First report of equine parvovirus-hepatitis and equine hepacivirus coinfection in horses in Korea
Abstract
Equine parvovirus-hepatitis (EqPV-H) and equine hepacivirus (EqHV) are etiologically associated with Theiler's disease (TD), causing fulminant equine hepatitis, but the transmission route and co-infection effect remain unclear. We determined EqPV-H and EqHV prevalence and coinfection rate in 160 serum and 114 faecal samples using nested polymerase chain reaction. Amino acid and nucleotide analyses were performed and phylogenetic trees were constructed. By measuring liver-specific parameters (AST, GGT, TBIL and A/G ratio), hepatopathological changes in viremia status were compared. We found a high prevalence (EqPV-H: 10.6% in serum, 5.3% in faeces; EqHV: 8.1% in serum) and coinfection rate (35.3% in EqPV-H) of TD-causing agents. The newly identified EqPV-H genomes showed high nucleotide and amino acid similarities with previously reported strains in the USA, China and Austria. In phylogenetic tree and recombination analysis, a natural recombination event was confirmed between Chinese and Korean strains. In the EqPV-H or EqHV viremic horses, AST was significantly elevated and at least two liver-specific parameters were outside the reference intervals in 43.5% (10/23) of horses. To our knowledge, this is the first prevalence field study of EqPV-H and EqHV using both serum and faeces, providing further evidence of faecal-oral transmission of TD. These epidemiologic and clinicopathologic analyses specify the risk factors of TD infection and promote disease prevention strategy.
1 INTRODUCTION
Equine serum hepatitis, also known as Theiler's Disease (TD), is a serious disease that can cause acute liver atrophy and parenchymatous hepatitis (Theiler, 1919). To date, several studies had been conducted to identify causative agents of TD: equine hepacivirus (EqHV), equine pegivirus (EPgV), TD-associated virus (TDAV) and equine parvovirus-hepatitis (EqPV-H) have been implicated (Burbelo et al., 2012; Chandriani et al., 2013; Divers et al., 2018; Kapoor et al., 2013). Although there is on-going debate regarding the connection between EPgV/TDAV and clinical signs of TD, both EPgV and TDAV have been shown to be not hepatotropic and are not related to hepatitis in horses (Tomlinson, Wolfisberg, et al., 2020). Presently, only EqPV-H and EqHV are considered potential etiologic agents of TD (Tomlinson, Van De Walle, et al., 2019).
EqPV-H is a non-enveloped, icosahedral, single-stranded DNA virus with a genome size of 4–6 kb and belongs to the species Ungulate copiparvovirus 6, in the genus Copiparvovirus of the family Parvoviridae (Divers et al., 2018; Mietzsch et al., 2019; Pénzes et al., 2020). In 2018, EqPV-H was first reported in a dead horse with fatal serum hepatitis after inoculation of tetanus antitoxin, and subsequent investigations have demonstrated that equine biological products are important sources of TD transmission (de Moraes et al., 2021; Divers et al., 2018; Meister et al., 2019; Tomlinson, Jager, et al., 2020; J. E. Tomlinson, Van De Walle, et al., 2019; Vengust et al., 2020). However, in previous surveillance studies of healthy horse populations in the USA, China, Germany, Austria and Brazil, the prevalence range of EqPV-H DNA was between 7.1 and 17%, and the seroprevalence range was between 15 and 34.7% (Altan et al., 2019; Badenhorst et al., 2021; Divers et al., 2018; Lu et al., 2018; Lu et al., 2020; Meister et al., 2019). These high EqPV-H prevalence rates imply that other transmission methods, such as natural horizontal or vertical transmission, and other types of iatrogenic routes, like stomach tubes or contaminated needles, are also possible alongside blood-origin administration (Tomlinson, Kapoor, et al., 2019). Recently, EqPV-H transmission after oral administration, and multiple routes of virus shedding (oral and nasal secretions, and in faeces) during the viremia period were demonstrated in experimentally inoculated horses (Tomlinson, Jager, et al., 2020). However, the entire range of transmission routes has not been fully elucidated and additional investigation is needed.
Hepacivirus is a single-stranded, positive-sense RNA virus in the family Flaviviridae (Simmonds et al., 2017). EqHV is the closest genetic homolog of the hepatitis C virus (HCV); the two viruses comprise the genus Hepacivirus (Pfaender et al., 2014; Thézé et al., 2015). EqHV is hepatotropic and shows HCV-like infection mechanisms with various clinical signs from subclinical to diffuse hepatocellular necrosis with virus elimination or chronic infection (Gather et al., 2016; Pfaender et al., 2015; Ramsay et al., 2015; Reuter et al., 2014; Scheel et al., 2015). In previous studies, the EqHV prevalence of horses ranged from 0.9 to 35.6% (Badenhorst et al., 2018; Elia et al., 2017; Figueir et al., 2018; Kim et al., 2017; Lyons et al., 2012; Matsuu et al., 2015; Pronost et al., 2017; Reichert et al., 2017; Tanaka et al., 2014).
