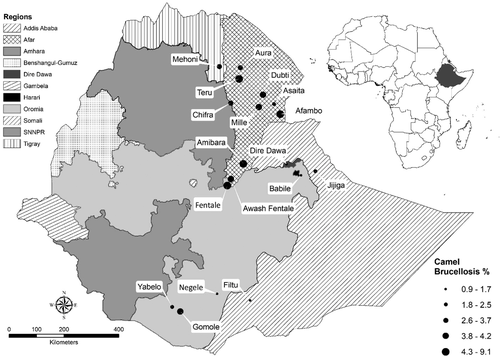
Figure 4 showed the dominance of reports from the pastoral areas of Ethiopia which included the whole of Afar, Borena Zone of Oromia, Somali and South Omo Zone of SNNPR State. Small ruminants in Afar Regional State were the most affected. Eight out of ten districts with a mean prevalence of 8.6% and above were located in Afar. Smaller prevalence reports predominate the rest of the country, especially, Somali Regional State. Six districts that reported only zero seropositive animals every time they were studied did not appear on the map. These included two districts in Somali, one district in Afar, one in Oromia, one in SNNPR and one in Tigray.
3.5 Animal-level meta-regression model
3.5.1 Univariable regression
In an animal-level regression analysis, the predictors used were more or less similar for all livestock species with little diversity. Predictors for cattle brucellosis included, year of study, sample size, breed of the animal, type of production system, screening technique, management system and administrative region. The model estimate in a univariable regression was analysed both with and without sample size considerations. Accordingly, the between-study variance noted for individual predictors ranged from zero for management system, administrative region and screening techniques to 9.3% for type of production system with sample size adjustment. For small ruminants, the predictors were year of study, sample size, species, type of production system, screening techniques and administrative regions. The corresponding model estimates of in-between study variation varied from 1.3% for year of study to 29.8% for administrative region. In camel, the predictors considered were year of study, sample size, screening technique and administrative region. Accordingly, the lowest estimate, zero was for year of study while the highest, 16.8% was for administrative region without sample size consideration (Table 2).
| Without sample size | With sample size | ||||
|---|---|---|---|---|---|
| Livestock species | Model | p value | p value | ||
| Cattle | Sample size | – | – | 3.9 | .001 |
| Year of study | 0.3 | .400 | 7.4 | .55 | |
| Breed | 0.6 | .110 | 6.0 | .023 | |
| Type of production system | 6.5 | .004 | 9.3 | .007 | |
| Management system | 0.0 | .629 | 2.9 | .872 | |
| Screening technique | 0.0 | .402 | 2.8 | .600 | |
| Administrative regions | 0.0 | .313 | 3.0 | .977 | |
| Small ruminants | Sample size | – | – | 5.9 | .019 |
| Year of study | 1.3 | .130 | 8.0 | .083 | |
| Type of Species | 1.6 | .145 | 10.2 | .040 | |
| Type of production system | 10.7 | .001 | 16.1 | .001 | |
| Screening technique | 16.7 | <.001 | 19.4 | <.001 | |
| Administrative regions | 24.2 | <.001 | 29.8 | <.001 | |
| Camel | Sample size | – | – | 1.9 | .311 |
| Year of study | 0.0 | .467 | 0.0 | .857 | |
| Screening technique | 0.1 | .305 | 3.5 | .249 | |
| Administrative states | 16.7 | .011 | 15.9 | .016 | |
In a subsequent multi-collinearity assessment, type of production system was collinear with screening technique (gamma = 0.6219), management system with type of production system (gamma = 0.7546), and breed with type of production system (gamma = 0.6033) in bovine brucellosis prevalence studies. In all the three cases, type of production system was considered for subsequent multivariable regression due to its biological plausibility over the others. In small ruminant and camel studies, none of the predictors were collinear at specified values (−0.6 to +0.6). Thus, all predictors from univariable regression were further subjected to multivariable regression analysis.
3.6 Multivariable regression
All non-collinear predictors were considered for the multivariable meta-regression model. For cattle, sample size, year of study, type of production systems and administrative regions were the potential predictors considered for analysis. However, in the final model sample size and type of production system were the two predictors observed to explain 17.0% of the in-between study variation (R2). In this analysis, it was noted that 10, 25 and 50 percentile of the studies were conducted on sample size less or equals to 79, 116 and 233 animals, respectively. As the sample size increased by 50%, the prevalence declined by half in the regression model (Figure 6a). Regarding type of production system, the prevalence of brucellosis declined by 29.4% as the production system changes from pastoral/agropastoral to the mixed crop-livestock system. Likewise, the decline in prevalence estimate for urban/periurban dairy production system was 32.2% compared to the reference category (Table 3).

