Revisiting Endoplasmic Reticulum Homeostasis, an Expanding Frontier Between Host Plants and Pathogens
ABSTRACT
The endoplasmic reticulum (ER) serves as the primary site for protein biosynthesis and processing, with ER homeostasis being essential for the survival of plant cells. Numerous studies have underscored the pivotal role of the ER as a battleground for host–pathogen interactions. Pathogens secrete effectors to subvert the host ER and manipulate ER-mediated defense responses, fostering an infection-permissive environment for their proliferation. Plants respond to these challenges by triggering ER stress responses, including the unfolded protein response (UPR), autophagy, and cell death pathways, to combat pathogens and ensure survival. Consequently, plants are faced with a life-or-death decision, directly influencing the outcomes of pathogen infection. In this review, recent advances in manipulating host ER homeostasis by pathogens are introduced, further key counteracting strategies employed by host plants to maintain ER homeostasis during infection are summarized, and finally, several pending questions the studies involving both parties in this evolving field are proposed.
1 Introduction
The endoplasmic reticulum (ER), a primary membrane system in plant cells, serves as the hub for protein synthesis, folding, and modification (Zeng et al. 2019). To ensure the accurate configuration and localization of proteins, plants employ an intricate ER quality control (ERQC) system to monitor protein folding (Howell 2013). Abiotic and biotic stressors can disrupt normal protein folding, resulting in ER stress and the accumulation of unfolded or misfolded proteins in the ER lumen (Guo et al. 2022). Thus, ER homeostasis is essential for maintaining normal life activities regarding the choice of survival or death, during which ER stress triggers a series of downstream responses. Transient or mild ER stress can be relieved by unfolded protein responses (UPRs), and those proteins that fail to fold properly may be degraded via an ER-associated degradation (ERAD) system. In contrast, prolonged or severe ER stress may ultimately trigger cell death (Howell 2013).
Recent studies have shown that ER is an important site for plant–pathogen interactions (Jing et al. 2016). Pathogen invasion often induces ER stress and promotes UPR activation by secreting effectors to suppress plant immunity. The UPRs have crucial roles in restoring ER homeostasis by triggering transcriptional reprogramming led by membrane-tethered transcription factors (TFs), accompanied by the activation of autophagic activity and ER stress-associated degradation. Otherwise, when ER stress remains unresolved, certain mechanisms that induce cell death may be initiated, which is potentially beneficial or detrimental to pathogens, depending on the timing of cell death and specific trophic specialization of pathogens. This review will outline a scenario encompassing several important aspects involved in plant–pathogen interaction in the ER, and explore some gaps in understanding the machinery in this specific battlefield further.
2 Pathogens Secrete Effectors to Manipulate the Host ER as Their Infection Target
Depending on whether they obtain nutrients from dead tissues, phytopathogens can be divided into three categories: biotrophic, hemibiotrophic, and necrotrophic pathogens (Fei and Liu 2023). Biotrophs depend entirely on living host cells for growth and reproduction and survive within the interstitial space between the cells (Fei and Liu 2023). Here, two common biotrophic pathogens are introduced. Blumeria hordei often colonizes economically important cereal crops, such as wheat and barley (Li et al. 2021), while, Bremia lactucae, a biotrophic oomycete, poses a major threat to lettuce (Meisrimler et al. 2019). Hemibiotrophs, including some oomycetes, fungi and bacteria, initially obtain nutrients from viable host tissues and then kill host cells in the later stages of the infection (Jing and Wang 2020). Phytophthora sp. are the best-studied oomycetes, such as P. capsici, P. parasitica, P. infestans, and P. sojae, that cause many destructive diseases in plants (Meng et al. 2014). P. capsici and P. parasitica have broad host ranges, including many agricultural crops, fruit trees, and ornamental plants (Quesada-Ocampo et al. 2023; Wang et al. 2024a), while P. parasitica is the main cause of tobacco black shank disease (Wang et al. 2024a). As the most well-investigated Phytophthora, P. infestans can cause potato and tomato late blight (Lin et al. 2022), while P. sojae infects only soybeans (Meng et al. 2014). Colletotrichum truncatum is an ascomycete fungus that causes anthracnose in pulse crops (Bhadauria et al. 2013), yams (Wang et al. 2024b), and Iris lacteal (Wang et al. 2022). Perhaps the most extensively investigated, Pseudomonas syringae, which infects many crop and fruit species, is a model for understanding bacterial pathogenicity, (Shao et al. 2021). In contrast, necrotrophs rely on absorbing nutrients from dead cells to accomplish infection (Bi et al. 2023; Chen et al. 2023). Botrytis cinerea is a necrotrophic pathogen (Chen et al. 2023) mainly depending on air flow dispersal of its conidia to cause gray mold decay in many crops, such as tomatoes, grapes, and beans. The rich toolbox of B. cinerea pathogenicity means that no plant shows complete resistance to B. cinerea (Bi et al. 2023). In addition to pathogens, pests also secrete effectors causing ER stress in plants. Meloidogyne incognita is an obligate biotrophic parasite that feeds on the roots of many crops and weeds, and it is also the most widespread and serious plant-parasitic nematode pest (Jaouannet et al. 2013). During infection by these pathogens or pests, their interaction with host plants largely relies on the secreted effectors or virulence proteins (Liang, Bi, and Sharon 2023a). These effectors may target interacting host proteins with different subcellular localization; here, the focus is on the effector factors targeting the ER.