Despite the high prevalence of the TD-causing agents, to our best knowledge, there has been no field investigation into virus transmission in the absence of equine biological products, virus shedding and pathological effects of coinfection. In Korea, none of the commercial equine blood products have received official government approval. Therefore, research into TD transmission in the absence of contaminated blood products is warranted.
This study reports the prevalence and coinfection rate of EqPV-H and EqHV among horses in Korea using serum and faecal samples. The genetic compositions of isolates, evolutionary history and natural recombination events were also analysed. In addition, we present the biochemical evidence of hepatopathy and possible risk factors of the viruses. Our findings strongly support the nonparenteral natural transmission of TD and expand the current knowledge for establishing a disease protection strategy.
2 MATERIALS AND METHODS
2.1 Sample collection
We collected 160 serum and 114 faecal samples (from a total of 253 horses) from two equine clinics of the Korea Racing Authority on Jeju Island, Korea. A total of 253 horses presented for medical treatment with clinical signs (n = 107), regular physical examination without clinical signs (n = 90) and blood doping test before racing (n = 56). From each of these horses, either one serum or one faecal sample was collected. From the other 21 horses, both serum and faecal samples were collected from each. Detailed information on the horses is presented in Table 1. Blood was collected via venipuncture into Vacutainer SST blood collection tubes (Becton-Dickinson, Franklin Lakes, NJ, USA) and sera were aliquoted after centrifugation. Faecal sampling was performed per rectum using sterile rectal gloves with lubrication (Kruuse, Langeskov, Denmark) or collected shortly after each horse defecated to reduce contamination. Faecal supernatant samples were harvested after 1 g of faeces and 10 mL of phosphate-buffered saline mixtures were vortexed and centrifuged at 3000 g for 30 min. All serum and faecal supernatant samples were placed in sterile microcentrifuge tubes and stored at −70℃ until further analyses.
| Breed | Country of Foaling | Prevalence | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Type | Purpose | Thorough-bred | Warm-blood | Jeju Horse | Other | Average age/years‡ (range) | Korea | USA | Other | EqPV-H(%) | EqHV(%) |
| Serum | Racing | 2/6/48† | – | 0/0/52 | 0/0/8 | 2.9 (0−11) | 2/6/108 | – | – | 2/108 (1.9) | 6/108 (5.6) |
| Breeding | 12/5/31 | – | – | 0/0/1 | 12.1 (3−22) | 1/2/8 | 11/3/23 | 0/0/1 | 12/32 (37.5) | 5/32 (15.6) | |
| Riding | 1/1/6 | 1/1/9 | 0/0/2 | 1/0/3 | 9.0 (2−18) | 2/1/12 | 0/0/1 | 1/1/7 | 3/20 (15) | 2/20 (10) | |
| Total | 15/12/85 | 1/1/9 | 0/0/54 | 1/0/12 | 5.5 (0−22) | 5/9/128 | 11/3/24 | 1/1/8 | 17/160 (10.6) | 13/160 (8.1) | |
| Faeces | Racing | 3/0/44 | – | 0/0/19 | 0/0/2 | 1.7 (0−12) | 3/0/65 | – | – | 3/65 (4.6) | 0/65 (0) |
| Breeding | 3/0/20 | – | 0/0/24 | – | 9.0 (2−17) | 0/0/34 | 3/0/10 | – | 3/44 (6.8) | 0/44 (0) | |
| Riding | – | 0/3 | 0/0/1 | 0/0/1 | 7.6 (3−16) | 0/0/4 | – | 0/0/1 | 0/5 (0) | 0/5 (0) | |
| Total | 6/0/64 | 0/3 | 0/0/44 | 0/0/3 | 4.8 (0−17) | 3/0/103 | 3/0/10 | 0/0/1 | 6/114 (5.3) | 0/114 (0) | |
- † Number of EqPV-H positive samples/EqHV positive samples/total samples.
- ‡ Age when samples were collected.
2.2 Virus detection
Viral DNA and RNA extraction was performed with a Patho Gene-Spin DNA/RNA kit (Intron Biotechnology, Seongnam, Korea) according to the manufacturer's guidelines using 150 μL of the serum or faecal sample supernatant eluted in 30 μL of RNase-free water.
The specific sequences of EqPV-H/EqHV were analysed through a nested polymerase chain reaction (PCR) process using Maxime PCR and RT-PCR PreMix kit (Intron Biotechnology). Two primer pairs used for each virus targeted the partial non-structural (NS) gene of EqPV-H and the partial NS3 gene of EqHV based on previously published data (Divers et al., 2018; Kapoor et al., 2011; Kim et al., 2017). PCR products were analysed by 1.5% agarose gel electrophoresis and the products of expected size were purified by gel extraction with the MEGAquick-spin Plus DNA Purification Kit (Intron Biotechnology), and then Sanger sequenced (Cosmo Genetech, Seoul, Korea).
