Efficacy and safety of phosphodiesterase 4 inhibitors in patients with asthma: A systematic review and meta-analysis
ABSTRACT
Phosphodiesterase 4 (PDE4) inhibitors are a novel medication approved for airway inflammatory diseases including chronic obstructive pulmonary disease. Their role and application in asthma are controversial and not defined.
A comprehensive search was performed in major databases (1946–2016) using the keywords: ‘phosphodiesterase 4 inhibitor’ or ‘roflumilast’ and ‘asthma’. Placebo-controlled trials reporting lung function, airway hyperresponsiveness by direct challenge, asthma control and exacerbations, and adverse events were included. Random or fixed-effects models were used to calculate odds ratios (OR) and mean differences between the two treatment groups. Statistical analyses were conducted using Mann–Whitney U-tests and Cochrane systematic review software, Review Manager.
Seventeen studies were included in the systematic review, of which 14 studies were included in the meta-analysis. Except for significant statistical heterogeneity in pre- and post-challenge predicted percentage of forced expiratory volume in 1 s (FEV1%; I2 = 72%, χ2 = 3.35, P = 0.06), there was no heterogeneity in outcome measures. Roflumilast (500 μg) significantly improved FEV1 (mean difference: 0.05, 95% CI: 0.01–0.09, Z = 2.50, P = 0.01), peak expiratory flow, asthma control and exacerbations, but showed variable effects on airway responsiveness to methacholine and a 20% fall in FEV1.Of note, PDE4 inhibitors were accompanied with significantly higher adverse events such as headache (OR: 3.99, 95% CI: 1.65–9.66, Z = 3.07, P = 0.002) and nausea (OR: 5.53, 95% CI: 1.38–22.17, Z = 2.41, P = 0.02).
In patients with mild asthma, oral PDE4 inhibitors can be considered as an alternative treatment to regular bronchodilators and inhaled controllers.
Abbreviations
-
- AHR
-
- airway hyperresponsiveness
-
- BDP
-
- beclomethasone dipropionate
-
- FEV1
-
- forced expiratory volume in 1 s
-
- FEV1%
-
- predicted percentage of FEV1
-
- FP
-
- fluticasone propionate
-
- MH
-
- Mantel–Haenszel
-
- PC20
-
- the provocation concentration of methacholine causing a 20% fall in FEV1
-
- PDE4
-
- phosphodiesterase 4
-
- PEF
-
- peak expiratory flow
INTRODUCTION
Asthma is a common chronic airway disease, and persistent airway inflammation is recognized as the cornerstone of clinical and pathological pathogenesis.1 A combination of inhaled corticosteroids and long-acting β2 agonists is a standard treatment to control asthma symptoms and reduce exacerbation risks due to their potent anti-inflammation and bronchodilation effects. However, in spite of the current standard and guideline-based treatment and management strategies, an annual prevalence and mortality of asthma worldwide remains as high as 2.4 million and 300 000, respectively, and leads to an excess cost of $1028.0 per patient.2-4 Moreover, it has been reported that approximately 10% of asthma patients present with corticosteroid insensitivity, which is typical of severe asthma.5
Phosphodiesterase 4 (PDE4) is expressed in smooth muscle cells and inflammatory cells; hence, it is a potential therapeutic target for patients with asthma.6 Selective PDE4 inhibitors are a novel second-generation medication, which targets the type 4 isoenzyme of PDE, interferes with the breakdown of cyclic AMP (cAMP) and reduces inflammation.7 The family of selective PDE4 inhibitors consists of roflumilast, cilomilast, rolipram and other emerging pharmaceuticals which are still under evaluation in clinical trials, such as BAY19-8004, MEM1414 and GSK256066.8 In patients with chronic obstructive pulmonary disease (COPD), oral roflumilast at 500 μg once per day reduces moderate-to-severe exacerbations, improves lung function and alleviates symptoms.9, 10 It is the only PDE4 inhibitor approved for clinical use and listed as an alternative choice for severe-to-very severe patients in addition to existing therapies in the guidelines of the Global Initiative for Chronic Obstructive Lung Disease.11
However, PDE4 inhibitors including roflumilast have not yet been recommended for patients with asthma due to insufficient evidence and controversial results from different trials. In a multicentre, randomized, double-blind, parallel-group study of 693 patients with mild-to-moderate asthma, roflumilast significantly improved forced expiratory volume in 1 s (FEV1) and peak expiratory flow (PEF), which was equivalent to the effects of beclomethasone dipropionate.12, 13 Nevertheless, researchers also found that PDE4 inhibitors did not have bronchodilator activity in early phase responses, did not improve lung function and did not inhibit allergen-induced early phase responses in patients with asthma.14, 15 Therefore, further evaluation of the effects of PDE4 inhibitors on clinical outcomes in patients with asthma is of considerable interest.