2.1 Pathogens Subvert Molecular Chaperone Functions to Interfere With ER Stress-Mediated Cell Death
Protein folding in the ER lumen is always accomplished via two well-investigated pathways, depending on whether they carry glycosylation sites: (1) the polypeptides carrying glycosylation sites are glycosylated by a multi-subunit oligosaccharide transferase and thus are involved in the calnexin/calreticulin (CNX/CRT) protein-folding cycle; (2) the other protein folding pathway primarily involves the lumen-binding immunoglobulin protein (BiP) and other molecular chaperones to form a large multiprotein complex (Howell 2013). In both folding processes, various enzymes and molecular chaperones facilitate protein folding, such as peptidyl-prolyl cis-trans isomerase (PPIase), Bip, CNX/CRT, glucose-regulated protein 94, and ER oxidoreductase (Fan et al. 2018). Increasing evidence has revealed that effectors may bind to the molecular chaperones responsible for protein folding and contribute directly to ER stress (Figure 1).
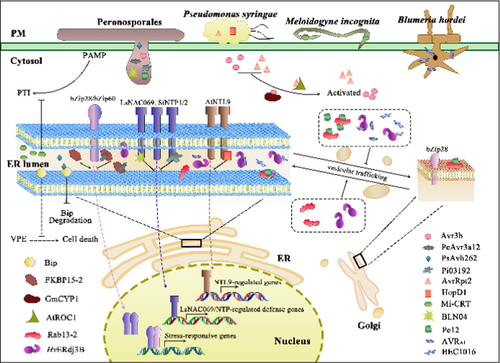
PPIases catalyze the cis-trans isomerization of proline peptide bonds, functioning as a rate-limiting step in protein folding. Comprising cyclophilins (CYPs), FK506-binding proteins (FKBPs), and parvulins, these enzymes are targeted by pathogens for their potential to block host enzyme activity and advance infection (Fan et al. 2018). In the early stage, following P. capsici infection, FKBP15-2 was a host target of PcAvr3a12, as PcAvr3a12 suppressed plant immunity by inhibiting the PPIase activity of FKBP15-2 (Fan et al. 2018). Nevertheless, some PPIases could stimulate the activity of effectors and thus promote pathogen infection. During P. syringae and P. sojae infections, the PPIase activities of cyclophilin AtROC1 (Rotamase CYP1) and GmCYP1 can enhance the activity of AvrRpt2 and Avr3b, respectively (Coaker, Falick, and Staskawicz 2005; Kong et al. 2015). AvrRpt2, an inactive cysteine protease, interacted directly with AtROC, resulting in its N-terminus cleavage by the PPIase activity of AtROC (Jin et al. 2003). Consequently, the activated protease AvrRpt2 subsequently cleaved RPM1 interacting protein 4 (RIN4), thus indirectly activating resistance to P. syringae 2 (RPS2)-guarded resistance (Coaker, Falick, and Staskawicz 2005). Similarly, the nudix hydrolase activity of Avr3b was required for the PPIase activity of GmCYP1 to produce an active virulence protein, while silencing of GmCYP1 attenuated cell death induced by Avr3b (Kong et al. 2015). C. truncatum exclusively secreted the effector CtNUDIX during the late biotrophic phase before necrotrophy, while the transiently expressed CtNUDIX elicited a hypersensitive response (HR)-like cell death in Nicotiana benthamiana leaves (Bhadauria et al. 2013). These data suggest that pathogens employ two distinct strategies to colonize hosts initially by inhibiting host PPIase activities, followed by adopting PPIase activity to activate their own hydrolase activity and promote the rapid expansion of lesion.
BiP is also a molecular target of pathogens. PsAvh262, an RxLR effector from P. sojae, mitigated ER stress-induced programmed cell death (ER-PCD) (Jing et al. 2016). Upon entry into host cells, PsAvh262 bound to the immunoglobulin/albumin-binding domain of host BiP and prevents its degradation, thus increasing the accumulation of BiP in the ER lumen, alleviating ER stress and suppressing ER-PCD. Conversely, PsAvh262 silencing resulted in reduced virulence because of diminished BiP accumulation. Additionally, the overexpression of BiP in soybeans partly restored the sensitivity to the PsAvh262-silenced P. sojae strain (Jing et al. 2016).