| Spp | Variables | Category/range | n | Pooled P (95% CI) | Coeff. | p* value | Overall p value | R2 |
|---|---|---|---|---|---|---|---|---|
| Cattle | Sample size | 38–2006 | – | – | −0.001 | <.001 | .001 | 17.02 |
| Production system | Pastoral/agropastoral | 34 | 4.8 (3.8–6.4) | ref | ||||
| Mixed crop livestock | 57 | 2.2 (1.7–2.8) | −0.747 | .001 | .001 | |||
| Urban/peri-urban dairy | 57 | 1.9 (1.4–2.5) | −0.879 | <.001 | <.001 | |||
| Small ruminants | Species | Sheep | 55 | 3.0 (2.3–3.9) | ref | 55.2 | ||
| Goat | 58 | 4.0 (3.1–5.1) | 0.365 | .145 | .064 | |||
| Region | Afar | 31 | 8.3 (6.3–10.8) | ref | ||||
| Somali | 19 | 2.0 (1.1–3.3) | −1.70 | <.001 | <.001 | |||
| Oromia | 29 | 2.8 (2.0–3.8) | −1.39 | <.001 | <.001 | |||
| SNNPR | 9 | 1.5 (0.7–2.9) | −1.81 | <.001 | <.001 | |||
| Amhara | 16 | 2.2 (1.4–3.7) | −0.91 | .001 | <.001 | |||
| Tigray | 5 | 2.5 (1.3–4.9) | −1.02 | .006 | .010 | |||
| Dire Dawa | 4 | 5.3 (2.7–10.1) | 0.27 | .294 | .505 | |||
| Screening test | RBPT-CFT | 91 | 2.9 (2.3–3.5) | ref | ||||
| RBPT-ELISA | 8 | 6.3 (4.5–8.9) | 1.16 | .052 | <.001 | |||
| ELISA | 14 | 8.9 (5.7–13.7) | 1.18 | <.001 | <.001 | |||
| Camel | Region | Afar | 18 | 4.4 (3.5–5.6) | ref | 29.2 | ||
| Oromia | 12 | 2.5 (1.6–4.1) | −0.47 | .024 | .038 | |||
| Somali | 10 | 2.1 (1.6–2.8) | −0.85 | .002 | .003 | |||
| Screening test | RBPT-CFT | 41 | 3.0 (2.4–3.8) | ref | ||||
| RBPT-ELISA | 3 | 1.9 (0.7–5.2) | −0.18 | .305 | .655 |
- n: number of study observations, coeff.: coefficient, P: prevalence, CI: confidence interval, p*: p value from univariable analysis, p: p value from multivariable regression analysis, R2: proportion of explainable heterogeneity, Spp: Species.
For small ruminants, all the predictors in univariable regression were subjected to multivariable regression; however, administrative regions and screening test used were found to explain 55.2 % of the heterogeneity among brucellosis reports in small ruminants. There was no significant APS difference between sheep and goats. Region-wise, the highest and the lowest pooled estimates were reported from Afar and SNNPR, respectively. Pooled estimates for all other regions were significantly lower (p < .05) than Afar except Dire Dawa City Administration. Screening test-wise, the lowest pooled estimate was reported for RBPT-CFT based studies, followed by RBPT-ELISA and ELISA based studies in ascending order. Accordingly, the overall pooled estimate rose by 76.3% for RBPT-ELISA, while 76.9% for ELISA over the reference RBPT-CFT.
Multivariable meta-regression analyses for camel studies were run with four predictors, namely, year of study, sample size, screening technique and administrative regions; however, only administrative region revealed statistically significant in-between study variation that explained 29.2% of the observed heterogeneity. The highest brucellosis APS was noted for Afar followed by Oromia and Somali regions (Table 3). Estimates could not be computed for Tigray and Dire Dawa as there were only two reports in each case.
3.7 Herd-level meta-regression model
3.7.1 Univariable meta-regression analysis
In a group-level analysis, the unit of interest was the herd/flock. A group was considered positive if a single animal was positive in a herd/flock. Like the animal-level analysis, predictors at herd level were also analysed both with and without considering the sample size in a univariable meta-regression. In cattle, sample size, year of study, production system, agroecology and administrative region were presumed to operate at herd level as well. The specific contribution of predictors for between-study variance varied from zero for agroecology and administrative regions to 69.9% for type of production system with no sample size consideration.
For small ruminants, the same types of predictors were presumed and the contribution of individual predictors ranged from zero for year of study and administrative region to 55.5% for type of production system. The corresponding value for camel herd Brucella survey heterogeneity also noted to vary from zero for administrative regions to 60.4% attributed to year of study with no sample size consideration. However, none of the three predictors considered revealed statistical significance (p < .05) in a multivariable regression with sample size adjustment (Table 4). The follow-up multicollinearity assessment revealed a lack of collinearity among predictors; hence, all were subjected to a multivariable regression.
| Without sample size | With sample size | ||||
|---|---|---|---|---|---|
| Livestock species | Variables | p value | p value | ||
| Cattle | Sample size | – | – | 26.4 | <.001 |
| Year of study | 0.19 | .235 | 31.7 | .027 | |
| Production system | 69.9 | <.001 | 54.8 | <.001 | |
| Agroecology | 0.0 | .402 | 26.5 | .125 | |
| Administrative region | 0.0 | .942 | 5.4 | .643 | |
| Small ruminants | Sample size | – | – | 0.0 | .899 |
| Year of study | 0.0 | .809 | 0.0 | .831 | |
| Production system | 55.5 | .003 | 48.3 | .004 | |
| Agroecology | 41.0 | .010 | 35.7 | .012 | |
| Administrative region | 0.0 | .467 | 0.0 | .461 | |
| Camel | Sample size | – | – | 20.0 | .218 |
| Year of study | 60.4 | .031 | 54.7 | .062 | |
| Administrative region | 0.0 | .967 | 5.3 | .586 | |
3.8 Multivariable regression on herd/flock-level data
In a multivariable meta-regression, sample size and type of production systems were again the two predictors that contributed to 53.8% of the in-between study variance of the reports at herd level. As the number of herds sampled increased by a unit it was noted that the herd-level prevalence reduced by half in the regression model (Figure 6b). For production system, the herd-level pooled estimate was reduced by 69.8% in mixed crop-livestock compared to pastoral/agropastoral production system. The corresponding figure for urban/peri-urban similarly decreased by 82.6%. In small ruminants, only production system remained statistically significant and contributed to 16.7% of the explainable heterogeneity in the final model. Accordingly, the flock-level brucellosis pooled estimate in the mixed crop-livestock system decreased by 77.5% from pastoral/agropastoral production system (Table 5). In camel studies, none of the predictors considered for herd-level analysis were statistically significant.
