Dynamics of cellular and humoral immune responses following duck Tembusu virus infection in ducks
Abstract
Duck Tembusu virus (DTMUV), an emerging avian pathogenic flavivirus, causes severe neurological disorders and acute egg drop syndrome in ducks. However, the effects of DTMUV on duck immunological components and functions remain largely unknown. In this study, the dynamics of cellular and humoral immune responses of DTMUV-infected ducks were investigated. The numbers of CD4+ and CD8+ T, B and non-T and B lymphocytes as well as the levels of neutralizing antibodies were quantified in parallel with DTMUV loads in blood and target organs. Our results demonstrated that DTMUV infection caused severe losses of non-T and B lymphocyte/myeloid cell subpopulation, and reduction in phagocytic activity during 3–5 days after infection. We also found that the numbers of T and B cells were increased during the first week of DTMUV infection. A significant negative correlation between the levels of CD4+ and CD8+ T, B and non-T and B lymphocytes and viral loads in blood and target organ (spleen) was observed during the early phase of infection. Additionally, DTMUV infection induced an early and robust neutralizing antibody response, which was associated with DTMUV-specific IgM and IgG responses. The presence of neutralizing antibody also correlated with reduction of viremia and viral load in the spleen. Overall, DTMUV elicited both cellular and humoral immune responses upon infection, in which the magnitude of these responses was correlated with reduction of viremia and viral loads in the target organ (spleen). The results suggested the critical role of both cellular and humoral immunity against DTMUV infection. This study expands our understanding of the immunological events following DTMUV infection in ducks.
1 INTRODUCTION
Duck Tembusu virus (DTMUV) is an emerging avian pathogenic flavivirus that causes severe neurological disorders and acute egg drop syndrome in ducks and other avian species, including geese and chickens (Zhang et al., 2017). DTMUV is an enveloped, single-stranded, positive-sense RNA virus, which is classified as a new genotype of Tembusu virus (TMUV) belonging to the genus Flavivirus of the family Flaviviridae (Su et al., 2011). Currently, DTMUV is widely distributed and is now endemic in duck populations in Asia, causing significant economic losses to the duck producing industry (Feng et al., 2020; Homonnay et al., 2014; Ninvilai et al., 2019; Peng et al., 2020). To develop an effective vaccine for preventing and controlling this emerging disease, a better understanding of the immunological mechanisms during DTMUV infection is required.
Several studies consistently showed that DTMUV predominantly causes retarded growth and severe neurological signs in young ducks, and decreased egg production in laying ducks (Li et al., 2015a; Lu et al., 2016; Sun et al., 2014). Our previous pathogenesis study in 4-week-old ducks demonstrated that DTMUV infection resulted in depression and loss of appetite as early as 2 days post-infection (dpi), followed by the development of severe neurological signs from 4 to 14 dpi. After showing neurological signs, some infected ducks died during 5–14 dpi, while surviving ducks continued to show mild clinical signs until the end of the observation period (21 dpi). Viremia in infected ducks was observed as early as 1 dpi, peaked at 3 dpi and then cleared by 9 dpi. Despite the absence of viremia after 7 dpi, relatively high viral loads were detected in the target organs, especially the brain, until 21 dpi. These findings indicated that although DTMUV induces a short-term viremia, the virus persists in tissues as well as oropharyngeal and cloacal swabs of DTMUV-infected ducks for a long period (Ninvilai et al., 2020). These observations indicate a complex interaction of DTMUV with the duck immune system. However, the immunological mechanisms involved in the control of DTMUV infection in ducks remain largely unknown. To date, studies on the immune responses against DTMUV in ducks have been limited to investigating the innate immune responses, which is essential for viral control in the early stage of infection (Li et al., 2015b, 2020). Little is known about the adaptive immune responses to DTMUV infection, especially the cellular immune responses, which play a crucial role in viral clearance and preventing reinfection (Slon Campos et al., 2018). In this study, we investigated the immunological changes in ducks following DTMUV infection. The dynamics of CD4+ and CD8+ T, B and non-T and B lymphocyte responses were examined by flow cytometric analyses. In addition, neutralizing antibodies and DTMUV-specific IgM and IgG antibody responses were evaluated.
