Convergent Isobilateral Leaves Increase the Risk for Mangroves Facing Human-Induced Rapid Environmental Changes
ABSTRACT
Understanding plant adaptations in extreme environments is crucial, as these adaptations often confer advantages for survival. However, a significant gap exists regarding the genetic mechanisms underlying these adaptations and their responses to human-induced rapid environmental change (HIREC). This study addresses the question of whether genetic convergence occurs among plants with similar adaptive features, specifically focusing on isobilateral leaves in mangrove species. Here, we analyse the genetic convergence of isobilateral leaves in mangroves that have independently adapted to coastal intertidal zones. Our findings reveal that genetic convergence is evident in gene families involved in leaf abaxial and adaxial development, with strong selection pressures identified in photosynthesis and leaf polarity pathways. Despite these adaptations, mangrove species with isobilateral leaves occupy narrower ecological niches and face diminishing suitable habitat areas projected under various HIREC scenarios. These results indicate that while convergent traits enhance local adaptation, they may also increase vulnerability to ongoing environmental changes. This research provides valuable insight into the interplay between genetic adaptation and environmental resilience, underscoring the necessity for targeted biodiversity conservation strategies that safeguard specific adaptive traits amid rapid environmental shifts.
1 Introduction
It is a ubiquitous phenomenon that phylogenetically distinct plant taxa experienced similar strong selective pressures under similar extreme environments will convergently evolved similar traits and adaptive strategies (Endler 1986; Losos 2011). However, species that closely match their phenotype to environment, such as ecological specialised species with narrow physiological tolerances or specific resource dependencies, are often more vulnerable to climate changes (Buckley and Kingsolver 2012; Grinder and Wiens 2023). Historically, phenotypic convergence has been driven primarily by natural selection acting on traits in response to specific environments, particularly when convergence provides a fitness advantage under similar environmental challenges (Stern 2013). Currently, human-induced rapid environmental changes (HIREC) are exerting strong selective pressures on similar functional traits, potentially creating a conflict between adaptation to past climates and rapid environmental change (Botero et al. 2015; Stanton et al. 2000). Functional traits that influence individual performance and fitness are crucial in modulating species' responses to climate change, thereby determining their vulnerability (Wang et al. 2021). Therefore, we hypothesise that species exhibiting convergent functional traits in extreme environments may be more vulnerable to HIREC.
Predicting changes in species habitat under rapid climate change is crucial for assessing their vulnerability (Pacifici et al. 2015). In response to past environments, species have shifted their distributions and abundances in various directions and extents (Davis and Shaw 2001; Williams, Jackson, and Kutzbach 2007). However, determining whether species can successfully colonise newly suitable climatic areas, especially as their current ranges become unsuitable, remains a challenge (Estrada et al. 2016). The most common method for estimating species' responses to climate change involves comparing future climatic conditions to current ones (Garcia et al. 2014). Ecological niche models are reliable tools widely used to quantify species' responses to climate change (Newbold et al. 2020). These models estimate a species' niche by correlating its geographic occurrences with environmental factors, allowing robust predictions of current distributions and potential impacts from environmental changes (Araújo et al. 2011; Carlson et al. 2022). Consequently, we employ ecological niche models to examine the responses of species with convergent functional traits facing HIREC.
Mangrove species, originating from independent inland ancestors, have successfully colonised in extreme intertidal environments characterised by high temperature, strong UV light and anaerobic soils (Xu et al. 2017; He et al. 2022). Physiological drought poses a common challenge for all intertidal plants due to high salinity. Hence, maintaining water balance is essential for successful growth of mangroves, particularly through functional adaptations in their leaves (Rodriguez et al. 2005). Unique adaptive features such as salt secretion and ultrafiltration have been identified, enabling mangroves to thrive in harsh environments (Nizam, Meera, and Kumar 2022). Leaf shapes and structures are crucial for plants to adapt to their environments (Adams and Terashima 2018). There are two different leaf displays found in mangroves, isobilateral and bifacial leaves (Das and Ghose 1996). Bifacial leaves, featuring palisade and spongy cells differentially occupy adaxial and abaxial sides respectively, are the dominant phenotype in eudicot plants. However, isobilateral leaves, which develop palisade tissues on both adaxial and abaxial sides (hence isobilateral leaf), are relatively common in mangrove but often overlooked. Anatomical studies suggest that these special features are adapted to saline environments (Das and Ghose 1996). Common traits of isobilateral leaves in mangroves may result from convergent evolution. However, whether this trait offers advantages for local adaptation and how it responds to environmental change warrant further study.
