CsCIPK20 Improves Tea Plant Cold Tolerance by Modulating Ascorbic Acid Synthesis Through Attenuation of CsCSN5-CsVTC1 Interaction
Dr. Lu Wang is responsible for correspondence and material distributions.
ABSTRACT
Low temperature is a limiting environmental factor for tea plant growth and development. CBL-interacting protein kinases (CIPKs) are important components of the calcium pathway and involved in plant development and stress responses. Herein, we report the function and regulatory mechanisms of a low-temperature-inducible gene, CsCIPK20, in tea plants. The overexpression of CsCIPK20 in Arabidopsis and its transient knockdown in tea plants confirmed its positive role in cold resistance. Notably, the ascorbic acid (AsA) levels increased in the overexpression lines and decreased in the CsCIPK20 knockdown tea plants under freezing stress. Transcriptomic analysis revealed that genes involved in flavonoid metabolism, glutathione metabolism, and AsA biosynthesis were significantly regulated by CsCIPK20. Furthermore, we found that CsCSN5, a key component of the COP9 signalosome, interacted with CsCIPK20 to mediate CsCIPK20 degradation. CsCSN5 interacted with CsVTC1, a key enzyme in AsA biosynthesis, and mediated CsVTC1 degradation. Knockdown of CsVTC1 in tea plants enhanced sensitivity to low temperature. Moreover, we demonstrated that CsCIPK20 competed with CsVTC1 to bind to CsCSN5, which protected CsVTC1 from degradation mediated by CsCSN5 and contributed to AsA accumulation. Overall, our findings uncovered a mechanistic framework through which the CsCIPK20-CsCSN5-CsVTC1 module mediated AsA accumulation and low-temperature resistance in tea plants.
1 Introduction
Extreme temperature fluctuations owing to climate change impose stress on plants. Low temperature, a major environmental factor, severely impedes plant physiology and development and reduces plant productivity (Pearce 2001). As one of the most popular health beverage plants, tea plants (Camellia sinensis (L.) O. Kuntze) have been cultivated in more than 60 countries and have significant economic values and social benefits worldwide (Türközü and Şanlier 2017). Due to originating from tropic or subtropical areas, tea plants are sensitive to low temperature (Yu 1986). Low temperature strictly limits the geographical distribution of tea plants and severely impedes tea yield and quality. Severe winters pose difficulties for the safe overwintering of tea plants. Additionally, cold spells in the spring are frequent and sudden, causing severe and devastating damage to the tea economy (Hao et al. 2018). In the past decade, more research has focused on omics analysis at the transcriptome, proteome, and metabolism levels in tea plants (Wang et al. 2012; Wang et al. 2022). Although a few functional genes involved in the cold stress response have been identified in tea plants, there is still an urgent need to uncover the regulatory mechanisms involved in the low-temperature response of tea plants, which will be of particular and vital significance for the tea industry.
Cold stress induces diverse response mechanisms in cold sensing and cellular signal transduction. One of the signaling molecules that has been reported to participate in nearly all abiotic stress is Calcium (Ca2+) (Kudla, Batistič, and Hashimoto 2010). Upon the perception of a cold stimulus, the influx of Ca2+ into the cytoplasm increased dramatically via Ca2+ channels for cold sensing in plants (Knight and Knight 2012). The Ca2+-binding proteins, calcineurin B-like proteins (CBLs), decode Ca2+ signals by modulating CBL-interacting protein kinases (CIPKs), which are plant-specific protein kinases (Shi et al. 1999). CIPKs constitute a signaling network that plays multiple roles in plant adaptive responses. They participate in plant cold tolerance. A study on rice showed that OsCIPK03 transgenic plants improved cold tolerance by significantly accumulating soluble sugars and proline (Xiang, Huang, and Xiong 2007). In another study, an OsCIPK7 mutant with a point mutation in the kinase domain generated via the TILLING procedure exhibited increased protein kinase activity accompanied by enhanced tolerance to chilling stress (Zhang et al. 2019). The knockdown of CaCIPK13 led to enhanced sensitivity to cold stress in pepper (Ma et al. 2021). In tea plants, the Ca2+ signaling pathway has been reported to play a vital role in the low-temperature response (Wang et al. 2012; Wang et al. 2019). Furthermore, the identification and analysis of the CsCIPK family showed that multiple CsCIPKs respond to low temperature (Wang et al. 2020a). However, the role and mechanism of CsCIPK in low-temperature responses remain largely unexplored.
Ascorbic acid (AsA) is an important antioxidant that contributes to plant growth, development, and stress responses by scavenging reactive oxygen species (ROS) produced as a by-product of photorespiration (Smirnoff 2018). Exogenous AsA can enhance chilling tolerance in plants (Lo'ay and El-Khateeb 2018). In tea plant leaves, genes related to AsA biosynthesis and AsA levels can be induced at low temperature (Li et al. 2018a). Moreover, exogenous AsA treatment can increase cold tolerance in tea plants via cell wall remodeling (Fu et al. 2023). The d-mannose/l-galactose pathway is the dominant AsA biosynthetic pathway in plants. Eight enzymatic steps participate in this pathway from the d-fructose-6-P conversion to GDP-d-mannose, and GDP-d-mannose conversion to AsA (Ishikawa et al. 2018). GDP-d-mannose pyrophosphorylase, a Vitamin C Defective 1 (VTC1) enzyme, catalyzes the formation of GDP-d-mannose in the eight enzymatic steps. Previous studies showed that the AsA level in Arabidopsis vtc1-1 mutant was reduced by 70%–75% compared to the wild-type, which revealed the important role of VTC1 in AsA biosynthesis (Conklin et al. 1999; Wang et al. 2013). VTC1 is modulated at the transcriptional, translational, and posttranslational levels (Sawake et al. 2015; Zhang et al. 2009; Zhang et al. 2012). CSN5B, a component of the photomorphogenic COP9 signalosome (CSN), can modulate AsA synthesis and subsequently modulate plant responses to abiotic stress by interacting with VTC1 and promoting its degradation under dark conditions, resulting in decreased AsA level (Wang et al. 2013). However, the regulatory mechanisms underlying AsA homeostasis at low temperature in tea plants remain largely unknown. Therefore, there is a critical need to understand the factors that regulate AsA biosynthesis and metabolism to increase the tolerance of tea plants to cold stress.