METHODS
The institutional ethics review boards approved study protocols of all trials included in this review. All participants provided written informed consent.
Search strategies
A comprehensive search spanning 1946 to August 2016 was performed in PubMed, Embase, Medline, Cochrane Central Register of Controlled Trials (CENTRAL), American College of Physician (ACP) Journal Club and ISI Web of Science. Our search strategy was the combination of keywords including ‘phosphodiesterase 4 inhibitor’ or ‘roflumilast’ and ‘asthma’, and a specific publication type of controlled clinical trial was limited. A review of references listed in the identified trials and a manual search of the related articles were conducted to identify all relevant and eligible studies in order to minimize potential publication bias.
Inclusion and exclusion criteria
Eligible clinical trials were defined on the basis of the following criteria: (i) study design was placebo-controlled clinical trials; (ii) participants were patients with asthma diagnosed by a history of asthmatic symptoms and a verification of airway hyperresponsiveness (AHR) by bronchial provocation test while they were not on treatment of inhaled long-acting bronchodilators or corticosteroids; (iii) intervention treatment was oral PDE4 inhibitors with or without corticosteroids, and control treatment was placebo with or without corticosteroids; and (iv) outcome measures included the changes from baseline in FEV1, predicted percentage of FEV1 (FEV1%), morning and evening PEF, provocation concentration of methacholine causing a 20% fall in FEV1 (PC20) and asthma symptom scores, as well as the use of rescue medication, level of blood tumour necrosis factor-α (TNF-α) and incidence of adverse events and asthma exacerbation. We did not include studies that were observational, cohort or case–control.
Study selection
Two consecutive phases were conducted by two independent investigators in the study selection. Initially, they discarded duplicated and non-controlled clinical trials through screening titles and abstracts. Afterwards, eligible studies were extracted by reviewing full texts according to the previously designed inclusion criteria. Any divergence was tackled by the involvement of a third investigator to reach a mutual consensus.
Data extraction
Two independent researchers separately retrieved relative data from each eligible study in a standardized Cochrane data extraction form16 including: first author or trial code, year of publication, study design, disease severity, population, patient demographic characteristics (age, gender, height, weight, etc.), details of interventions (drug name, dose, frequency, administration route, treatment duration and follow-up) and outcome measures and study results. Differences in opinions were resolved by the presence of a third investigator until a unanimous agreement was achieved.
Quality assessment
A standard bias tool recommended by Cochrane was used to assess the risk of potential biases in the methods and outcomes reported by each enrolled study, which included: (i) random sequence generation (selection bias); (ii) allocation concealment (selection bias); (iii) blinding of participants and personnel (performance bias); (iv) blinding of related outcomes assessment (detection bias); (v) incomplete outcome data (attrition bias); (vi) selective reporting (reporting bias); and (vii) other bias.16 Identical procedures were performed by two individual investigators, and mutual consensus was reached with a third investigator if any disagreement presented.
Statistical analysis
Statistical analysis was conducted by an independent statistician, who was blinded to the study protocol, using Cochrane systematic review software Review Manager (RevMan; Version 5.3.5; The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark, 2014).
We evaluated the clinical, methodological and statistical heterogeneities by chi-square test with P < 0.1 and I2 > 50% indicating significance. Sensitivity analysis was also conducted to substitute alternative decisions or ranges of values for decisions that were arbitrary or unclear.
Continuous data was reported as mean ± SD, while dichotomous variables were presented as frequency and proportion. For pooled data, we performed the meta-analysis; otherwise, a systematic review was conducted. In the meta-analysis, both fixed and random-effects models were fitted as a sensitivity analysis. For dichotomous data we calculated odds ratio (OR) and 95% CI, while for continuous data we calculated mean difference and 95% CI. Inverse-variance weighting (IVW) approach was used for the analysis of mean difference and the Mantel–Haenszel (MH) method for pooling the OR. Statistical analyses were conducted using Mann–Whitney U-tests, and a two-sided P-value of <0.05 was rendered as significant difference.