Interestingly, certain pathogens and parasitic nematodes can simulate ER functionality to secrete molecular chaperone-like proteins and manipulate ER-associated immune response within host cells. M. incognita is capable of secreting M. incognita-calreticulin (Mi-CRT) to target the host ER and Golgi apparatus, facilitating its successful infection (Jaouannet et al. 2013). The suppression of Mi-CRT impaired the capacity of nematodes to infect plants, while Mi-CRT overexpression in host plants augmented the susceptibility to M. incognita and P. parasitica. Upon secretion into plants, Mi-CRT may function to alleviate ER stress akin to host CRT. Although the pattern-triggered immunity (PTI) associated genes were suppressed and calcium signaling may also be disrupted, the mechanism by which Mi-CRT precisely suppresses defense responses remains elusive. Upon P. capsici infection, Pc12, a virulent RxLR effector, instigated cell death independent of the effector-triggered immunity (ETI) mediated by nucleotide-binding leucine-rich repeat proteins. It was discovered that Pc12 obstructed Rab13-2 recycling and amplified the interaction between Rab13-2 and Rab escort protein (REP) by simulating an inactive state of Rab13-2 (Kim et al. 2023). Given that Rab13-2 plays a pivotal role in intracellular vesicle trafficking, the interaction with Pc12 also stabilized the Rab13-2-REP complex and arrested vesicle formation in the ER-Golgi trafficking pathway. Consequently, the Pc12 targeting caused excessive accumulation of proteins in the ER lumen, resulting in the inevitable demise of the cell when the host was incapable of mitigating ER stress, ultimately triggering the transition of the life cycle of P. capsici from biotrophy to necrotrophy (Kim et al. 2023). These findings elucidated the essential role of Pc12 as a molecular switch for the trophic cycle of P. capsici, further convoluting its infection strategy.
Notably, some effectors may hijack components in the ERQC system. AVRA1 and BEC1016, two effectors secreted by B. hordei, targeted the ERQC component Hc of Hordeum vulgare (Li et al. 2024). The overexpression of these two effectors and silencing of HvERdj3B disturbed normal trafficking of the ER-derived marker protein RFP-AFVY and caused its retention in the ER. Given that HvERdj3B is located in the ER lumen and it has previously been documented that AVRA1 may be recognized by MLA1, these results suggested a probable escaping strategy employed by B. hordei. Intriguingly, when P. infestans and Hpa effectors invariably bear a signal peptide, the delivery of these two effectors to the ER lumen did not depend on their signal peptides, but likely relied on the Sec. 61 translocon complex (Li et al. 2024), further underscoring that pathogens may exploit host membrane trafficking for their own sake.
2.2 Pathogens Target ER-Anchored NAC TFs to Suppress Plant Immunity
Genomic studies have unveiled that P. infestans effectors invariably harbor either or both of an “RxLR motif” and a “DEER motif” (Jones, Staskawicz, and Dangl 2024). Based on protein topology and bioinformatics analysis, it was reported that a conserved C-terminal transmembrane domain (TMD) or tail-anchor domain might be accountable for the targeting of effectors from oomycetes to host ER. In contrast, a majority of these effectors targeted membrane-anchored NAC (NAM, ATAF1/2, CUC2) TFs (Chi et al. 2017; Breeze et al. 2023).
Recent studies have demonstrated that certain effectors may interact with ER-residing NAC-TFs and suppress host defense responses (Figure 1). Type III effectors of P. syringae and RxLR effectors of P. infestans are among the most extensively investigated effectors. The P. syringae type III effector HopD1 targeted AtNTL9 in the ER membrane to suppress ETI and promote pathogenesis (Block et al. 2014). Similarly, P. infestans secreted Pi03192, another RxLR effector, to target the host NAC proteins, namely NAC Targeted by Phytophthora (NTP) 1 and NTP2 (McLellan et al. 2013). The suppression of Pi03192 reduced the pathogenicity of P. infestans on wild-type N. benthamiana plants. In contrast, this pathogenicity was reinstated in the NTP1 or NTP2-silenced plants, attributable to the release of both NAC TFs from the ER membrane upon Pi03192 silencing. Notably, multiple effectors may have synergistic or overlapping actions on identical molecular targets within hosts. B. lactucae is capable of secreting several RxLR effectors, namely BLN04, BLR05, BLR08, and BLR09, to co-opt LsNAC069 and impede its relocation to the nucleus (Meisrimler et al. 2019). The RxLR effectors interacted with LsNAC069, relying on the C-terminal region (including the TMD) rather than the NAC/NAM domain. Predictably, these B. lactucae effectors were also capable of interacting with StNTP1, StNTP2, and certain tail-anchored NACs in Arabidopsis, while LsNAC069 can also interact with the P. infestans effector Pi03192 (Meisrimler et al. 2019). These findings indicate that the interaction between NACs and effectors may be highly conserved and primarily depend on the C-terminal domain of NACs to obstruct stress-induced relocalization to the nucleus. Notably, although it has been reported that some effectors may be internalized into host cells by clathrin-mediated endocytosis (Wang et al. 2023), it remains unclear how these effectors target the ER following their entry when host proteins are synthesized and secreted from the ER following the secretory pathway.
3 ER Stress Responses: The Initiator for a Process of Self-Salvation
Upon the perception of attack, plant cells do not wait for their doom but devise diverse strategies to repel pathogen invasion, among which the ER stress response serves as an important self-salvation way. In this process, various regulatory elements dictate the ultimate fate of host cells.