In our previous study, several CsCIPK genes were reported to respond to cold stress on a genome-wide scale. Moreover, CsCIPK20 exhibited a specific response to cold stress in tea plants subjected to different abiotic stresses, indicating that CsCIPK20 may mediate cold stress signaling in tea plants (Wang et al. 2019; Wang et al. 2020a). However, the role of CIPK20 in the regulation of cold stress response in plants remains unclear. Here, we investigated the function of CsCIPK20 under cold stress via its overexpression in Arabidopsis and downregulation in tea plants and elucidated a novel mechanism through which CsCIPK20 attenuates the degradation of CsVTC1 mediated by CsCSN5B, which contributes to AsA accumulation and confers cold tolerance to tea plants.
2 Results
2.1 CsCIPK20 Was Induced by Low Temperature in Tea Plants and Improved Low Temperature Tolerance in Arabidopsis
The spatial expression in buds and leaves of ‘Longjing 43 (LJ43)’ showed that CsCIPK20 expression was higher in mature leaves and relatively lower in buds, indicating an increase in CsCIPK20 expression with increasing leaf maturity (Figure 1A). The transcript levels of CsCIPK20 were detected in two tea plant cultivars (‘Zhenong 12 (ZN12)’ and ‘Damianbai (DMB)’) during natural cold acclimation in winter. The results showed that the expression level of CsCIPK20 remained high during the cold acclimation period (December, January, and February) and was low during the non-acclimation (October and November) and de-acclimation (March) periods (Figure 1B). Following 4°C treatment, CsCIPK20 expression was induced at 6 h and significantly elevated at 3 days in the one bud with one leaf (1B1L) and mature leaf (ML) of ‘LJ43’ (Figure 1C). These results revealed that CsCIPK20 expression was significantly upregulated at low temperature. Subcellular localization analysis of CsCIPK20 showed that CsCIPK20-GFP fluorescence was present in the nucleus and plasma membrane of Nicotiana benthamiana leaves (Fig. S1). CsCIPK20 exhibited a typical nonspecific subcellular localization similar to that of the empty 35S-GFP protein.
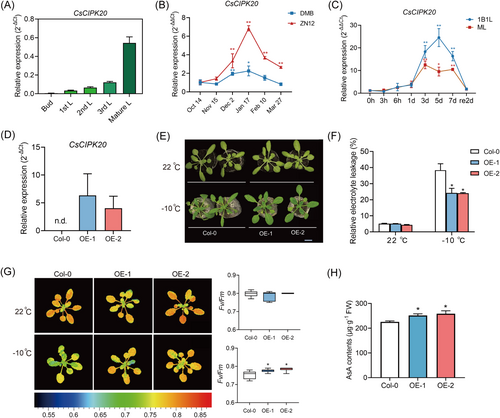
Given that a stable genetic transformation system for tea plants has not yet been established, to further elucidate the function of CsCIPK20 in the low-temperature stress response, overexpression (OE) vector of super promoter-CsCIPK20 was constructed and transfected into Arabidopsis. Two independent CsCIPK20 transgenic lines, OE-1 and OE-2, were generated and used for further analysis. Quantitative real-time PCR (qRT-PCR) assay was performed to confirm the overexpression efficiency in the two lines (Figure 1D). Under normal culture conditions, there were no significant differences between the phenotypes of CsCIPK20-OE and wild-type (Col-0) plants. However, when subjected to −10°C for 7.5 h and recovery at 22°C for 6 days, the leaves of Col-0 showed more severe wilting compared to the CsCIPK20-OE transgenic lines (Figure 1E). Consistent with these phenotypes, after the freezing treatment, the relative electrolyte leakage (REL) of the CsCIPK20-OE lines was significantly lower than that of the Col-0 plants (Figure 1F). The maximum efficiency of photosystem II photochemistry (Fv/Fm) value and AsA levels were significantly higher in the CsCIPK20-OE lines than in Col-0 plants under freezing conditions (Figure 1G,H). These findings indicate that the overexpression of CsCIPK20 conferred enhanced low-temperature tolerance in transgenic Arabidopsis.
2.2 Knockdown of CsCIPK20 in Tea Plant Leaves Resulted in Low-Temperature Sensitivity
In recent years, gene suppression by antisense oligonucleotides has become common for gene functional research of tea plants (Zhao et al. 2020). We further generated CsCIPK20-suppressed tea plants using the gene-specific antisense oligodeoxynucleotide (AsODN) suppression strategy, as described in a previous study (Zhao et al. 2020). The efficiency of the antisense was confirmed using qRT-PCR assays, which showed that the transcript level of CsCIPK20 in tea leaves treated with CsCIPK20-AsODN was significantly reduced (decreased by 64%) compared to tea leaves treated with sense oligodeoxynucleotides (CsCIPK20-sODN) (Figure 2A). This indicated that transient suppression of CsCIPK20 in tea leaves was achieved using the AsODN method.
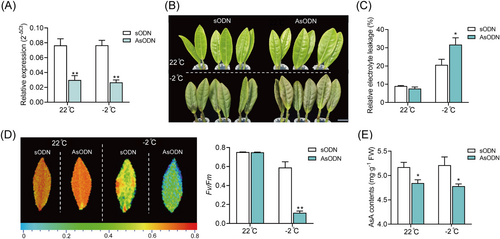
The CsCIPK20-AsODN leaves were more sensitive to low temperature than sODN leaves (Figure 2B). Physiological indices of the CsCIPK20-sODN and CsCIPK20-AsODN tea plants were assayed before and after low-temperature treatment. Compared with CsCIPK20-sODN tea plants, higher REL values were detected in the CsCIPK20-AsODN plants (Figure 2C), and lower Fv/Fm values and AsA levels were detected in the CsCIPK20-AsODN tea plants (Figures 2D,E). These results demonstrated that the CsCIPK20-suppressed tea plants were more sensitive to low-temperature stress than the control group. Based on these results, CsCIPK20 played a positive role in regulating low-temperature tolerance in tea plants.