RESULTS
A total of 1145 records were identified from electronic databases (n = 1132) and reference lists review (n = 13) (Fig. 1) Through titles and abstract screening, 1123 studies were discarded for non-controlled clinical trial (n = 1083), duplication (n = 31) and patients without asthma (n = 9). After thorough review of the remaining 22 studies in full text, we included 17 trials14, 15, 17-23 in our final analysis due to four studies not comparing PDE4 inhibitors with placebo and one study not reporting expected outcome measures. The remaining 17 studies14, 15, 17-23 were all included in the systematic review and 14 studies14, 17, 18, 20-23 were included in the meta-analysis.
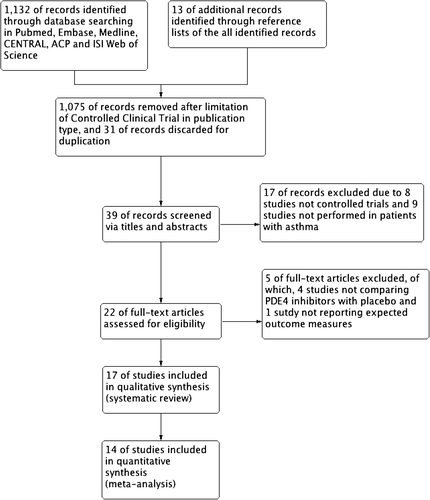
Study description
Thirteen studies14, 18, 20-23 were performed in patients with mild-to-moderate asthma, while three studies15, 19, 22, 23 enrolled moderate-to-severe asthmatic patients. Allergic or atopic asthmatic patients were further specified in four studies,14, 18, 20, 21 and only one study included patients with exercise-induced asthma,17 but the remaining 12 studies did not specify asthma phenotypes. All included studies were placebo-only controlled trials compared with PDE4 inhibitor monotherapy, except for three studies22, 23 in which a combination treatment of beclomethasone or fluticasone was prescribed. In the trials with roflumilast, a dose range of 125–1000 μg was used; while in the trials with other PDE4 inhibitors, different doses were applied. PDE4 inhibitors were orally administered once daily, but the treatment and follow-up duration varied from 7 days to 24 weeks. With regard to outcome measures, changes of spirometry including FEV1 and PEF were reported in 14 studies,14, 15, 17, 19, 20, 22, 23 AHR alleviations defined as changes of methacholine PC20 were presented in 2 studies20, 21 and asthma controls identified as the changes of symptom scores and use of rescue medications were shown in 10 studies.19, 22, 23 In addition, two trials detected the concentrations of blood TNF-α,15, 17 three studies reported the incidence of asthma exacerbations22, 23 and all studies provided data about adverse events. Details of patients’ characteristics, intervention strategies and outcomes are summarized in Table 1.