Two complete viral coding sequences (CDS) of EqPV-H, including NS and viral particle (VP) proteins, were acquired by the primer walking method with five PCR amplicons. The full genomic sequences were assembled using SeqMan software (DNASTAR, Madison, WI, USA).
All sequenced genes of EqPV-H (two complete CDS and 11 partial NS genes) and EqHV (13 partial NS3 genes) were deposited into the GenBank database (accession numbers OK032478 to OK032502). The primer sets and PCR information are listed in Supporting information Table S1.
2.3 Blood biochemistry and liver biopsy
For clinical pathology analysis, biochemical analysis was performed using the VetScan VS2 chemistry analyser (Abaxis, Union City, CA, USA) with 23 EqPV-H- and/or EqHV-positive serum samples and 32 negative serum samples as a control for the detection of liver-related parameters including aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), total bilirubin (TBIL) and albumin/globulin ratio (A/G ratio). One serum sample of an EqPV-H-positive horse was excluded because it was found after several hours of death and the parameter levels were out of the measurement ranges. We divided the horse population above into four groups; EqPV-H–/EqHV– (n = 32), EqPV-H+/EqHV– (n = 10), EqPV-H–/EqHV+ (n = 7) and EqPV-H+/EqHV+ (n = 6). And then, parameter levels were compared among the four divided groups or with the reference intervals (Riond et al., 2009; Vengust et al., 2020). In addition, a liver sample, with suspected liver damage in appearance, was partially resected during abdominal surgery and soaked in 10% formalin for further pathological analysis by a commercial laboratory (IDEXX, Westbrook, ME, USA).
2.4 Phylogenetic tree and similarity assessment
To evaluate the evolutionary history of the identified virus sequences, we used Molecular Evolutionary Genetics Analysis software version X (MEGA-X, https://www.megasoftware.net). The sequences were aligned using MUSCLE and the phylogenetic trees were built with the maximum likelihood method. The bootstrapping value was 500 and the substitution models were determined with the best DNA/Protein model finding option of MEGA-X. In addition, to compare the similarity of the amino acid and nucleotide sequences between identified strains in this study and other genus Copiparvovirus members, representative strains were aligned using MUSCLE and calculated p-distances.
2.5 Recombination event analysis
Recombination Detection Program (RDP) version 4.101 was employed for EqPV-H recombination event detection. Two newly discovered EqPV-H Korean strains and 16 other corresponding complete CDS were aligned with the MUSCLE program in the MEGA-X. Seven methods (RDP, GENECONV, BootScan, MaxChi, Chimera, SiScan and 3Seq) in RDP were used for recombination breakpoint determination. The significance level was p <.01 with standard Bonferroni correction. Standard similarity plots between recombinant and parent strains were generated with Simplot version 3.5.1 for further identification of putative recombination sequences.
2.6 Statistical analysis
To assess the impact of virus infection on the liver-specific parameter levels, a one-way analysis of variance, or Kruskal–Wallis test was applied after a normality test (Shapiro-Wilk) and homogeneity of variances test (Levene's). The Tukey method was used for the post-hoc comparisons and p < .05 was set as the statistical significance. All analyses were performed in Jamovi software (The Jamovi Project 2021, version 1.6.23).
3 RESULTS
3.1 Prevalence of EqPV-H and EqHV
In total, 274 serum and faecal samples were tested for EqPV-H and EqHV. Among 160 serum samples, 17 were EqPV-H DNA-positive (10.6%) and 13 were EqHV RNA-positive (8.1%). Among 114 faecal samples, six were EqPV-H DNA-positive (5.3%), while EqHV RNA was not detected in any faecal samples. Regarding EqPV-H DNA prevalence, breeding horses from the USA showed the highest rate of EqPV-H infection with 11 of 23 (47.8%) in serum and 3 of 10 (30%) in faecal samples; in contrast, Jeju horses, an indigenous Korean horse breed, were all negative (Table 1). Among the 17 EqPV-H DNA-positive horses (serum), five were also tested using faecal samples, with four (80%) also testing EqPV-H DNA-positive (Table 2). Regarding EqHV, breeding horses born in Korea had the highest positive rate (2/8, 25%), followed by breeding horses from the USA (3/23, 13%) (Table 1). Unlike the EqPV-H, the group of racing horses foaled in Korea showed a relatively higher prevalence of EqHV RNA (6/108, 5.6%) in serum (Table 1). About the horse purpose regardless of country of foaling, the breeding horse population (the oldest group; average age: 12.1 years) also had the highest prevalence rates of EqPV-H DNA (12/32, 37.5% in serum and 3/44, 6.8% in faecal samples) and EqHV RNA (5/32, 15.6% in serum) (Table 1).
