Leptospira noguchii associated to reproductive disease in ruminants
Luiza Aymée and Maria Isabel Nogueira Di Azevedo contributed equally to this article.
Abstract
Leptospirosis is known to determine reproductive disorders on livestock, and Leptospira interrogans and Leptospira borgpetersenii are the most frequently reported species. Leptospira noguchii is an emerging pathogen, but its association with reproductive disease is unclear. We have detected L. noguchii as the agent of an outbreak with reproductive disorders in a Brazilian dairy goat flock. In the kidding season, five out of 10 Saanen had abortions in the final month of pregnancy and two newborn kids had acute clinical signs. After necropsy of three foetuses and one newborn kid, fragments of liver, lung and kidney were submitted to lipL32-PCR. It yielded positive results in at least one fragment from each animal. After, a nested secY-PCR, followed by sequencing, could identify L. noguchii, with 99–100% of identity with sequences obtained from cattle in the same region. For the first time, L. noguchii was detected in goats and, most importantly, the association of this leptospiral species with reproductive failures in ruminants has been demonstrated.
1 INTRODUCTION
Leptospirosis is an important zoonotic disease and it is prevalent in ruminants worldwide, leading to important economic hazards (Ellis, 2015; Loureiro et al., 2019). Leptospiral infection in adult ruminants leads to a reproductive syndrome, while infections in young animals could cause systemic disease (Ellis, 2015; Loureiro & Lilenbaum, 2020). Leptospires can be harboured by maintenance reservoirs and shed in environment by urine and other bodily fluids, where the infection of other animals occurs (Putz & Nally, 2020). Ruminants are known to be maintenance hosts of Leptospira interrogans genotype Hardjoprajtino and L. borgpetersenii genotype Hardjobovis, both strains from Sejroe serogroup (Loureiro & Lilenbaum, 2020). Therefore, the reproductive disease in ruminants is associated to Hardjo strains or L. santarosai genotype Guaricura (Loureiro et al., 2016). Nonetheless, Leptospira noguchii has been isolated from cattle in the past few years and can be considered an emerging pathogen (Martins et al., 2015; Zarantonelli et al., 2018). Furthermore, L. noguchii has been reported in humans with clinical signs (Silva et al., 2009), domestic animals such as dogs and sheep (Costa et al., 2021; Silva et al., 2007) and wild animals (Ballados-González et al., 2018; Medeiros et al., 2020).
In those reports, this agent was recovered from asymptomatic animals, except for a single report of a calf with systemic clinical signs of the disease (Zarantonelli et al., 2018). In the same paper, the authors raised a question about the nature of the clinical manifestations that this leptospiral species leads in ruminants. Additionally, Loureiro and Lilenbaum (2020) recently emphasized genital leptospirosis as an important syndrome in ruminants that leads to reproductive signs such as embryonic death and abortions. Even though L. noguchii has been frequently reported, little is known about the role of this agent in livestock concerning to reproductive disorders. Therefore, this study aims to associate L. noguchii to reproductive failure in ruminants, with genetic characterization of the agent in an abortion outbreak in a Brazilian goat flock.
2 MATERIALS AND METHODS
2.1 The flock
This study was performed with samples of animals from a small dairy flock located in Southeast Brazil, with the coordinates −21.53606547651664 and −42.64495696200207. The flock was composed by 90 caprine (Capra aegagrus hircus) of Saanen breed in intensive production system. Animals were submitted to a fulltime confinement and had no access to pasture. Goats were fed with corn silage, elephant grass (Pennisetum purpureum), concentrate of corn fluor and soybean, mineral salt and well water. Breeding was proceeded through natural mating and all goats were reared in this flock. No other caprine was acquired for the flock in the last months.
Aside rodents, no other animals had access to the installations, according to the flock owners. Due to the broken feeders, the flock owners reported the increased presence of rodents in the facilities. Although there is a river next to the facilities, there was no history of flood before the kidding season. The flock had no history of previous abortions outbreaks caused by leptospirosis or other diseases. Animals were vaccinated against clostridiosis and had never received vaccines with leptospiral antigens.
After the abortions outbreak, the goats were treated by the practitioner with streptomycin (25 mg/kg). Animals were vaccinated against leptospirosis with a commercial polyvalent vaccine with bacterins L. interrogans serovar Pomona, Wolffi, Hardjoprajtino, Icterohaemorrhagiae, Canicola, Copenhageni, Bratislava and L. borgpetersenii serovar Hardjobovis (Bovigen® Lepto 8, Virbac, Brazil). The dosage used was recommended by the manufacturer to sheep.
2.2 Animals
At the beginning of the kidding season, five (50%; five out of 10) of lactating goats had abortions. Abortion episodes occurred in the final month of pregnancy. In addition, two goats (20%; two out of 10) gave birth to two kids that showed clinical signs (24 h after birth) of weakness, pale mucous membranes, lung crepitation, hypothermia, haematuria, petechiae and skin suffusions. Despite treatment with vitamins, fluid therapy, flunixin meglumine (1.0 mg/kg), penicillin (8.000 IU/kg) and streptomycin (4.0 mg/kg), both the newborn kids died, 2 and 4 days after beginning of the clinical signs.