| Author (year) | Study design | Patients | Population (I/C) | Intervention | Control | Routine | Duration | Follow-up | Outcomes† | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Dose | Frequency | |||||||||
| Timmer (2002)17 | Randomized, placebo-controlled, double-blind, two-period crossover trial | Exercise-induced asthma (FI%: 10–20%) | 16 (16/16) | Roflumilast | 500 μg | Once daily | Placebo | Oral | 28 days | 28 days | ②⑧⑨ |
| van Schalkwyk (2005)18 | Double-blind, placebo-control, three-period crossover study | Mild allergic asthma (FEV1%: ≥70%) | 23 (23, 21/22) | Roflumilast | 500/250 μg | Once daily | Placebo | Oral | 7–10 days | 9–19 weeks | ⑨ |
| Louw (2007)14 | Randomized, double-blind, two-period, crossover study | Mild allergic asthma (FEV1%: ≥70%) | 13 (13/13) | Roflumilast | 1000 μg | Once daily | Placebo | Oral | NM | NM | ②⑨ |
| Gauvreau (2011)20 | Two-centre, double-blind, randomized, placebo-controlled, crossover study | Mild atopic stable asthma (FEV1%: ≥70%) | 25 (25/25) | Roflumilast | 500 μg | Once daily | Placebo | Oral | 14 days | 8–12 weeks | ①⑤⑨ |
| FK1 011 (1999)22, 23 | Placebo-controlled, double-blind, parallel-group studies | Mild-to-moderate asthma (FEV1%: 50–80%) | 46 (24/22) | Roflumilast | 500 μg | Once daily | Placebo | Oral | 4 weeks | 4 weeks | ①③④⑥⑦⑨ |
| FK1 004 (1997–1998)22, 23 | Placebo-controlled, double-blind, parallel-group studies | Mild-to-moderate asthma (FEV1%: 60–90%) | 69 (45/24) | Roflumilast | 500 μg | Once daily | Placebo | Oral | 6 weeks | 6 weeks | ①③④⑥⑦⑨ |
| FK1 003 (1997–1998)22, 23 | Placebo-controlled, double-blind, parallel-group studies | Mild-to-moderate asthma (FEV1%: 50–85%) | 257 (170/87) | Roflumilast + BDP | 500 + 500 μg | Once daily | Placebo + BDP | Oral | 6 weeks | 6 weeks | ①③④⑥⑦⑨ |
| FHP 031 (2001–2004)22 | Placebo-controlled, parallel-group study | Mild-to-moderate asthma (FEV1%: ≥60%) | 42 | Roflumilast | 125/250 μg | Once daily | Placebo | Oral | 12 weeks | 12 weeks | ⑨ |
| FK1 021 (2002–2004)22, 23 | Placebo-controlled, double-blind, parallel-group studies | Mild-to-moderate asthma (FEV1%: 50–85%) | 692 (221, 237/234) | Roflumilast | 125/250 μg | Once daily | Placebo | Oral | 12 weeks | 12 weeks | ①③④⑥⑦⑨ |
| FK1 020 (2001–2003)22, 23 | Placebo-controlled, double-blind, parallel-group studies | Mild-to-moderate asthma (FEV1%: 50–85%) | 495 (252/243) | Roflumilast | 250 μg | Once daily | Placebo | Oral | 12 weeks | 12 weeks | ①③④⑥⑦⑨ |
| M2 012 (2003–2005)22, 23 | Placebo-controlled, double-blind, parallel-group studies | Mild-to-moderate asthma (FEV1%: 60–80%) | 770 (257, 257/256) | Roflumilast | 250/500 μg | Once daily | Placebo | Oral | 24 weeks | 24 weeks | ①③⑥⑦⑨⑩ |
| M2 023 (2003–2005)22, 23 | Placebo-controlled, double-blind, parallel-group studies | Moderate-to-severe asthma (FEV1%: 45–85%) | 771 (253, 265/253) | Roflumilast | 250/500 μg | Once daily | Placebo | Oral | 24 weeks | 24 weeks | ①③④⑥⑦⑨ |
| M2 014 (2002–2003)22, 23 | Placebo-controlled, double-blind, parallel-group studies | Mild-to-moderate asthma (FEV1%: 50–80%) | 649 (313/336) | Roflumilast + FP | 500 + 250 μg | Once daily | Placebo + FP | Oral | 24 weeks | 24 weeks | ①③④⑥⑨⑩ |
| M2 013 (2003–2005)22, 23 | Placebo-controlled, double-blind, parallel-group studies | Mild-to-moderate asthma (FEV1%: 50–80%) | 740 (374/366) | Roflumilast + FP | 500 + 400 μg | Once daily | Placebo + FP | Oral | 24 weeks | 24 weeks | ①③④⑥⑦⑨⑩ |
| Grootendorst (2003)15 | Double-blind, randomized, placebo-control, crossover study | Moderate-to-severe asthma (FEV1%: 40–80%) | 7 (7/7) | BAY19-8004 | 5 mg | Once daily | Placebo | Oral | 7 days | 24–32 days | ①⑧⑨ |
| Lu (2009)19 | Multicentre, randomized, placebo-controlled, double-blind, crossover phase II study | Moderate-to-severe asthma (FEV1%: 50–80%) | 88 (88/88) | MK-0359 | 15 mg | Once daily | Placebo | Oral | 14 days | 55 days | ①③④⑥⑦⑨ |
| Leaker (2014)21 | Two-centre, double-blind, randomized, placebo controlled, crossover study | Mild allergic asthma (FEV1%: >75%) | 16 (16/16) | MEM1414 | 600 mg | Once daily | Placebo | Oral | 7 days | 4–12 weeks | ⑤⑨ |
- † Outcome measures include: ① Change from baseline in FEV1; ② Change from baseline in FEV1%; ③ Change from baseline in morning PEF; ④ Change from baseline in evening PEF; ⑤ Change of baseline in PC20; ⑥ Change of asthma symptom scores; ⑦ use of rescue medication; ⑧ Blood TNF-α; ⑨ Incidence of adverse events; ⑩ Incidence of asthma exacerbation or days to first severe exacerbation.