| ID† | Sample type | Age‡ | Sex | Breed | Purpose | CC | PV-H§ | HV | AST(U/L) | GGT (U/L) | TBIL (mg/dL) | A/G |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14 | Serum/Faeces | 4 m | M | TB | Racing | Diarrhoea | + / + | 196 | 20 | 5.3 | 1.0 | |
| 20 | Serum | 3 y | F | TB | Breeding | Anorexia | + | 355 | 20 | 3.2 | 0.9 | |
| 23 | Serum | 19 y | G | WB | Riding | Colic | + | + | 242 | 16 | 2.6 | 1.1 |
| 24 | Serum | 6 y | F | TB | Breeding | – | + | + | 289 | 16 | 1.4 | 0.9 |
| 25 | Serum | 4 m | F | TB | Racing | Umbilical hernia | + | 241 | 9 | 1.7 | 1.2 | |
| 28 | Serum | 2 y | M | TB | Racing | Lameness | + | 324 | 17 | 1.5 | 1.0 | |
| 31 | Serum | 12 y | F | TB | Breeding | – | + | 257 | 16 | 1.8 | 0.8 | |
| 51 | Serum | 9 y | F | TB | Breeding | Dystocia | + | + | 283 | 17 | 2.8 | 1.3 |
| 65 | Serum | 12 y | F | WB | Breeding | Colic | + | 216 | 23 | 2.6 | 1.2 | |
| 72 | Serum | 9 y | F | TB | Riding | – | + | 304 | 16 | 2.7 | 1.4 | |
| 77 | Serum | 10 y | F | TB | Riding | – | + | 346 | 33 | 1.8 | 1.4 | |
| 79 | Serum/Faeces | 1 y | M | TB | Racing | Colic | + / - | 370 | 30 | 2 | 0.8 | |
| 96 | Serum | 12 y | F | Mixed | Riding | Cachexia (Deceased) | + | – | – | – | – | |
| 100 | Serum/ Faeces | 16 y | F | TB | Breeding | Colic | + / + | 527 | 20 | 2.6 | 1.2 | |
| 101 | Serum/Faeces | 2 y | M | TB | Racing | Colic | + / - | 318 | 12 | 3.4 | 0.8 | |
| 103 | Serum | 4 m | F | TB | Racing | Colic | + | + | 320 | 31 | 2.1 | 0.7 |
| 141 | Serum/Faeces | 11 y | F | TB | Breeding | – | + / + | 442 | 21 | 1.3 | 1.0 | |
| 147 | Serum | 13 y | F | TB | Breeding | Colic | + | 372 | 20 | 7.9 | 1.1 | |
| 151 | Serum | 13 y | F | TB | Breeding | Dystocia | + | 296 | 16 | 4.5 | 1.2 | |
| 157 | Serum/Faeces | 11 y | F | TB | Breeding | Colic | + / + | 352 | 13 | 4.6 | 0.7 | |
| 159 | Serum | 12 y | F | TB | Breeding | Colic | + | 274 | 17 | 8.3 | 1.1 | |
| 173 | Serum/Faeces | 15 y | F | TB | Breeding | Colic | + / - | + | 388 | 18 | 10.7 | 1.3 |
| 174 | Serum | 17 y | F | TB | Breeding | Colic | + | + | 270 | 13 | 3.2 | 1.0 |
| 179 | Serum | 2 y | M | TB | Racing | Colic | + | 353 | 18 | 3.4 | 1.6 | |
| 207 | Faeces | 3 m | M | TB | Racing | – | + | – | – | – | – | |
| 274 | Faeces | 2 y | M | TB | Racing | OCD | + | – | – | – | – |
- † Co-infected horses indicated in bold letters.
- ‡ Age when samples were collected (m, month; y, years).
- § Notation order: serum/faeces.
- Abbreviations: A/G, albumin/globulin ratio; AST, aspartate aminotransferase; CC, chief complaint; GGT, gamma-glutamyl transferase; HV, equine hepacivirus; M/F/G, male/female/gelding; OCD, osteochondritis dissecans; PV-H, equine parvovirus-hepatitis; TB, thoroughbred; TBIL, total bilirubin; WB, warmblood.
3.2 Coinfection rate of EqPV-H and EqHV
In total, 17 horses were identified as EqPV-H DNA-positive with serum samples, six horses were also co-infected with EqHV (35.3%) and the average age was 11 years (range: 4 months to 19 years) (Table 2, Figure 1A). Among the six co-infected horses, four were thorough-bred breeding horses, one was a warm-blood riding horse, and one was a 4-month thorough-bred foal for the racing purpose (Table 2). Four horses were imported (three from the USA and one from Australia) and two, including one foal, were born in Korea. Most co-infected horses presented to equine clinics with colic signs (four cases) and one horse was dystocia, while the other did not show any clinical signs (Table 2).
