Toxoplasmosis in a free-ranging hairy dwarf porcupine (Sphiggurus spinosus) with a potential novel genotype
Alessandra Loureiro Morales dos Santos and Pedro Enrique Navas-Suárez contributed equally to the work.
Abstract
Toxoplasmosis is a zoonotic disease caused by the ubiquitous coccidia Toxoplasma gondii. Rodents play an important role in maintaining its life cycle, as they are one of the main diet sources for felids (wild and domestic), the unique definitive hosts. However, reports of toxoplasmosis in porcupines (Order Rodentia) are uncommon, with gaps concerning its pathophysiology. South America is the continent with the greatest genetic diversity of rodents and T. gondii. A free-ranging hairy dwarf porcupine was admitted to a wildlife rescue centre with a history of trauma. During rehabilitation, the animal presented neurological symptoms (sporadic episodes of hind limbs paresis) and died 5 months later. The main findings during necropsy were brain congestion and severe incisor overgrowth associated with maxillary perforation. The histopathological exam showed moderate encephalitis, with variable-sized round cysts, positive for PAS stain and immunohistochemistry for T. gondii. Additionally, two cysts were observed in the medulla of the adrenal gland. Molecular techniques were performed to characterize the parasite load by qPCR (Cq = 30) and the genotype by PCR-RFLP with 11 markers, which revealed a potential new genotype. This case adds to the body of knowledge in comparative pathology of Neotropical Rodentia and reports a new potential genotype circulating in South America.
1 INTRODUCTION
Toxoplasmosis is a zoonotic disease caused by the protozoan Toxoplasma gondii, and its distribution is worldwide. Felids (Family Felidae) are its only definitive host, and rodents (Order Rodentia) play an essential role in maintaining their life cycle (Su et al., 2010); however, there is information demonstrating that T. gondii can infect almost all warm-blooded animal species. Recently, molecular studies show a wide diversity of genotypes worldwide, with Brazil being a hub of great genetic diversity (Pena et al., 2011). Within the order Rodentia, the family Erethizontidae includes the South American porcupines. Although these species have a wide distribution in the Americas, due to their cryptic habits, the knowledge on their ecological and sanitary aspects are limited. The hairy dwarf porcupine Sphiggurus spinosus (F. Cuvier, 1823) has a wide distribution in northeastern Argentina, eastern Paraguay, northern Uruguay and southeastern Brazil, including the Cerrado, the Pantanal and the Atlantic Forest biomes (Voss, 2015). The hairy dwarf porcupine is categorized as Least Concern (LC) according to the red list of the International Union for Conservation of Nature (IUCN) and is part of Appendix III of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) since 1976.
There are few reports of toxoplasmosis in porcupines. Microscopic and immunohistochemical features were described in a captive Sphiggurus mexicanus in Costa Rica (Morales et al., 1996), and T. gondii was molecularly diagnosed in three Chaetomys subspinous in Brazil (Bezerra et al., 2015). To the best of our knowledge, this is the first report describing the molecular, immunohistochemical and pathological features of this condition in neotropical porcupines, including the report of a potential new genotype.
2 MATERIAL AND METHODS
An adult female hairy dwarf porcupine (Sphiggurus spinosus, F. Cuvier, 1823) was admitted to a wildlife rescue centre with a history of trauma. It was found in a periurban area of the metropolitan region of São Paulo, between the Atlantic rainforest and densely urbanized area. The animal was kept under medical care for almost 5 months, and during the rehabilitation, the animal presented sporadic episodes of bilateral hind limb paresis (Supplementary material 1). Days before the natural death, a purulent discharge in the right nostril was observed. Necropsy was performed, and tissue fragments (brain, heart, lungs, liver, spleen, kidney, adrenal glands, small intestine, uterus and urinary bladder) were collected and fixed in 10% neutral buffered formalin, and fresh brain and liver fragments were frozen at −20°C. The samples were referred to Adolfo Lutz Institute within the framework of the wildlife epidemiological and laboratorial surveillance project: potential for the emergence and re-emergence of infectious diseases (SES-PRC-2020/32339 – PROC. 28/2020) in order to detect potential zoonotic diseases in wildlife.
For histopathology, the tissues were processed routinely, embedded in paraffin-wax, sectioned at 4-μm-thick and stained with haematoxylin and eosin (H&E) and periodic acid of Schiff (PAS), for routine microscopic analysis. Immunohistochemical and molecular methods are fully described in the Appendix. The location where the porcupine was found was recorded. The location where the porcupine was found was recorded (Appendix).