- BDP, beclomethasone dipropionate; FEV1, forced expiratory volume in 1 s; FEV1%, predicted percentage of FEV1; FI%, the percentage fall index of FEV1; FP, fluticasone propionate; I/C, intervention/control; NM, not mentioned; PC20, the provocation concentration of methacholine causing a 20% fall in FEV1; PEF, peak expiratory flow.
A total of 5357 patients were included in our systematic review, of which 3039 patients received PDE4 inhibitors with/without corticosteroids and 2506 patients received placebo with/without corticosteroids. A total of 4361 patients were included in our meta-analysis, of whom 2540 and 1914 patients were assigned to receive PDE4 inhibitors or placebo with/without corticosteroids. The ratio of male to female patients was approximately 1.4 (n = 3136; n = 2221), and the majority of patients were non-smokers (76.24%). Details of baseline characteristics of patients in studies are shown in Table 2.
| Author (year) | No. | Age (year, SD) | Sex (male, %) | Height (cm, SD) | Weight (kg, SD) | Smoker (%) | FEV1 (L, SD) |
FEV1% (%, SD) | Morning PEF (L/min, SD) | PC20 (mg/mL) | Asthma symptom score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Timmer (2002)17 | 16 | 23 (range: 20–30) | 16 (100) | NM | 69 (range: 60–98) | NM | NM | NM | NM | NM | |
| van Schalkwyk (2005)18 | 23 | 28 (range: 20–44) | 11 (48) | 168 (10.7) | 76 (19.3) | 4 (17.39) | 3.17 (0.82) | 89 (10) | NM | 4.09 (3.79) | NM |
| Louw (2007)14 | 13 | 24 (5.5) | 13 (100) | NM | NM | 1 (7.69) | NM | 86 (7.6) | NM | 1.62 (4.1) | NM |
| Gauvreau (2011)20 | 25 | 28.7 (range: 18–54) | 10 (40) | NM | NM | 0 (0) | NM | 92.8 (range: 75.9–117.1) | NM | 4.0 (range: 0.3–14.4) | NM |
| Grootendorst (2003)15 | 7 | 46.4 (10.7) | 4 (57) | NM | NM | NM | NM | 69.5 (9.3) | NM | NM | NM |
| Lu (2009)19 ‡ | 88 | 30.4 (8.1) | 51 (58.0) | NM | NM | NM | 2.55 (0.47) | 64.6 (8.18) | NM | NM | NM |
| Leaker (2014)21 | 16 | 33 (7) | 6 (37.5) | NM | NM | NM | 3.2 (0.57) | 94.89 (12.46) | NM | NM | NM |
- † Data reported in all patients receiving PDE4 inhibitors.
- ‡ Data reported in patients receiving sequence MK-0359/placebo.
- FEV1, forced expiratory volume in 1 s; FEV1%, predicted percentage of FEV1; NM, not mentioned; No., numbers; PC20, the provocation concentration of methacholine causing a 20% fall in FEV1; PDE4, phosphodiesterase 4; PEF, peak expiratory flow.
Quality assessment
Quality assessment of the 17 studies showed no biases in data attrition and reporting, but only one study22, 23 did not blind participants and personnel (performance bias) as well as outcome assessment (detection bias). Six trials14, 15, 17, 19-21 met the criteria of random sequence generation and allocation concealment (selection bias), but other biases are not directly stated. No studies were excluded for low quality and no publication bias was detected in the Funnel plot.