| Spp | Variables | Category/range | n | APS (95% CI) | Coff. | p* Value | Over all p Value | R2 |
|---|---|---|---|---|---|---|---|---|
| Cattle | Sample size | 3–270 | – | – | −0.011 | <.001 | <.001 | 53.8 |
| Production system | Pastoral/agropastoral | 17 | 37.1 (23.8–47.5) | ref | ||||
| Mixed crop-livestock | 29 | 13.3 (9.7–19.3) | −0.841 | .001 | .002 | |||
| Urban/peri-urban | 34 | 11.4 (8.5–14.9) | −1.56 | <.001 | <.001 | |||
| Small ruminants | Year | 2012–2020 | 24 | – | 0.118 | .809 | .211 | 16.7 |
| Production system | Pastoral/agropastoral | 10 | 19.7 (13.3–28.3) | ref | ||||
| Mixed crop-livestock | 13 | 7.9 (4.3–14.3) | −1.23 | .046 | .023 |
- n: number of study observations, Coeff.: coefficient, APS: apparent pooled seroprevalence, CI: confidence interval, p*: p value from univariable analysis, p: p value from multivariable regression analysis, R2: proportion of explainable heterogeneity, Spp: species.
3.9 Pooled estimate in human and public health perception
The pooled prevalence estimate of brucellosis in humans was 5.0% (95% CI: 3.3–7.3). In a random effect model analysis, the heterogeneity chi-square test (Q) of the estimate was 228.9 (df = 30, p = .000). The Higgin's statistics value (I2) along the between-study variation (τ2) were 86.9%, and 0.9836, respectively. All the estimates were calculated based on a forest plot (Figure 7).
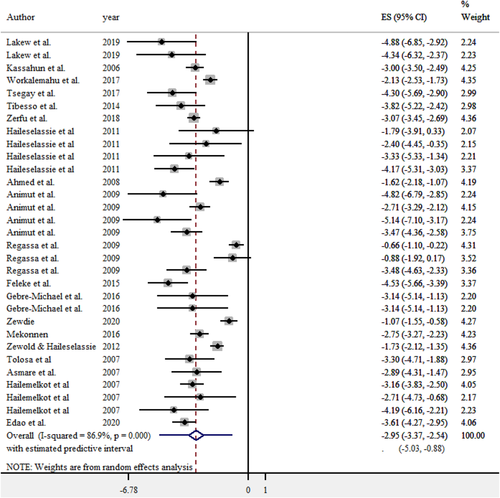
As described in Table 6, the potential predictors considered for heterogeneity were administrative regions where the individuals were living, occupation, exposure risk level, clinical status and screening techniques used in addition to the year of study and sample size. However, only screening test revealed a statistically significant (p < .05) difference and contributed to 8.2% heterogeneity with sample size adjustment. Following multicollinearity assessment of all predictors, the noncollinear ones were subjected to multivariable regression; however, only type of screening test, again, revealed a significant level of heterogeneity among studies in the final model (R2 = 0.833). The screening test considered were the agglutination test, RBPT-CFT and LFA. RBPT-cELISA and RBPT-ME were excluded from the analysis for lack of sufficient observations for comparison. In the final model, the APS estimate of LFA was higher than the reference test (Table 7).
| Model | Without sample size | With sample size | ||
|---|---|---|---|---|
| p value | p value | |||
| Sample size | – | – | 0.6 | .337 |
| Year of study | 0.0 | .603 | 0.0 | .821 |
| Administrative region | 0.0 | .639 | 2.6 | .312 |
| Occupation | 0.0 | .847 | 0.0 | .653 |
| Type of screening test | 6.8 | .071 | 8.2 | .064 |
| Clinical status | 0.0 | .619 | 0.0 | .529 |
| Exposure risk level | 0.0 | .523 | 0.0 | .466 |
| Variables | Category/range | n | P (95% CI) | Coff. | p* Value | Over all p value | R2 |
|---|---|---|---|---|---|---|---|
| Year | 2006–2020 | – | – | −0.038 | .501 | .071 | 8.3 |
| Screening test | Agglutination | 6 | 2.9 (1.2–6.7) | ref | |||
| RBPT-CFT | 19 | 4.5 (2.9–6.9) | 0.426 | .435 | .419 | ||
| LFA | 3 | 16.6 (3.9–49.2) | 1.85 | .013 | .023 |
- n: number of observations, coeff.: coefficient, P: prevalence, CI: confidence interval, p*: p value from univariable analysis, p: p value from multivariable regression analysis, R2: proportion of explainable heterogeneity. LFA: lateral flow assay, RBPT: Rose Bengal Plate Test, CFT: complement fixation test.
3.10 Public health awareness (knowledge, attitude, practice)
Data on different aspects of public awareness of brucellosis and risk factors for human infections were obtained from 47 published studies. Among them, 40 studies were conducted on knowledge, attitude and practice assessments as part of studies conducted on human or animal brucellosis; hence, the studies were dominated by farmers in the mixed crop-livestock, urban and peri-urban dairy farming, pastoral and agro-pastoral production systems, human patients with fever and those with direct contact with livestock. Seven of the studies were conducted primarily on public health awareness of zoonotic diseases or factors that may expose people to food-borne zoonoses. Accordingly, summarized results showed that public recognition of brucellosis as a zoonotic disease was low (<20%) while factors predisposing individuals to the disease were common (Table 8).
| Yes | No | ||||||
|---|---|---|---|---|---|---|---|
| Knowledge, attitude and practice | No. respondents† | No. | % | No. | % | Range (%) | No. of studies |
| Know brucellosis is zoonotic | 3874 | 712 | 18.4 | 3162 | 81.6 | 0.0–70.0 | 32 |
| Raw milk consumption | 5475 | 4122 | 75.3 | 1353 | 24.7 | 9.3–100 | 38 |
| Contact with abortion materials | 3290 | 2467 | 75.0 | 823 | 25.0 | 11.3–100 | 24 |
| Raw meat consumption | 2746 | 1426 | 51.9 | 1320 | 48.1 | 0.2–100 | 17 |
| Assisted animal delivery | 1597 | 910 | 57.0 | 687 | 43.0 | 9.0–100 | 11 |
- † Number of respondents are not equal as some of the factors present in some of the studies were not present in others.