2.3 Transcriptome Analysis of CsCIPK20-Regulated Cold-Responsive (CsCOR) Genes
To assess the molecular networks underlying the CsCIPK20-mediated response of tea plants to low temperature, we performed RNA-seq analysis of CsCIPK20-sODN and CsCIPK20-AsODN tea leaves incubated at 4°C and −2°C, respectively. The differentially expressed genes (DEGs) (fold change ≥ 2 and adjusted p < 0.05) were identified.
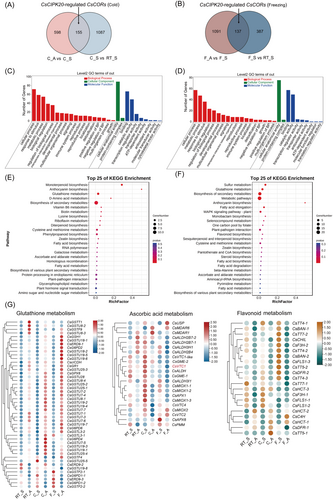
A total of 1242 (155 + 1087) and 524 (137 + 387) DEGs in CsCIPK20-sODN tea leaves were identified following the 4°C and −2°C treatments, respectively, compared to the 22°C group (Figure 3A,B). A total of 753 and 1228 DEGs were identified between the CsCIPK20-AsODN and CsCIPK20-sODN tea leaves under 4°C and −2°C treatments, respectively. Of these, 155 DEGs were recognized as CsCIPK20-regulated CsCOR genes following the 4°C treatment and 137 DEGs were recognized as CsCIPK20-regulated CsCOR genes following the −2°C treatment (Figure 3A,B). Gene ontology (GO) enrichment analysis suggested that these CsCIPK20-regulated CsCOR genes were highly enriched in items related to the biological processes of ‘cellular process’, ‘response to stimuli’, and ‘signaling’, cellular component of ‘cellular anatomical entity’, and the molecular functions of ‘binding’, ‘catalytic activity’ and ‘ATP-dependent activity’ (Figure 3C,D), which were associated with response to stress. The KEGG analysis revealed that the CsCIPK20-regulated CsCOR genes were involved in multiple pathways, including the ‘glutathione metabolism’, ‘biosynthesis of second metabolites’, ‘ascorbate and aldarate metabolism’ and ‘flavonoid metabolism’ pathway among others (Figure 3E,F).
A detailed expression analysis of the DEGs revealed that the expression of most DEGs involved in the ‘glutathione metabolism’ pathway was upregulated in the CsCIPK20-AsODN tea leaves compared to the CsCIPK20-sODN tea leaves (Figure 3G). Moreover, the expression of most DEGs involved in the ‘ascorbate and aldarate metabolism’ and ‘flavonoid metabolism’ pathways was downregulated in the CsCIPK20-AsODN tea leaves than in the CsCIPK20-sODN tea leaves (Figure 3G). Thus, these results revealed an obvious transcriptome reprogramming of CsCORs mediated by CsCIPK20 downregulation at low temperature and revealed that genes involved in the GSH-AsA cycle and flavonoid metabolism may contribute to the low-temperature sensitivity of CsCIPK20 downregulated tea plants.
2.4 CsCSN5 Interacted With CsCIPK20 to Degrade CsCIPK20
To elucidate the mechanism by which CsCIPK20 responds to cold stress, yeast two-hybridization (Y2H) screening was performed using a tea plant cDNA library with CsCIPK20 as the bait. CsCSN5 was identified as an interacting protein through the Y2H screening. Sequence analysis revealed that CsCSN5 was closely related to AtCSN5A and AtCSN5B, which encoded a component of CSN, and we named it CsCSN5 (Supporting Information S1: Figure S2). qRT-PCR showed that the spatial expression of CsCSN5 was higher in buds and lower in mature leaves (Figure 4A), in contrast to that of CsCIPK20 (Figure 1A). The expression of CsCSN5 in ‘ZN12’ and ‘DMB’ tea plants was also significantly upregulated owing to low temperature during winter (Figure 4B). Following the 4°C treatment, CsCSN5 expression was significantly elevated at 5 days in the 1B1L and rapidly induced at 3 h in the ML (Figure 4C). These results suggested that CsCSN5 participated in cold tolerance in the tea plants. Moreover, the subcellular localization of CsCSN5 was consistent with that of CsCIPK20, which was distributed in the nucleus and plasma membrane (Supporting Information S1: Figure S1).
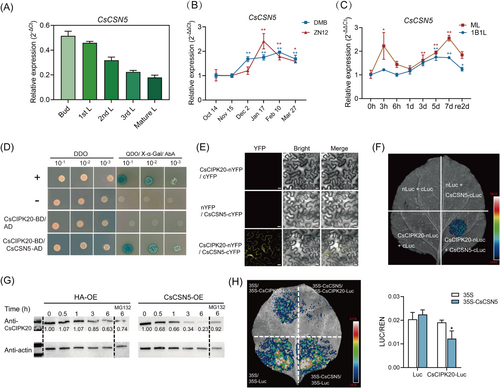
To confirm the screening result, the Y2H experiment was performed. The results showed that only the yeast cells co-expressed with CsCIPK20 and CsCSN5 could grow on the QDO screening medium with X-α-Gal and AbA, demonstrating that CsCIPK20 interacted with CsCSN5 (Figure 4D). The bimolecular fluorescence complementation (BiFC) assay showed fluorescence indicative of heterodimer formation of CsCIPK20-nYFP and CsCSN5-cYFP in the cytomembrane (Figure 4E). Consistent with the BiFC assay, the luciferase complementation imaging (LCI) assay revealed a luminescence signal when CsCIPK20-nLuc and CsCSN5-cLuc proteins were co-expressed (Figure 4F). Altogether, these results indicated that CsCIPK20 interacted with CsCSN5.