To investigate that isofacial leaf development in mangrove plants results from convergent evolution, we conducted comparative genomic analyses to identify signals of convergence in the genome. Simultaneously, we employed ecological niche models to predict how mangrove species with isobilateral leaf respond to HIREC. Our findings indicate that isobilateral leaves in mangrove plants are a product of convergent evolution, offering advantages for local adaptation. However, mangrove with such trait may also increases vulnerability for mangrove plants to HIREC.
2 Materials and Methods
2.1 Plant Materials and Leaf Anatomy
Mangrove leaves used for anatomy analysis were collected from Xiatanwei Wetland Park, Xiamen, Fujian Province in China. Mangrove plants Sonneratia caseolaris and Avecinia marina were selected as typical isobilateral and bifacial leaves, respectively. Leaf anatomy by freezing sections were done with following main steps: (1) Preparation of fixative solution by dissolving 3 g of Paraformaldehyde (CAS: 30525-89-4, Sangon Biotech, Catalogue # A500684-0500) and 4 g of EDAC (CAS: 25952-53-8, Sangon-Biotech, Cat # C600433-0100) in 80 mL of deionized water, heat at 37°C for 24 h, add 100 μL of Triton X-100, top up to 100 mL with more deionized water, store at 4°C. (2) Tissue fixation by picking fresh leaves and cut fresh leaf tissue into pieces, immerse in fixative (10:1 ratio), vacuum for 20 min, let stand 30 min, refrigerate at −4°C for 24 h. (3) Discard the fixative, rinse with distilled water on a shaker for 10 min, repeat three times. (4) Tissue embedding: using tweezers to remove the leaf, blot dry with filter paper, embed in Tissue-Tek O.C.T. compound (Sakura, Cat # 4583) and freeze, avoiding bubbles. (5) Slice with a Leica CM1900 cryostat at 8 μm thickness. Select even and intact slices for photography under a stereo microscope.
2.2 Phylogenetic Analysis and Divergent Time Estimation
Phylogenetic relationship of three isobilateral leaf mangrove plants S. caseolaris, Laguncularia racemosa, Kendalia obovata and additional closely related 8 species with available whole-genome sequences, including Combretum micranthum from Combretaceae, and Arabidopsis thaliana from Brassicales, Carallia brachiata, Bruguiera gymnorrhiza, Ceriops tagal from Rhizophoraceae, Glycine max from Leguminosae, A. marina from Verbenaceae, and Trapa natans from Lythraceae were constructed (Zhu et al. 2023; Shearman et al. 2022; He et al. 2022; Chang et al. 2013; Xu et al. 2017; Table S1). Highly conserved single-copy genes inferred from OrthoFinder and employed MAFFT v.7.515 to align orthologous proteins and PAL2NAL v.14. to generate codon sequences against aligned protein sequences (Katoh and Standley 2013; Emms and Kelly 2019; Suyama, Torrents, and Bork 2006). Sequences shorter than 150 bp and gaps were removed using Gblocks. Finally, 1531 high-confidence orthologous single-copy genes were retained and used for phylogenetic tree reconstruction using RAxML v.8.2.12 with the GTR + GAMMA + I model and 1,000 bootstrap replicates with A. marina as the outgroup (Stamatakis 2014).
To estimate the evolutionary timescale for isobilateral leaf mangrove plants, species tree was dated by MCMCTREE from the PAML package (Yang 2007). Three known time points according to the fossil record were set for calibration: (i) recent ancestors of Rhizophoreae have appeared in early Eocene (56 to 47.8 Ma), (ii) recent ancestor of Rhizophora has appeared in late Eocene (37.8–33.9 Ma; Graham 2006), (iii) the common ancestor of A. marina and A. thaliana was placed at ~115 Mya (Graham 2013).