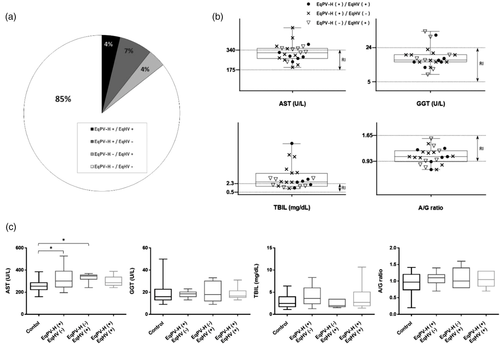
3.3 Clinical pathology of liver damage
We divided the horse population into four groups; EqPV-H–/EqHV– (n = 32), EqPV-H+/EqHV– (n = 10), EqPV-H–/EqHV+ (n = 7) and EqPV-H+/EqHV+ (n = 6) to compare AST, GGT, TBIL and A/G ratio levels with reference intervals and for intergroup evaluation of hepatopathy. Among the total 23 EqPV-H and/or EqHV nucleic acid-positive horses, 10 horses (43.5%) had more than two liver parameters with values outside the reference ranges (Figure 1B). We then performed an intergroup comparison between the control (EqPV-H–/EqHV–) and the other groups. There was statistically significant AST elevation (p < 0.05) in the EqPV-H+/EqHV– and EqPV-H–/EqHV+ groups compared to the control group, while the coinfection group (EqPV-H+/EqHV+) did not show a significant difference from the control (Figure 1C). In addition, we found hepatocellular necrosis containing infiltrates of neutrophils, and a mild degree of biliary hyperplasia with infiltrates of lymphocytes, plasma cells and neutrophils in the liver specimens of an EqHV RNA-positive horse (Supporting information Figure S1).
3.4 Phylogenetic analysis and sequence similarity
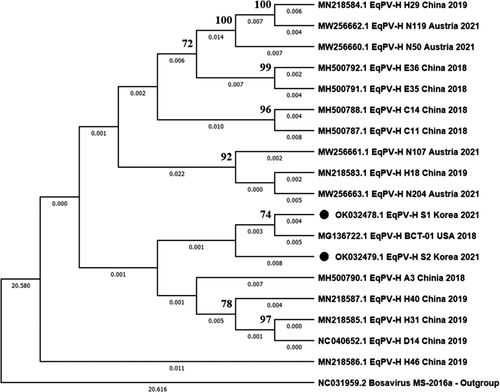
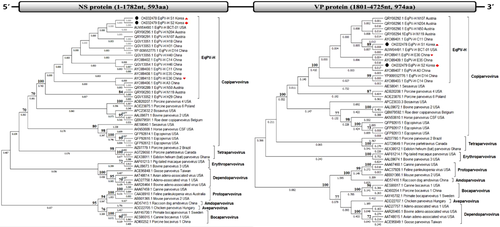
The two newly identified complete genomes of EqPV-H, 10 partial NS genes of EqPV-H and 13 partial NS3 genes of EqHV were genetically evaluated. In the phylogenetic tree analysis of the EqPV-H complete nucleotide sequences, the Korean strains (S1 and S2) were the most closely related to the USA isolate (BCT-01) (Figure 2). The EqPV-H genome encoded two large ORFs composed of NS and VP proteins. Each NS and VP protein was compared with the subfamily Parovirinae including the genus Copiparvovirus. Both ORFs clustered the U. copiparvovirus 6 species with other EqPV-H strains. At the species level, NS proteins of the two Korean strains were also closely related to BCT-01 (USA) similar to in the nucleotide analysis, while each of the VP proteins were clustered with BCT-01 and A3 (China) strains, respectively (Figure 3). Other equine parvovirus species (EqPV-cerebrospinal fluid, Eqcopivirus), which were also isolated from the horses, were clustered with Sesavirus for the NS region and bovine parvovirus 2 or Roe deer copiparvovirus for the VP region, rather than with EqPV-H strains (Figure 3). Phylogenetic trees of the partial NS genes of EqPV-H and NS3 genes of EqHV are also shown in Supporting information Figures 2 and 3.
We also compared the amino acid and nucleotide similarity between EqPV-H and other Copiparvovirus species. The demonstrated NS proteins of EqPV-H strains in Korea showed 97.7−99.4%, 48.0−53.3% and 51.0−54.8% nucleotide identities, and 98.1−99.3%, 26.6−29.2% and 29.7−35.9% amino acid identities with the previously identified EqPV-H strains, other equine parvovirus species and other copiparvoviruses, respectively (Table 3). Regarding the VP proteins of the Korean strains, there were 95.9−98.9%, 51.7−52.9% and 50.7−55.2% nucleotide identities, and 95.9−97.9%, 29.8−32.8% and 28.4−36.9% amino acid identities with the previously identified EqPV-H strains, other equine parvovirus species and other copiparvoviruses, respectively (Table 4).
