SmHSFA8 Enhances the Heat Tolerance of Eggplant by Regulating the SmEGY3-SmCSD1 Module and Promoting SmF3H-mediated Flavonoid Biosynthesis
ABSTRACT
High temperature (HT) is a major environmental factor that restrains eggplant growth and production. Heat shock factors (HSFs) play a vital role in the response of plants to high-temperature stress (HTS). However, the molecular mechanism by which HSFs regulate heat tolerance in eggplants remains unclear. Previously, we reported that SmEGY3 enhanced the heat tolerance of eggplant. Herein, SmHSFA8 activated SmEGY3 expression and interacted with SmEGY3 protein to enhance the activation function of SmEGY3 on SmCSD1. Virus-induced gene silencing (VIGS) and overexpression assays suggested that SmHSFA8 positively regulated heat tolerance in plants. SmHSFA8 enhanced the heat tolerance of tomato plants by promoting SlEGY3 expression, H2O2 production and H2O2-mediated retrograde signalling pathway. DNA affinity purification sequencing (DAP-seq) analysis revealed that SmHSPs (SmHSP70, SmHSP70B and SmHSP21) and SmF3H were candidate downstream target genes of SmHSFA8. SmHSFA8 regulated the expression of HSPs and F3H and flavonoid content in plants. The silencing of SmF3H by VIGS reduced the flavonoid content and heat tolerance of eggplant. In addition, exogenous flavonoid treatment alleviated the HTS damage to eggplants. These results indicated that SmHSFA8 enhanced the heat tolerance of eggplant by activating SmHSPs exprerssion, mediating the SmEGY3-SmCSD1 module, and promoting SmF3H-mediated flavonoid biosynthesis.
1 Introduction
As the frequency and severity of extreme weather events increase, global climate change poses a considerable threat to agricultural production (Zandalinas, Fritschi, and Mittler 2021; Wang et al. 2024). Among them, extreme heat waves and rising temperatures impact plant growth and productivity (Yue et al. 2023). High-temperature stress (HTS) occurs when the ambient temperature exceeds the appropriate temperature for the normal growth and development of plants (Wahid et al. 2007; Shekhawat et al. 2022; Khan et al. 2023). HTS disturbs plant cellular homoeostasis, including the cell membrane system, antioxidant system, osmotic adjustment and photosystem, resulting in severe retardation of plant growth and development (Djanaguiraman et al. 2018; Niu and Xiang 2018; Abdelrahman et al. 2020; Jacott and Boden 2020; Janni et al. 2020).
To cope with HTS, plants have evolved a series of adaptive mechanisms, including genetic and epigenetic regulation (Liu et al. 2015; Jiang et al. 2017; Ohama et al. 2017; Gong et al. 2020). The accumulation of heat shock proteins (HSPs) is an effective way for plants to alleviate or prevent HTS damage (Shekhawat et al. 2022; Jahan, Nie, and Yan 2023). As molecular chaperones, HSPs play essential roles in protein folding, degradation and translocation, and protect organisms against damage under various stresses (Jacob, Hirt, and Bendahmane 2017; Farkas et al. 2018; Aghaie and Tafreshi 2020). HSPs are activated by heat shock factor (HSF)-dependent systems upon the perception of HTS via various sensors (Kotak et al. 2007; Hua 2009; Chen, Galli, and Gallavotti 2022). HSFs are the central regulators of the heat stress response (HSR) and play key roles in high temperature (HT) responsiveness (Larkindale and Vierling 2008; Yeh et al. 2012; Ohama et al. 2017). Under HTS, HSFs are able to activate the expression of downstream genes by binding heat shock cis-elements (HSEs; nGAAnnTTCn or nTTCnnGAAn) (Littlefield and Nelson 1999; Andrási, Pettkó-Szandtner, and Szabados 2021). HSFs are divided