2.3 Sampling
Three foetuses and one newborn corpse were necropsied. Fragments of kidney, liver and lungs of each animal were collected and sent to the Laboratory of Veterinary Bacteriology of Federal Fluminense University. Besides, two urine samples were collected from the newborn kid through natural urination. The samples were submitted only to leptospirosis diagnosis.
2.4 Molecular and phylogenetic analysis
DNA extraction from the tissue samples was performed using commercial Kit DNeasy Blood and Tissue Kit (QIAamp, Qiagen, Courtaboeuf, France) as recommended by the manufacturer. Initially, specific primers from the lipL32 gene, reported to be present in pathogenic leptospires (Stoddard et al., 2009), were used for the PCR reactions, amplifying a 242 bp fragment. The reactions were done as previously described in Hamond et al. (2015). Posteriorly, lipL32 positive samples were subjected to a nested PCR targeting a partial region of the secY gene (410 bp), according to Di Azevedo et al. (2020). As reaction negative and positive control, ultrapure water and DNA from L. interrogans serovar Copenhageni (Fiocruz L1-130) were used, respectively. Electrophoresis was performed in a 2% agarose gel to PCR products analysis, and visualized under UV light after gel red staining. The secY amplicons were directly sequenced using BigDye Terminator v.3.1 Cycle Sequencing Ready Reaction Kit (Applied Biosystems) in a 3100 automated DNA sequencer according to the manufacturer's instructions.
Pairwise/Blast/NCBI, SeqMan v. 7.0, ClustalX v 2.0 (Larkin et al., 2007) and BioEdit v. 7.0.1 (Hall, 1999) software were used for the editing and sequence analysis. A maximum likelihood (ML) tree was constructed using the Tamura-Nei model (TN92) in MEGA X software (Kumar et al., 2018), as it was determined to be the best-fitting model of DNA substitution using the Bayesian information criterion. Genetic distances were calculated using the Kimura-2-parameters (K2P) model on MEGA X.
3 RESULTS
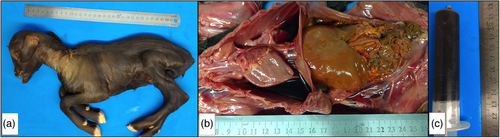
The macroscopic lesions found in necropsy were coppery liver (50%), dark reddish liver (50%), pulmonary haemorrhage (25%), serosanguineous content in thoracic cavity (75%), clots in urinary bladder (25%), clots in the left heart ventricle (25%), darkened kidneys (75%) or icteric kidneys (25%), and icteric intestines (50%). Results of each animal are described in Table 1. Macroscopic lesions from the newborn kid are shown in Figure 1 and lesion from the aborted foetus 1 (G2) are shown in Figure 2.
| Animal | Organ | lipL32-PCR | secY sequencing | Macroscopic pathology |
|---|---|---|---|---|
| Newborn kid (G1) |
Liver Lung Kidney Urine |
POS NEG POS POS |
L. noguchii L. noguchii LSQ |
Focal pulmonary haemorrhage, jaundice, coppery liver, clots and bloody fluid inside urinary bladder. |
| Aborted foetus 1 (G2) |
Liver Lung Kidney |
POS NEG POS |
L. noguchii LSQ |
Thoracic cavity with serosanguineous content, cupric clots in heart's left ventricle, jaundice, coppery liver, dark reddish kidneys. |
| Aborted foetus 2 (G3) |
Liver Lung Kidney |
POS NEG NEG |
L. noguchii | Subcutaneous oedema, thoracic cavity with serosanguineous content. Liver, kidneys, lungs, heart and intestines with dark reddish aspects. |
|
Aborted foetus 3 (G4) |
Liver Lung Kidney |
POS NEG POS |
LSQ L. noguchii |
Subcutaneous oedema, thoracic cavity with serosanguineous content. Liver, kidneys, lungs, heart and intestines with dark reddish aspects. |
- LSQ: low sequence quality. G1, G2, G3 and G4: identification of the animals in the phylogenetic tree.
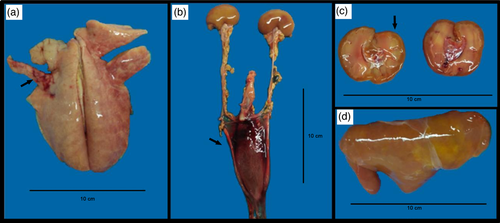
All the four liver and three out of four kidney samples were positive to lipL32-PCR, while all lung samples were negative. One of the two urine samples was positive (Table 1). All lipL32-PCR positive samples (n = 8) were later submitted to secY amplification and sequencing, and from those, five provided good nucleotide sequences for analysis (Table 1). Sequences were deposited on GenBank under accession numbers MZ682610-MZ682614.