2.3 Gene Family Evolution
Gene family analysis was performed in three isobilateral leaf mangrove plants described above and with closely related bifacial leaf plants including C. tagal, C. micranthum and T. natans. Protein sequences of the 6 species were aligned with DIAMOND v.0.9.25 (Buchfink, Xie, and Huson 2015). Gene families were clustered using OrthoFinder v.2.5.4 (Emms and Kelly 2019). Gene families involved in leaf development identified by assigning A. thaliana homologues using the TAIR10 database (https://www.arabidopsis.org/).
2.4 Estimation of the Strength of Natural Selection in Isobilateral Leaves
To identify candidate genes for isobilateral leaf formation, the strength of natural selection were evaluated. High-confidence orthologous single-copy genes from three isobilateral leaf mangrove plants with their closely related bifacial leaf plants from OrthoFinder were aligned and filtered described above to perform positive and relaxed seleciton analysis in PAML v.4.9j and HyPhy v.3.1 (Yang 2007; Wertheim et al. 2015). In positive selection, we used classical branch-site model to determine if amino acid sites in the lineage of isobilateral leaf mangrove plants have undergone positive selection (foreground ω > 1) compared with bifacial leaf plants (background). Branch-site model A (model = 2, Nssite = 2, fix_omega = 1, omega = 0) and branch-site model null (model = 2, Nssite = 2, fix_omega = 1, omega = 2) were done in PAML. The significance of likelihood ratio tests (LRT) was calculated as 2 ∗ (lnLHA – lnLH0) to identify positively selected sites between model A and model null. The Bayes Empirical Bayes (BEB) algorithm method was used to calculate posterior probabilities of positive selected sites (Yang 2005; Allouche, Tsoar, and Kadmon 2006). Similarly, C. micranthum and all three bifacial leaf plants were as foreground branch with the remaining species as the background branch to confirm these candidate genes only positively selected in isobilateral leaf mangrove plants. Moreover, we used orthologous single-copy genes to run RELAX in HyPhy for testing relaxd selection (Wertheim et al. 2015). RELAX including three Dn/Ds rate categories in alternative model to test and reference branch sets and infers selection intensity by introducing the parameter K. K < 1 is indicative of relaxed natural selection and K > 1 would indicate an intensified selection on test lineages compared to the reference set.
There are 63 leaf polarity genes have been verified to play a key role in formation of leaf polarity in Arabidopsis (Sun et al. 2024). To test whether leaf polarity genes are subjected to stronger or weaker selection pressures in isobilateral leaf mangrove plants, we reciprocal blastp (-outfmt 6 and E-value < 1e-5) these Arabidopsis proteins with six species described above, to search for orthologous of polarity genes and nonpolarity genes in leaf development. Then, leaf polarity and nonpolarity genes in three species pairs including K. obovata versus C. tagal, S. caseolaris versus T. natans and C. micranthum versus L. racemosa were aligned by MAFFT v.7.515, and rates of nonsynonymous (Dn)/substitutions synonymous substitutions (Ds) of all single copy homologous gene pairs were calculated using NG method in KaKs_Calculator v.2.0 software (Zhang et al. 2006).
2.5 Leaf Water and Chlorophyll Content Measurements
The leaves of three isobilateral leaf mangrove plants including S. caseolaris, L. racemosa and K. obovata and three bifacial leaf mangrove plants, A. marina, Bruguiera gymnorrhiza and Aegiceras corniculatum in Xiatanwei Wetland Park were selected to measure leaf water and chlorophyll content every month from January to December in 2023. 10–15 plants per species with three mature leaves from each plant were collected. The fresh weight (FW) of sampled mangrove leaves were obtained using a electronic scale with a 0.01 mg measurement error. Sampled mangrove leaves were dried in an oven at 70°C until a constant dry weight (DW) was reached (approximately 48 h), and leaf water content rate calculated using the equations: (FW − DW)/FW ∗ 100% = leaf water content rate. The leaf chlorophyll contents were measured using chlorophyll metre SPAD-502Plus (Konica Minolta Inc.).