into three subfamilies on the basis of the structural characteristics of the oligomerization domain region: A, B and C (Nover et al. 2001; von Koskull-Döring, Scharf, and Nover 2007; Scharf et al. 2012). Within the HSFA subfamily, HSFA1s are the master regulators and directly regulate the expression of HSR genes, including DEHYDRATION-RESPONSIVE ELEMENT BINDING PROTEIN 2A (DREB2A), HSFA2, HSFA7a, HSFBs and MULTIPROTEIN BRIDGING FACTOR 1 C (MBF1C), thereby activating transcriptional networks in response to HTS (Yoshida et al. 2011). HSFA2, HSFA3, HSFA6 and HSFA7 also play critical roles in the plant response to HTS. In tall fescue, FaHSFA2c enhances the heat tolerance of plants by activating HSR genes (Wang et al. 2017). In Arabidopsis thaliana, AtHSFA3 regulates plant tolerance to HTS by inducing DREB2A (Yoshida et al. 2008; Schramm et al. 2008). In wheat, TaHSFA6e regulates heat tolerance by activating HSPs (Wen et al. 2023). In tomato, SlHSFA7 affectes heat tolerance by inducing SlHSFA1 (Mesihovic et al. 2022). Heat tolerance is also mediated by HSFB subfamily members. HSFBs positively regulate heat tolerance in the wild tomato Solanum peruvianum and grape (Bharti et al. 2004; Chen et al. 2023a, 2023b) but negatively regulate heat tolerance in A. thaliana and maize (Ikeda, Mitsuda, and Ohme-Takagi 2011; Qin et al. 2022). In addition, HSFC2 positively regulates the heat tolerance of plants by activating the expression of HSPs and HSFs in both wheat and lily (Zhuang et al. 2018; Wu et al. 2024).
Eggplant, an important Solanum vegetable crop species, was widely cultivated around the world (Taher et al. 2017). Statistics from the Food and Agriculture Organization of the United Nations (FAO, https://www.fao.org, accessed on 9th May 2024) indicated that the global harvest area of eggplant reached 1.962 million hectares in 2021, with a total output of approximately 59 million tons. The most suitable temperature for eggplant growth and development is 22°C–30°C. When the temperature exceeds 35°C, the growth and development of eggplant at the seedling, flowering and fruit stages are inhibited (Zhang and Huang 2011; Wu et al. 2020). During HTS, the cell membrane system, antioxidant machinery and photosynthetic system of eggplant are destroyed (Hannachi et al. 2022), and the nutrient contents of eggplant fruits, including polyphenols (Chumyam et al. 2013), chlorogenic acids (Šilarová et al. 2019) and anthocyanins (Lv et al. 2019; Zhang et al. 2019), are lost. The yield and quality of eggplant are severely reduced by HTS. In addition, relative electrolytic leakage (REL) and malondialdehyde (MDA) content are the important choices for rapidly identifying the heat tolerance of eggplant, which can effectively evaluate cell membrane damage and oxidative stress degree (Faiz et al. 2020). However, the molecular mechanism by which HSFs regulate heat tolerance in eggplants has not been reported.
In previous studies, SmEGY3 was shown to play positive roles in the heat tolerance of eggplant by promoting SmCSD1-mediated H2O2 production and H2O2-mediated retrograde signalling pathway (Liu et al. 2024). In this study, we demonstrated that SmHSFA8 directly regulated SmEGY3 expression and enhanced the function of SmEGY3 by interacting with it. SmHSFA8 was identified as a central regulator of the HT response in eggplants and positively regulated the heat tolerance of eggplant by activating SmHSPs expression, mediating the SmEGY3-SmCSD1 module and promoting SmF3H-mediated flavonoid biosynthesis. These findings help elucidate the regulatory mechanisms underlying heat tolerance in eggplants.
