OsPIL15-Induced Delay in Rice Heading Date via Direct Binding to the OsLF Promoter is Dependent on Functional Phytochrome B
ABSTRACT
Heading date of rice (Oryza sativa) is a key factor determining rice production and regional adaptability. We analysed the molecular mechanism of OsPIL15, encoding phytochrome-interacting factor-like protein, in delaying rice heading date. Overexpression of OsPIL15 delayed rice heading date by upregulating Hd1 and inhibiting Hd3a and RFT1 expression. OsLF, encoding one rice heading repressor, was found to be the putative candidate regulated by OsPIL15 through a chromatin immunoprecipitation sequencing assay and a transcriptome sequencing assay. OsPIL15 could directly bind to the OsLF promoter and activated its expression. Knocking-out OsLF in OsPIL15-overexpressing lines resulted in flowering 2–3 days earlier, partially rescuing the delayed phenotype. This indicates that overexpression of OsPIL15 overexpression delays heading date partially through OsLF. Protein–protein interaction assay of OsPIL15 or OsPIL15-∆APB (OsPIL15 lacking the active phytochrome B [phyB]-binding [APB] motif) with PHYB showed that the APB motif was required for the interaction between OsPIL15 and PHYB. Furthermore, overexpression of either OsPIL15-∆APB in the wild type or OsPIL15 in the phyB mutant did not delay rice heading date under natural long-day conditions, suggesting that phyB influences OsPIL15-mediated delay in rice heading date.
1 Introduction
In rice (Oryza sativa L.), heading date is a vital agronomic trait that determines the adaption of rice varieties to specific regions and seasons, ultimately affecting rice yield (Izawa 2007; Sun et al. 2022; Osnato 2023). Various external and internal signals, such as photoperiod, temperature, and hormonal cues, influence heading. Among the factors, photoperiod is considered the most critical environmental determinant of the heading date in rice (Zhou et al. 2021; Qiao et al. 2023). After the vegetative growth stage, rice becomes sensitive to photoperiod. Under conditions of inductive day length, the leaves recognise the photoperiod and generate a florigen, which is a mobile signal. The florigen is then transported from the leaves to the shoot apex and is perceived by the shoot apical meristem to activate downstream floral identity genes, thereby inducing transition to the flowering phase (Zhou et al. 2021). There are two florigen genes in rice: HEADING DATE 3a (Hd3a), an ortholog of the FLOWERING LOCUS T (FT) gene in Arabidopsis, and RICE FLOWERING LOCUS T1 (RFT1), which is unique to rice. To date, numerous genes regulating the rice heading date have been identified and functionally characterised using molecular genetic approaches, and these genes affect the heading date through the regulation of Hd3a and RFT1 expression (Qiao et al. 2023). Photoperiod flowering in rice is believed to be controlled mainly by the crosstalk between two modules: HEADING DATE 1 (Hd1)-mediated conservative evolutionary GIGANTEA (GI)-Hd1-Hd3a/RFT1 pathway and EARLY HEADING DATE 1 (Ehd1)-centred monocotyledon-specific Ehd1-Hd3a/RFT1 pathway (Zhou et al. 2021; Zong et al. 2021; Wang and Han 2022; Qiao et al. 2023). As hub genes of the photoperiod pathway regulating the heading date, Hd1 and Ehd1 are regulated by many genes, as reported in previous studies (Sun et al. 2014; Zuo and Li 2014; Wei et al. 2020; Zhou et al. 2021; Zong et al. 2021). For example, Ehd1 is positively regulated by Ehd2/RID1, Ehd3, Ehd4,/ELF3, DTH8, and OsCOL4 and negatively regulated by Ghd7, OsLFL1, bZIP42, Dhd1, and OsRE1, and so on (Peng et al. 2007; Matsubara et al. 2008; Wei et al. 2010; Wu et al. 2008; Matsubara et al. 2011; Zhao et al. 2012; Gao et al. 2013; Nemoto et al. 2016; Brambilla et al. 2017; Zhang et al. 2019; Chai et al. 2021). In addition, Hd1 and Ghd7 form complexes and repress Ehd1 expression (Nemoto et al. 2016). The genes GI, CONSTANS (CO), and FT constitute a circadian clock–controlled flowering pathway that is conserved in both Arabidopsis and rice. In rice, OsGI regulates the expression of Hd1, an ortholog of Arabidopsis CO, thus controlling the transcription of Hd3a and affecting the heading date. LATE FLOWERING (OsLF), which encodes an atypical HLH protein, may repress the expression of OsGI and Hd1, thereby delaying heading in rice under short-day (SD) conditions. The OsGI-Hd1-Hd3a/RFT1 and Ehd1-Hd3a/RFT1 pathways are not independent.