2 MATERIALS AND METHODS
2.1 Virus
DTMUV strain DK/TH/CU-1 isolated from DTMUV infected ducks in Thailand was used in this study (Thontiravong et al., 2015). This virus is classified as DTMUV cluster 2, which is the predominant DTMUV cluster circulating in Asia (Ninvilai et al., 2019). DTMUV was propagated and titrated in 9-day-old embryonated duck eggs as previously described (Ninvilai et al., 2020).
2.2 Animal study
To evaluate the dynamics of cellular and humoral immune responses in ducks following DTMUV infection, samples from DTMUV-infected and non-infected control ducks, including whole blood, serum and spleen, were obtained from the previously described pathogenesis study (Ninvilai et al., 2020). Briefly, 4-week-old specific pathogen free (SPF) ducks (n = 35) were inoculated with 105 50% embryo lethal dose (ELD50)/ml of DTMUV (strain DK/TH/CU-1) in a total volume of 1 ml via the intranasal (0.5 ml) and intramuscular (0.5 ml) routes. Another group of 35 SPF ducks, inoculated intranasally and intramuscularly with allantoic fluid from SPF duck eggs, served as the non-infected control group. All ducks were obtained from healthy breeder duck flocks that were reared in a private research farm operated with high biosecurity standard and routinely confirmed to be free from common duck viruses, including DTMUV, by virus-specific RT-PCR/PCR and serological assays. Heparinized whole blood and spleen samples were collected for immunological studies at 0, 3, 5, 7, 9, 14 and 21 dpi, while serum samples were collected for serological study at 1, 3, 5, 7, 9, 14 and 21 dpi. Spleen samples were kept in the complete RPMI medium (RPMI-1640 supplemented with 10% foetal bovine serum [FBS], 2 mM glutamax-I, 100 U/ml penicillin and 100 μg/ml streptomycin [Invitrogen, CA, USA]) until further processed. In addition, blood samples and target organs, including brains and spleens, were collected for viral load determination at 1, 3, 5, 7, 9, 14 and 21 dpi. DTMUV loads in blood and target organs (brain and spleen) were quantified by DTMUV E-specific qRT-PCR in parallel with immunological analyses, and these data were retrieved from our previous DTMUV pathogenesis study (Ninvilai et al., 2020). The animal experiment was conducted under the approval of the Chulalongkorn University Animal Care and Use Committee (approval number 1673012).
2.3 Isolation of peripheral blood mononuclear cells and splenocytes
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized whole blood samples by density gradient centrifugation using Isoprep separation medium (Robbins Scientific Co., CA, USA) according to the manufacturer's protocol. Single splenocyte suspension was prepared by gently teasing splenic tissue through a 70 μM cell strainer (Corning Inc., NY, USA). Splenocytes were subsequently isolated by density gradient centrifugation for 20 min at 1000 × g using Isoprep separation medium as described previously (de Geus et al., 2012; Hao et al., 2020; Jansen et al., 2013). The cells were resuspended in a complete RPMI medium and subjected to immunofluorescent staining or determining of in vitro phagocytic activity.
2.4 Serum neutralization test
The presence of DTMUV-specific neutralizing antibodies in the serum samples was determined by the previously described serum neutralization (SN) test (Tunterak et al., 2018). Briefly, triplicate serial twofold dilutions of heat inactivated sera were incubated with 100 TCID50 of DK/TH/CU-1 for 1 h at 37°C. Following the incubation, the virus-serum mixture was added into a 96-well plate containing BHK-21 cells. The cells were then incubated at 37°C and were examined for the presence of cytopathic effects (CPE) daily for 5 days. Reference DTMUV seropositive and negative sera, uninfected BHK-21 cells and back titration of used virus served as controls. DTMUV-specific neutralizing antibody titres were expressed as the reciprocal of the highest serum dilution that inhibited CPE.