3.1 Translocation of Membrane-Bound TFs in Response to ER Stress
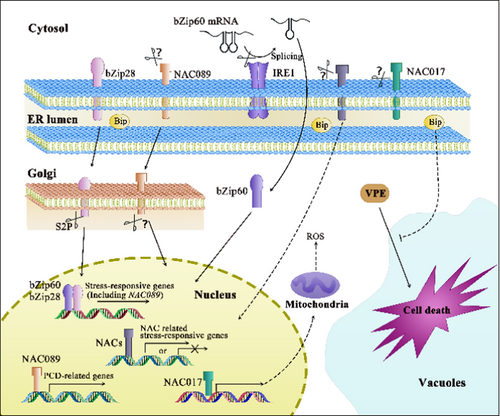
Protein translocation has played a pivotal role in the signaling network since the inception of ER stress (Figure 2). Upon external stimuli at a specific threshold, the UPR is promptly initiated as a conserved protective signaling cascade to mitigate ER stress under adverse conditions. Currently, several lines of evidence have been provided for the UPR in plants: (1) Alternative splicing instigated by inositol-requiring enzyme 1 (IRE1). IRE1 splices the mRNA of basic leucine zipper (bZIP) 60 to generate the activated form, which subsequently transcriptionally upregulates the expression of downstream UPR genes. In addition, the ribonuclease activity of IRE1 necessitated for inducing autophagic activity upon ER stress has been documented (Bao et al. 2018). (2) Release of bZIP17/28, two membrane-associated TFs. Under ER stress, bZIP17/28 are activated and released from the ER membrane and subsequently translocated to the Golgi apparatus. After cleavage by site 2 protease (S2P), the active TF domains are released into the cytosol and ultimately migrate into the nucleus to activate stress-response genes (Howell 2013). bZIP28 is the primary contributor to the upregulation of UPR genes, whereas the roles of bZIP17 in the UPR remain ambiguous and it may share certain functional redundancy with bZIP28 (Kim, Yamaguchi-Shinozaki, and Shinozaki 2018).
Accumulating evidence has shown that there are commonalities and differences in the ER stress responses between animals and plants. In addition to the signaling triggered by XBP1 and ATF6 in animal cells (the homologs in plants, bZIP28 and bZIP60, have been identified), PKR-like ER kinase (PERK) is also a sensor of the UPR that phosphorylates eukaryotic initiation factor 2 alpha (eIF2α), leading to a rapid decrease in the overall protein synthesis rate. Although no PERK homolog has been reported in plants thus far, the Arabidopsis eIF2α homolog was still phosphorylated upon ER stress (Izquierdo et al. 2018), suggesting the possibility for the existence of a PERK-like component or other kinases with similar functions.
In addition to the two conserved routes through the UPR, NAC TFs serve as UPR transducers in plants (Fuchs et al. 2022; Meng et al. 2022). As documented previously, the induced expression of a nucleus-localized NAC in the bZIP28 and bZIP60 double mutant (zip28zip60) notably enhanced ER stress resilience (Yang et al. 2014a). These NAC proteins frequently comprise three segments: the N-terminal NAC domain, the middle regulatory domain and the C-terminal TMD (Yang et al. 2014b). When the translocation of the NAC proteins necessitates removing the TMD, the TF function greatly relies on the NAC, also referred to as the NAM domain (Ai et al. 2021; Yang et al. 2014b). An excellent example was attributed to two potato NAC TFs, StNTP1, and StNTP2, which were released from the ER membrane to the nucleus after treatment with the filtrate (CF) from in vitro cultivated P. infestans. The silencing of NTP1 or NTP2 in N. benthamiana augmented the susceptibility of the host plants to P. infestans. These results collectively indicated that NTPs positively regulated the resistance to Phytophthora spp. (McLellan et al. 2013). Additionally, two ER membrane proteins, NAC017/AtNTL7 and NAC089, have been identified as regulators of ER stress in Arabidopsis. NAC017/AtNTL7 was localized in the contact site between the ER and F-actin under stress conditions. However, it was cleaved by a rhomboid protease at the cleavage site preceding the TMD, and further translocated into the nucleus to induce downstream ER stress-responsive genes. The overexpression of NAC017/AtNTL7 led to enhanced ER stress resistance (Chi et al. 2017). Similarly, NAC089, whose expression is governed by bZIP28 and bZIP60, contributes to the ER stress response through the dissociation from the ER membrane, subsequently regulating the expression of PCD-related genes (Yang et al. 2014b). NAC089 overexpression resulted in cell death after the removal of its TMD, while NAC089 knock-down conferred ER stress tolerance. Ai et al. (2021) reported that treatment with P. capsici CF and flg22 or inoculation with P. syringae pathovar tomato DC3000 could promote the translocation of NAC089 to the nucleus, confirming that NAC089 positively contributed to host resistance against these pathogens as a common mechanism. Identical to the case of NAC017/AtNTL7, the overexpression of NAC062 lacking the TMD also induced the expression of UPR downstream genes, and the upregulation of NAC062 under ER stress conditions depends directly on bZIP60 (Yang et al. 2014a).