3 RESULTS AND DISCUSSION
The main gross findings were purulent discharge in the right nostril extending to the frontal sinus; dental overgrowth of the lower right incisor associated with a solution of continuity in the maxillary and the right infraorbital regions, and marked congestion of the cortical blood vessels in the brain. No lesions were observed in other organs. Histologically, mild-to-moderate multifocal non-suppurative encephalitis, characterized by mononuclear perivascular cuffs with two to four layers of lymphocytes, plasma cells and macrophages, associated with mild-to-moderate multifocal gliosis, occasional necrosis of neuropil, randomly in white and grey matter. Multifocal protozoan cysts ranging from 66.3 × 44.9 to 126.7 × 136.0 μm, PAS-positive, were observed (Figure 1). Protozoan cysts showed immunolabeling for T. gondii at IHC (Figure 1). Two protozoan cysts were found in the medulla of the adrenal gland.
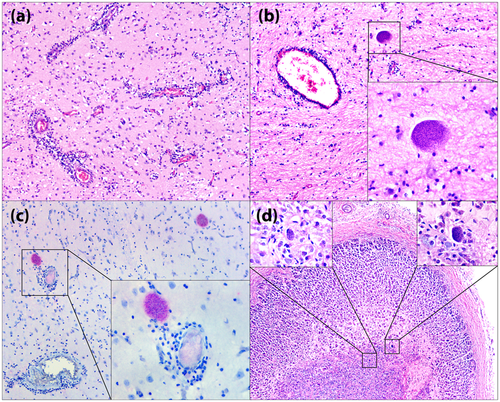
In rodents (mainly mice and rats), it is hypothesized that in latent infections, there is a neuroanatomical tendency of parasite cysts to be more abundant in amygdalar structures, resulting in impaired learning and memory and behavioural abnormalities, associated with loss of fear from predators, which could favour the biological cycle of the T. gondii (Hari Dass & Vyas, 2014). In this porcupine, we observed T. gondii cysts exclusively in the central nervous system and in the medulla of the adrenal gland, the latter with a neuroectodermal origin. We believe that this case corresponds to a latent infection due to the absence of tachyzoites in other tissues, such as the liver and spleen, the predominance of large cysts and rare inflammatory infiltrates around cystic structures.
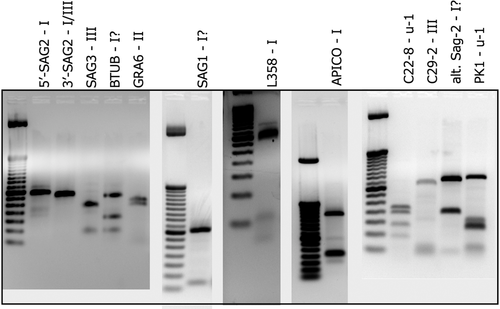
Molecular confirmation through qPCR showed a moderate parasite load (Cq = 30). In ToxoDB database platform, different genotypes (#1–#278) of T. gondii in human and animal samples are registered. This sample is likely a new genotype, with similarity to other known genotypes, since 9 out of 11 markers matched to ToxoDB PCR-RFLP genotypes #6, #80, #126 and #244 (Table 1 and Figure 2). The genotype #6 (also known as BrI and Africa 1) is the major virulent lineage previously reported in Brazil, and it was previously identified in domestic and wild Brazilian animals like chickens, dogs, cats, sheep, goats, pigeons and capybaras (Dubey et al., 2012). The genotype #6 is also observed in HIV-immunosuppressed humans in Brazil, with severe and diffuse cerebral toxoplasmosis (Ferreira et al., 2011). The genotypes #80 and #126 are described in Brazilian cats. Information about genotype #244 was not found in the literature. Beyond that, the only genotyping study on Sphiggurus spinosus showed 4/7 markers similarity (Richini-Pereira et al., 2016).
| SAG1 | 5-3SAG2 | alt.SAG2 | SAG3 | BTUB | GRA6 | c22-8 | c29-2 | L358 | PK1 | Apico |
|---|---|---|---|---|---|---|---|---|---|---|
| I? | I | I? | III | I? | II | u-1 | III | I | u-1 | I |
- Multilocus genotyping of Toxoplasma gondii strain from a hairy dwarf porcupine (Sphiggurus spinosus) by PCR-RFLP analysis. Potential new genotype, with comparison to strains indexed into TOXO-DB.
4 CONCLUSION
Herein, we describe the pathologic, IHC and genotyping features of toxoplasmosis in a free-ranging hairy dwarf porcupine. To the best of our knowledge, this is the first report describing the molecular, immunohistochemical and pathological features of this condition in neotropical porcupines, including the report of a potential new genotype. It reinforces the importance of wildlife surveillance to detect zoonotic diseases, especially in urban areas.