The CsCIPK20 protein purified from prokaryotic expression showed no kinase activity and CsCIPK20 was unable to phosphorylate CsCSN5 in vitro (Supporting Information S1: Figure S3). Since the CSN complex regulates cullin-RING E3 ubiquitin ligase, which mediates protein stability (Schwechheimer and Isono 2010), a cell-free degradation assay was performed to examine whether CsCSN5 could affect CsCIPK20 protein stability. An immunoblot analysis showed that the CsCIPK20-GST fusion protein levels decreased and then nearly disappeared after incubation with the total protein extracts of Nicotiana benthamiana leaves infiltrating the 35S-CsCSN5-HA construct for 6 h, compared with the control group. When MG132 was added, the degradation of the CsCIPK20-GST proteins was inhibited (Figure 4G). Therefore, CsCIPK20 interacted with CsCSN5 to promote the ubiquitin-mediated degradation of CsCIPK20 via the 26S proteasome pathway. To further confirm the degradation of CsCIPK20 by CsCSN5 in vivo, a protein degradation assay was performed in Nicotiana benthamiana. The control group showed no significant differences between the 35S-CsCSN5-HA/35S-Luc and 35S-HA/35S-Luc. Compared with the fluorescence intensity in the areas of 35S-HA/35S-CsCIPK20-Luc, the 35S-CsCSN5-HA/35S-CsCIPK20-Luc areas exhibited decreased fluorescence intensity (Figure 4H). This suggested that CsCSN5 mediated CsCIPK20 degradation in vivo.
2.5 CsCSN5 Interacted With CsVTC1 to Degrade CsVTC1
AsA is a vital plant-derived antioxidant that eliminates ROS, and VTC1 is crucial for its biosynthesis (Conklin et al. 1999). A previous study reported that AtCSN5B interacted with AtVTC1 and promoted AtVTC1 ubiquitination and degradation through the 26S proteasome pathway (Wang et al. 2013), reflecting that CSN5 participated in modulating AsA synthesis by affecting VTC1 activity. Considering that CsCIPK20 affected the AsA content in tea plants and Arabidopsis (Figures 1 and 2), we wondered whether CsCSN5 could interact with CsVTC1 to influence CsVTC1 stability in the tea plants. qRT-PCR showed that the spatial expression of CsVTC1 in the leaves was consistent with that of CsCIPK20. A higher expression was detected in mature leaves and a lower expression was detected in buds (Figure 5A). The expression of CsVTC1 in ‘ZN12’ and ‘DMB’ tea plants was significantly upregulated during natural cold acclimation in the winter (Figure 5B). The CsVTC1 expression was significantly upregulated at 5 days in the 1B1L and at 7 days in the ML following the 4°C treatment (Figure 5C). Consistent with the subcellular localization of CsCSN5 and CsCIPK20, CsVTC1 was located in the nucleus and plasma membrane (Supporting Information S1: Figure S1).
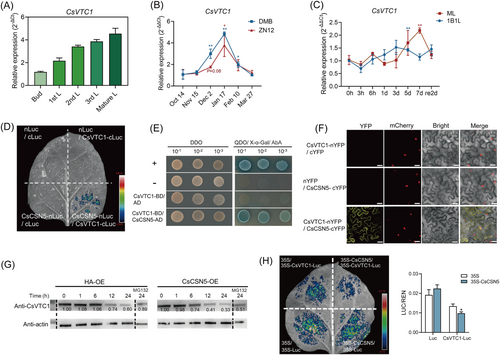
LCI analysis revealed that luminescence signals were visualized in areas where CsCSN5-cLuc and CsVTC1-nLuc were co-expressed (Figure 5D). However, no luminescence signal was observed in areas where CsVTC1 and CsCIPK20 were co-expressed (Supporting Information S1: Figure S4). This suggested that CsVTC1 interacted with CsCSN5 but not CsCIPK20. The yeast cells co-expressed with CsVTC1 and CsCSN5 could grow on the QDO screening medium (Figure 5E). BiFC analysis showed that the interaction fluorescence of CsVTC1-nYFP and CsCSN5-cYFP was observed in the cytomembrane and nucleus (Figure 5F).
Degradation assays were performed to examine whether CsCSN5 could interact with CsVTC1 to degrade it. Cell-free degradation assay showed that the CsVTC1-His fusion protein levels decreased after incubation with the total protein extracts of 35S-CsCSN5-HA, compared with the control group. MG132 treatment inhibited the degradation of the CsVTC1 (Figure 5G). An in vivo degradation assay showed that compared to the fluorescence intensity in areas in which 35S-HA was co-expressed with 35S-CsVTC1-Luc, the areas in which 35S-CsCSN5-HA was co-expressed with 35S-CsVTC1-Luc exhibited a decreased signal (Figure 5H). This suggested that CsCSN5 mediated CsVTC1 degradation in vivo and in vitro.
2.6 Knockdown of CsVTC1 in Tea Plant Leaves Enhanced Sensitivity to Low Temperature
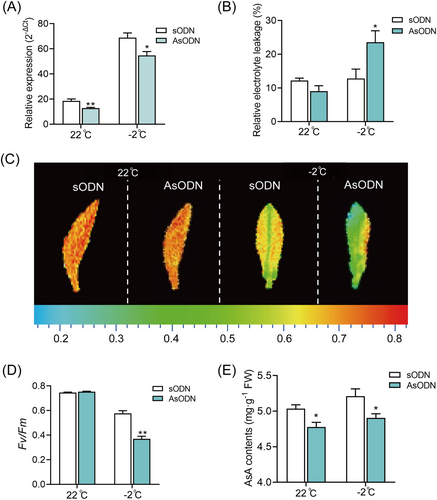
To further investigate whether CsVTC1 could promote freezing tolerance in tea plants, CsVTC1 expression was suppressed in young tea leaves using the gene-specific AsODN suppression strategy. The relative expression of CsVTC1 was effectively suppressed before and after freezing stress (Figure 6A). Under freezing treatment, the REL of CsVTC1-AsODN leaves was significantly higher than that of sODN leaves (Figure 6B). Moreover, the Fv/Fm and AsA levels were significantly downregulated in CsVTC1-AsODN leaves (Figure 6C–E). These results indicated that CsVTC1 contributed to freezing tolerance in the tea plants.