Heterogeneity
For the studies and outcomes in the meta-analysis, we did not find significant statistical heterogeneities in the change of FEV1 from baseline (I2 = 0%, χ2 = 7.55, P = 0.58) or adverse events especially headache (I2 = 0%, χ2 = 0.87, P = 0.83) and nausea (I2 = 0%, χ2 = 0.00, P = 1.00), whereas significant statistical heterogeneity was noticed in pre- and post-challenging FEV1% (I2 = 72%, χ2 = 3.35, P = 0.06). On the basis of the results showing that I2 was equal to 0 in most cases, the random-effects model was also used as a sensitivity analysis to further screen for heterogeneities among different studies but resulted in a similar outcome.
Outcomes
Lung function
FEV1 and PEF were the most commonly used parameters to evaluate asthma control.1 In our meta-analysis, we found a significant increase of FEV1 in patients receiving 500 μg of roflumilast (Z = 2.50, P = 0.01), but not in those with 250 μg of roflumilast (Z = 0.30, P = 0.77) (Fig. 2) Moreover, 500 μg of roflumilast as an add-on therapy to corticosteroids did not provide additional improvement in FEV1 (Z = 1.65, P = 0.10). Two studies14, 19 reported the change of FEV1 in the treatment of other PDE4 inhibitors, and the results showed that MK-0359 rather than BAY19-8004 also improved FEV1 compared with placebo. Furthermore, the change of FEV1% was similar between roflumilast and placebo (Z = 1.07, P = 0.28).
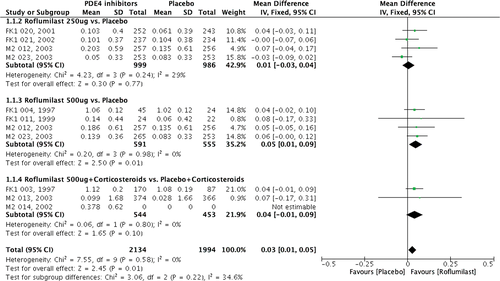
In terms of the change of PEF from baseline, 500 μg of roflumilast significantly increased PEF compared with placebo, but 250 μg of roflumilast resulted in an equivocal outcome. In studies of FK1 021 and M2 023, there was a numerical decrease in PEF, but it was found to be significantly increased in the studies of FK1 020 and M2 012 with 250 μg of roflumilast.
Airway responsiveness
Two studies reported the effects of PDE4 inhibitors on methacholine PC20, which was widely measured as an indicator for AHR. In the study by Gauvreau et al.,20 14 days’ treatment with 500 μg of roflumilast did not change the methacholine PC20 compared to treatment with placebo (P > 0.05), which was similar to the findings in Leaker et al.’s21 study using 7 days’ treatment with 600 mg of MEM1414 (P = 0.38). However, Gauvreau et al.’s study20 further showed a significant attenuation in allergen-induced fall in AHR by 500 μg of roflumilast from 5.64 ± 1.26 mg/mL in pre-allergen to 3.65 ± 0.81 mg/mL in 24-h post-allergen compared with placebo (P = 0.004).
Asthma control
Assessment of asthma control consisted of daytime and night-time symptom control, rescue use of relievers and activity limitations. In terms of asthma symptom control, the asthma control questionnaire was used in 10 studies and the results showed significant improvement with treatment using roflumilast regardless of the dose or add-on treatment of inhaled corticosteroids, as well as in the treatment of 15 mg of MK-0359 (−0.21, 95% CI: −0.33 to −0.09, P ≤ 0.001) (data derived from original study). A similar pattern was observed in the use of rescue medications in the patient group with roflumilast and 15 mg of MK-0359 (−0.70, 95% CI: −1.12 to −0.27, P ≤ 0.01) (data derived from original study).
Blood TNF-α and asthma exacerbation
The effect of PDE4 inhibitors on lipopolysaccharide (LPS)-stimulated blood TNF-α level was contradictory. The median blood TNF-α level decreased significantly during 500 μg of roflumilast treatment (15 472 pg/mL vs 12 176 pg/mL, P = 0.009) compared with placebo (14 194 pg/mL vs 13 531 pg/mL, P = 0.298),17 while no change was found when treated with 5 mg of BAY19-8004 (19.3 ± 7.8 ng/mL vs 14.4 ± 8.4 ng/mL, P > 0.3)15 (data and statistical analysis shown here were originally obtained from the original studies).