Light is an important environmental signal that modulates plant growth and development, including the heading date. Through a group of photoreceptors, including phytochromes, cryptochromes, and phototropins, plants perceive a range of light signals. As chromoproteins, phytochromes regulate the expression of many genes responsive to light, thus influencing a wide range of photomorphogenic events. In rice, all three phytochrome (PHY) genes—PHYA, PHYB, and PHYC—are involved in the regulation of flowering time (Takano et al. 2005; Osugi et al. 2011; Lee, Yi, and An 2016). Hd1 functions as either an activator or suppressor of Hd3a depending on the phytochrome, since Hd1 promotes flowering under any photoperiodic condition in mutations in phytochrome chromophore synthesis (se5) or phyB (Andrés et al. 2009; Ishikawa et al. 2011).
Phytochrome-interacting factors (PIFs), which can directly interact with phytochromes, function as negative regulators of light responses. Moreover, PIF and PIF-like (PIL) proteins function as cellular signalling hubs that integrate various signals to regulate the transcriptional network, driving numerous aspects of downstream morphogenesis (Li et al. 2022). While the functions and regulatory networks of PIFs have been well-characterised in Arabidopsis, their physiological roles in rice remain largely unknown (Ji et al. 2019). In rice, six OsPILs in the genome have been identified, named as OsPIL11 to OsPIL16 (Nakamura et al. 2007). They belong to the basic helix–loop–helix (bHLH) subfamily, which contains a conserved active phytochrome B-binding (APB)-motif (the active phytochrome motif) and a bHLH domain (Nakamura et al. 2007). Among these OsPILs, OsPIL15 has been studied in detail to regulate seedling growth, tiller angle, seed size, stomatal aperture and resistance of sheath blight (Zhou et al. 2014; Ji et al. 2019; Xie et al. 2019; Li et al. 2022; Yuan et al. 2023), indicating that it functions in rice growth under abiotic and biotic stresses. However, whether OsPIL15 is involved in determining the heading date remains unknown.
Our previous studies showed that OsPIL15 regulates rice seedling growth dependent on light (Zhou et al. 2014). This study aimed to investigate the molecular mechanism by which OsPIL15 delays the rice heading date. We found that the overexpression of OsPIL15 delayed rice heading under natural long-day (LD) conditions. Molecular evidence showed that OsPIL15 can bind to the promoter region of OsLF, which is reported to repress flowering and upregulate OsLF, thus inducing a delayed heading date. Moreover, the delayed heading date induced by OsPIL15 was observed to be dependent on the functional phyB.
2 Materials and Methods
2.1 Plant Materials and Growth Conditions
The phyB mutant (Takano et al. 2005), OsPIL15-overexpressing (OsPIL15-OX) transgenic lines (OsPIL15-OX and OsPIL15-enhanced green fluorescent protein-OX (OsPIL15-eGFP-OX)) (Zhou et al. 2014), OsPIL15-knockout (OsPIL15-KO) lines (Sun et al. 2012), and atypical HLH gene OsLF-overexpressing (OsLF-OX) transgenic lines (Zhao et al. 2011) have been described in previous studies. In this study, all the rice plants were in an O. sativa L. cv. Nipponbare background, excluding the OsLF-OX transgenic lines, which were in a Zhonghua 11 (O. sativa L. subsp. japonica) background.
Seeds were sown in mid-May, and the seedlings were transplanted to a paddy field in Jinan, China (lat 36°40′N, long 117°00′E) in late June with a planting density of 20 cm × 25 cm. The plants were cultivated under standard field management practices.
2.2 Generation of Transgenic Rice Plants
The generation of OsLF-KO lines was carried out by Biogle (Hangzhou, Zhejiang Province, China) using CRISPR/Cas9. The single-guide RNA (sgRNA) sequence was 5′-TGCCAGCCGAGCTGCGGCCCCGG-3′. The designed sgRNA was cloned into the BGK03 vector containing the Cas9 gene. The construct was transformed into Nipponbare rice plants using Agrobacterium-mediated transformation, as described previously (Toki et al. 2006).
OsLF-KO lines with an OsPIL15-OX background (oslf/OsPIL15-OX) were constructed by crossing OsLF-KO homozygous lines (#6 and #9, Supporting Information S1: Figure S6a) with OsPIL15-OX lines (#13, #7, and #16). Among of the oslf/OsPIL15-OX lines, #13 was homozygous by crossing OsLF-KO-#6 with OsPIL15-OX-#13; #7 was homozygous by crossing OsLF-KO-#6 with OsPIL15-OX-#7; #16 was homozygous by crossing OsLF-KO-#9 with OsPIL15-OX-#16.