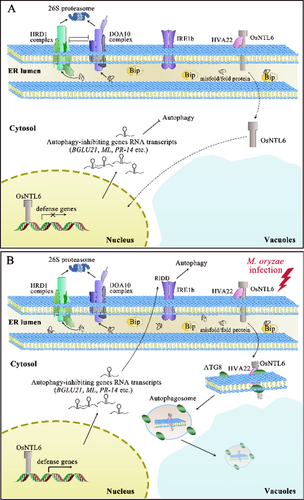
Nevertheless, the roles of NAC proteins may not remain consistent in certain circumstances, contingent upon the precise trophic specialization of pathogens. The silencing of LsNAC069 augmented the resistance of transgenic lettuce to the necrotrophic pathogen P. cichorii rather than the biotrophic oomycete B. lactucae; however, B. lactucae could influence the relocalization of LsNAC069 via secretion of effectors (Meisrimler et al. 2019). This could be elucidated by the compensation of other NAC family proteins demonstrating redundant function to LsNAC069. Furthermore, the relocalization of LsNAC069 required Ser/Cys-protease for proteolytic cleavage (Meisrimler et al. 2019), suggesting that regulation of this hydrolase activity may be pivotal for modulating pathogen–host interactions in this context. Conversely, a similar case reported that OsNTL6 suppressed the immune response and negatively regulated the resistance of rice seedlings to Magnaporthe oryzae (Liang et al. 2023b). A follow-up study pinpointed the function of OsHLP1 (HVA22-like protein 1), an unexplored gene induced by M. oryzae infection, which interacted with OsNTL6 to regulate disease resistance. The overexpression of OsHLP1 compromised resting ER homeostasis and triggered ER-phagy to dismantle OsNTL6, eventually enhancing resistance (Liang et al. 2023b; Meng et al. 2022) (Figure 3). In terms of the ER stress induced by M. oryzae infection, some uncertainties remain. The effectors or elicitors that contribute to the damage of normal ER morphology and activation of resistance are unknown, while the machinery for inducing OsHLP1 overexpression also requires further dissection. Moreover, no protease has been identified to cleave OsNTL6 and activate its translocation. The answers to these questions may inevitably provide more insights into the regulations on ER stress sensors. Moreover, the NAC TFs execute multifaceted roles in plant growth, development and defense responses. They may also influence multiple hormone signaling pathways, while the interplay between ER stress and hormone signaling still requires further dissection.
Studies on ER stress and the UPR in plants lag behind those in animals. Although some UPR components in plants may have conserved roles similar to those in animals, their mechanism of action may be different. For example, as a regulator of cell death in animals, Bax inhibitor 1 (BI-1) interacts with and inhibits IRE1 kinase activity in response to ER stress. At the same time, BI-1 has no inhibitory activity on IRE1 during ER stress in plants (Ishikawa et al. 2011). This may be partly explained by the genome duplication events that occurred independently, resulting in differential contributions from redundant UPR components to stress resilience under respective cellular contexts.
3.2 Degradation Related to ER Stress
As an important biomolecular system following ER stress, ERAD is a principal proteolytic cascade for the efficient elimination of misfolded proteins. It constitutes a cytosol-localized ubiquitin–proteasome system, involving two major complexes: HMG-CoA REDUCTASE DEGRADATION 1 (HRD1) complex and DEGRADATION OF ALPHA2 10 (DOA10) complex, both indispensable for protein homeostasis (Sun et al. 2021). The majority of components within the HRD1 complex and DOA10 complex exhibit ubiquitinase activities, necessitating stringent regulation of the enzymatic activity for optimal functionality of the ERAD system (Liu et al. 2021). Misfolded or unfolded proteins are transported from the ER lumen to the cytosol via these complexes, where they are subsequently degraded by 26S proteasomes (Liu et al. 2021; Sun et al. 2021) (Figure 3).
The HRD1 complex in Arabidopsis involves HRD1, OSTEOSARCOMA9/EMS bri1 SUPPRESSOR6 (OS9/EBS6) (Su et al. 2012), HRD3/EBS5 (Su et al. 2011), DER1 (DEGRADATION IN ER 1) (Qian et al. 2018), EBS7 (Liu et al. 2015), and PAWH1/2 (PROTEIN ASSOCIATED WITH HRD1-1/2) (Lin et al. 2019). HRD1A and HRD1B, two ER membrane-anchored E3 ubiquitin ligases, serve as pivotal components of the Arabidopsis ERAD system (Liu et al. 2015). OS9 is an ER luminal lectin that directly interacts with AtHRD3, subsequently recognizing a unique asparagine-linked glycan on misfolded proteins (Su et al. 2012). Additional elements in this complex may interact with HRD1, thus colocalizing within the ER to regulate the protein stability of HRD1 under ER stress (Lin et al. 2019; Su et al. 2012).