2.5 Indirect immunofluorescence assay
DTMUV-specific IgM and IgG antibodies in the serum samples were determined using indirect immunofluorescence assay. To assess the levels of DTMUV-specific IgM and IgG antibodies, serial twofold dilutions of serum (1:10 to 1:10,240) were added to a 96-well-plate containing fixed DTMUV-infected BHK-21 cells and incubated for 1 h at room temperature. Cells were washed three times with PBS, incubated with an anti-duck IgM-fluorescein isothiocyanate (FITC) secondary antibody (Nordic-MUbio, Susteren, The Netherlands) or an anti-duck IgG-FITC secondary antibody (KPL, MD, USA) and then evaluated under the inverted fluorescence microscope. DTMUV-specific IgM and IgG antibody titres were expressed as the reciprocal of the highest dilution of serum with positive staining. Negative and positive control samples were included in all assays. All samples were simultaneously tested against infected and uninfected control cells in a triplicate manner.
2.6 Immunofluorescent staining and flow cytometric analysis
Frequencies of CD4+ and CD8+ T, B, non-T and B lymphocyte subpopulations in the splenocytes and PBMC were assessed by flow cytometric analyses as described previously (Kothlow et al., 2005). Briefly, fresh splenocytes and PBMC were plated in a 96-well round-bottom plate at a density of 1 × 106 cells/well in the complete RPMI medium and then washed twice with FACS buffer (calcium- and magnesium-free phosphate-buffered saline supplemented with 1% FBS and 0.1% sodium azide). Subsequently, the cells were stained with antibodies to identify duck CD4+ lymphocytes (rat anti-human CD3ε conjugated with Alexa Fluor 647 (clone CD3-12, isotype rat IgG1) and mouse anti-duck CD4 (clone Du CD4-2, isotype mouse IgG2a), duck CD8+ lymphocytes (rat anti-human CD3ε conjugated with Alexa Fluor 647 (clone CD3-12, isotype rat IgG1) and anti-duck CD8α (clone Du CD8-1, isotype mouse IgG2b) and duck B lymphocytes (mouse anti-duck IgY light chain (clone 14A3, isotype mouse IgG1) and rat anti-human CD3ε conjugated with Alexa Fluor 647 (clone CD3-12, isotype rat IgG1) for 30 min at 4°C in the dark. All antibodies were obtained from Bio-Rad (CA, USA). After washing with FACS buffer, isotype-specific secondary antibodies, including goat anti-mouse IgG2a FITC, goat anti-mouse IgG2b PE-Cy7 and goat anti-mouse IgG1 PE (Southern Biotech, AL, USA), were added to the cells and incubated in the dark at 4°C for 30 min. Cells stained with isotype control antibodies were included as the background cut-off. The fluorescence minus one (FMO) staining controls were also performed during the establishment and validation of the assay. Data of assay validation are available upon request. The cells were gated at least 5 × 104 cell/events for each analysis. Samples were run through a FC 500 MPL (Beckman Coulter, CA, USA) and analyzed with FlowJo software (Tree Star Inc., OR, USA). PBMC and splenocytes were gated into CD3+CD4+ (CD4+ T cells), CD3+CD8+ (CD8+ T cells), CD3–IgY+ (B cells) and CD3–IgY– (non-T and B lymphocytes) subpopulations. To determine the actual changes induced by DTMUV infection, the absolute numbers of each subpopulation in the PBMC were calculated by using percentages obtained from flow cytometry combined with total PBMC count as described previously (Solomos et al., 2016).
2.7 In vitro phagocytic activity
Fresh PBMC (5 × 106 cells/well) were incubated with the complete RPMI medium at 37°C for 2 h. The attached cells, referred to as myeloid cells, were subsequently cultured with FITC-conjugated Escherichia coli (Molecular Probes, Invitrogen) at 1:50 ratio of attached cells: E. coli, at 37°C for 10 min with shaking. Cold PBS was added to stop phagocytic activity, and the cells were subsequently washed four times with PBS. The cells were then resuspended in FACs buffer for flow cytometric analysis.