2 Materials and Methods
2.1 Plant Materials and Growth Conditions
The thermotolerant eggplant line ‘E390’, intermediate heat-tolerant eggplant line ‘19009’ and thermosensitive eggplant line ‘HM’ were identified in a previous study (Liu et al. 2023). The eggplant line ‘19009’ was used for the virus-induced gene silencing (VIGS) assay, tobacco (Nicotiana benthamiana) was used for the functional verification assays and the A. thaliana Columbia (Col) ecotype and tomato (Solanum lycopersicum cv. Micro-Tom) were used to generate transgenic plants. Eggplant and tomato seedlings were grown under a 25/18°C day/night temperature with a 16/8 h light/dark photoperiod, a relative humidity of 60%–70% and a light intensity of 6000 lux. Tobacco seedlings were grown under a 25/18°C day/night temperature with a 16/8 h light/dark photoperiod, a relative humidity of 60%–70% and a light intensity of 3000 lux. A. thaliana seedlings were grown under a 22/18°C day/night temperature with a 16/8 h light/dark photoperiod, a relative humidity of 60%–70% and a light intensity of 3000 lux.
2.2 HT, Exogenous H2O2, and Exogenous Flavonoid Treatments
For high-temperature treatment (HTT), several plants of the eggplant lines ‘E390’, ‘19009’ and ‘HM’ with three or four true leaves were transferred into a growth chamber at different temperatures (25, 33, 38 or 42°C) with a 16/8 h light/dark photoperiod, a relative humidity of 60%–70% and a light intensity of 6000 lux for 72 h. The roots, stems and second true leaves (from the bottom to the top) of the eggplant line ‘19009’ not subjected to HTT were collected for tissue-specific analysis. The second true leaves of the eggplant line ‘19009’ were collected after treatment at different temperatures for 24 h, and the second true leaves of the eggplant lines ‘E390’ and ‘HM’ were collected at 0, 1, 6, 12, 24, 48 and 72 h post-42°C HTT for gene expression analysis.
For H2O2 treatment, water, 50 mM and 100 mM H2O2 solution was used to spray the leaves of the eggplant line ‘19009’ until three or four true leaves were dripping. The second true leaves of eggplant seedlings were collected at 24 h post-H2O2 treatment for gene expression analysis.
For flavonoid treatment, according to a previous study (Kang et al. 2022), rutin (0, 0.25, 0.75 and 2.5 g) was separately weighed and dissolved in 0.5 L of ethanol, and the rutin ethanol solution was sonicated in a water bath with ice for 30 min before application. The solution was used for spraying the leaves of the eggplant line ‘19009’ with three or four true leaves until the leaves were dripping. After spraying for 12 h, the treated plants were subjected to HTT (42/42°C day/night temperature, 16/8 h light/dark photoperiod) for 4 days. After HTT for 4 days, the heat injury index was measured. The eggplant heat injury index was rated as follows: 0, healthy; 1, first true leaf wilted; 2, second true leaf wilted; 3, third true leaf wilted; 4, all leaves wilted; 5, the stem wilted. The heat injury index was equal to Σ (incidence grade×the number of corresponding grade plants)/(highest grade×total plants) × 100. The treated plants were subsequently subjected to phenotypic observation and determination of the chlorophyll fluorescence and photosynthetic parameters, and the leaves were collected for determination of the REL and MDA content. Three biological replicates and three technical replicates of each biological replicate were included for all the samples. All the samples were placed in liquid nitrogen and then stored at −80°C for further experiments.
2.3 Bioinformatics Analysis
DNA and amino acid sequence alignment were performed using DNAMAN software. The phylogenetic evolutionary trees were generated using MEGA X via the neighbour-joining method and visualised and optimised using iTOL V5 (Letunic and Bork 2021). Gene collinearity analysis among the HSFA8 genes from A. thaliana, eggplant, tomato, pepper and potato were conducted with MCScanX tools (Wang et al. 2012) and visualised via multiple synteny plot tools in TBtools software (Chen et al. 2020). Information on the HSFA8 genes from A. thaliana, eggplant, tomato, pepper and potato was derived from the genome databases TAIR10 (https://www.arabidopsis.org/), S. melongena HQ-1315 (http://eggplant-hq.cn/Eggplant/home/index), ITAG4.0 (https://solgenomics.net/organism/Solanum_lycopersicum/genome/), Capsicum annuum zunla (http://peppersequence.genomics.cn/) and DM 1-3 516 R44-v6.1 (http://spuddb.uga.edu/dm_v6_1_download.shtml). The 1500 bp nucleotide sequences upstream of the start codon of SmHSFA8 were uploaded to the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) with default parameters to predict cis-elements (Lescot 2002).