Incidence of severe exacerbations or asthma exacerbations requiring oral or parenteral steroids were numerically fewer in the roflumilast treatment groups compared with the placebo groups regardless of the medication doses or add-on treatment with corticosteroids. Moreover, the days to first severe exacerbation were significantly higher in 250 μg of roflumilast alone (35 days vs 30 days, P = 0.0443) and 500 μg of roflumilast plus 400 μg of beclomethasone dipropionate (51 days vs 36 days, P = 0.0259) compared with placebo.
Adverse events
In general, PDE4 inhibitors were well tolerated and no clinically relevant alterations in 12-lead resting electrocardiograms, blood pressures, heart rate and routine laboratory tests including haematology, biochemistry and urinalysis were observed. However, one study reported that one subject treated with 500 μg of roflumilast was withdrawn from the study due to a serious adverse event—depression.
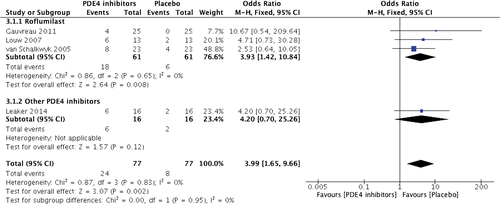
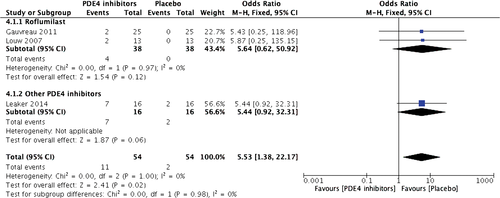
In total, 41 mild adverse events were reported in different studies, of which nervous system disorders including headache and dizziness, and gastrointestinal disorders including nausea, diarrhoea, vomiting and dyspepsia were the most commonly reported adverse events. The majority of side effects occurred in participants receiving PDE4 inhibitors. Headache and nausea were significantly more common in the roflumilast treatment group (18 of 61 vs 6 of 61, OR: 3.93, 95% CI: 1.42–10.84, Z = 2.64, P = 0.008) (Fig. 3). There was a similar incidence of nausea in both groups (4 of 38 vs 0 of 38, OR: 5.64, 95% CI: 0.62–50.92, Z = 1.54, P = 0.12) (Fig. 4). However, in pooled effects, PDE4 inhibitor treatment had a significantly higher incidence of both headache (OR: 3.99, 95% CI: 1.65–9.66, Z = 3.07, P = 0.002) and nausea (OR: 5.53, 95% CI: 1.38–22.17, Z = 2.41, P = 0.02).
DISCUSSION
In our review, we found that roflumilast (500 μg) and MK-0359 could significantly increase FEV1 compared with placebo, and 500 μg of roflumilast also significantly improved PEF, attenuated asthma symptoms, reduced rescue use of medication and asthma exacerbations, and decreased blood TNF-α. However, PDE4 inhibitors showed variable effects on airway responsiveness. Furthermore, PDE4 inhibitors treatment was associated with more adverse events such as headache and nausea.
Variability in expiratory flow limitation is a hallmark clinical manifestation for asthmatic patients, and FEV1 and/or PEF are the most commonly used measurements in predicting risk of asthma exacerbations and asthma control.24 Previous studies showed a dose-dependent treatment efficacy of roflumilast in improving FEV1 and PEF, of which 500 μg of roflumilast was most likely to improve these parameters and presented a significantly higher increase from baseline in FEV1 by 400 mL, morning PEF by 20 L/min and evening PEF by 16 L/min.12, 13 This review has similar findings to individual studies: roflumilast and other PDE4 inhibitors improve lung function.7 However, no additional synergistic effects were observed when PDE4 inhibitors including roflumilast were added to inhaled corticosteroid treatment. Therefore, 500 μg of roflumilast cannot be recommended for combined use with inhaled corticosteroids but might be considered as a potential alternative for asthmatic patients with contraindications to corticosteroid use.25, 26 Nevertheless, whether other asthma controllers (such as long-acting β2 agonists or montelukast) can be used effectively with PDE4 inhibitors needs to be addressed in future investigations.