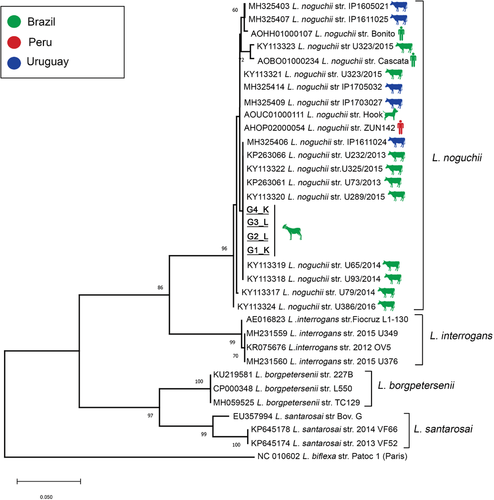
Pairwise/Blast/NCBI comparisons with the GenBank secY gene dataset identified all of them as L. noguchii, with 99–100% of identity. Phylogenetic analysis based on ML-TN92 tree including secY sequences from GenBank confirmed the species identification, with sequences from the present study included in a high supported cluster (bootstrap = 96%) with L. noguchii sequences, distant from other common pathogenic species (Figure 3). The genetically close group (K2P = 0.01, 99% of similarity) includes sequences from bovines from the same region (Figure 3). Moreover, a high genetic identity with strains of L. noguchii obtained from other hosts from South America, including humans and dogs, was also observed (Figure 3).
4 DISCUSSION
Although L. noguchii has already been reported in other ruminants, to our knowledge this is the first time that this species is characterized in goats. Except in one case, all of these reports were occasional findings of apparently healthy animals in slaughterhouses. In one report, Zarantonelli and collaborators (2018) isolated this Leptospira species of a calf with acute signs. The authors raised the following question: ‘Are L. noguchii strains a relevant cause of acute disease or reproductive problems in cattle?’ Therefore, the clinical manifestations presented in this study could contribute to the debate of this question.
Although L. noguchii can be considered an incidental strain to ruminants, the adult goats had not presented other signs besides reproductive disorders. After the abortions, these goats were apparently healthy. This indicates that possibly the previous occasional reports of L. noguchii in cattle could be associated with a silent and chronic infection—characteristic of genital leptospirosis. In contrast, the newborn kid clearly presented severe systemic disease, as demonstrated by the necropsy findings and PCR positive results on different organs.
The mechanism of how this pathogen was introduced in the flock could not be determined. In Atlantic Forest, the biome where the flock is located, a previous study genetically identified L. noguchii from kidney fragments of opossum (Didelphis albiventris) (Vieira et al., 2019). However, since little is known about L. noguchii in wild animals, the opossum cannot be confirmed as a reservoir. Furthermore, the owners of the flock declared that only rodents had been seen in contact of the goats. In a study performed in Cambodia, Kudo and collaborators (2018) identified L. noguchii in urine and bladder tissue of wild rodents from urban settlements. Likewise, more evidences are needed to affirm that rodents can be reservoirs of this leptospiral species.
The control of leptospirosis in the flock was proceeded according to Martins and Lilenbaum (2017) with the triad treatment, vaccination and environmental control. The goats that presented abortions were treated with streptomycin and all adult animals were vaccinated. Although there are no studies regarding treatment of L. noguchii infection and the commercial vaccine does not have this leptospiral species in that composition, the flock did not present new cases of abortions after those procedures. In addition, the broken feedlots were fixed and an appropriated control of rodents was implemented.
It is important to highlight the importance of those findings in a One Health context. Besides the proximity to cattle strains, the present sequences were also very close to those obtained from humans. Besides animal infection, the zoonotic aspect of this emerging pathogen has been suggested. A study in Nicaragua correlated L. noguchii infection in domestic animals with cumulative incidence of human cases (Flores et al., 2017); this species has also been associated to leptospiral disease in humans, especially in rural workers, in Brazil and Ecuador (Barragan et al., 2016; Silva et al., 2009). Additionally, in a molecular-based study, the zoonotic potential of L. noguchii has been discussed (Loureiro, et al., 2019). Since rural workers had close contact with livestock animals, fluids and abortions contents of possible infected animals, this category has major risk to contracting this zoonosis.
5 CONCLUSION
L. noguchii was characterized as the agent of an outbreak of leptospirosis in goats, leading to abortions and systemic disease in newborn kids. The zoonotic potential of the species was discussed and its role as an important pathogen associated to reproductive disorders in ruminants could be confirmed.
ACKNOWLEDGEMENTS
The authors are grateful for the help of Felipe Seabra Cardoso Leal, Mirela Balistrieri Dias and Paula Cortat for assistance in the field. W. L. is a FAPERJ and CNPq fellow, M. I. N. D. A. is a FAPERJ fellow. We also thank the Program for Technological Development in Tools for Health-PDTIS/FIOCRUZ for the use of its facilities.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICAL STATEMENT
Animal ethical statement is not applicable since the samples were received for diagnose procedures.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