To analyse the relationship between OsPIL15 and phyB, we constructed the OsPIL15-∆APB expression vector, which was similar to the OsPIL15-OX vector but lacks the active phytochrome B binding (APB) motif (sequence: TCCGACGGCAACGACTTCGCCGAGCTGCTGTGGGAGAACGGCCAGGCGGTGGTGCACGGGAGGAAGAAGCACCCG) (Nakamura et al. 2007). Using the full-length OsPIL15 cDNA as a template, the ORF region of OsPIL15-∆APB was PCR-amplified. The sequences of the primer pair used for the amplification and the introduction of the restriction sites are as follows: 5′-AACTAGTATGCAGCCGGCCTTCCCGCCGTTC-3′ (SpeI site underlined) and 5′-ATGGTCACCTTATGTTTCAGCCCCATCTC-3′ (BstEII site underlined). The PCR product was digested using SpeI and BstEII restriction enzymes, followed by subcloning into the p1390-Ubi vector (Li et al. 2011). The insertion was placed between the maize ubiquitin promoter and the nos terminator. The plasmid was introduced into the Agrobacterium tumefaciens strain EHA105 (Hood et al. 1993) via electroporation. Rice (O. sativa L. cv. Nipponbare) was transformed using the agroinfection method described by Hiei et al. (1994).
OsPIL15-OX transgenic lines in a phyB background were generated by transforming the expression vectors of OsPIL15-OX into phyB via agroinfection methods (Hiei et al. 1994).
2.3 Heading Date Statistics
Heading dates were recorded daily from sowing to the first panicle appearance of individual plants. The number of days to heading was averaged over 30 plants to determine the phenotype of heading date.
2.4 Quantitative Real-Time (qRT)-PCR Analysis
Total RNA was isolated from rice plants using RNAiso (Takara, Dalian, China). qRT-PCR analysis was performed using SYBR Premix Ex Taq on an ABI 7500 Fast Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). The relative expression level of each target gene was quantified (Jain et al. 2006), with the rice OsEF-1a gene (AK061464) as an internal control. Three biological experiments were performed. Details of the primers used in this assay are listed in Supporting Information S1: Table S1.
2.5 Transcriptome Sequencing Analysis
The latest expansion leaves of Nipponbare and OsPIL15-OX line (#7) were sampled at 10 AM and 10 PM, respectively. Six leaves of individual plants were pooled as one biological replicate and frozen immediately in liquid nitrogen, with three biological replicates from each treatment group.
The RNA-SEQ experiments were conducted by Novogene Bioinformatics Institute (Beijing, China). Differential expression analysis was performed using the DESeq R package controlling the false discovery rate. Genes with an adjusted p-value (using the Benjamini and Hochberg's approach) < 0.05 found by DESeq were assigned as differentially expressed genes (DEGs).
2.6 Chromatin Immunoprecipitation (ChiP) and qRT-PCR (ChIP–qPCR) Analysis
ChIP–qPCR was performed using OsPIL15-eGFP transgenic plants as described previously (Sun et al. 2020). The details of the primers used in this assay are listed in Supporting Information S1: Table S1.
2.7 Dual-Luciferase Reporter Assay
Full-length cDNA of OsPIL15 was cloned into pGreen II 0029 62-SK vector (SK; Hellens et al. 2005) as the effector. Wild-type or mutant promoter regions of OsLF with a fragment length of approximately 2k were inserted into pGreen II 0800-LUC vector (LUC; Hellens et al. 2005) as the reporter, respectively. The mutant promoter sequences were obtained through artificial synthesis (Songon Biotechnology, Co. Ltd.) The mutant sequences are listed in Supporting Information S1: Table S1. Different combinations of constructs were co-transformed in rice protoplasts mediated by PEG, as described previously (Bart et al. 2006). Briefly, 2-week-old seedlings are cut into 0.5 mm pieces and digested in the cellulase and macerozyme solution to isolate rice protoplasts. For transformation, 40% PEG was added to the protoplasts containing combinations of vector constructs and incubated at 28°C in the dark overnight. Firefly luciferase and Renilla luciferase activities were detected using a Promega GloMax96 instrument and the Dual-Luciferase Reporter Assay System (Promega). Dual-luciferase assays were performed with five biological replicates, and the ratio of LUC activities (firefly LUC/Renilla LUC) was calculated to normalise each assay.