2.6 Species Occurrence Data
Species occurrence data collected from field investigations and the Global Biodiversity Information Facility (GBIF). Species names ‘Sonneratia caseolari’, ‘Kandelia obovata’, ‘Avicennia marina’, ‘Aegiceras corniculatum’, ‘Bruguiera gymnorrhiza’, ‘Lumnitzera racemosa’ were used as keywords to search plant collection records from 2000 to 2024 in Asia on GBIF (https://www.gbif.org). Incomplete, duplicated, and ambiguous occurrence records were abandoned. Remaining records were projected on a global map with a resolution of 2.5 arc-min using ArcGIS 10.5 (Environmental Systems Research Institute), irrational points discarded (i.e., on rivers, oceans, or outside the known ranges) and only one point was retained in a cell. Finally, 732 occurrence records retained (55 of S. caseolari, 161 of K. obovata, 189 of A. marina, 62 of A. corniculatum, 145 of B. gymnorrhiza and 119 of L. racemosa; Table S6).
2.7 Environmental Variables
Ninteen bioclimatic variables and monthly climate data for minimum, mean, and maximum temperature, precipitation, solar radiation, wind speed, water vapour pressure, total 108 environmental variables were downloaded from WorldClim (a database of high spatial resolution global weather and climate data, http://worldclim.org/) with 2.5 arc-min resolution (Fick and Hijmans 2017). Pearson's correlation coefficient R < 0.75 and variance inflation factor > 10 were used to avoid collinearity between variables. Ultimately, six environmental variables were retained including Temperature Seasonality (Bio4), Max Temperature of Warmest Month (Bio5), Annual Precipitation (Bio12), Precipitation of Warmest Quarter (Bio18), Precipitation of Warmest Quarter (Bio19), and solar radiation of February (Srad2).
2.8 Ecological Niche Models and Niche Identity
To construct the distribution of six mangrove species and leaf morphology at current conditions, three optimal models and 100 replicates of each model in R package ‘Biomod2’ were selected and assembled according to receiver operating characteristic (ROC) and true skill statistic (TSS) values (Allouche, Tsoar, and Kadmon 2006; Fawcett 2006; Thuiller et al. 2009). Finally, fandom forest, generalised boosted models (GBM), and Maximum Entropy Model (Maxent) were selected for having highest values of ROC and TSS values (Figure S1). A total of 500 layers for each species and leaf morphology were assembled with a threshold of TSS ≥ 0.9 according to proportion in R package ‘Biomod2’. Ensembled layers were constructed and then mapped by ArcGIS 10.5. The value of the cutoff that was generated when the finish model running was used as the threshold to determine the extent of suitable distribution regions. The niche breadth of six mangrove species and leaf morphology were calculated in the R ackage ‘ENMTools’ based on Levins' B values and generated using layers based on the GBM model by 100 bootstrap sampling (Warren, Glor, and Turelli 2010; Levins 1968).
2.9 Prediction of Habitat Changes of Isobilateral and Bifacial Leaves Under HIREC
The above environment variables in future climate scenarios were used to predict habitat changes of isobilateral and bifacial leaves under HIREC. Two future emission scenarios including shared socioeconomic pathways (SSPs) scenarios 245 and 585, three general circulation models (GCMs), Australian Community Climate and Earth-System Simulator Coupled Model 2 (Bi et al. 2020; ACCESS-CM2), Beijing Climate Centre Climate System Model version 2 Medium Resolution (BCC-CSM2-MR) and Centro Euro-Mediterraneo sui Cambiamenti Climatici Earth System Model version 2 (Wu et al. 2019; Lovato et al. 2022, CMCC-ESM2), a total of six future climate scenarios in two times at 2050 (average of 2041–2060) and 2090 (average of 2081–2100) were adopted. Species occurrence data of isolateral and bifacial leaf mangrove species were combined as isolateral and bifacial leaves occurrence data, respectively. The above optimal models are assembled in R package ‘Biomod2’ to predict habitat changes of isolateral and bifacial leaves under different future climate scenarios, suitable habitat change were calculated using ArcGIS 10.5 (Environmental Systems Research Institute).