DOA10, EMR (ERAD-mediating RING finger protein) and UBIQUITIN-CONJUGATING ENZYME 32 (UBC32) are key players regulating the function of the DOA10 complex in Arabidopsis (Cui et al. 2012; Park et al. 2018; Sun et al. 2021). EMR localizes to the ER membrane juxtaposed to the cytosol and forms a complex with UBC32. Notably, all other constituents in the DOA10 complex are E3 ubiquitin ligases, with the exception of UBC32. UBC32 is an E2 ubiquitin-conjugating enzyme functioning in the DOA10 complex. Recent studies revealed that UBC32 was a crucial factor in regulating the interplay between the HRD1 complex and the DOA10 complex. Under normal circumstances, the UBC32 abundance was sustained at a relatively low level through proteasome-dependent degradation orchestrated by the HRD1 complex (Chen et al. 2016). Meanwhile, UBC32 negatively regulated the stability of OS9, a component in the HRD1 complex (Chen et al. 2017) (Figure 3A). Conversely, the disruption of negative regulation between these two complexes enhanced the ERAD capacity under ER stress (Figure 3B); therefore, a fine-tuned ubiquitin–proteasome system is crucial for confronting the proteotoxic stress associated with the ER.
Autophagy constitutes a universally conserved cellular process to sequester cytoplasmic contents for subsequent disposal and recycling, while it can also inadvertently promote cell death under certain circumstances (Howell 2013). Autophgy-Related 8 (ATG8) lipidation is important for autophagosome maturation and is frequently employed as a universal indicator of autophagosomes (Zeng et al. 2019); however, the mechanism underlying the autophagic decision between survival and death remains elusive, potentially depending on the severity and persistence of stress. The ER is a vital membrane reservoir and a potential target of autophagy, for which ER-associated regulators have been identified, including IRE1b, nck-associated protein 1, phosphate deficiency response 2 and low phosphate response 1 (Naumann et al. 2019; Wang et al. 2016). IRE1b is indispensable for initiating autophagy upon ER stress; however, it is not the direct instigator of autophagy (Bao et al. 2018). It mediates the connection between ER stress and autophagy by dismantling the RNA transcripts of specific factors that impede autophagy induction. The nucleotide binding and RNase activity of IRE1b, rather than its protein kinase activity or splicing target bZIP60, are essential for ER stress-induced autophagy (Bao et al. 2018). These observations suggest that autophagy mitigates ER stress independent of the UPR, further complicating the regulatory roles of autophagy in ER stress.
ER-phagy is a specialized self-renewal process to eliminate the misfolded proteins or a compromised ER to alleviate ER stress, which is mediated by ATGs and ER-phagy receptors (Liang et al. 2023b; Yang et al. 2021a). AtSec. 62 is colocalized with ATG8 and indispensable for ER-phagy, effective as an ER-phagy receptor under ER stress conditions (Hu et al. 2020). The overexpression of AtSec. 62 augmented the resistance to ER stress, paralleled by increased colocalization with ATG8. Maize reticulon proteins (Rtn) 1 and Rtn2 are also ER membrane proteins that interacted with ATG8a and functioned as ER-phagy receptors. In response to ER stress, the binding of Rtn proteins to ATG8 increased, thus safeguarding aleurone cells against ER stress (Zhang et al. 2020). Nevertheless, the roles of ER-phagy in host-pathogen interaction have only been explored recently. A compelling study reported OsHLP1, a rice ER-phagy receptor, bound to OsATG8b and facilitated ER-phagy to degrade OsNTL6, thus contributing to disease resistance against M. oryzae (Liang et al. 2023b) (Figure 3). AtHVA22J, a homologous protein of OsHLP1 in Arabidopsis, showed a comparable function (Liang et al. 2023b). Thus, the HVA22 family proteins might be a conserved protein family that serve as ER-phagy receptors in higher plants.
3.3 Regulators Contribute to the Decision for Cell Death
When the adaptive capacity cannot restore ER homeostasis, UPR signaling may trigger cell death through certain mechanisms that remain enigmatic in plants. PCD, an intrinsic mechanism governing cellular disruption, perceives and tracks developmental and environmental signals, thus assuming a crucial role in plant development and immunity (Lord and Gunawardena 2012). Based on the accompanying morphological attributes, PCD in plants can be classified into two primary categories: vacuolar cell death and necrosis (van Doorn et al. 2011). As one of the most common examples, HR, a cell death modality exhibiting features of necrosis and vacuolar cell death, plays a critical role in pathogen–host interactions (van Doorn et al. 2011). During pathogen–host interactions, the occurrence of plant cell death is not invariably advantageous to hosts, but depends on whether the pathogens obtain nutrients from dead tissues. Hence, the regulation of cell death in plants is remarkably precise (Figure 2).