2.8 Statistical analysis
Data were analyzed using two tailed, unpaired Student's t test. Correlations were evaluated by Pearson's correlation analysis. All statistical analyses were performed using the GraphPad Prism 5.0 software (GraphPad Software Inc. La Jolla, CA). All p < .05 were considered statistically significant.
3 RESULTS
3.1 Dynamics of cellular immune responses in ducks following DTMUV infection
To evaluate the dynamics of cellular immune responses following DTMUV infection, the frequencies and absolute numbers of CD4+ and CD8+ T, B and non-T and B cell subpopulations in the splenocytes and PBMC were analyzed by flow cytometry. Representative flow plots for CD4+ T cells (CD3+CD4+ cells), CD8+ T cells (CD3+CD8+ cells), B cells (CD3–IgY+ cells) and non-T and B lymphocytes (CD3–IgY– cells) are shown in Figure 1a. Following DTMUV infection, the frequencies of CD4+ T cells in the splenocytes of infected ducks increased significantly as early as 3 dpi, then decreased rapidly and returned to the level, similar to that of the non-infected ducks by 9 dpi (Figure 1b). Likewise, B-cell frequencies in the splenocytes of infected ducks followed the same trend of the CD4+ T cells, peaked at 3 dpi and then quickly declined to baseline level by 9 dpi (Figure 1b). The frequencies of CD8+ T cells in the spleen were markedly reduced at 3 dpi, increased at a later time point (5 dpi) and returned to the baseline level by 21 dpi (Figure 1b). Interestingly, reduction of non-T and B lymphocytes, referred to as putative myeloid cell subpopulation, in the splenocytes of infected ducks was evident throughout the observation period (Figure 1b).
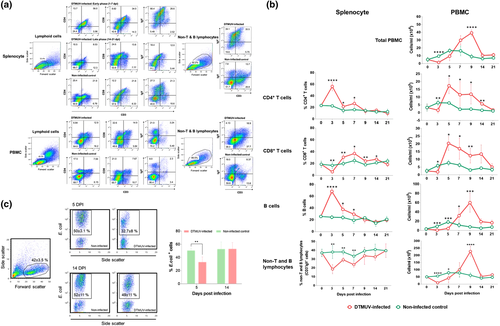
To investigate the cellular immune responses in the periphery of DTMUV-infected ducks, the numbers of cellular subsets in the circulation were measured. As shown in Figure 1b, DTMUV infection caused leucopenia during an early stage of infection, as observed by a significant reduction in the total PBMC count during 3–5 dpi. This finding was consistent with a significant decrease in numbers of T, B and non-T and B lymphocyte subpopulations in the PBMC of infected ducks at the early time points after infection (3–5 dpi) (Figure 1b). In contrast to decreased T- and B-cell numbers in the splenocytes during 5–7 dpi, the numbers of CD4+ and CD8+ T cells in the PBMC of infected ducks increased significantly at 5 dpi and sustained through 14 dpi (Figure 1b). A significant increase in numbers of B cells was detected at a later time point (7 dpi), peaked at 9 dpi and gradually declined thereafter (Figure 1b).
In contrast to the kinetic of non-T and B lymphocytes in the spleens, significant decreases in numbers of non-T and B lymphocytes in the PBMC of infected ducks were observed only during 3–5 dpi, then gradually increased and peaked at 9 dpi, and returned to the baseline level by 14 dpi (Figure 1b). In addition, the phagocytic activity of non-T and B lymphocyte subpopulation in the infected ducks significantly decreased at 5 dpi and restored at 14 dpi (Figure 1c).