3 Results
3.1 Leaf Structures
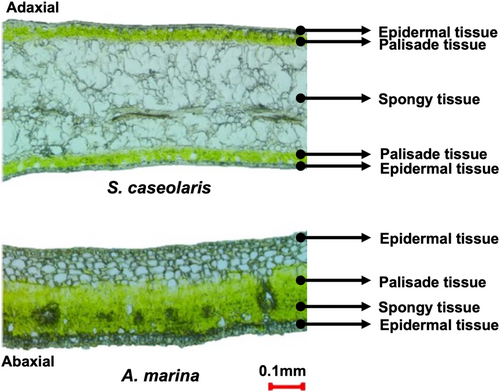
The obvious difference of leaf structures between isobilateral and bifacial leaves in mangroves, larger spongy tissue and more palisade tissue are found in isobilateral leaves (Figure 1). In S. caseolari with isobilateral leaf, almost symmetrical palisade tissues on both adaxial and abaxial sides of the leaf and a large spongy tissue exists between palisade tissues. While in A. marina of bifacial leaf, the palisade tissue appears only on the adaxial side and the spongy tissue exists on the abaxial side.
3.2 Origin of Isobilateral Leaves in Mangroves
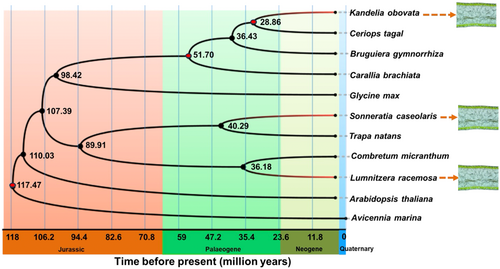
To investigate the occurrence of isobilateral leaves in mangroves, we constructed phylogenetic relationships of three mangrove plants with isobilateral leaves based on whole-genome sequence data. The phylogenetic relationships and divergence times among and within the main clades are consistent with previous findings (He et al. 2022; Xu et al. 2017). K. obovata within the Rhizophoraceae diverged from C. tagal at 26.61 Mya, while S. caseolaris was estimated to share a common ancestor with C. micranthum at 40.43 Mya, and L. racemosa divided with T. natans at 32.29 Mya (Figure 2). These results suggest that isobilateral leaves in mangroves distributed across different evolutionary lineages and originated independently.
3.3 Evolution of Gene Families Associated With Isobilateral Leaf Development
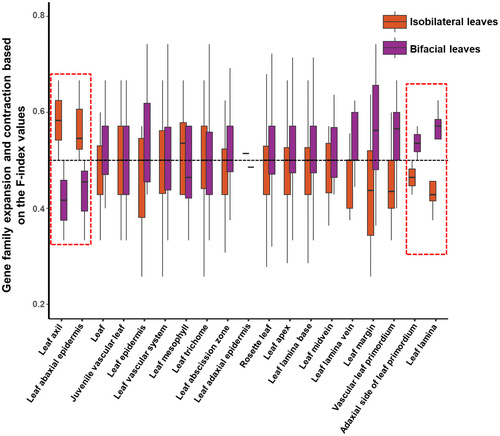
To detect the genetic convergence of isobilateral leaf in mangroves, we conducted gene family analyses on three isobilateral leaf mangrove species with their closest related bifacial leaf counterparts. From a total of 23,940 gene families, 4982 are related to leaf development, based on their homology to the model plant of Arabidopsis. In isobilateral leaf mangrove plants, gene families associate with leaf axil and abaxial epidermis development are expanded, whereas gene families relate to the adaxial side of the leaf primordium, leaf lamin are contracted compare to bifacial leaf mangrove plants (Figure 3). Convergent expansion or contraction of key gene families for leaf development are common strategy in isobilateral leaf mangrove plants. In plants, the establishment of abaxial and adaxial sides is the basis of leaf morphological diversity (Fukushima and Hasebe 2014; Reinhardt et al. 2005). Consistent with previous studies, gene families relate to leaf abaxial and adaxial side development have convergent expansion or contraction in isobilateral leaf manrove plants (Figure 3). These findings suggest that changes in gene family sizes are essential in the formation of isobilateral leaves in mangroves.