2.8 Electrophoretic Mobility Shift Assay (EMSA)
EMSA was performed as described previously (Ma et al. 2009). The His-tagged fusion proteins with OsPIL15 were purified as described previously (Sun et al. 2020). Using a Biotin 30 End DNA Labelling Kit (Beyotime, Shanghai, China), the E-box, N-box, and PBE-box oligonucleotide sequences were synthesised and biotin-labelled individually. DNA probes were obtained by annealing two complementary oligonucleotides using an EMSA kit (Beyotime). The specific details of the primers used in this assay are provided in Supporting Information S1: Table S1.
3 Results
3.1 OsPIL15 Delayed the Rice Heading Date Under Natural LD Conditions
Here, we investigated the effect of OsPIL15 on the heading date. OsPIL15-OX lines had a delayed heading date by approximately 5–7 days in a paddy field under natural LD conditions (Figure 1a,b, Supporting Information S1: Figure S1a).
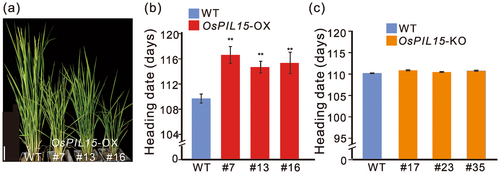
We constructed OsPIL15-KO lines using the CRISPR/Cas9-system. However, no significant difference in heading date was observed between the OsPIL15-KO lines and Nipponbare (Figure 1c, Supporting Information S1: Figure S1b,c), suggesting that some factors had redundant functions with OsPIL15 in regulating the rice heading date.
3.2 OsPIL15 Upregulated Hd1 and Downregulated Hd3a and RFT1
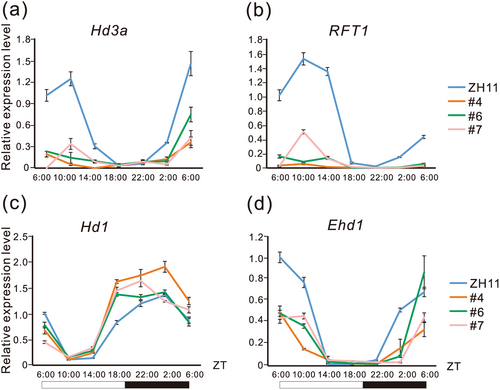
To elucidate the molecular mechanism underlying heading date regulation by OsPIL15, we analysed the expression patterns of essential genes involved in the flowering pathway. Hd3a and RFT1 were downregulated significantly in OsPIL15-OX lines compared to the wild type (WT) plants (Figure 2a,b). Meanwhile, Hd1 was upregulated significantly in OsPIL15-OX lines compared to in the WT (Figure 2c), whereas no apparent difference in the transcription level of Ehd1 was found between the two (Figure 2d). These results indicate that OsPIL15 delayed the heading date by upregulating Hd1 and downregulating Hd3a and RFT1.
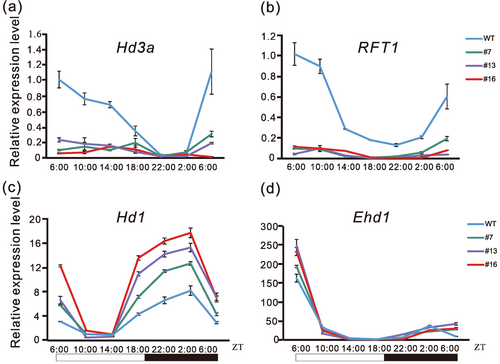
As Hd1, which is involved in the conserved circadian clock-dependent flowering pathway, was upregulated in OsPIL15-OX lines, we determined whether OsPIL15 is involved in the pathway. The transcription levels of rice circadian regulators LATE ELONGATED HYPOCOTYL (OsLHY) and PSEUDORESPONSE REGULATOR1 (OsPRR1) in the OsPIL15-OX lines were analysed. Interestingly, the levels of both were not significantly different between the WT and OsPIL15-OX lines (Supporting Information S1: Figure S2). This suggests that OsPIL15 regulates the heading date via a circadian clock-independent flowering pathway.