To investigate the role of cellular immune responses on DMTUV control, the correlations between cellular frequencies in the PBMC and DTMUV loads in the blood and target tissues were analyzed. The results demonstrated that increased numbers of CD4+ and CD8+ T, B and non-T and B lymphocytes in the peripheral blood coincided with reduced viremia and viral loads in the spleens during the early phase of infection (Figure 2a). Supporting this finding, the numbers of CD4+, CD8+ T, B and non-T and B lymphocytes in the PBMC of infected ducks exhibited a significant negative correlation with DTMUV genome copy numbers in blood and spleen during the first week of infection; however, no significant correlation was observed between the numbers of these cells and viral load in the brains (Figure 2a,b).
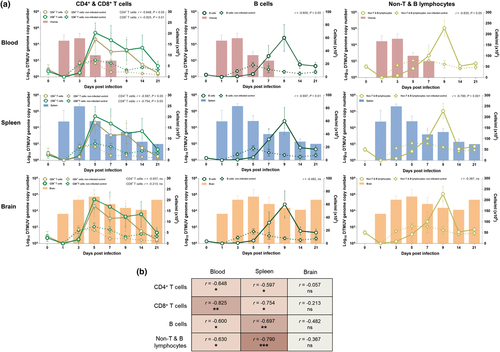
Altogether, these observations suggested that DTMUV induced T and B lymphocyte responses within the first week of infection and the induced cellular responses contributed to the reduction of viral loads in blood and spleen, but not in the brain, of the infected ducks.
3.2 Dynamics of humoral immune responses in ducks following DTMUV infection
To investigate the kinetics of DTMUV-specific humoral immune responses in the infected ducks, the levels of DTMUV-specific neutralizing antibodies and DTMUV-specific IgM and IgG antibodies were determined. As shown in Figure 3a, DTMUV-specific neutralizing antibodies were detected as early as 5 dpi, peaked at 7 dpi and maintained at a high level until the end of the observation period (21 dpi). Consistent with neutralizing antibodies, DTMUV-specific IgM and IgG antibodies were first detected on 5 dpi and peaked at 9 and 14 dpi, respectively (Figure 3a). The level of IgM gradually decreased from 9 dpi, while the level of IgG remained high until the end of the observation period, 21 dpi (Figure 3a). It should be noted that the kinetics of DTMUV-specific IgM and IgG positively correlated with that of the neutralizing antibodies (Figure 3c).
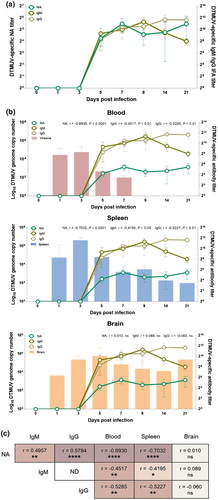
To evaluate the association of viral-specific humoral immune responses with DTMUV control, the correlations between the levels of neutralizing, IgM and IgG and DTMUV loads in blood and target tissues were analyzed. The results showed that induction of neutralizing antibodies coincided with reduced viral loads in blood and spleen (Figure 3b). Supporting this finding, the levels of neutralizing antibody titres negatively correlated with viral loads in blood and spleen, but not in the brain (Figure 3b,c). Consistent with neutralizing antibodies, the levels of viral-specific IgM and IgG negatively correlated with the viral loads in blood and spleen, but not in the brain (Figure 3b,c).
Taken together, our results indicated that induction of DTMUV-specific antibodies was associated with reduction of the systemic viral loads in blood and spleen of infected ducks. However, they could not completely eliminate viruses in the brain.
4 DISCUSSION
In the present study, we evaluated the dynamics of cellular and humoral immune responses in ducks following infection with DTMUV. Our results revealed that DTMUV infection induced cellular and humoral immune responses, which were characterized by the generation of T- and B-cell responses and neutralizing antibodies. Notably, the magnitude of these responses correlated well with reduction of viremia and viral loads in the spleen. These findings implied that these immunological factors played an important role in reducing viral loads in DTMUV-infected ducks. In addition, we demonstrated that DTMUV infection caused severe losses of the non-T and B lymphocyte/myeloid cell subpopulation, and reduction in phagocytic activity during the early stage of infection (3–5 dpi). It should be noted that the presence of DTMUV in target organs, especially the brain, was still observed in the infected ducks until the end of an observation period. This suggested that other viral immune evasion mechanisms as well as immune compartmentalization could possibly contribute to the persistence of DTMUV in the target tissues, which warrants further investigation. A schematic diagram of the dynamics of cellular and humoral immune responses in DTMUV-infected ducks is shown in Figure 4. To the best of our knowledge, this is the first study providing a comprehensive profile of cellular and humoral immune responses following DTMUV infection in ducks.