3.4 Evidence of Natural Selection for Isobilateral Leaves
To investigate whether shared biological pathways of isobilateral leaf mangrove plants undergo similar natural selection in leaf development, we conducted positive and relaxed selection analyses in three pairs of species (Figure 4a). After comparing the alternative hypothesis against the null model, 98 out of 1531 (6.48%) orthologous single-copy genes in isobilateral leaves exhibit strong evidence at least one site that under positive selection based on foreground ω value and likelihood ratio tests (LRTs, p < 0.05, Table S2). Most of the positive selection genes are associated with leaf development (71.43%, Figure 4b). These positive selection genes of leaf development are mainly related to photosynthesis process in GO terms, such as response to photooxidative stress, photorespiration, chlorophyll biosynthetic process, and cellular metabolism process like in RNA modification (Figure 4c). There are 392 and 113 genes under positive selection when C. micranthum and all three bifacial leaf plants as target species (Table S3 and S4), they are primarily in DNA break repair, cell activity process, but not related to photosynthesis (Figure S1a,b). Relaxed selection may occur when pressure of natural selection is reduced, causing accumulations of deleterious mutations (Lahti et al. 2009). Using the RELAX model to detect selection intensity of leaf development genes in isobilateral leaf mangrove plants, more than 370 genes experience significantly relaxed selection that accounts for 90.71% of the total relaxed genes in isobilateral leaf mangrove plants (p < 0.05; k < 1). These genes are mainly involved in DNA metabolism pathways at DNA damage response, replication and repair, carotenoid and pigment synthesis, starch biosynthetic synthesis and metabolic processes (Figure 4d).
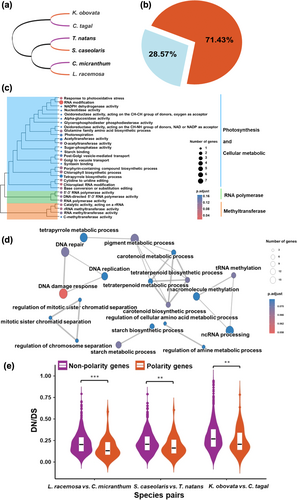
There are 46 orthologous leaf polarity gene pairs in K. obovata versus C. tagal, 51 in S. caseolaris versus T. natans and 43 in C. micranthum versus L. racemosa (Table S5). Mean values of Ka/Ks in leaf polarity gene and nonpolarity genes are 0.24 versus 0.29, 0.18 versus 0.22 and 0.14 versus 0.18, respectively (Figure 4e), suggesting that leaf adaxial-abaxial polarity genes all subjected to stronger purification selection in isobilateral leaf mangrove plants.
The outcomes of positive and relaxed selections suggest that the development of isobilateral leaves in mangroves correlates with occurrences of positive or relaxed selection in shared biological pathways, although we still could not determine whether these selected pathways occur before or after the formation of isobilateral leaves. Moreover, convergently purified selection of leaf adaxial-abaxial polarity genes are common in convergent evolution of isobilateral leaf mangrove species.
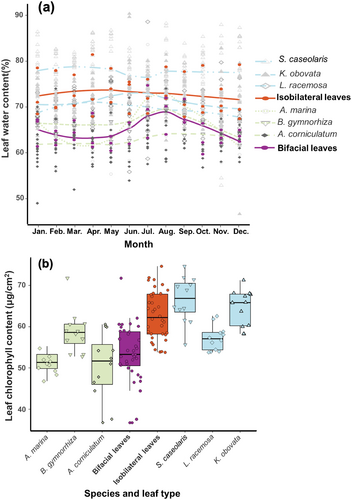
3.5 High Leaf Water and Chlorophyll Contents in Isobilateral Leaves
To test physiological functions of isobilateral leaves, we measured leaf water and chlorophyll contents over a year in three isobilateral leaf mangroves, S. caseolaris, L. racemosa and K. obovata, and three bifacial leaf mangroves, A. marina, B. gymnorrhiza and A. corniculatum. Nearly all isobilateral leaves in different mangrove species consistently exhibit higher levels of leaf water content compared to that of bifacial leaf species, and maintain elevated levels across all months (Figure 5a). Leaf water contents in bifacial leaves were significantly higher in June to September (Summer) compare to other months, consistent with the results of other bifacial leaf plants (Xu et al. 2021; Gond et al. 1999). The average chlorophyll contents in isobilateral leaves are also higher than bifacial leaves but variable in different bifacial leaf mangroves, such as B. gymnorrhiza has significantly higher chlorophyll contents than other two species of bifacial leaves (Figure 5b).