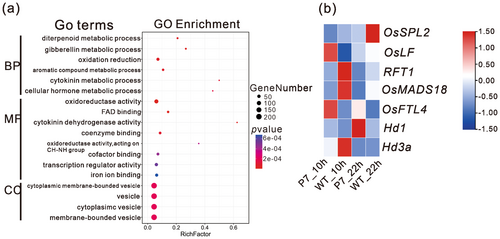
To explore the molecular mechanism via which heading date is regulated by OsPIL15, we analysed of transcriptomes of WT and OsPIL15-OX. As the expression of genes-related heading date mostly exhibit diurnal rhythms, we sampled the leaves of 60-day-old plants at 10 AM and 10 PM under natural long day conditions, respectively. Correlation analysis demonstrated that the Pearson correlation coefficient between replicates in WT and OsPIL15-OX plants was > 0.88, indicating our RNA-seq data had high confidence (Supporting Information S1: Figure S3a). Genes with a padj value < 0.05 and fold change ≥ 2 or fold change ≤ 0.5 between WT and OsPIL15-OX plants were identified as DEGs. We identified 922 DEGs, out of which 441 were upregulated and 481 were downregulated in OsPIL15-OX plants sampled at 10 AM (Supporting Information S1: Figure S3b, Supporting Information S2: Table S2). Four hundred and five DEGs were identified, out of which 233 were upregulated and 172 were downregulated in OsPIL15-OX plants sampled at 10 PM (Supporting Information S1: Figure S3c, Supporting Information S2: Table S2). Eighty-three DEGs were overlapping at both time points (Supporting Information S1: Figure S3d). Further GO analysis demonstrated that all DEGs (1245) were enriched mainly in pathways related to diterpenoid metabolic process, oxidation reduction, cytokinin metabolic processing, coenzyme binding, transcription regulator activity, vesicle (Figure 3a). However, no GO terms were enriched for overlapping genes (data not shown). Among the DEGs, at least at one time point, seven genes were reported to regulate rice heading date, including florigen genes (RFT1 and Hd3a) (Figure 3b). Besides, bHLH family had the most members, accounting for 6.9% of the DEGs (Supporting Information S2: Table S2). The above results indicate that transcription factors and hormone might be involved in the effects on heading date mediated by OsPIL15.
3.3 OsPIL15 Activated OsLF Expression by Directly Binding to Its Promoter
We determined whether the DEGs are involved in delaying heading date by OsPIL15 via ChIP using OsPIL15-eGFP seedlings. The heading date of OsPIL15-eGFP was similar to that of OsPIL15-OX, significantly later than that of Nipponbare (Supporting Information S1: Figure S4). The results suggest that OsPIL15-eGFP is functional. Among the direct target genes regulated by OsPIL15, OsLF, an atypical HLH transcription factor, was selected for further investigation in the present study, because it was upregulated in OsPIL15-OX lines when compared with that in Nipponbare in transcriptome profiling (Figure 3d). To confirm whether OsPIL15 directly binds to the promoter of OsLF, ChIP-qRT-PCR was performed with OsPIL15-eGFP lines. Previous studies have reported that bHLH family members can bind E-, N-, and PBE-box (Murre, McCaw, and Baltimore 1989; Atchley and Fitch 1997; Atchley, Terhalle, and Dress 1999; Li et al. 2006; Pires and Dolan 2009; Carretero-Paulet et al. 2010; Ji et al. 2019). Thus, fragments A, B, C, and D, each harbouring two or three E/N/PBE-box motifs, were subjected to enrichment analysis in the 2 kb sequence upstream of the OsLF promoter. Fragment M, positioned 1394 bp upstream of the OsLF transcription start site, was used as a negative control (Figure 4a). As shown in Figure 4b, compared with the negative control fragment M, fragments A, B, C, and D were enriched in the OsPIL15-eGFP plants. To determine whether OsPIL15 directly binds to the OsLF promoter through the E-box, PBE-box, or N-box elements, an EMSA was conducted using the purified OsPIL15-His recombinant protein and OsLF probes containing both normal and mutated E-box, PBE-box, and N-box elements (Supporting Information S1: Table S1). The results showed that OsPIL15 specifically bound to the OsLF promoter in all four fragments with normal E-box, PBE-box, and N-box sequences (Figure 4c). In addition, in the presence of unlabelled competitor probes A, B, C, and D, the binding efficiencies of the four fragments decreased significantly, while there was no decrease in binding with unlabelled probes containing mutated E-box, PBE-box, and N-box sequences (Figure 4c).

Subsequently, transient expression assays were conducted using a Dual-luciferase reporter system to confirm the binding results in vivo. p35S:OsPIL15 effector and pOsLF:LUC or pOsLF-mut:LUC reporter were constructed in to pGreen II 0029 62-SK and pGreen II 0800-LUC vector, respectively (Figure 4d). Rice protoplast were transfected with A. tumefaciens strains containing the p35S:OsPIL15 effector and pOsLF:LUC or pOsLF-mut:LUC reporter plasmids. Co-expression of pOsLF:LUC reporter with p35S:OsPIL15 effector led to significant enhancement of LUC reporter activity, when comparing with that in the empty vector control (Figure 4e). In addition, the pOsLF-mut:LUC reporter was constructed with mutant of the E-, N-, and PBE-box to confirm whether the binding elements were indispensable. As expected, cotransformation of pOsLF-mut:LUC and p35S:OsPIL15 resulted in a notable decrease in LUC activity, when compared with pOsLF-:LUC and p35S:OsPIL15 (Figure 4e). Moreover, OsPIL15 lost its activity to enhance the transactivation activity of mutant of OsLF (Figure 4e). Furthermore, the expression level of OsLF in OsPIL15-OX transgenic lines was measured. As shown in Figure 4f, OsLF was upregulated significantly in OsPIL15-OX transgenic lines. Overall, the results demonstrate that OsPIL15 can activate OsLF expression in vivo by directly binding to the promoter E-box, PBE-box, and N-box elements.