2.4 DNA Affinity Purification Sequencing (DAP-Seq) Analysis
Genomic DNA was extracted from mature leaves of the eggplant line ‘E390’ using a MICH DNA Clean Beads (NGS-0201-100, Bluescape Hebei Biotech, Baoding, China) with two biological replicates. The construction of the library was carried out via the MICH TLX DNA-Seq Kit (NGS 0602-24, Bluescape Hebei Biotech, Baoding, China). DAP-seq experiments were performed according to a published protocol (Bartlett et al. 2017). Specifically, SmHSFA8 protein was prepared using the TNT SP6 Coupled Wheat Germ Extract System (L5030, Promega, Beijing, China), then immobilised on Magne HaloTag Beads (G7281, Promega, Beijing, China) and incubated with the constructed DNA library. Unbound DNA fragments were removed by washing the beads three times. The SmHSFA8-binding DNA fragments were then eluted and amplified with the indexed primers. Subsequent sequencing was performed with an Illumina NovaSeq instrument (NovaSeq. 6000, Illumina, San Diego, USA). The reads were mapped to the eggplant genome ‘HQ-1315’ using Bowtie2 (Langmead and Salzberg 2012). To identify SmHSFA8-binding sites, DAP-seq peaks were screened with MACS2 (fold enrichment > 2 and q < 0.05) (Zhang et al. 2008). Next, the locations of the DAP-seq peaks upstream or downstream of the transcription start site within the 2000 bp region were analysed via Homer software (Heinz et al. 2010). The SmHSFA8-targeted genes were identified via BEDtools (Quinlan and Hall 2010). Motif identification was performed using the Meme-ChIP suite 5.0.5 (Machanick and Bailey 2011).
2.5 RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT‒PCR)
The total RNA of the roots, stems and second true leaves of eggplant and the leaves of A. thaliana and tomato were isolated and extracted using an Eastep Super Total RNA Extraction Kit (LS1040, Promega, Beijing, China). The primers for qRT‒PCR were designed using Primer 5.0 and listed in Table S1. All qRT‒PCR analyses were performed using ChamQ Universal SYBR qPCR Master Mix (Q711–02/03, Vazyme, Nanjing, China). The relative gene expression was calculated via the 2−ΔΔCt method (Livak and Schmittgen 2001).
2.6 Subcellular Localisation Assay
The coding sequences (CDSs) of SmHSFA8 without the termination codon were cloned and inserted into the pGreenII-C18 vector. The recombinant plasmids and nuclear localisation signal (NLS-DsRed) were transformed into Agrobacterium tumefaciens GV3101 (psoup) strain (AC1002, Weidi Biotechnology, Shanghai, China), which was subsequently mixed at a 1:1 ratio and injected into the leaves of 5- to 6-week-old tobacco. After 72 h, the tobacco leaves were collected for fluorescence microscopy observation (BX53F, Carl Zeiss, Jena, Germany).
2.7 Transcriptional Activation Region Analysis
The CDSs of SmHSFA8 were truncated according to the conserved domain. The segmented sequences were cloned and inserted into the pGBKT7 (BD) vector. The recombinant plasmids were subsequently transformed into Y2HGold yeast strain (YC1002, Weidi Biotechnology, Shanghai, China). Positive transformants were selected by culturing on SD/-Trp and SD/-Trp-Ade-His media. The positive transformants were subsequently stained with X-α-Gal (SL0940, Coolaber, Beijing, China).
2.8 Transcriptional Activity Analysis
Transcriptional activation analysis assays were performed using a Dual-Luciferase Reporter Assay System (E1910, Promega, Beijing, China). The CDSs of SmHSFA8 were ligated into the pEAQ-PBD vector. The empty vector, reporter gene (GAL-LUC) and generated constructs were separately transformed into Agrobacterium tumefaciens GV3101 strain (AC1001, Weidi Biotechnology, Shanghai, China). The Agrobacterium tumefaciens strain harbouring the empty vector or generated construct and reporter at a 1:1 ratio was coinfiltrated into the leaves of 5- to 6-week-old tobacco. After 72 h, the tobacco leaves were collected, and the activities of REN and LUC were measured according to the protocol of the Dual-Luciferase Reporter Assay System.