The translocation of NAC family proteins from the ER to the nucleus may answer the question of whether the initiation of cell death signaling occurs in the ER, a captivating topic that has garnered substantial interest. Severe ER stress triggers the re-localization of NAC089 from the ER membrane to the Golgi apparatuses and subsequent migration to the nucleus, thus inducing caspase-like activity and the expression of downstream PCD-associated genes, such as the genes encoding metacaspase, BCL2-associated athanogene, NAC094 and WRKY33 (Yang et al. 2014b). Cell death hinges upon a functional NAC089 localized to the nucleus, as the overexpression of truncated NAC089 lacking the TMD also induced PCD (Ai et al. 2021; Yang et al. 2014b). Notably, the expression of NAC089 was regulated by bZIP28 and bZIP60, implying that both pro-survival and pro-death signals are instigated by bZIP28 and bZIP60 under ER stress. These activities also address the importance of crosstalk between signaling in the ER, Golgi apparatus and nucleus under ER stress. Moreover, reactive oxygen species (ROS) contribute significantly to disease resistance and the regulation of cell death, as ROS bursts are frequently observed during cell death (Chen et al. 2021; Zhao et al. 2023). Of paramount importance to ROS production, the appropriate functionality of mitochondria is intrinsically tied to cell death (Lord and Gunawardena 2012). Another ER-localized NAC family protein, NAC017, also migrated from the ER to the nucleus under ER stress, thus orchestrating mitochondrial retrograde signaling in Arabidopsis (Fuchs et al. 2022). Coincidently, the nac017 mutant exhibited reduced ROS levels and impaired mitochondrial performance. In response to hydrogen peroxide (H2O2), soybean GmNTL1 was released from the ER and migrated to the nucleus upon H2O2-mediated posttranslational modification on cysteine, further activating the expression of RESPIRATORY BURST OXIDASE HOMOLOG B (GmRbohB) in return (Zhang et al. 2024a). Consequently, this feed-forward loop facilitated the accumulation of H2O2 and further amplified H2O2 signaling.
During these intricate processes wherein plants regulate cell death, caspase-like proteases assume a crucial role. Some scholars have proposed that the caspases may act as “initiators” and “executors” for triggering cell death (Yang et al. 2021b), albeit no authentic caspase has yet been delineated in plants (Lord and Gunawardena 2012). The vacuolar processing enzyme (VPE) possesses a caspase-1 activity that is instrumental in cell death. VPE-induced cell death depends on its enzymatic activity, whereby it functions to activate diverse vacuolar proteins and hydrolytic enzymes to induce vacuolar collapse (Hatsugai et al. 2004). These vacuolar proteins, including VPE, are synthesized on the ER as inactive proprotein precursors that are then transported to vacuoles. After self-catalyzing, VPE is converted into the active form to cleave other vacuolar proteins to generate their respective mature proteins (Hiraiwa, Nishimura, and Hara-Nishimura 1999). In addition to the roles in modulating immune responses, the VPE-dependent PCD pathway is also involved in the responses to a variety of stress inducers and the development of various tissues (Hatsugai et al. 2015). Evidence from soybean indicated that GmNAC81 and GmNAC30 directly bound to the promoter of VPE and activated its transcription under ER stress (Mendes et al. 2013). The silencing of VPEs in N. benthamiana leaves abrogated the hypersensitive cell death incited by tobacco mosaic virus (TMV) (Hatsugai et al. 2004). Following the colonization of Arabidopsis thaliana roots, Piriformospora indica induced ER stress and VPE/caspase 1-like-mediated cell death but impeded the adaptive UPR. In Arabidopsis vpe null mutants, P. indica showed reduced colonization and a decreased incidence of cell death (Qiang et al. 2012). Similarly, the SlVPE3 RNA interference (RNAi) tomato fruit demonstrated delayed ripening and higher susceptibility to B. cinerea, a notorious necrotrophic pathogen that causes tremendous postharvest losses (Chen et al. 2023; Wang et al. 2017). A serine protease inhibitor, Kunitz trypsin inhibitor 4 functions downstream of SlVPE3 to regulate the resistance of tomato fruit, however, specific effectors and the precise mechanism for activating VPE cleavage in host cells remain unknown.
After realizing that some membrane-associated TFs, such as NACs and BAG7, may be cleaved and further translocated to the nucleus to regulate gene expression, the transcriptional reprogramming triggered by these TFs and specific downstream genes remains largely unresolved. Moreover, how they are correlated with the transcription of the UPR genes activated by bZIP28 and bZIP60 is unknown. Predictably, the integration of multi-omics data and machine learning-based network models may provide new evidence for understanding the maintenance of ER homeostasis.