2.7 CsCIPK20 Attenuated the Interaction Between CsCSN5 and CsVTC1
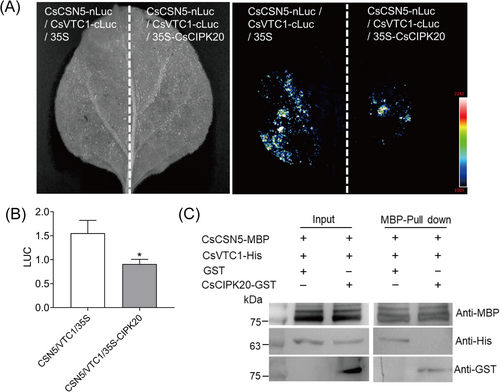
To confirm our hypothesis that CsCIPK20 can regulate plant low-temperature tolerance via the CsCSN5–CsVTC1 complex, which plays an important role in AsA level modulation, an LCI assay was performed. When CsCIPK20-HA was co-transformed with CsCSN5-cLuc and CsVTC1-nLuc, the qualitative and quantitative analyses showed that the luminescence signal was dramatically decreased in the Nicotiana benthamiana leaves (Figure 7A,B). Therefore, the interaction between CsCSN5 and CsVTC1 was attenuated by CsCIPK20. We further investigated whether CsCIPK20 and CsVTC1 could competitively interact with CsCSN5 using a pull-down assay. The results showed that the purified CsCSN5-MBP protein pulled down the CsVTC1-His protein (Figure 7C). When CsCSN5-MBP was incubated with CsVTC1-His protein and CsCIPK20-GST protein, the ability of CsCSN5-MBP to combine with CsVTC1-His was repressed by CsCIPK20-GST (Figure 7C). This indicated that CsVTC1 and CsCIPK20 competitively interacted with CsCSN5.
3 Discussion
Cold stress limits tea plant growth and development. Still, there is a critical need to understand more regarding the regulatory mechanisms underlying cold tolerance in tea plants. CIPK has been identified as an important component of the Ca2+ signaling pathway in response to abiotic stress (Ma et al. 2021; Zhang et al. 2019). However, the roles of CsCIPKs in tea plants have not yet been clarified. In this study, we reported that CsCIPK20 can play a positive role in the cold tolerance of tea plants, and explored the regulatory mechanisms of CsCIPK20 under low-temperature stress.
3.1 CsCIPK20 Functions as a Positive Regulator of Cold Tolerance by Improving the Asa Levels
CIPKs are broadly involved in plant development and stress responses through decoding distinct Ca2+ signals (Mao et al. 2022). In the current study, we confirmed that CsCIPK20 was a cold-inducible gene (Figure 1B,C), suggesting that it was closely related to the low temperature response of tea plants. We preliminarily found that CsCIPK20 positively regulated the cold tolerance of plants by overexpressing CsCIPK20 in Arabidopsis and knocking down CsCIPK20 in tea plants (Figure 1 and Figure 2). Notably, the AsA content of plants was significantly regulated by CsCIPK20 under freezing stress (Figure 1H and Figure 2E), suggesting that the modulation of the AsA content played an important role in the regulation of cold resistance by CsCIPK20. AsA is a vital nonenzymatic antioxidant that can scavenge ROS generated by abiotic and biotic stresses (Hu et al. 2016; Smirnoff 2000). Exogenous AsA treatment contributes to cold resistance of tea plants by reducing lipid peroxidation and improving their Fv/Fm (Fu et al. 2023). The transcriptome analysis further indicated that the glutathione metabolism, AsA biosynthesis, and flavonoid metabolism pathways were involved in CsCIPK20-mediated cold tolerance improvement in tea plants (Figure 3). The regulation of the AsA biosynthesis pathway by CsCIPK20 may partly explain the modulation of AsA content by CsCIPK20 in plants.
As an important economic crop, tea plants are rich in secondary metabolites, such as flavonoids, which not only confer healthy properties to the tea, but also protect tea plants from various abiotic stresses by acting as unique antioxidants, phytoalexins and signal molecules (Wang et al. 2022). In this study, we found that the repression of CsCIPK20 downregulated the expression of flavonoid metabolism genes, which may be responsible for the decreased freezing tolerance of CsCIPK20-AsODN plants. In apples, MdCIPK20 participates in anthocyanin biosynthesis by regulating the stability of RGL2a (An et al. 2023). In this study, the ‘anthocyanin biosynthesis’ pathway was significantly enriched in CsCIPK20-regulated CsCORs (Figures 3E,F), indicating the potential role of CsCIPK20 in regulating anthocyanin biosynthesis in tea plants. Anthocyanin accumulation contributes to hydrogen peroxide (H2O2) scavenging under low temperature, and promotes plant cold resistance (Xie et al. 2018). It is speculated that CsCIPK20 may also regulate cold tolerance by regulating anthocyanin synthesis. Moreover, the regulation of the ‘glutathione metabolism’ and ‘ascorbate and aldarate metabolism’ pathways exhibited opposite results regarding CsCIPK20 (Figure 3G). We speculate that there may be a feedback regulation of the AsA-GSH cycle (Li, Liu, and Zhang 2010), which requires further investigation.