3.4 OsLF Delayed the Rice Heading Date by Downregulating Hd3a and RFT1
OsLF delays flowering under SD and LD conditions (Zhao et al. 2011) and can directly regulate Hd1 expression by binding to its promoter (Wang et al. 2013). OsLF might repress OsGI and Hd1 to delay flowering under SD conditions (Zhao et al. 2011); however, the molecular mechanism by which OsLF delays the heading date under LD conditions remains unknown. Firstly, we confirmed the delayed heading date of OsLF under the natural long day conditions in Jinan, Shandong province (Supporting Information S1: Figure S5). Subsequently, we analysed the expression patterns of essential genes involved in the photoperiodic flowering pathway in OsLF-OX transgenic lines. As shown in Figure 5, Hd3a and RFT1 were downregulated significantly in OsLF-OX lines compared with in the WT (ZH11) (Figure 5a,b). In addition, Hd1 and Ehd1, which are hub factors in the photoperiodic flowering pathway, were upregulated and downregulated significantly, respectively, in the OsLF-OX lines compared to ZH11 (Figure 5c,d).
Furthermore, OsLF-KO transgenic lines were constructed using the CRISPR/Cas9 system. However, no significant difference in heading date was observed between OsLF-KO and ZH11 plants in the paddy fields (Supporting Information S1: Figure S6).
3.5 OsPIL15 Delayed the Heading Date Partially via OsLF
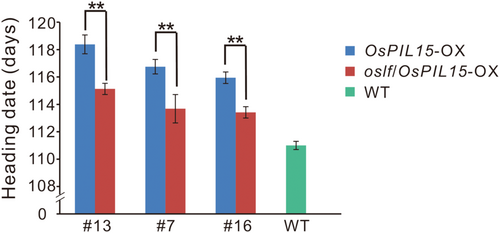
To examine the genetic relationship between OsPIL15 and OsLF in the regulation of heading date under LD conditions, OsLF-KO lines were constructed in an OsPIL15-OX background and a homozygous mutant of OsLF in an OsPIL15-OX background (oslf/OsPIL15-OX) was obtained. Under natural LD conditions, the heading date of oslf/OsPIL15-OX (#13, #7, #16) was significantly earlier than that of OsPIL15-OX, by approximately 3 days, but was still significantly later than that of the WT (p value is 2.22869E−10, 0.016, and 0.013, respectively), by approximately 3–5 days (Figure 6). This result indicates that OsPIL15 partially delayed the heading date via OsLF.
Based on the above results, we speculated the mechanism by which OsPIL15 regulates the heading date under LD conditions in rice. OsLF delays rice heading by downregulating Ehd1 and upregulating Hd1, thereby downregulating Hd3a and RFT1. OsPIL15 directly binds to the promoter of OsLF and activates its expression, leading to a delayed heading date.