2.9 VIGS Assay
To identify the functions of SmHSFA8 and SmF3H in the eggplant response to HTS, SmHSFA8 and SmF3H were knocked down through a VIGS assay. A 300 bp fragment of SmHSFA8 or SmF3H was ligated into the pTRV2 vector, respectively. The Agrobacterium tumefaciens GV3101 strain harbouring pTRV1 was mixed with the Agrobacterium tumefaciens GV3101 strain harbouring pTRV2-SmHSFA8 or pTRV2-SmF3H plasmid at a 1:1 ratio and then incubated in the dark at 28°C for 2 h. The mixed bacterial solution was injected into the cotyledons of 2-week-old eggplant seedlings, which were placed at 20°C in the dark for 48 h. Then, the eggplant seedlings were transferred to a standard culture chamber under a 25/18°C day/night temperature with a 16/8 h light/dark photoperiod, a relative humidity of 60%–70% and a light intensity of 6000 lux. After 2 weeks, the second true leaves of the transformed plants were collected for gene expression analysis. The SmHSFA8-silenced and SmF3H-silenced eggplant plants were subsequently subjected to HTT (42/42°C day/night temperature, 16/8 h light/dark photoperiod) for 4 days. The treated plants were first subjected to phenotypic observation, and then the leaves were collected for determination of the REL, MDA content, flavonoid content and gene expression.
2.10 Genetic Transformation of Tomato
To overexpress SmHSFA8 in tomato, the CDSs of SmHSFA8 without the termination codon were ligated into the BG-plant-GFP vector. The generated construct was introduced into Agrobacterium tumefaciens GV3101 strain, which was subsequently transformed into tomato plants via a previously described method (Fan et al. 2018). The positive transformants were identified according to the transcript level of SmHSFA8 and three independent positive transformed lines (T2) were used for subsequent experiments. The wild-type (WT) and OE-SmHSFA8 transgenic tomato plants (4-week-old) were subjected to HTT (42/42°C day/night temperature, 16/8 h light/dark photoperiod) for 4 days. After HTT for 4 days, the heat injury index was measured. The tomato heat injury index was rated as follows: 0, healthy; 1, single leaf wilted; 2, a branch of the leaves wilted; 3, multiple branches of the leaves wilted; 4, all leaves wilted; 5, the stem wilted. The tomato heat injury index was equal to Σ (incidence grade×the number of corresponding grade plants)/(highest grade×total plants) × 100. The treated plants were subjected to phenotypic observation and determination of the chlorophyll fluorescence and photosynthetic parameters, and the leaves were subsequently collected for determination of the REL, MDA content, H2O2 content, flavonoid content and gene expression.
2.11 Genetic Transformation of A. thaliana
To overexpress SmHSFA8 in A. thaliana, the CDSs of SmHSFA8 without the termination codon were ligated into the pBI121-GFP vector, whereas the 1500 bp nucleotide sequences upstream of the start codon of SmHSFA8 were ligated into the CP013 vector. The generated constructs were subsequently introduced into Agrobacterium tumefaciens GV3101 strain. Transgenic A. thaliana lines were generated via the floral-dip method (Clough and Bent 1998) and the transgenic A. thaliana plants were screened using 1/2 MS media supplemented with 50 mg/L kanamycin. The seeds of the T2 transgenic A. thaliana lines were harvested for subsequent experiments. WT and OE-SmHSFA8 transgenic A. thaliana plants were planted in the soil for PCR and gene expression analysis, and they were subsequently subjected to HTT (43/43°C day/night temperature, 16/8 h light/dark photoperiod) for 24 h and recovery treatment (22/22°C day/night temperature, 16/8 h light/dark photoperiod) for 5 d. The phenotypes of the plants were observed, and the flavonoid content and gene expression were determined after HTT for 24 h, whereas the seedling survival rate and fresh weight were determined after HTT and recovery treatment for 5 d. In addition, WT and OE-SmHSFA8 transgenic A. thaliana plants were seeded into MS media, and they were subsequently subjected to HTT (44°C) for 50 min and recovery treatment (22/22°C day/night temperature, 16/8 h light/dark photoperiod) for 5 or 7 days. The seedling survival rate was measured after HTT and recovery treatment for 5 days, whereas the number of lateral roots was counted after HTT and recovery treatment for 7 days. HT phenotypes of the plants cultivated in the soil were analysed using 27 seedlings (n = 27), whereas those in MS media were analysed using 50 seedlings (n = 50). Three and four repeated experiments were performed, respectively.