3.2 CsCIPK20 Attenuates the Interaction Between CsCSN5 and CsVTC1, Contributing to AsA Accumulation
Temperature and light coordinately modulate plant response to low temperature. It is well known that some key components of light signaling play important roles in plant response to low temperature. For example, PIF1, PIF3, PIF4, PIF5, and PIF7 negatively regulate cold responses in plants (Jiang et al. 2020; Lee and Thomashow 2012). phyB and HY5 are stabilized by cold stress and positively regulate freezing tolerance in plants (Jiang et al. 2020; Li et al. 2021). In the present study, we identified the photomorphogenic factor CsCSN5 in tea plants. CSN5 is a subunit of the CSN complex that regulates the activity of cullin-RING E3 ubiquitin ligases (Chen et al. 2010; Dohmann, Kuhnle, and Schwechheimer 2005). The CSN complex regulates protein turnover through 26S proteasome, and the CSN5 subunit is the activity center of the CSN (Nezames and Deng 2012; Wei, Serino, and Deng 2008). In Arabidopsis, AtCSN5 comprises AtCSN5A and AtCSN5B (Gusmaroli, Feng, and Deng 2004). However, whole-genome and protein sequence alignment analyses showed that there was only one CsCSN5 in tea plants that was homologous to AtCSN5A and AtCSN5B in Arabidopsis (Supporting Information S1: Figure S2), suggesting that CsCSN5 may simultaneously possessed functions similar to AtCSN5A and AtCSN5B (Gusmaroli, Feng, and Deng 2004; Wang et al. 2013). Moreover, we demonstrated that CsCSN5 was a low temperature-inducible gene, and CsCSN5 can interact with CsCIPK20 (Figure 4), indicating its potential role in the response to low-temperature stress in tea plants. Further degradation analysis revealed that CsCSN5 mediated CsCIPK20 degradation, which was consistent with the opposite expression pattern observed in leaves of different maturities (Figure 1 and Figure 4). This suggested that CsCSN5 may be involved in the response to low temperature by degrading CsCIPK20 in tea plants. However, despite repeated tries, we were unable to obtain an effective AsODN to inhibit the expression of CsCSN5 in tea plants. In a recent study, SlCSN5A was involved in regulating the stability of chloroplast proteins and negatively regulated the tolerance of tomato plants in response to low temperature stress at night (Lu et al. 2024). We speculated that CsCSN5 may have similar function, warranting further investigation.
The regulatory mechanism of AsA biosynthesis in plants has garnered increasing attention and is beneficial for improving plant resilience and optimizing food quality. Moreover, the regulation of VTC1 is important for AsA biosynthesis and adaptation to abiotic stress in plants. AtERF98 and SlHZ24 activated the transcription of VTC1 to modulate AsA biosynthesis and enhanced salt tolerance of Arabidopsis (Hu et al. 2016; Zhang et al. 2012). The F-box protein MdAMR1L1 modulated MdVTC1 to regulate AsA biosynthesis in apples (Ma et al. 2022). AtCSN5B negatively regulated AsA synthesis through interacting with AtVTC1 (Wang et al. 2013). The tomato C2H2 zinc-finger protein SlZF3 interacted with CSN5B and participated in regulating AsA synthesis and salt tolerance (Li et al. 2018b). Consistent with this, we further demonstrated that CsCSN5 interacted with CsVTC1 in tea plants and mediated its degradation (Figures 5G,H), indicating a conserved mechanism of CSN5 in degrading VTC1 in plants. The opposite expression patterns of CsCSN5 and CsVTC1 in leaves of different maturities also supported this observation (Figure 4A and Figure 5A). In addition, the CsVTC1-downregulated plants exhibited lower tolerance to freezing stress (Figure 6), and the CsVTC1-AsODN plants exhibited lower AsA content than the sODN plants under the freezing treatment (Figure 6E). This indicated that CsVTC1 can mediate freezing tolerance through the regulation of AsA synthesis. Furthermore, CsCIPK20 and CsVTC1 were competitively bound to CsCSN5 (Figure 7), implying that this competition inhibited the degradation of CsVTC1. This indicates that CsCIPK20 can regulate the AsA levels in tea plants via CsCSN5-CsVTC1.
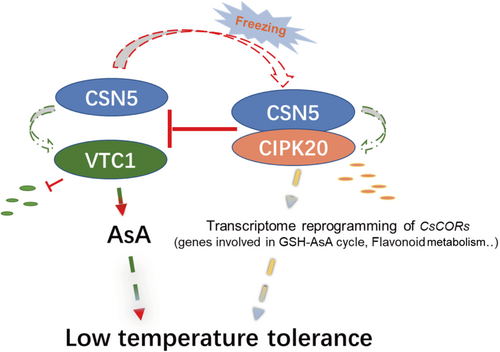
Altogether, our data revealed a regulatory role of CsCIPK20 in the regulation of AsA biosynthesis in tea plants under low-temperature stress. Under low temperature, CsCIPK20 regulates glutathione metabolism and flavonoid metabolism to enhance the resistance to low temperature. Additionally, CsCIPK20 competes with CsVTC1 to interact with CsCSN5, thereby inhibiting the degradation of CsVTC1 and resulting in increased AsA accumulation and freezing tolerance (Figure 8).
4 Materials and Methods
4.1 Plant Materials and Growth Conditions
Potted tea plants of ‘Longjing 43’ (‘LJ43’) were used in this study. For tissue expression analysis, the buds, first leaves (1st L), second leaves (2nd L), third leaves (3rd L), and mature leaves were sampled from 5-year-old potted ‘LJ43’ tea plants. For time-course expression analysis, 5-year-old ‘LJ43’ tea plants were grown in a chamber (12 h/12 h, 22°C light/20°C dark) and were transferred to 4°C for cold treatment. One bud with one leaf (1B1L) and mature leaf (ML) of the tea plants were harvested at 0 h, 3 h, 6h, 1 day, 3 days, 5 days and 7 days posttreatment and after 2 days of recovery. For winter expression analysis, two tea plant cultivars, ‘Damianbai (DMB)’ and ‘Zhenong 12 (ZN12)’, grown in the field at the Tea Research Institute of the Chinese Academy of Agricultural Sciences, Hangzhou, China were used in this study (Wang et al. 2019). The first three apical mature leaves of ‘DMB’ and ‘ZN12’ were sampled. Three independent biological replicates were used in each experiment. For gene suppression, the branches of the ‘LJ43’ tea plants used for the suppression were cultivated in a climatic chamber for 2 days under 12 h (22°C)/12 h (20°C) light/dark conditions for adaptation.