4 Crosstalk Between the ER and Other Organelles During the Biotic Stress Response in Plants
The ER contains multiple contact sites that can tether both non–membrane-bound and membrane-bound organelles (Lee et al. 2020). Membrane contact sites (MCSs) are established via protein–protein or protein–lipid interactions, that physically connect the ER to other membrane-bound organelles (Bian et al. 2023). In addition to the crosstalk between the ER, Golgi apparatus and nucleus, crosstalk between the ER and mitochondria through ER–mitochondrial contact sites (EMCSs) have been reported to regulate autophagy, Ca2+ transfer, lipid metabolism and other vital activities (Li et al. 2022). The Ca2+ channels at EMCSs, such as the mitochondrial outer membrane-localized voltage-dependent anion-selective channel and the ER membrane-anchored inositol triphosphate-dependent calcium channel IP3R, are believed to mediate the transport of Ca2+ between the ER and mitochondria in response to ER stress (Lee and Min 2018). Multiple EMCSs form a unique cellular compartment, named the “mitochondria-associated ER membranes” (MAMs), which are enriched in enzymes involved in lipid biosynthesis (Parakh and Atkin 2021). Cardiolipin (CL), a specific phospholipids family of mitochondrion, plays an important role in protecting plants against PCD-induced stresses (Pan, Jones, and Hu 2014; Pineau et al. 2013). At early signaling events, the interaction between cytochrome c and CL seems an essential element (Petereit et al. 2017). However, there are only limited evidence about the regulation of CL in plants, which might be a potential research area in plant PCD. The plasma membrane (PM) is associated with the ER through ER–PM contact sites (EPCSs), which have been reported to be involved in the immune responses during plant–fungus interactions (Kim et al. 2016) and plant–virus interactions (Levy, Zheng, and Lazarowitz 2015). ER stress could increase EPCS formation, altering the local membrane lipid composition to enhance the proteolytic processing of the PM-anchored ANAC062, allowing the cleaved ANAC062 to mitigate ER stress by functioning as a TF in the nucleus (Seo et al. 2010). The chloroplasts are unique organelles to plants and algae. The MCSs between chloroplasts and the ER are involved in lipid transport and can regulate systemic acquired resistance against pathogens (Michaud and Jouhet 2019). The modulation of the chloroplast-synthesized methylerythritol cyclodiphosphate signal or the expression of chloroplast genes (SSI2 and SAL1) could affect the expression of ER stress marker genes, such as IRE1, bZIP60, and Bip3, subsequently affecting ER homeostasis in plant cells (Benn et al. 2016; Iwata et al. 2018; Xi et al. 2016). In addition, contact sites exist between the ER and vacuole; however, only a few reports are currently available in plants (Bian et al. 2023).
5 Concluding Remarks and Perspectives
The past decades of research on the maintenance of ER homeostasis have produced rapid advances. Transient or mild ER stress can be mitigated by the UPR, whereas sustained or severe ER stress may trigger autophagy and cell death upon exposure to adverse conditions. Nevertheless, some pending questions in the context of host–pathogen interactions remain:
IRE1, a sensor for ER stress, participates in orchestrating the UPR, cell death and autophagy (Bao et al. 2018; Howell 2013); however, the mechanism by which it is activated remains enigmatic. Apart from IRE1 and bZIP28, is there any specific sensor in the ER stress induced by pathogen invasion? What are the exact triggers that activate HLP1 and other similar ER homeostasis regulators? Could the mild UPR be harnessed by manipulating ER stress sensors and thus enhancing plant resilience to resist pathogen invasion? Future endeavors in identifying and manipulating ER stress sensors in response to pathogen invasion may aid in addressing these questions and thus improve crop performance under adverse conditions.
Second, there potentially exists a specific threshold for accurately and timely triggering the UPR, autophagy, or cell death. Could this threshold ultimately decide the outcome of the UPR to be survival or cell death? These inquiries require in-depth dissection through the integration of molecular tools and modifications using metabolic engineering. Precise control over this threshold may steer future endeavors in molecular design and improvement of crops to circumvent unforeseen economic loss under biotic and abiotic stresses.
Third, in conjunction with autophagy, ERAD is extensively implicated in plant growth and responses to diverse stress factors (Liu et al. 2021). Although scant data are available on the roles of ERAD in the context of host–pathogen interactions, scrutinizing how plants coordinate downstream components in these pathways for maintaining ER homeostasis upon varied abiotic and biotic stress would be intriguing.
Fourth, as a highly dynamic membranous architectures, the ER shares numerous contact sites with the PM, mitochondria, Golgi apparatus, and other organelles (Lee et al. 2020). A reciprocal interaction in response to pathogen invasion plays a pivotal role in determining the fate of plant cells. A recent study employed structured illumination microscopy in combination with an optimized self-supervised denoising framework to examine the fine ER structures in Arabidopsis (Zhang et al. 2024b). A meticulous dissection of the dynamics of ER and contact events is likely to yield more insights, given that artificial intelligence and machine-learning techniques have emerged as driving forces in this field.
Finally, to the authors' knowledge, plants likely utilize the same suite of UPR components to harmonize growth and stress responses (Srivastava et al. 2018). How do they discern diverse developmental or environmental cues to adjust the degree of UPR activation precisely? The underlying mechanism still necessitates further elucidation. Furthermore, it may be a promising strategy to augment pathogen resistance by manipulating the ER proteins in host plants to evade targeting by effectors and thus maintain their essential functions.
Acknowledgements
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDA0440303) and the National Natural Science Foundation of China (Grant No. 32430082 and 32372777).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.