| S1 (Korea) | S2 (Korea) | BCT01 (USA) | A3 (China) | N50 (Austria) | EqPV-CSF | Eqcopivirus | Sesavirus | BPV2 | PPV4 | RDCV | PPV6 | Bosavirus | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 99.3 | 99.4 | 98.8 | 97.8 | 53.3 | 48.1 | 52.1 | 53.4 | 54.6 | 52.1 | 52.9 | 51.1 | |
| S2 | 99.7 | 98.9 | 98.4 | 97.7 | 53.2 | 48.0 | 52.1 | 53.4 | 54.8 | 52.0 | 52.8 | 51.0 | |
| BCT01 | 99.2 | 98.8 | 98.5 | 97.8 | 53.1 | 48.1 | 51.9 | 53.4 | 54.4 | 52.1 | 52.8 | 51.1 | |
| A3 | 99.3 | 99.0 | 99.2 | 97.6 | 53.1 | 48.3 | 51.9 | 53.6 | 54.9 | 52.4 | 52.8 | 51.4 | |
| N50 | 98.3 | 98.1 | 98.1 | 99.0 | 53.3 | 48.1 | 52.5 | 53.3 | 54.3 | 52.0 | 53.1 | 51.1 | |
| EqPV-CSF | 29.4 | 29.2 | 29.5 | 29.6 | 29.6 | 56.6 | 47.8 | 51.7 | 46.9 | 49.4 | 47.0 | 50.5 | |
| Eqcopivirus | 26.6 | 26.6 | 26.6 | 26.8 | 27.0 | 37.5 | 46.4 | 47.9 | 44.7 | 46.0 | 41.3 | 47.3 | |
| Sesavirus | 30.0 | 30.0 | 29.7 | 29.8 | 29.6 | 27.2 | 25.9 | 47.0 | 46.3 | 44.0 | 45.3 | 46.3 | |
| BPV2 | 35.9 | 35.9 | 36.1 | 35.7 | 35.7 | 30.3 | 27.2 | 28.9 | 49.9 | 54.5 | 47.8 | 51.5 | |
| PPV4 | 34.7 | 34.6 | 34.9 | 34.9 | 34.6 | 31.0 | 30.0 | 27.6 | 37.6 | 47.7 | 56.2 | 48.5 | |
| RDCV | 29.7 | 29.7 | 29.8 | 30.0 | 30.4 | 28.1 | 25.9 | 25.8 | 49.1 | 33.7 | 48.2 | 50.0 | |
| PPV6 | 34.0 | 34.0 | 34.1 | 34.2 | 34.2 | 30.6 | 30.5 | 28.0 | 35.1 | 58.9 | 34.5 | 47.2 | |
| Bosavirus | 31.8 | 32.0 | 32.1 | 32.1 | 32.0 | 24.6 | 27.8 | 26.4 | 32.8 | 35.3 | 33.1 | 36.5 |
- Abbreviations. BPV, bovine parvovirus; EqPV-CSF, equine parvovirus species-cerebrospinal fluid; PPV, porcine parvovirus; RDCV, Roe deer copiparvovirus.
- EqPV-H strains indicated by grey shading.
| S1 (Korea) | S2 (Korea) | BCT01 (USA) | A3 (China) | N50 (Austria) | EqPV-CSF | Eqcopivirus | Sesavirus | BPV2 | PPV4 | RDCV | PPV6 | Bosavirus | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 98.4 | 98.9 | 98.2 | 95.9 | 51.7 | 52.9 | 53.7 | 50.7 | 55.2 | 51.7 | 53.4 | 51.6 | |
| S2 | 97.4 | 98.1 | 98.5 | 96.3 | 51.8 | 52.5 | 53.5 | 51.0 | 55.2 | 51.9 | 53.8 | 51.6 | |
| BCT01 | 97.9 | 97.4 | 98.2 | 96.1 | 51.9 | 52.6 | 53.9 | 50.8 | 55.2 | 51.7 | 53.4 | 51.6 | |
| A3 | 96.9 | 97.4 | 97.5 | 96.2 | 52.0 | 52.7 | 53.6 | 51.0 | 55.3 | 51.9 | 53.6 | 51.7 | |
| N50 | 95.9 | 96.3 | 96.6 | 96.6 | 51.3 | 51.8 | 52.4 | 50.3 | 55.0 | 51.2 | 53.7 | 51.2 | |
| EqPV-CSF | 29.8 | 30.1 | 29.9 | 30.2 | 29.8 | 50.8 | 46.9 | 48.2 | 49.6 | 47.1 | 43.4 | 48.3 | |
| Eqcopivirus | 32.8 | 32.8 | 32.6 | 32.7 | 32.6 | 35.8 | 44.3 | 46.1 | 51.7 | 45.7 | 44.2 | 48.4 | |
| Sesavirus | 34.8 | 34.7 | 34.8 | 34.3 | 34.7 | 30.3 | 27.0 | 47.0 | 49.0 | 45.2 | 46.6 | 48.7 | |
| BPV2 | 30.1 | 30.2 | 29.8 | 30.1 | 29.9 | 32.8 | 30.3 | 28.8 | 48.3 | 50.3 | 45.2 | 47.5 | |
| PPV4 | 36.5 | 36.9 | 36.7 | 36.7 | 36.9 | 35.5 | 36.9 | 37.5 | 33.4 | 47.1 | 51.1 | 51.9 | |
| RDCV | 28.4 | 28.5 | 27.9 | 27.8 | 27.8 | 26.9 | 27.5 | 28.2 | 36.8 | 29.3 | 46.3 | 47.6 | |
| PPV6 | 32.7 | 32.1 | 32.2 | 32.2 | 31.5 | 28.1 | 26.8 | 32.0 | 28.6 | 42.7 | 27.5 | 47.2 | |
| Bosavirus | 31.2 | 31.1 | 31.2 | 31.1 | 31.2 | 27.9 | 28.4 | 33.7 | 27.8 | 37.6 | 29.5 | 29.9 |
- Abbreviations. BPV, bovine parvovirus; EqPV-CSF, equine parvovirus species-cerebrospinal fluid; PPV, porcine parvovirus; RDCV, Roe deer copiparvovirus.