3.6 OsPIL15-Mediated Delay in the Rice Heading Date is Dependent on phyB
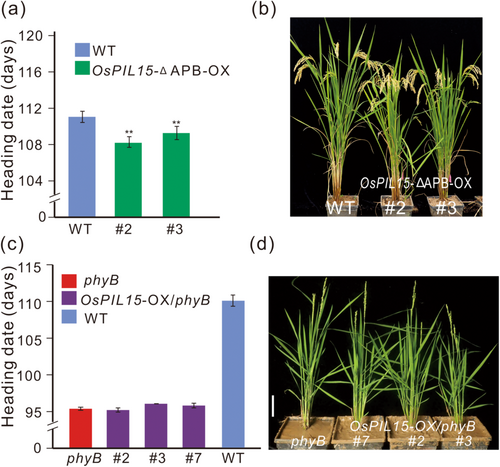
The interaction between PIFs and phyB requires the presence of the APB motif in Arabidopsis (Khanna et al. 2004). As OsPIL15 belongs to the bHLH subfamily, which contains the APB motif and bHLH domains (Nakamura et al. 2007), we determined whether phyB is involved in the OsPIL15-mediated delay in the rice heading date. A protein-protein interaction assay with OsPIL15/OsPIL15-∆APB (OsPIL15 lacking the APB motif) and PHYB using a yeast two-hybrid system revealed that OsPIL15 could interact with PHYB whereas OsPIL15-∆APB could not (Supporting Information S1: Figure S7). This indicates that the APB motif is indispensable for the interaction between OsPIL15 and PHYB. We obtained OsPIL15-∆APB-OX lines and found that they had an earlier heading date, by approximately 2 days, compared with Nipponbare (Figure 7a,b, Supporting Information S1: Figure S8a), suggesting that the APB motif is required for the OsPIL15-mediated delay in the rice heading date. To elucidate the role of phyB in regulation of the heading date by OsPIL15, we constructed OsPIL15-OX transgenic lines in a phyB mutant background (OsPIL15-OX/phyB) and examined the heading date under natural LD conditions. As expected, the heading date of OsPIL15-OX/phyB lines did not significantly differ from that of phyB (Figure 7c,d, Supporting Information S1: Figure S8b,c). These results indicate that the OsPIL15-mediated delay in rice heading is dependent on the functional phyB. Thus, we speculated on the working model of OsPIL15-regulated heading date under LD conditions in rice, as shown in Figure 8.

4 Discussion
Rice heading date is a key factor determining rice production and regional adaptability. In the present study, we analysed the role of OsPIL15 in delaying rice heading date under LD conditions. Overexpression of OsPIL15 delayed rice heading, partially by directly activating OsLF expression to repress Hd3a and RFT1. OsPIL15-OX lines delayed rice heading by upregulating Hd1 and inhibiting the expression of Hd3a and RFT1.
4.1 Putative Functional Redundant Factors With OsPIL15 in the Regulation of Rice Heading Date Are Present
Although OsPIL15-OX exhibited a delayed heading date under natural conditions (Figure 1a,b), no significant difference in heading date was found between OsPIL15-KO lines and Nipponbare (Figure 1c, Supporting Information S1: Figure S1b), suggesting that some factors have redundant functions with OsPIL15 in regulating the rice heading date. Members of the same family have similar biological functions in the regulation of plant growth. There are six PILs in the rice genome, named OsPIL11 to OsPIL16 (Nakamura et al. 2007). Based on amino acid sequence alignment, they can be clustered into three groups: OsPIL11 and OsPIL12 cluster together, OsPIL13 and OsPIL14 cluster together, and OsPIL15 and OsPIL16 cluster together. This is consistent with previous clustering analysis results of PILs in rice and Arabidopsis (Nakamura et al. 2007). Thus, we speculated whether OsPIL16 also had the function in regulating rice heading. Subsequently, we analysed the heading date of OsPIL16-OX transgenic lines and Nipponbare under natural long-day conditions. OsPIL16-OX delayed the heading date significantly when compared with that of the WT (Supporting Information S1: Figure S9). The results suggest that OsPIL15 has redundant functions in regulating rice heading with OsPIL16. Moreover, OsPIL16 was upregulated in OsPIL15-KO lines (Supporting Information S1: Figure S10). Thus, OsPIL15 and OsPIL16 may have a complex relationship, besides the redundant functions in regulating the heading date, which warrants further investigation.
4.2 OsLF Was not the Sole Target of OsPIL15 in the Regulation of Heading Date
OsLF has been reported to repress heading date under LD and SD conditions (Zhao et al. 2011). In the present study, OsPIL15 could bind to the promoter of OsLF to activate its expression in OsPIL15-OX lines (Figure 4), indicating that OsPIL15 might delay rice heading via OsLF. However, knockout of OsLF in OsPIL15-OX plants resulted in a heading date that was 3 days earlier than that of OsPIL15-OX but still later than that of WT, by approximately 3–5 days (Figure 6). Moreover, as shown in Figure 2, OsPIL15 did not affect the Ehd1-dependent flowering pathway; however, Ehd1 was downregulated in OsLF-OX lines (Figure 5d). These results indicate that other factors, in addition to OsLF, are involved in the OsPIL15-mediated regulation of the heading date.