3.11 Brucella species isolated from livestock in Ethiopia
Three published studies conducted on isolation of Brucella spp. using the classical microbiological methods identified B. melitensis and B. abortus from samples collected from goats and cows, respectively. None of the studies identified the biovars of the isolates. Brucella abortus was isolated from three of 46 samples collected from abortion materials from cows in Asela and Bishoftu towns in central Ethiopia; however, isolates were recovered only from cattle at Asela (Geresu et al., 2016b). Two of the studies that reported B. melitensis, one from slaughtered seropositive (n = 2/14) (Sintayehu et al., 2015) and the other from clinical samples (n = 8/64) of aborted goats (Tekle et al., 2019) also confirmed the identities of the isolates using molecular methods. Eight B. melitensis reported from aborting goats in the Amibara district of Afar demonstrated a band pattern typical of B. ovis in the Bruce-ladder multiplex PCR. The isolates lack the 1682 bp band typical for B. melitensis but are reported to have typical microbiological and serum agglutination properties of B. melitensis. Human isolates are yet to be reported from patients in Ethiopia.
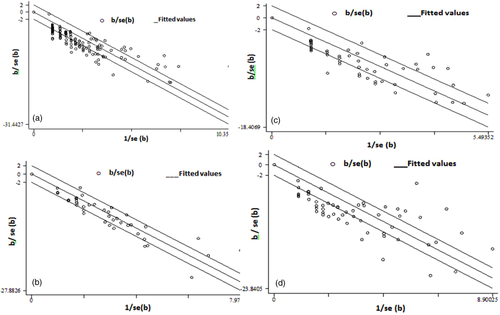
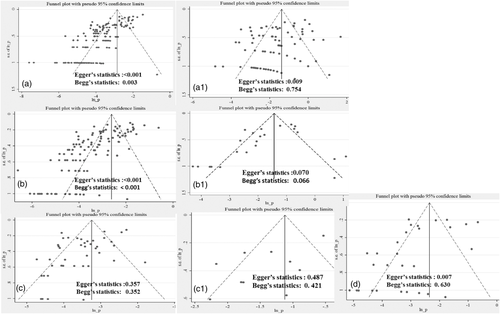
3.12 Literature bias and sensitivity analyses
For all livestock and human pooled prevalence estimates, sensitivity analyses were made in statistical and graphical methods. The test statistics used were Egger's statistic (weighted regression) and Begg's statistic (rank correlation method), while the graphical analyses were run in Funnel plots. Accordingly, for cattle and small ruminants, the statistical estimate and graphical analysis show the presence of bias at the individual animal level. However, the flock-level estimate for small ruminants revealed the absence of bias, while the cattle herd estimate was inconclusive. The finding on camel data did not have any evidence of bias both at individual and herd levels. On the other hand, the levels of bias on logit estimate given for human data were inconclusive as well (Figure 8). No individual study had an adverse influence on the robustness of the pooled estimate based on influence plot analyses (data not shown).
4 DISCUSSIONS
This comprehensive review revealed that brucellosis is endemic in livestock in Ethiopia. The APS estimate of brucellosis was 2.6% (95% CI: 2.2–3.0) for cattle, 3.0% (95% CI: 2.3–3.9) for sheep, 3% (95% CI: 2.4–3.7) for camel and 4.0% (95% CI: 3.1–5.1) for goats. Relatively, the infection burden in all four species was low to moderate; nevertheless, the occurrence across the country is variable and widely distributed among the herds and flocks managed in different production systems. The spatial distribution maps depicted the presence of some hotspots and areas of markedly low prevalence. On the other hand, there were also areas with no data. The prevalence differences noted and the absence of data for some of the regions dictate the need for designing contextualized intervention strategies and further investigations. Indeed, the pooled estimates given for all four livestock species are of great relevance; however, as serological evidence is not conclusive of the type of circulating Brucella species, it would be difficult to differentiate spillover from principal hosts. Thus, sufficient microbiological studies are indispensable to use the available information for pragmatic intervention.
Direct comparison of brucellosis studies between countries and regions is difficult for a range of technical reasons, including in study methodologies, diagnostic tests used, livestock species and herd structures, herd management, presence or absence of control programs and many more factors. However, a similar pooled prevalence report was available for cattle in China between the years 2013–2018 (Ran et al., 2019). In most of the African countries, such pooled estimates or estimates that cover the whole nation are rarely available; yet, in Tanzania, a higher pooled prevalence estimate, 8.2% (95% CI: 6.5−10.2), at a national level was reported (Alonso et al., 2016). Likewise, a higher prevalence range of 9.9–15% was also reported from pastoral cattle herds of northern Kenya (Njeru et al., 2016) and 31% from cattle in Bahr el Ghazal region of South Sudan (Madut et al., 2018). A large study in Nigeria also reported a higher prevalence of 26.3% from cattle reared in northern Nigeria (Mai et al., 2012). In the Middle East, where brucellosis is widely known among nomads, national- or regional-level reports are noted to range from 7.0% to 16.7% (Al-Majali et al., 2009; Dahl, 2020; Holt et al., 2011; Selim et al., 2019). Indeed, the comparisons made are not sufficient, but they provide an idea on the variability of brucellosis seroprevalence from country to country that call for attention to introduce working intervention strategies that account for local contexts.