- EqPV-H strains indicated by grey shading.
3.5 Natural recombination analysis
For putative recombination event detection within the sequences of the novel Korean EqPV-H strains, RDP analysis was conducted and the available complete genomes of EqPV-H strains from the USA, China and Austria were employed as possible parent strains. The results confirmed by seven methods in the RDP program revealed that the newly discovered Korean S2 strain is a potential recombinant strain produced by a major parent strain S1 (Korea) and minor parent strain E36 (China) (Figure 4A). The breakpoints of recombination were located at the 1039 nucleotide (beginning point) and 3611 nucleotides (ending point) of the S1 strain (Figure 4B).
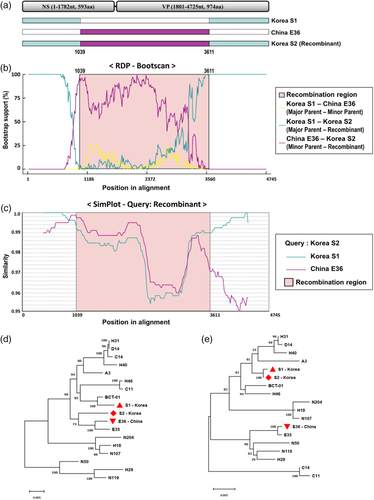
To confirm the recombination detection by RDP, a standard similarity plot was analysed (Figure 4C). The Korean S2 strain showed high similarity with the S1 strain, except between two breakpoints detected in RDP, while the Chinese E36 strain had a relatively high similarity with the putative recombination region.
Two phylogenetic trees based on putative recombination region and non-recombination region were also constructed to understand the relationship between recombination-related strains (Figure 4D and E). In the phylogenetic tree on putative recombinant genomic regions, Korean S2 and Chinese E36 showed the closest relationship (Figure 4D). However, in the analysis with non-recombinant genomic regions, Korean strains, S1 and S2, had the closest relationship (Figure 4E).
4 DISCUSSION
Despite the fact that four viruses (EqPV-H, EqHV, EPgV and TDAV) have been indicated as the causative agents of TD since 2011, only EqPV-H and EqHV are related to equine hepatitis (Tomlinson, Kapoor, et al., 2019; Tomlinson, Wolfisberg, et al., 2020). However, information on these two viruses, including worldwide prevalence, transmission mechanism and pathogenesis remains limited (Tomlinson, Kapoor, et al., 2019). Recently, we reported the first clinical case of EqPV-H-related TD in Asia, describing clinical course of the EqPV-H infection as well as oral and nasal shedding of viral DNA (Yoon et al., 2021). Moreover, an experimental study demonstrated the virus shedding via multiple routes (nasal and oral secretions, and in faeces) and infectivity after oral inoculation of EqPV-H (Tomlinson, Jager, et al., 2020); however, extensive field evidence about virus shedding and transmission is still lacking. Here, we investigated the prevalence and coinfection rate of EqPV-H/EqHV among horses in Korea using both serum and faecal samples to determine the possibility of faecal-oral transmission (FOT) of each virus, which, to the best of our knowledge, has not been previously verified in a field study. Furthermore, we analysed clinical pathology data to estimate the effect of the viremia and coinfection of EqPV-H and EqHV. Finally, we generated phylogenetic trees of both viruses, and described the history and epidemiological relationship of the identified viruses in this study.
As we conjectured, the results showed a high prevalence rate of EqPV-H (10.6% with serum and 5.3% with faeces samples) and EqHV (8.1% with serum and 0% with faeces samples). As far as we are aware, this is the first field-based evidence collected from faecal samples and it supports FOT of EqPV-H, which was found to be uncommon in EqHV. A lower prevalence rate in the faeces than in the serum is consistent with a previous experimental study that described low-level faecal shedding of EqPV-H around the viremia period (Tomlinson, Jager, et al., 2020). Despite the detection of HCV RNA in the faeces of chronically infected humans (Beld et al., 2000; Monrroy et al., 2017), EqHV was not detected in the horse faeces, indicating that FOT is not the main transmission route of EqHV infection. However, we cannot rule out faecal shedding of EqHV because the horses in this study did not show hepatitis-specific clinical signs; therefore, additional research into clinically symptomatic horses is required. The breeding horse population, with the oldest average age (12.1 years), had the highest prevalence rates of EqPV-H DNA (12/32, 37.5% in serum and 3/44, 68% in faecal samples) and EqHV RNA (5/32, 15.6% in serum). In support of this finding, a previous study reported that the risk of EqPV-H infection increases with age (Badenhorst et al., 2021). Furthermore, it is also suspected that breeding horses have more contact opportunities with humans and stallions through the breeding procedure, thus, contaminated instruments or semen can be a potential source of TD infection.