4.3 OsPIL15 Did Not Affect the Circadian Clock Pathway
The endogenous circadian clock in higher plants coordinates daily external environmental changes with internal metabolic and energy processes, contributing to their fitness (Wei et al. 2020). In Arabidopsis, the central circadian clock oscillator consists of multiple interlocked transcriptional feedback loops, including the transcription factors CIRCADIAN CLOCK ASSOCIATED1 (CCA1), LHY, and their reciprocal regulator TIMING OF CAB EXPRESSION1 (TOC1or PRR1) (Wang et al. 2020). However, how the circadian clock plays a role in the photoperiodic flowering of rice remains unclear (Sun et al. 2021). OsGI-Hd1-Hd3a/RFT1, the circadian clock-dependent flowering pathway, is conserved in Arabidopsis and rice, and Hd1 was upregulated in OsPIL15-OX lines; OsLF is the target gene of OsPIL15, and the OsLF protein might interfere with the function of the OsPRR1 protein by competing with OsPIL13 and OsPIL15, leading to a late flowering phenotype (Zhao et al. 2011). Thus, we examined whether OsPIL15 is involved in the circadian clock-dependent flowering pathway. The transcription levels of the rice circadian regulators, OsLHY and OsPRR1, which are homologous to Arabidopsis CCA1/LHY and TOC1, respectively (Wang et al. 2020), were analysed in the OsPIL15-OX lines. Notably, the expression levels of both did not differ between the WT and OsPIL15-OX lines (Supporting Information S1: Figure S2), suggesting that OsPIL15 regulates the heading date via a circadian clock-independent flowering pathway. Moreover, the transcriptomic results of OsPIL15-OX and WT indicated no circadian clock-related DEGs were identified (Supporting Information S2: Table S2). These results indicates that a complex network is involved in the OsPIL15-mediated flowering pathway.
4.4 Both APB Motif and phyB Are Necessary for Regulation of Heading Date in Rice by OsPIL15
We found that OsPIL15-OX lines had a delayed heading date by approximately 5–7 days under natural LD conditions (Figure 1a,b), whereas OsPIL15-∆APB-OX lines had an earlier heading date by approximately 2 days when compared with Nipponbare (Figure 7a,b). This suggests that the APB motif is indispensable for OsPIL15-mediated delay in the rice heading date. In contrast, overexpression of OsPIL15 in a phyB-deficient background did not affect heading date (Figure 7c,d). These results suggest that both APB motif and phyB are necessary for OsPIL15-mediated regulation of heading date.
Rice OsPILs belong to the bHLH subfamily and contain a DNA-binding motif at the N-terminus of the bHLH domain. By binding to the G-box (CACGTG) and/or E-box (CANNTG) motifs in their promoters, PIFs directly regulate the expression of downstream genes (Chaudhary and Skinner 1999; Moon et al. 2008; Zhang et al. 2022). In the present study, we confirmed that OsPIL15 binds to the E-, N-, and PBE-box in the OsLF promoter (Figure 4). Moreover, OsPIL15 has potential transcriptional activation activity, with an active site located at 1-380 amino acids in the N-terminus (Supporting Information S1: Figure S11). Furthermore, the APB motif is required for the interaction between OsPIL15 and PHYB (Supporting Information S1: Figure S7). Thus, the APB motif might affect the role of OsPIL15 in regulating the rice heading date, either by interacting with phyB or by reducing the activating ability of OsPIL15.
In summary, we speculate the working model of OsPIL15-mediated delay in rice heading date under natural long-day conditions. In wild type, OsPIL15 can interact with PHYB depending on the APB motif. Overexpression of OsPIL15 can delay the rice heading date under natural long day conditions partially via directly binding to the E-box, N-box, or PEB-box of the OsLF promoter and activate its transcription. OsLF can directly bind to the promoter of Hd1 (Wang et al. 2013) to upregulate its expression, and then inhibit the expression of Hd3a and RFT1, thus inducing a delayed heading date. Additionally, OsLF also can slightly downregulate the expression level of Ehd1 under natural long-day conditions. However, overexpression of OsPIL15-∆APB promotes heading date under natural long-day conditions in wild type, indicating APB motif is required for the role of OsPIL15 in delaying heading date. As APB motif locates in the transcription activation domain of OsPIL15, loss-of APB motif might reduce the transcription activation ability of OsPIL15, thus leading to the earlier heading date in OsPIL15-∆APB-OX lines. Besides, as the domain necessary for interacting with PHYB, APB motif might affect the OsPIL15 functions dependent on PHYB. All possibilities are waiting for being validated through experiments.
Acknowledgements
We thank Dr. Zhenying Shi (Institute of Plant Physiology and Ecology, the Chinese Academy of Science) for providing OsLF-OX seeds. We also thank Wiley Editing Services (https://wileyeditingservices.com/en/article-preparation/english-language-editing) for editing of the English text of a draft of this manuscript. This work was supported by grants from National Natural Science Foundation of China (Youth Program, 31700251; General Program, 32070216), Natural Science Foundation of Shandong Province of China (General Program, ZR2022MC180 and ZR2021MC057), National Natural Science Foundation of China (Youth Program, 32301857).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.