The pooled estimate of brucellosis in cattle varies among the production systems and regions. Cattle kept in the pastoral/agropastoral production system had significantly higher pooled seroprevalence compared to cattle kept in the sedentary mixed crop-livestock production. The lowest pooled estimate was computed for urban/peri-urban dairy production systems that are managed intensively or semi-intensively. The relatively higher prevalence of pastoral/agropastoral production system could be due to large herd sizes and mobility compared to a small number and less mobile livestock keeping practice implemented by farmers in the crop-livestock production system (Ducrotoy et al., 2017). In the former production system, the rate of herd-level contact due to migration and congregation both at pasture and watering point is high. Moreover, the mobility and associated practices have also predisposed the less mobile group of animals in the adjacent sedentary mixed crop-livestock system. On the other hand, in the urban/peri-urban dairy system, herd contact rates are low. There is also evidence that farmers practice informal culling of Brucella seropositive cows and cows with abortions regardless of the etiologies, as a result of the awareness created by the veterinary authorities (Geresu et al., 2016a; Tesfaye et al., 2011). The results of such measures could be observed in cities like Addis Ababa where the seroprevalence of brucellosis decreased from 10% in 2002–2003 (Eshetu et al., 2005) to 7.1% in 2008–2009 (Haile et al., 2010) and then to 0.1% or less in 2016–2017 (Edao et al., 2018).
The other sources of variability in bovine studies were the sample sizes both at individual animal- or herd-level reports. In this review, we observed an inverse relationship between sample size and seroprevalence estimates. Perhaps this may be attributed to cross-sectional studies conducted in areas where prior knowledge of reproductive disorder exists or studies done in areas where the history of abortion or stillbirth are frequently reported. Such targeted investigative approaches are common in farms and are conducted with limited sample sizes.
Brucellosis is endemic and occurs at a moderate level among small ruminants in the country. However, noticeable seroprevalence differences were observed among regions and the type of screening tests used. Indeed, the regional differences noted possibly are dictated by the type of production system and associated technicalities.
Based on the accrued evidence, over 78% (n = 113/145) of the studies on small ruminant brucellosis were conducted in arid and semiarid regions where pastoralism/agropastoralism is practiced. Afar Regional State had a significantly higher (p < .05) brucellosis burden compared to all the other regions except Dire Dawa City Administration. The reasons for this difference were not clear even with in the same type of production system. A study comparing differences in animal husbandry between Afar and Somali pastoralists observed larger herd/flock congregations originating from wider geographic areas to be common among Afar pastoralists as opposed to Somali pastoralists where only livestock owned by members of the same clan had access to pasture and water resources within the borders of the clan which might have significantly reduced the disease burden in the later (Teshale et al., 2006). Such differences were noticed in China where higher pooled prevalence estimates were reported in different provinces compared to the national pooled prevalence of 2.30% (95% CI: 2.00−2.60) (Ran et al., 2018).
The second source of heterogeneity in small ruminant brucellosis was the type of screening tests used. In the current review, it was observed that tests where ELISAs (indirect ELISA or competitive ELISA) were used alone or as confirmatory tests for RBPT, detected more seropositive animals (p < .05) than the most commonly used serial RBPT and CFT. Several other studies described higher sensitivity and specificity of ELISAs over RBPT and CFT based tests (Godfroid et al., 2010; Gürbilek et al., 2017; Gusi et al., 2019; Jacques et al., 1998). For this reason, some investigators recommend the use of ELISAs as a standalone or combined in series with RBPT as a confirmatory test replacing CFT (Gürbilek et al., 2017; Jacques et al., 1998).
The burden of brucellosis in camels depends on the prevalence of the disease in other livestock species kept alongside camels (Gwida et al., 2012; OIE, 2018). The control of the disease in this species also depends on the control of the disease in the main co-existing reservoir hosts. Camel studies in Ethiopia showed the presence of significant variation in the distribution of the disease among the regions. Afar Region with an APS of 4.4% (95% CI: 3.5–5.6) had the highest estimate compared to camel rearing areas of Oromia and Somali regions. The differences are mirrored in the distribution of the disease observed for small ruminants in Afar and adjacent areas of Oromia and that observed for cattle in Borena pastoral areas in southern Oromia. We, therefore, suggest that camels might be spillover hosts of the disease from small ruminants in Afar and support the suggestion by Megersa et al. (2011), that cattle could be the reservoir hosts for brucellosis in Borena. Our suggestion is based on the relative prevalence of the disease in small ruminants and a recent report of B. melitensis isolated from aborting goats in Afar (Tekle et al., 2019). Borena pastoralists were socio-culturally cattle herders for centuries and only recently started diversifying their livestock to browsing species following consecutive droughts that killed cattle and invasion of rangelands by bush encroachment. Camels and small ruminants were introduced into Borena from neighbouring Somali areas where pastoralists culturally kept these species and where the prevalence of the disease remained low to date (Megersa et al., 2008; Megersa et al., 2014; Tolera & Abebe, 2007). Therefore, microbiological investigation of the agent in the areas will help in sorting out the principal host which will be the primary targets for interventions.
At a herd level, few seropositive animals were distributed in more than 16% of the cattle herds or >12% of small ruminant flocks at a national level. The figure computed for camel herds was close to 20%. Camel, being one of the dairy animals in arid and semiarid regions of the country, the highest figure noted for camel herd-level brucellosis is a clear indication of the prevailing zoonotic brucellosis threat. Thus, if no action is taken, the prevalence will become higher and the effect of the diseases both on animals and people will become far more serious.
The herd-level dynamics of the diseases were seen to vary across production systems and the number of herds tested. In cattle, significantly more cattle herds (p < .05) in the pastoral/agro-pastoral areas (37.1%: 95% CI: 23.8–47.5) of the country tested positive to the presence anti-Brucella antibodies compared to herds in the mixed crop-livestock (13.3%: 95% CI: 9.7–19.3) and the urban/peri-urban dairy production systems (11.4%: 95% CI: 8.5–14.9). Similar findings were reported from different production systems in other African countries (Asakura et al., 2018; Magona et al., 2009; Mai et al., 2013; Njeru et al., 2016; Osoro et al., 2015). In our pooled estimate analysis against herd numbers tested, it was noted that the seroprevalence declined as the number of tested herds increased. Perhaps, the same reason given above for individual animal-level brucellosis works in this case as well.