Arabidopsis ecotype Columbia-0 (Col-0) was used as the background for overexpressing CsCIPK20. Col-0 and CsCIPK20-OE lines were grown under 10 h (22°C)/14 h (20°C) light/dark conditions before cold acclimation. The 3-week-old seedlings were subjected to 4°C for 7 days, followed by freezing treatment at −10°C for 7.5 h.
The tobacco genotypes used in this study were the Nicotiana benthamiana (wild tobacco) and the transgenic Nicotiana benthamiana, which expressed the H2B-RFP nuclear marker (Li et al. 2017). Wild tobaccos were used for LCI and protein expression analyses. Transgenic tobaccos (H2B-RFP) were used for subcellular localization and BiFC assays. They were cultivated in a chamber under 16 h (22°C)/8 h (20°C) light/dark conditions.
4.2 Quantitative Real-Time PCR (qRT-PCR) Analysis and RNA-Seq Assays
Total RNAs were extracted from the tea plants using the RNAprep Pure kit (Tiangen Beijing, China), followed by reverse transcription using a PrimeScript RT reagent kit (TaKaRa, Shiga, Japan). Then, qRT-PCR assays were performed on a Light Cycler 480 machine (Roche, Switzerland) using SYBR Green I Master Mix (Roche, Switzerland). The primer sequences used for qRT-PCR are listed in Supporting Information S1: Table S1. CsPTB was used as the reference gene (Hao et al. 2014). For RNA-seq analysis, after injecting the tea leaves with sODN and CsCIPK20-AsODN solutions for 15 h, they were treated at 4°C for 3 h and −2°C for 3 h, respectively. Then, the tea leaves were collected, and RNA was extracted. Three independent biological replicates were performed for each experiment.
The libraries were sequenced on an Illumina NovaSeq. 6000 (Illumina, USA) at Novogene (Beijing, China). Raw reads in the fastq format were firstly processed using in-house Perl scripts from Novogene (Beijing, China). Then, the reads were mapped to the tea plant genome with default parameters (Wang et al. 2020b). The FPKM of each gene was calculated using HTSeq (v0.6.1) with the union model. Differential expression analysis was performed using the DESeq R package (v1.10.1) with the default parameters. Significant DEGs were identified as those with a p value of differential expression above the threshold (|log2 fold-change | > 1, adjusted p < 0.05). GO and KEGG enrichment analyses were performed with the accession numbers of the significant DEGs using the OmicShare platform. The heatmap of differentially expressed genes was generated by TBtools software (version: 2.034) and performed using Log2FPKM values. Meanwhile, data in each row were normalized and compared separately and were clustered by rows.
4.3 Knockdown of CsCIPK20 and CsVTC1 in Tea Plants
Antisense oligonucleotide (AsODN) and sense oligonucleotide (ODN) sequences were designed using Soligo software by inputting the full length of CsCIPK20 and CsVTC1 (Ding 2003). The oligonucleotide sequences of CsCIPK20 and CsVTC1 are listed in Table S2. For CsCIPK20 and CsVTC1 suppression, the first leaves of the tea plants sampled in May were treated with 1 mL of 20 μM AsODN or sODN solution for 15 h. Then, they were subjected to −2°C for 3 h. The tea leaves were harvested before and after freezing stress for REL and Fv/Fm measurements, and were frozen in liquid nitrogen for RNA extraction and the assessment of AsA content. The phenotype was recorded during recovery for 2 h at 22°C after freezing stress.
4.4 Measurement of REL, Fv/Fm, and AsA Content
The REL of the tea plants and Arabidopsis was determined using a conductivity meter, Orion 5 Star (ThermoFisher Scientific, Massachusetts, USA), as previously described (Wang et al. 2019; Yao et al. 2020). The Fv/Fm of the leaves was measured using FluorCam 7 (Photon Systems Instruments, Brno, Czech Republic) after 20 min of dark adaptation, with ten independent replicates from each treatment. The AsA content of the leaves was assessed using an AsA content test kit (Comin, Suzhou, China), following the manufacturer's instructions.
4.5 Y2H Assay
For the matchmaker gold Y2H Library screening system, the CsCIPK20 and CsVTC1 fragments were ligated with the pGBKT7 vector to generate bait vectors (CsCIPK20-BD and CsVTC1-BD), and CsCSN5 was ligated with the pGADT7 (CsCSN5-AD). All primer sequences used for vector construction are listed in Supporting Information S1: Table S3. Recombinant vectors were introduced into the yeast AH109-chemically competent cell according to the manufacturer's protocol (Weidi, Shanghai, China). The AH109 cells co-transformed with pGADT7-T and pGBKT7-p53 were used as the positive control (+). The AH109 cells co-transformed with pGADT7-T/pGBKT7-lam, the CsCIPK20-BD/AD empty vector, and the CsVTC1-BD/AD empty vector were used as the negative control (−). Positive transformants were grown on an SD/-Leu/-Trp (deficient in leucine and tryptophan) dropout medium and screened on an SD/-Leu/-Trp/-His/-Ade (without tryptophan, leucine, adenine, and histidine) dropout medium. They were then cultured at 30°C for 5 days.
4.6 BiFC and LCI Assays
For the BiFC assays, CsCIPK20, CsCSN5, and CsVTC1 were fused with nYFP, cYFP, and nYFP, respectively. The fused vectors were carried by Agrobacterium GV3101 strains (Weidi, Shanghai, China). Different combinations of vectors were transiently expressed in the leaves of transgenic tobacco plants (H2B-RFP). CsCIPK20-nYFP/cYFP, nYFP/CsCSN5-cYFP, and CsVTC1-nYFP/cYFP were used as the control groups. CsCIPK20-nYFP/CsCSN5-cYFP and CsVTC1-nYFP/CsCSN5-cYFP were used as the experimental groups. After infiltration for 3 days, the fluorescence of YFP was recorded using a Zeiss LSM710 confocal laser scanning microscope (Zeiss, Oberkochen, Germany).