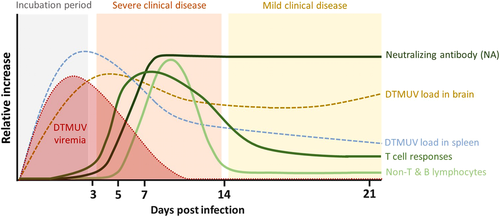
Analysis of T- and B-cell subpopulations revealed that both T- and B-cell responses were elicited early within the first week of DTMUV infection, suggesting that DTMUV had minimal effect on the cellular immune compartment. Our findings also demonstrated that the number of T and B cells increased earlier in the spleen (3 dpi) followed by the peripheral blood (5–7 dpi). It is possible that these T- and B-cell subsets were initially activated in spleen and/or secondary lymphoid associated tissues and subsequently migrated to the peripheral tissues via the blood circulation. In addition, we observed significant increases in the numbers of B cells following the increase of CD4+ T cells in the peripheral blood of DTMUV-infected ducks. This finding supported the crucial role of activated CD4+ T cells on B-cell activation, cellular trafficking, and virus-specific antibody production (Liang et al., 2019; Slon Campos et al., 2018; Suthar et al., 2013). Importantly, increasing numbers of T and B lymphocytes occurred simultaneously and correlated with the reduction of viral loads in blood and spleens during the first week of infection. Altogether, our findings suggested that cellular immune response, elicited following DTMUV infection, contributed to viral control, particularly during the first week of infection. Consistent with our findings, several previous studies clearly showed that cellular immune responses, including CD4+ and CD8+ T-cell responses, played a critical role in controlling flavivirus replication, viral clearance and protecting against infection (Bassi et al., 2015; Ng et al., 2019; Sitati & Diamond, 2006). Further studies are needed to characterize the specificity and functions of these T- and B-cell subpopulations in controlling DTMUV infection.
Notably, despite expansions of T and B lymphocytes following infection, the persistence of viruses in the brains and severe clinical diseases were still observed in DTMUV-infected ducks. One possible explanation for this finding is that DTMUV spread rapidly to the target organs, including brains, as early as 1 dpi before the induction of cellular immune responses, thereby potentially leading to severe clinical outcomes in the infected ducks. These observations raise the possibility that early priming of adaptive immune responses prior to infection may mitigate viral spreading and severe clinical signs. Therefore, DTMUV vaccines that can induce viral-specific adaptive immune responses, particularly neutralizing antibodies, prior to the infection are expected to be effective in preventing DTMUV infection in ducks. Another possible explanation is that the detrimental effects on the innate immune compartment during the early stage of DTMUV infection might result in uncontrollable viral persistence and severe clinical outcomes. Our results showed that DTMUV induced severe losses of non-T and B lymphocyte/myeloid cell subpopulation, and reduction in phagocytic activity during the early stage of infection. Supporting our findings, previous studies demonstrated that DTMUV could infect monocyte/macrophage, leading to extensive inhibition of several antiviral innate immune responses, including the induction of type I interferons and phagocytic activity (Li et al., 2020; Liang et al., 2021; Liu et al., 2019; Ma et al., 2019). It is possible that the vaccine platforms that can prime adaptive immune responses may be effective for preventing DTMUV infection and spreading through reducing the viral effects on the innate immune functions.