AHR is a pathophysiological feature in asthma development, and it is associated with asthma severity, progression and control.27 Methacholine PC20 is a widely measured indicator of AHR in clinical settings, and a majority of clinical trials consistently showed significant improvement in allergen-induced AHR after treatment with PDE4 inhibitors.14, 28 In our analysis, we also found that PDE4 inhibitors could improve AHR to different allergen provocations, but it did not attenuate methacholine PC20 from baseline without allergen challenge, even if lung function improved. This might result from two possible causes: (i) the variable relationship between AHR and lung function and AHR often persisting, even when lung function returns to normal1; or (ii) different asthma phenotypes such as obese patients with asthma in whom AHR was ameliorated after roflumilast treatment.29
Asthma control is important and the risk of future exacerbations has been demonstrated.1 In our review, we found significant relief of asthma symptoms and reduction of rescue reliever medication use. Moreover, the incidence of severe exacerbations after treatment with PDE4 inhibitors was also reduced. Individual studies have also demonstrated that the PDE4 inhibitors were not inferior and not better than inhaled corticosteroids or montelukast in controlling asthma symptom and attenuating time to first asthma exacerbation.13, 25, 26
TNF-α is an important inflammatory mediator and reportedly related to AHR in asthma.30 Theoretically, it can be a marker for activity of PDE4 inhibitors because its synthesis and release can be inhibited by cAMP.31 In animal experiments, PDE4 inhibitors were reported to inhibit TNF-α synthesis by up to 85%.32 This review found a significant decrease of LPS-induced blood TNF-α by 500 μg of roflumilast compared with placebo, which was similar to the findings by Murad et al.’s study,33 which compared roflumilast and formoterol together with fluticasone.
PDE4 inhibitors were generally well tolerated in patients with asthma, but they caused mild adverse events such as headache and nausea. These central nervous system and gastrointestinal side effects of PDE4 inhibitors have been attributed to (i) the mismatching of high-affinity and low-affinity PDE4 receptors, of which high-affinity PDE4 inhibition is associated with emesis and gastric responses, whereas low-affinity PDE4 inhibition is related to potential anti-inflammatory effect34; (ii) the different PDE4 isoforms, as PDE4D is considered to be the main isoform associated with central nervous and gastrointestinal adverse events35; and (iii) administration routes, especially with systemic administration of PDE4. On the basis of these findings, adverse events of PDE4 inhibitors might be minimized by either measuring the individual ratio of high-affinity and low-affinity PDE4 or developing inhaled preparations. With the aim of minimizing side effects of PDE4 inhibitors and improving patient acceptability and tolerability, Vestbo et al. conducted a double-blind, placebo-controlled trial of an inhaled PDE4 inhibitor, UK-500, in 209 patients with moderate-to-severe COPD.36 There was only a slightly higher incidence of treatment-related adverse events in the group which received inhaled PDE4 inhibitors, although no improvement was observed in trough FEV1 and clinical symptom scores. Development of alternative inhaled formulations of PDE4 inhibitors and more studies of inhaled PDE4 inhibitors in combination with inhaled bronchodilators or corticosteroids are warranted.
Several limitations of our review are noted: (i) not all studies were randomized trials although all were placebo-controlled studies; (ii) heterogeneity testing in our analysis was underpowered due to a very small number of studies; (iii) the outcome data of some studies were either too complex to retrieve or not reported, hence some outcomes were not analysed in the meta-analysis which might affect the accuracy and applications of our results; (iv) the patients in each study mainly had mild asthma, thus general conclusions of effects of PDE4 inhibitors in the full severity spectrum of asthma patients could not be made; and (v) medication dose and duration were inconsistent among different studies but sub-analyses of optimal dose and duration were not feasible.
In conclusion, oral PDE4 inhibitors including 500 μg of roflumilast may be an alternative treatment to regular bronchodilators and inhaled controllers in patients with mild asthma. Oral PDE4 inhibitors improve lung function and asthma control, and decrease asthma exacerbations. However, this occurs at the expense of increased adverse events. Future studies in asthma of different severities and in combination with other asthma controllers will be of interest.
Acknowledgements
We thank Professor Dongtao Lin (College of Foreign Languages, Sichuan University) with expertise in biomedical writing and for copyediting this manuscript.