In Korea, commercial equine blood products known to be a main transmission source of TD and EqHV transmission have not been permitted to date. Consequently, iatrogenic infection is uncommon. Additionally, vertical transmission is not possible in EqPV-H, in contrast to EqHV, which is frequently transmitted vertically (Gather et al., 2016; Tomlinson, Jager, et al., 2020). Considering this unusual situation in Korea, it is speculated that EqPV-H and EqHV have been introduced from abroad to Korea, and that FOT plays an important role in spreading EqPV-H. This is consistent with the results of the horses from the USA, the largest horse exporter in Korea, which showed a much higher prevalence rate of EqPV-H (45.8% in serum and 30% in faeces)/EqHV (12.5% in serum) than those born in Korea. Besides, the serum EqHV infection rate (9/128, 7.0%) was much higher than that of EqPV-H (5/128, 3.9%) among horses born in Korea, in contrast to the imported horses (EqHV; 4/32, 12.5% and EqPV-H; 12/32, 37.5%). It is suspected that the vertical transmission of EqHV resulted in the higher rate of EqHV infection among horses born in Korea.
Interestingly, the Korean indigenous Jeju horse breed tested negative for EqPV-H and EqHV in both serum and faeces. Jeju horses are usually raised apart from other horse breeds. This environment may act as a barrier against exotic viruses. However, considering the high prevalence of EqPV-H/EqHV and geographical limitation of Jeju Island, other factors, such as genetic character and physiological differences, also should be analysed.
We measured four liver-specific parameters (AST, GGT, TBIL and A/G ratio) to evaluate the hepatopathy. At least two parameters of numerous EqPV-H- or EqHV-positive horses (43.5%) showed values lying outside the reference ranges. We found substantial AST elevation in EqPV-H or EqHV positive horses compared to both negative groups (Figure 1B and C). However, regarding EqPV-H or EqHV coinfection in horses, there were no significant differences (Figure 1C). In previous studies (Divers et al., 2018; Lu et al., 2018; Tomlinson, Jager, et al., 2020), natural EqPV-H or EqHV infections without clinical hepatopathy were common, even though liver enzyme elevation and histopathological changes occurred in an experimental study. Furthermore, a significant number of horses in this comparison were diagnosed with metabolic diseases such as colic (15/32 of control and 12/23 of positive groups, Table 2), and thus, the metabolic disorder-related liver function could have affected the results (Underwood et al., 2010). Also, in this study, the viruses were confirmed by conventional PCR and no viral load was determined. The relationship between clinical signs and virus titre would provide a better understanding of viral pathogenicity. Consequently, additional broad range studies and virus quantification analysis along with clinical signs are needed to confirm the pathological effect of TD and to identify the elements affecting TD pathology.
In previous studies, the EqPV-H gene has been shown to be well conserved with low genetic diversity (Divers et al., 2018; Lu et al., 2018). Similarly, the two Korean strains of EqPV-H in the present study had low genetic diversities in both NS and VP regions (<3% and <5%, respectively) (Tables 3 and 4). Recently, a natural recombination of EqPV-H between Chinese and American strains was reported in China (Lu et al., 2020). Considering the geological location and international relationship between China and Korea, a recombination event or transmission of a recombinant strain might be also possible in Korea. In the present study, each NS and VP protein of the newly identified Korean strains showed distinct evolutionary histories, indicating recombination. Additional investigation into the possibility of recombination between EqPV-H strains confirmed the putative recombination event between Korean and Chinese strains. This result provides clear evidence of frequent natural recombination between EqPV-H strains. However, the effects of recombination, such as virus transmission, virulence and infectivity remain unclear, and additional research is, therefore, warranted.
5 CONCLUSIONS
In conclusion, to the best of our knowledge, this is the first field study providing evidence of the FOT of EqPV-H, and high prevalence and coinfection rate of EqPV-H and EqHV in Korea. Natural recombination between EqPV-H strains was also discovered. The clinical pathogenesis of the viruses is not obvious and further investigation regarding the clinicopathology is, therefore, warranted. These findings can facilitate the development of disease prevention strategies by tracing the virus-spreading pathway.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The animal protocols for this study were approved by the Institutional Animal Care and Use Committee of Korea Racing Authority (KRA IACUC-2106-AEC-2106).
FUNDING
This paper was supported by Konkuk University Researcher Fund in 2021 (Grant number: 2021-A019-0300).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