3.6 Distribution and Niche of Isobilateral Leaf Mangroves at Current Enviroments
To detect the distribution and suitable habitat of mangrove plants with isobilateral and bifacial leaves at the current environment, three optimal ecological niche models were assembled (Figure S2). The primary distribution regions of isobilateral leaf mangrove plants are the southern coasts of Asia, including regions of Malaya, Sri Lanka, and the South China Sea. Bifacial leaf mangrove plants have broader distribution ranges that extend to the coasts of the Persian Gulf and the Arabian Sea (Figure 6a). Consistent with broader distribution ranges in bifacial leaf mangrove plants, they also have more suitable habitat areas at current environments (Figure 6b). However, suitable habitat areas varied greatly among different mangrove species, such as A. marina has the largest suitable habitat area (105.9 × 104 km2) and K. obovata has the least suitable habitat area (15.3 × 104 km2) in all six mangrove species (Figure 6b), even K. obovata has most abundance in isobilateral leaf mangrove plants (Table S6), supporting the notion that ecological niche models are not species occurrence dependent (Costa et al. 2010).
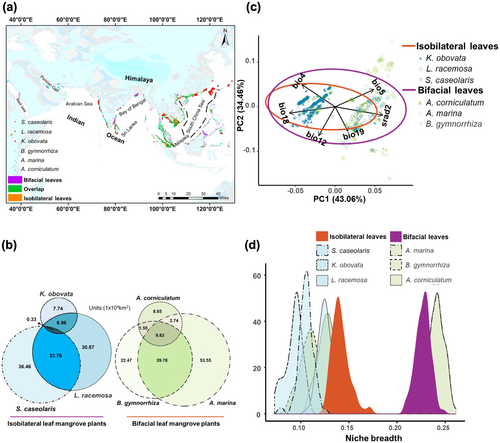
The variation in environmental factors among isobilateral leaf mangrove plants are relatively small, whereas greater variation observed in bifacial leaf mangrove plants according to the PCA of six environmental variables (Figure 6c). Consistent with the PCA result, isobilateral leaf mangrove plants occupy similar and narrow niche, bifacial leaf mangrove plants occupy broader and variable niche (Figure 6d). Combine all isobilateral leaf and bifacial leaf mangrove plants together for abiotic niche analysis severally, narrower abiotic niche in isobilateral leaf mangroves (the dark orange density peak) compare with bifacial leaf mangroves still maintained (the purple density peak, Figure 6d).
3.7 Suitable Habitat Changes of Isobilateral Leaf Mangrove Plants Under HIREC
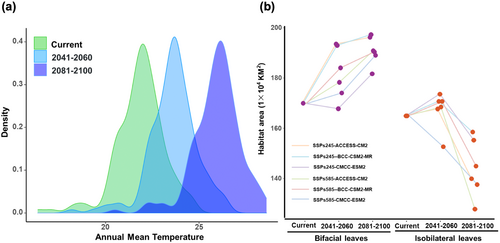
To predict the response of isobilateral leaf mangrove plants under HIREC, two SSP (Shared Socioeconomic Pathways) scenarios (SSPs245 and SSPs585) and three GCMs (General Circulation Models) for years 2050 (average of 2041–2060) and 2090 (average of 2081–2100) are used as environmental variables of HIREC to analyse habitat changes of isobilateral leaf mangrove plants. Environmental factors are projected to change more dramatically in 2090 compared to 2050, with a significant increase in ambient temperatures expected (Figure 7a). Habitat areas of mangrove plants with bifacial leaf are increasing from current environment in 2050 under all modelling scenarios, and more dramatically increase in 2090 (Figure 7b). In contrast, habitat areas of isobilateral leaf mangrove plants increase from current to 2050 lightly, but rapid declined from 2050 to 2090 under all scenarios and GCMs (Figure 7b). This indicates that mangrove plants with isobilateral leaf may be sensitive to environment changes and more vulnerable to the impacts of HIREC.