Livestock production systems were also responsible for the heterogeneity of brucellosis prevalence among small ruminant studies. Small ruminants in the pastoral/agropastoral system had higher (p < .05) seroprevalence (19.7%) than (7.9%) those in the mixed crop-livestock production system. The reason for this difference could be the difference in herd sizes and the extent of mixing at communal pasture and watering sites between the two production systems. Large herd sizes and extensive mixing of animals from herds of several families and villages are characteristics of pastoral/agropastoral systems in Africa as compared to the mixed crop-livestock systems (Otte & Chilonda, 2002). Studies conducted on small ruminant brucellosis in Jordan (Musallam et al., 2015a) and Niger (Boukary et al., 2013) found that herd size and communal grazing were important risk factors for brucellosis. Larger flocks were associated with a higher density of animals that facilitate close contact and environmental contaminations that lead to higher chances of transmission, especially, during cases of abortion and parturition (Constable et al., 2017).
A pooled seroprevalence of brucellosis in humans in Ethiopia was 5.0% (95% CI: 3.3–7.3) over the last two decades. The reported apparent prevalence of the disease ranged from zero to 34.1% in different geographic areas of the country. Afar pastoralists and communities living in southern pastoral areas of Borena (Oromia Region) and South Omo (SNNPR) zones bordering northern Kenya had a higher seroprevalence of the disease. All of the six (n = 6/31) districts reporting a higher estimate range of 10.6–34.1% were found in these areas of the country. On the lowest end, 10 out of 31 individual-level studies in this review reported a zero seroprevalence including three each from Amhara, Oromia and Tigray regions and one from Somali Region. These figures were reflected in the distribution of brucellosis in livestock in the respective areas. Similar observations were made in Kenya (Kairu-Wanyoike et al., 2019; Osoro et al., 2015), Sudan (Omer et al., 2010), South Sudan (Madut et al., 2018) and Kyrgyzstan (Bonfoh et al., 2012) where the burden of brucellosis in livestock was reflected in the burden of the disease in humans. In these countries, higher levels of brucellosis in livestock and humans were reported from pastoral areas. This agrees with the general understanding that in endemic areas, the incidence of human brucellosis is associated with the prevalence of the disease in livestock (McDermott & Arimi, 2002; Musallam et al., 2015a). Therefore, pastoral areas need to be prioritized for control of brucellosis both in humans and livestock. Factors recognized as common among communities with a higher incidence of brucellosis would be discussed later in this section.
As opposed to livestock, brucellosis in humans is one of the most neglected zoonotic diseases in Ethiopia. It is not a reportable disease. There were only a few well-structured epidemiological studies on human brucellosis. The studies used for analysis in the current review were mostly done as part of brucellosis studies in livestock. The inclusion of humans in the studies was simply to observe the zoonotic existence of the disease. In most cases, the sample sizes were small and human selections were made based on the chance that the farms were selected for animal studies and voluntary owners or animal attendants were included for sampling with support from local human health professionals. In other human studies, samples were obtained from clinical samples conducted at regional health institutions or from symptomatic people with non-specific signs as part of screening for multiple diseases. As a result, the APS estimate in this review would only provide information on the occurrence and extent of the disease in these high-risk groups of individuals included in the studies.
In humans, LFA-based studies reported significantly higher (p < .05) seropositivity to Brucella exposure than RBPT-CFT serial and serum agglutination tests. Lateral flow assay is generally easy to perform test used in poor rural settings where better-equipped laboratories are not available. The test was described to be more sensitive than the serum agglutination test (Smits et al., 2003; Zeytinoğlu et al., 2006). Comparative studies conducted to evaluate the relative importance of serological tests in humans in Ethiopia were not available. However, studies conducted elsewhere demonstrated that RBPT based tests were good at detecting anti-Brucella antibodies in people with no regular exposure to the agent but poor in people in endemic areas where repeated exposures existed. It was also poor in detecting antibodies in people who were infected and received treatment during the previous 12 months (Al Dahouk & Nöckler, 2011; Ruiz-Mesa et al., 2005). Enzyme-linked immunosorbent assays were considered to be more sensitive and specific than other serological tests and suggested to be used both as screening and confirmatory test in one step (Al Dahouk et al., 2003). Only in two studies, ELISA was used as a confirmatory test for RBPT positive sera. In the current review, 22 out of 31 individual-level studies were conducted using RBPT as a screening test. Together with six reports that used less sensitive serum agglutination test for diagnosis, the overall pooled prevalence of anti-Brucella antibodies might have been underestimated.
Public awareness of brucellosis was low in the majority of studied populations in Ethiopia. Only 18.4% (range 0.0–70.0%) of the respondents knew that brucellosis is a zoonotic disease. Five studies that reported average or above-average knowledge of the disease were from major urban and peri-urban areas of the country (data not shown). Studies on farmers from rural areas of the country practicing mixed-crop livestock agriculture, pastoralists or agro-pastoralists and residents of small towns consistently reported low levels of awareness of the disease. Low awareness of zoonotic brucellosis in the current review is in agreement with studies from Tajikistan where 85% of the respondents had never heard of the disease (Lindahl et al., 2015) and Sri Lanka where only 2.6% of the farmers knew that brucellosis is zoonotic (Kothalawala et al., 2018). Our finding also indicated that the level of knowledge on zoonotic brucellosis in Ethiopia is less than the global average of 37.6% (Zhang et al., 2019). Better knowledge of brucellosis as a zoonosis was reported from farmers in Egypt (Hegazy et al., 2009; Hegazy et al., 2016) and Jordan (Musallam et al., 2015b) and pastoralists in Uganda (Kansiime et al., 2014) and Kenya (Njenga et al., 2020). Higher knowledge of the disease in countries like Egypt and Jordan could be associated with awareness creation campaigns conducted during brucellosis vaccinations programs that have been ongoing for many years (Musallam et al., 2016). Lack of standardized animal and human health extension services, high rate of illiteracy among farmers/pastoralists, presence of multiple infectious diseases of perceived economic and public health importance that gained priorities both in public and animal health sectors, and absence of brucellosis control programs in the country might have contributed to low awareness of the disease in Ethiopia.