ACKNOWLEDGEMENTS
The authors would like to give special thanks to Doctor Chunlei Su for the precious support in electrophoresis analysis. Thanks to all the Wildlife Division (Cemacas) staff, Biological Sample Management Center (NGAB) and Pathology Center, Adolfo Lutz Institute. Thanks to Vinícius Gomes da Silva and Silvana de Melo Pereira da Silva for technical support. Doctor Hilda Fátima de Jesus Pena for pattern strains. Doctor Roberto Mitsuyoshi Hiramoto for the positive control and Vera Lúcia Pereira-Chioccola for the technical guidance on genotyping. The authors thank all fundings: Support Group to Prevention and Protection to the Health (GAPS/FESIMA grant #28/2020 and #040/2019), the Wildlife and to Coordination for the Improvement of Higher Education Personnel (CAPES; grant # 88887.374652/2019-00), the financial support for Santos ALM Master's study and the National Research Council (CNPq; grant #304999-18) for Catão-Dias JL fellowship. The funders had no role in study design, data collection, analysis, decision to publish or manuscript preparation.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required for this specific study.
CONFLICT OF INTERESTS
The authors declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
APPENDIX: IMMUNOHISTOCHEMICAL AND MOLECULAR METHODOLOGIES
Immunohistochemistry (IHC)
Deparaffinized 3-μm sections of formalin-fixed paraffin-embedded (FFPE) tissues silanized slides were submitted to antigen retrieval (citric acid solution 10 mM pH 6.0 in a pressure cooker, for 3 min, at 120°C). Endogenous peroxidase was blocked with 6% hydrogen peroxide for 30 min followed by overnight incubation with Polyclonal Rabbit Anti-Toxoplasma gondii (Dako Carpinteria, CA, USA) at a concentration of 1/50. Signal was amplified by the micropolymers conjugated with alkaline phosphatase enzyme (MACH 4® Universal AP Polymer Kit, Biocare Medical, CA, EUA) for 60 min, and visualization was achieved by chromogenic substrate (Warp-red®, Biocare Medical, CA, EUA) for 3 min. The samples were counterstained with Harris Haematoxylin for 20 s followed by dehydration and slide mounting with synthetic resin. Tissue fragments known to be positive and confirmed by PCR for the agent were used as positive controls and tissue sections in which the primary antibodies were replaced by non-immune serum of those species where antibodies were raised served as negative controls.
Molecular study
For quantitative analysis, real-time PCR (qPCR) assays were performed. DNA was extracted from frozen samples of the central nervous system using a commercial kit (Bio Gene, Bioclin Quibasa, DNA/RNA Viral Extraction), according to the manufacturer's instruction. The lyse buffer and proteinase K were added to 20 mg of tissues and incubated at 56°C overnight. The lysate was transferred to the silica column, washed and eluted with the elution buffer in a final volume of 60 μl. qPCR assays for T. gondii were carried out using primer pairs forward (TCC CCT CTG CTG GCG AAA AGT)/Reverse (AGC GTT CGT GGT CAA CTA TCG ATT G) and the probe (FAM - TCT GTG CAA CTT TGG TGT ATT CGC AG – BHQ1) and the Taqman Universal PCR Master Mix (Applied Biosystems, ThermoFisher Scientific, Warrington, UK) in a final volume of 25 μl. The expected product sizes were 98 bp (Lin et al., 2000). The cycle threshold value (CT), indicative of the quantity of target gene at which the fluorescence exceeds a preset threshold, was determined.
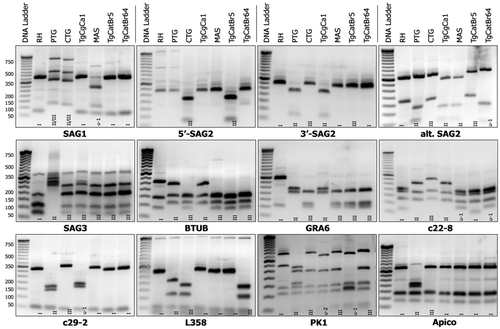
The genotype of this strain was determined by multilocus PCR-RFLP using 11 different markers: apico, PK1, L358, SAG1, SAG2, alt.SAG-2, SAG3, BTUB, GRA6, c22-8, c29-2 (Su et al., 2010). The PCR-RFLP products were examined by electrophoresis in 3% agarose gel, and compared to the pattern strains (RH, PTG, CTG, TgCgCa1, MAS, TgCatBr5, TgCatBr64) gel images (Figure A1).
Location of the porcupine
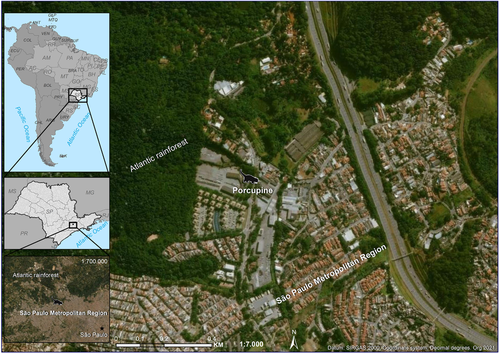
We geocoded the address using Geographic Information System ArcGIS 10.2.2. and its online imagery base maps only for location purposes (Figure A2).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Authorea at https://doi.org/10.22541/au.163590389.97218429/v1.