4 Discussion
4.1 Convergent Evolution for Adapting to Extreme Environments
Isobilateral leaves are common phenomena in mangrove plants and occur in multiple species with higher leaf water content and chlorophyll content (Figure 5), leading to higher photosynthesis and hence plant growth (Li et al. 2018; Wang et al. 2022). Phylogenetic analysis shows that isobilateral leaves in mangroves are an independent origin events and the results of adaptive convergent evolution (Figure 3). Actually, convergent phenotypes are more common in mangroves like vivipary, aerating roots, salt secretion and so on (Nizam, Meera, and Kumar 2022), great deal of efforts have been devoted to elucidating the molecular mechanisms underlying these adaptive traits (Hao, Su, and Li 2021; Qiao et al. 2024), but genetic convergence analysis for those phenotypes are still limited. In this study, we used public genomic data to investigate genetic convergence of isobilateral leaves in mangroves, the results suggest that isobilateral leaf mangrove plants tend to contract or expand gene families related to leaf development, especially in leaf adaxial and abaxial sides development, and those genes are crucial for leaf morphology and function in plants have been proved (Sun et al. 2024; Wang et al. 2021).
Genetic convergence of gene families could be a familiar tactic for plants in extreme environments. Another example that gene family changes involve in tolerance to salt stress was found in the P. euphratica lineage (Ma et al. 2013). In fact, mangroves have become classic cases for studying convergent evolution, numerous signals of convergent evolution at different genetic hierarchy were found, such as genetic convergence at a specific site of a gene, gene structure, the same genetic pathway, or genomic constitution (Xu et al. 2020), suggesting that convergent phenotypes or adaptive strategies can be driven by multiple genetic hierarch (Wu, Wang, and Xu 2020). Similarly, genetic convergent signal in isobilateral leaf mangrove plants were found at some genetically shared pathways by positive and relaxed selection analysis, such as response to photooxidative stress, photorespiration, chlorophyll biosynthetic process experienced a strong positive selection but relaxed selection involved in carotenoid and pigment synthesis (Figure 4c,d). In general, extreme environments can promote the occurrence of phenotypic convergence, more detection methods and multiple genetic hierarchy for genetic convergence of convergent phenotype are necessary.
4.2 Species With Convergent Functional Traits Respond to Climate Change in Extreme Environments
Phenotypic convergence of functional traits in mangroves can provide advantages for local adaptations. An intriguing question is that convergent traits confer local adaptive advantages for plants to adapt to past environment, whether species with convergent traits are also more resistant to future environmental changes. Currently, an compelling example show that stronger local adaptation can exacerbate the challenges faced by alpine plants in response to rapid climate warming (Li et al. 2023). In this study, a noteworthy finding is that mangrove plants with isobilateral leaves have advantages for local adaptation in the intertidal zone. However, the variation in abiotic environments for species with isobilateral leaves is less than that of bifacial leaves, and their abiotic niches are also narrower (Figure 6c,d). Further research confirms that habitat areas for mangrove plants with isobilateral leaves significantly decrease under various future abiotic environmental scenarios (Figure 7b), indicating that these plants are more sensitive to environmental changes. Climate changes are undeniable reality, and human activities are accelerating such environmental shifts (Franklin et al. 2016), creating a conflict between adaptation to past climates and HIREC for plants. A strong alignment between phenotypes and environments may increase the risk under climate change (Boulangeat et al. 2012). To survive in a rapidly changing environment, it is crucial to strike a balance between local adaptation and the capacity to withstand environmental alterations. Especially for plants that adapt to extreme environments often convergence on functional traits (Xu et al. 2020). Such convergence may be worsening their situation when facing HIREC in the future. Therefore, greater emphasis should be placed on understanding how species in extreme environments can respond to climatic and environmental changes, enabling the development of more effective conservation strategies.
In summary, isobilateral leaves are proven to be a convergent evolutionary phenomenon for genetic convergence of shared biological pathways in some mangrove plants. While it can provide adaptive advantages for mangroves and promote local adaptation, it also increases the risk when facing HIREC in the future. This study is helpful for understanding of convergent evolution and provides a new perspective for convergent functional traits in extreme environments under environmental change conditions.
Acknowledgements
We thank Haidong Qu, Ziwei Zhao and Wenting Ouyan for technical supports, and students from ours and Dr. Shengchang Yang's labs for leaf water and chlorophyll content measurements. This work was supported in part by funding from the Xiamen Institute of Ocean Research.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.