On the other hand, high-risk practices that expose individuals to Brucella infections such as consumption of raw milk and raw meat, provision of assistance to animals during delivery or contact with aborting animals or abortion fluids were common among respondents in Ethiopia. These practices were also found to be common even among countries where farmers/pastoralists were reported to have better knowledge of the disease (Hegazy et al., 2016; Kansiime et al., 2014; Musallam et al., 2015b; Njenga et al., 2020). In some of the studies, it was shown that the knowledge of brucellosis among farmers/pastoralists was not complete; especially, there were gaps in understanding the modes of disease transmission from animals to humans (Hegazy et al., 2016; Musallam et al., 2015b; Njenga et al., 2020). In many pastoral areas, perceived wholesomeness, nutritional, remedial and cultural values attributed to raw milk outweigh the risk of getting any infection (Amenu et al., 2019; Buttigieg et al., 2018; Edao et al., 2020). A low level of brucellosis awareness in the presence of high level of risk factors for Brucella exposure and unregulated trade in animals and animal products in Ethiopia, calls for the implementation of brucellosis control and prevention interventions. ‘One Health’ approach would, ideally, be the best way forward to achieve a reduction in disease burden both in humans and livestock (Bagheri Nejad et al., 2020; Buttigieg et al., 2018).
There were only three published studies so far reporting Brucella spp. isolated from livestock in Ethiopia (Geresu et al., 2016b; Sintayehu et al., 2015; Tekle et al., 2019). Of the three studies, one was conducted on samples from aborting cows in Asela and reported the isolation of B. abortus from three cows (Geresu et al., 2016b). The other two studies reported B. melitensis from goats, either from slaughtered seropositive animals and confirmed it with species-specific primers (Sintayehu et al., 2015) or from milk and vaginal swabs of aborting goats (Tekle et al., 2019). In the latter study, Bruce-ladder multiplex PCR was used to confirm the identity of the isolates obtained by the classical microbiological methods. The agarose gel band patterns of the Bruce-ladder multiplex PCR of the isolates lack the 1682 bp fragment and, therefore, do not conform to the typical B. melitensis; rather, they showed band patterns typical of B. ovis (López-Goñi et al., 2008). The authors, citing the fact that the isolates were cultured from goats following abortions, smooth colonial morphology of the isolates, biochemical reactions and agglutinations with M-specific antisera, concluded that the isolates could be strains of B. melitensis that have not yet been characterized and recommended further characterization using whole-genome sequencing. Most studies in West Africa described B. abortus to be the predominant cause of brucellosis in cattle (Sanogo et al., 2017). In this region of Africa, it was isolated from cattle, aborting sheep and horses (Bertu et al., 2015; Ocholi et al., 2004). In North Africa and the Middle East, although the presence of B. abortus was reported in many countries, the predominant species was B. melitensis (Musallam et al., 2016). In East Africa, only a few studies on the isolation of Brucella spp. are available. While these studies are not sufficient to determine the predominant circulating species, they, however, established the presence of B. abortus and B. melitensis in Kenya (Muendo et al., 2012) and Sudan (Musa et al., 2008; Omer et al., 2010), and B. abortus in cattle in Tanzania (Mathew et al., 2015). Both B. melitensis and B. abortus were isolated from humans in Kenya and Tanzania (Bodenham et al., 2020; Njeru et al., 2016). To the best of our knowledge, no Brucella isolate was reported in humans from studies conducted in Ethiopia. However, few studies are reporting B. melitensis from immigrants of Ethiopian origin in the United States (Rhodes et al., 2016) and Norway (Johansen et al., 2018), and Swedish patients with a history of travel to Ethiopia and Somalia (Sacchini et al., 2019). Similarly, there is no report on Brucella species infecting camels, equines, dogs and wildlife in the country.
Like any other review, the strength of this review is dependent of the quality and completeness of the information acquired from the primary studies. In this regard, this manuscript has tried to organized relevant data to present the overall status of brucellosis in people and livestock based on the study reports accrued over the last twenty years. Nevertheless, there are critical data and information gaps this manuscript could not provide and are worth further investigation. The most crucial ones include the data gap on circulating Brucella species and biotypes and the failure of identifying principal and spillover hosts throughout the country. Moreover, there are also regions where there were insufficient or very limited study reports. In this regard, the vast area eastern and southeastern areas of the Somali region, the whole areas of Benishangul Gumuz, and Gambella regional states, and parts of Amhara, SNNPR and Tigray regions need special attention. The other challenge faced was related to the type of study design researchers used and the way results were presented. Lack of clear operational definition for some of the presumed risk factors including age category, flock/herd size category and agroecology has limited our data depth and possible implications in revealing additional epidemiological features. The review also lacks information on swine, equines and wildlife brucellosis and their contribution to the epidemiology of the infection. In sensitivity analyses of the extracted data, it was observed that some of the pooled estimate given for cattle, small ruminants and human brucellosis has shown sign of bias that potentially could influence the figures. Much of the bias was believed to have originated from small sample sizes and low prevalence reports as shown on the funnel plots.
Like any other zoonotic diseases prevention and control endeavors, a brucellosis control venture in Ethiopia should begin with the establishment of a national-level ‘One Health’ team which is at least composed of experts from regulatory sectors including animal health and production sectors, wildlife authorities, public health and environmental health sectors. The team should conduct scenario analysis for the development and implementation of strategic interventions that involve policy matters, human resources, technical facilities and logistic issues (WHO/MZCP, 1988). Once such prerequisites are in place, the finding of this comprehensive review is instrumental in designing the actual control intervention targeting the diseases from people and the livestock perspective.