For the LCI assays, CsCIPK20-nLuc, CsCSN5-cLuc, CsCSN5-nLuc, and CsVTC1-cLuc vectors were constructed. The vectors were carried by Agrobacterium GV3101 strains (Weidi, Shanghai, China). Different vector combinations were transiently expressed in the leaves of the wild tobacco plants. nLuc/cLuc, CsCIPK20-nLuc/cLuc, nLuc/CsCSN5-cLuc, CsCSN5-nLuc/cLuc, and nLuc/CsVTC1-cLuc were used as the control groups. CsCIPK20-nLuc/CsCSN5-cLuc, CsCSN5-nLuc/CsVTC1-cLuc, and CsCIPK20-nLuc/CsVTC1-nLuc were used as the experimental groups. After infiltration for 3 days, the luciferin solution was sprayed onto the injected leaves and images were recorded using a Tanon 5200 apparatus (Tanon, Shanghai, China).
The LCI assays were used for competitive binding. Based on the LCI assay of CsCSN5-nLuc and CsVTC1-cLuc, the CsCIPK20-HA recombinant vector and HA empty vector were transformed into Agrobacterium GV3101 strain, and equal volumes of Agrobacterium carrying the corresponding construct pairs (CsCSN5-nLuc/CsVTC1-cLuc/HA and CsCSN5-nLuc/CsVTC1-cLuc/CsCIPK20-HA) were injected into wild-type tobacco leaves. Images were recorded using a Tanon 5200 apparatus (Tanon, Shanghai, China). The luminescence intensity was quantified using GloMax 20/20 system (Promega, Wisconsin, USA).
4.7 Dual-Luc Assays
The Dual-Luc assay was carried out to analyze the stability of the CsCIPK20 and CsVTC1 proteins, as previously described (Xu et al. 2018). The CsCIPK20 and CsVTC1 were placed under the control of the 35S promoter and were inserted into pGreenII 0800-Luc to generate 35S-CsCIPK20-Luc and 35S-CsVTC1-Luc constructs. The 35S-CsCSN5-HA and 35S-HA empty vectors co-expressed with the 35S-Luc vector in wild tobacco were used as the control groups. The 35S-CsCSN5-HA and 35S-HA empty vectors co-expressed with 35S-CsCIPK20-Luc and 35S-CsVTC1-Luc vectors in wild tobacco, respectively, were set as the experimental groups. After 2 days, chemiluminescence images were acquired using a Tanon 5200 system (Tanon, Shanghai, China). The luminescence intensity was quantified by GloMax 20/20 system (Promega, Wisconsin, USA). Six independent biological replicates were taken.
4.8 Cell-Free Degradation
The CsCIPK20-GST protein and CsVTC1-His were expressed in Escherichia coli BL21(DE3) cells and purified using Pierce Glutathione Agarose (ThermoFisher Scientific, California, USA) and BeyoGold His-tag Purification Resin (Beyotime, Shanghai, China), respectively. The total proteins of the wild tobacco overexpressed 35S-HA or 35S-CsCSN5-HA were extracted using an extraction buffer [10 mM ATP, 10 mM MgCl2, 10 mM NaCl, 25 mM Tris-HCl (pH 7.5), 5 mM DTT, and 0.1% Triton X-100]. The protein concentration was determined using a BCA protein assay kit (Kangwei, Beijing, China). Each reaction system contained 100 ng of CsCIPK20-GST (or CsVTC1-His) and 500 μg of total protein of wild tobacco-overexpressed 35S-HA or 35S-CsCSN5-HA. For the proteasome inhibitor experiments, 30 min before the experiment, 20 μM of MG132 was added. The reaction system was incubated at 22°C. Protein samples of CsCIPK20-GST were harvested at 0, 0.5, 1, 3, and 6 h. Protein samples of CsVTC1-His were harvested at 0, 1, 6, 12, and 24 h. Actin was used as the reference protein. An SDS-PAGE loading buffer was used to stop the reactions by boiling at 100°C for 10 min. The results were recorded using a Tanon 5200 apparatus (Tanon, Shanghai, China). The protein amounts were normalized by the reference protein and quantified to demonstrate the degradation of CsCIPK20 and CsVTC1.
4.9 Pull-Down Assays
The full-length coding sequences of CsCSN5, CsVTC1, and CsCIPK20 were ligated with the pMAL vector with a MBP tag (New England Biolabs, Massachusetts, USA), the pET vector with a His tag (Yeasen, Shanghai, China), and the pGEX vector with a GST tag (GE Healthcare Bio-Sciences, Uppsala, Sweden), respectively. The fusion recombinant proteins were induced and expressed in Escherichia coli strain BL21 (DE3). CsCSN5-MBP was incubated with MBP magnetic beads (New England Biolabs, Beijing, China) at 4°C for 3 h, and CsVTC1-His/GST or CsVTC1-His/CsCIPK20-GST proteins were added and incubated as indicated at 4°C overnight. After washing, the immunoprecipitated proteins were detected via immunoblot analysis with anti-MBP (M20051S), anti-His (M20001S), and anti-GST (M20007S) antibodies (Abmart, Shanghai, China).
4.10 Statistical Analysis
All experimental data with more than three replicates were analyzed using the IBM SPSS Statistics 21 software. Student's t-test was used to calculate significant differences between pairs.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (U22A20499 and 32372774), the special project of Zhejiang province (2020R52036), the China Agriculture Research System of MOF and MARA (CARS-19-01A), and the Chinese Academy of Agricultural Sciences through an Innovation Project for Agricultural Sciences and Technology (CAAS-ASTIP-2021-TRICAAS).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All data supporting the current findings are available in the paper and its Supplementary Material. The raw data used for RNA-seq analysis were uploaded to the NCBI (BioProject ID. PRJNA1080729).