Our results demonstrated that ducks infected with DTMUV developed an early and robust viral neutralizing and DTMUV-specific IgM and IgG antibody responses, which were shown to be correlated with reduction of viremia and viral load in spleens. This finding indicated that neutralizing activity is likely to be associated with both IgM and IgG antibodies, which was in agreement with the previous studies reporting the correlations between IgM and IgG and neutralizing antibody responses in other flavivirus-infected cases (Libraty et al., 2015; Puschnik et al., 2013). Our observations suggested that similar to other flaviviruses (Pestka et al., 2007; Slon Campos et al., 2018), neutralizing antibodies likely played an important role in viral clearance from blood and limiting viral dissemination in ducks during DTMUV infection. However, it is noted that despite the presence of robust viral-specific neutralizing antibodies in the peripheral blood, DTMUV remained persisted in the brains of infected ducks through the end of the observation period, indicating that high levels of circulating neutralizing antibodies could not clear the viruses from the brains of infected ducks. Consistently, a previous study demonstrated that DTMUV could spread to the brain without disruption of blood–brain barrier integrity during the early stage of infection, preventing the recruitment of immune effectors from the periphery to the brain (Yang et al., 2021). These observations raise the possibility that pre-existing viral-specific antibodies may be required to prevent viral spreading to the brain. Our findings supported development of the vaccine that can promote both cellular and humoral immune responses against DTMUV. However, it is also possible that other vaccine platforms that primarily promote humoral immune responses and elicit sufficiently high and sustained neutralizing antibody titres may also be effective for preventing DTMUV infection by promptly terminating viremia and preventing viral dissemination to the target organs. Supporting this notion, previous studies showed that flavivirus vaccines that efficiently induced either viral-specific antibodies or both types of adaptive immune responses were shown to be effective in protection against flaviviruses, including Japanese encephalitis virus and yellow fever virus (Scherwitzl et al., 2017; Slon Campos et al., 2018; Yau et al., 2020). Further studies will be required to define the exact immune correlates of protection for DTMUV infection and compare the effectiveness of both types of vaccine against DTMUV infection.
In conclusion, our data collectively indicated that both cellular and humoral immune responses were elicited following DTMUV infection in ducks. Increasing numbers of CD4+ T cells, CD8+ T cells, B cells and viral-specific neutralizing antibodies correlated well with the reduction of viremia and viral loads in the spleens. These findings highlight the importance of both cellular and humoral immune responses for the control of DTMUV infection. The findings expand our understanding of the immunological events in ducks following DTMUV infection and provide insights for the design and development of effective DTMUV vaccines.
ACKNOWLEDGMENTS
We would like to thank the staff and graduate students of the Virology Unit, Department of Veterinary Microbiology, Department of Pathology, Faculty of Veterinary Science, and Chulalongkorn University Laboratory Animal Center (CULAC), Chulalongkorn University. We also would like to acknowledge Drs. R. Tantilertcharoen, S. Wannaratana, S. Munyahongse, N. Yurayat, B. Limchareon, K. Limpavithayakul, N. Nonthabenjawan, C. Sirisereewan, S. Sooksong and C. Chokboonmongkol for technical assistance with the animal experiment. We would also like to thank CPF (Thailand) Public Company Limited for contributing to experimental ducks and equipment required for the animal experiment. This work was supported by the Agricultural Research Development Agency (PRP6305030260), Chulalongkorn University: CU-GR_61_015_31_0047 and the 90th Anniversary of Chulalongkorn University Scholarship. We would also like to thank Chulalongkorn University for its financial support to the Center of Excellence for Emerging and Re-emerging Infectious Diseases in Animals, the Animal Vector-Borne Disease Research Unit and the Thailand Research Fund for its financial support to the TRF Senior Scholar to AA (RTA6080012).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ETHICAL APPROVAL
The authors confirm that the ethical policies of the journal, as noted in the journal’s author guidelines, have been adhered to and the appropriate ethical review committee. Animal experiment was approved and conducted in accordance with the ethical guidelines of the Chulalongkorn University Animal Care and Use Committee (approval number 1673012).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