The efficacy of brucellosis control or eradication effort in the country or a region is basically dependent on prior knowledge of infection level in the herds or flocks, type of livestock species, type of husbandry, economic resources, public health impacts, and potential international trade implications (FAO, 2003). The low overall pooled estimate of brucellosis in all the four types of livestock species is of great advantage to introduce control interventions. This is because reducing the prevalence is one of the objectives of control in countries where the prevalence is high before screening and culling is effected economically for ultimate eradication (Constable et al., 2017; Minas, 2006; Smits, 2013). However, the absence of a clear picture of the type of circulating Brucella species is the critical hurdle that needs to be cleared before the presumed control interventions are in place. This is because identification of the agent type helps in defining the target livestock species which is indispensable. Once it is sorted out, be it vaccination or screening and culling or both along with regulatory movement restrictions can be implemented with a better chance of success. The other leverage seen in facilitating control intervention in Ethiopia is the prevalence variability seen among production systems. Thus context-based intervention approach is advisable in line with the course of production systems and geographically delineated regions.
A pragmatic control strategy can be organized from regional administration and production systems perspectives. The regional approach can cluster the country into regions of high prevalence, low prevalence and regions with limited or no information. In regions where data are limited or lacking further investigation is warranted before control interventions are in effect. From the production system perspective, the system with low prevalence and intermediate prevalence can be handled separately. In line with this, there are four marked categories. These are (I) Afar and adjacent areas of Amhara, Tigray and Oromia regional states; (II) southern and southeastern areas of Somali, Benshangul-Gumuz, Gambella and western parts of Amhara and Tigray regional states; (III) the vast areas of the northwestern and southeastern highland system where crop-livestock production system is practiced; (IV) the urban and periurban dairy production systems. Accordingly, the intervention proper can be launched with the available information and identification of circulating Brucella species for the first and the last categories, while well-designed surveys in all livestock species are needed for category II. The third category can wait until the required prevalence data and microbiological information is acquired from the second and third categories. Since this part takes the most densely populated region of the country, the respective intervention plan could be prepared following the required additional data and feedback from the preceding intervention.
The prevalence of brucellosis in small ruminants (8.3%) and dromedary camels (4.4%) in Afar and adjacent regions are the highest in the country. Therefore, after identification of the principal host, vaccination of the target species is acceptable to reduce the level of infection and associated impact (MZCC, 1986). In this part of the country, however, brucellosis data on cattle are scarce and their status as spillover or a principal host should be sorted out. Production system wise, the prevalence of brucellosis in urban and peri-urban dairy cattle was 1.9% and low compared to crop-livestock and pastoral/agropastoral systems. Hence, screening and culling could be considered due to economic, logistic and regulatory justification; that is, the animals are superior (high producing improved breeds) in terms of economic traits and the number of sero-reactors animals alleged for compensation is expected to be low. Moreover, the animals in this management are less mobile and implementations of regulatory restrictions are relatively easier. Thus, the test-and-slaughter policy recommended is justified on economic, public health, technical and regulatory grounds (European Commission, 2001). For the other two production systems, additional microbiological data and survey reports are required to launch the control intervention at the country level. Above all, the authors believe such a phased approach will help the success of national-level intervention from resource, logistic and experience perspectives.
5 CONCLUSIONS
Brucellosis is endemic in Ethiopia. It is widely distributed in livestock in all major livestock-producing regions of the country. Although the overall apparent pooled seroprevalence estimate of brucellosis, 2.6% in cattle, 4.0% in goats, 3.0% each in sheep and camels can be considered low to moderate, the herd/flock-level pooled prevalence estimates of the disease were high, ranging from 12.1% in goat flocks to 19.7% in camel herds. The disease burden was found to be higher in pastoral and agropastoral production systems of the country compared to the sedentary extensive mixed crop-livestock and urban/peri-urban dairy production systems. Small ruminants and camels in the Afar region had significantly higher pooled seroprevalence of brucellosis compared to other regions of the country. Despite the limited data available on Brucella isolates in Ethiopia, B. melitensis and B. abortus were isolated from goats in pastoral areas and dairy cows of central Ethiopia, respectively. The available serological evidence on human brucellosis was also indicative of infection among high-risk professionals and febrile patients in Ethiopia. Nevertheless, public health awareness on its zoonosis relevance was low and high-risk practices that expose the population to Brucella infections were highly prevalent. Screening and confirmatory tests used both in livestock and humans had significant variations in their ability to detect anti-Brucella antibodies.
In fact, the evidence accrued is not exhaustive; however, it is good enough to conclude the presence of Brucella infection both in people and livestock in Ethiopia. Therefore, we suggest a concerted one health approach to implement contextual brucellosis control intervention in the country. Accordingly, the strategy for intervention proper needs to account for the production systems and regional contexts to institute meaningful intervention both from economic and technical perspectives. In line with, vaccination of small ruminants in Afar and adjacent areas (Southern Tigray, Eastern Amhara and bordering areas of Oromia) could be launched following determination of the predominant Brucella species circulating in the area. Urban and peri-urban dairy production systems could start testing and removal of seropositive cattle with the imposition of strict movement control on dairy cattle. The intervention in mixed crop-livestock systems and other pastoral regions could be addressed based on the lessons drawn from the preceding approaches. Besides, tests to be used for screening and confirmation of seropositive animals need to be validated for use in Ethiopia. There is also a need for public education for awareness creation on the disease and its risk factors in humans and livestock for successful control.
ACKNOWLEDGEMENTS
The authors would like to thank all researchers whose works were used in this review.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required as this is a review article with no original research data.
Open Research
DATA AVAILABILITY STATEMENT
Data is made available as used for the final statistical analyses (Supplementary Table 5). Original extracted raw data is available with the authors and may be requested if needed.