2.12 GUS Activity Staining Assay
A GUS activity staining assay was performed using CP013-ProSmHSFA8-GUS transgenic A. thaliana lines. The plants were stained using a GUS stain kit (SL7160, Coolaber, Beijing, China), and GUS expression was observed after decolorisation.
2.13 Determination of Physiological and Biochemical Indices
The REL of the leaves was determined using a conductivity metre (DDS-307, Shanghai instrument, Shanghai, China) and the MDA content was measured via a reaction with thiobarbituric acid (TBA) (Sun et al. 2012). The flavonoid content of the leaves was determined via a flavonoid content determination kit (HTC-1-W, Comin, Suzhou, China) and the H2O2 content was measured via a H2O2 content determination kit (H2O2–1-Y, Comin, Suzhou, China). Chlorophyll fluorescence and photosynthetic parameters were evaluated via a chlorophyll fluorescence imager (PlantExplorer, PhenoVation, Wageningen, Netherlands) (Liang et al. 2024). The plants were dark-adapted for 10–30 min. Then, the chlorophyll fluorescence of the entire plant was measured from the top. The fluorescence intensity was measured by applying a saturating pulse of 3000 μmol photons m−2s−1 for 1 s, and the fluorescence intensities were stored at a 12-bit resolution. In addition, the leaf area of plants was determined via a ImageJ software.
2.14 Yeast One-Hybrid (Y1H) Assay
The CDSs of SmHSFA8 were recombined into the pGADT7 (AD) vector and the truncated segment sequences upstream of the start codons of SmHSP70, SmHSP21, SmHSP70B, SmF3H and SmEGY3 were separately recombined into the pAbAi vector. The Y1H assay was performed according to the manufacturer's instructions (PT4087-1, Clontech, Takara, China). The AD-SmHSFA8 vector was transformed into Y1HGold yeast strain (YC1001, Weidi Biotechnology, Shanghai, China) harbouring the pAbAi-recombinant plasmid. Then, they were spotted onto SD/-Leu and SD/-Leu + Aureobasidin A (AbA) (60231ES03, YEASEN, Shanghai, China) plates.
2.15 Electrophoretic Mobility Shift Assay (EMSA)
The CDSs of SmHSFA8 were cloned and inserted into the pMAL-c2X vector, and upon expression, SmHSFA8-MBP fusion protein was obtained. Biotin-labelled probes for SmHSP70, SmHSP21, SmHSP70B, SmF3H and SmEGY3 were synthesised by Sangon Biotech (Shanghai, China). The probe sequences were listed in Table S1. The protein and biotin-labelled probes were incubated as previously described (Wu et al. 2022). EMSAs were performed using a LightShift Chemiluminescent EMSA kit (20148, Thermo Scientific, Waltham, USA) according to the manufacturer's protocols.
2.16 Dual-Luciferase Reporter (DLR) Assay
The CDSs of SmHSFA8 and SmEGY3 were inserted into the pEAQ vector as effectors, whereas the promoter sequences of SmHSP70, SmHSP21, SmHSP70B, SmF3H, SmEGY3 and SmCSD1 were recombined into the pGreenII-0800 vector as reporters. The Agrobacterium tumefaciens GV3101 strain harbouring the pEAQ/pEAQ-SmHSFA8 plasmid mixed with the Agrobacterium tumefaciens GV3101 (psoup) strain harbouring the pGreenII-0800-ProSmHSP70/-ProSmHSP21/-ProSmHSP70B/-ProSmF3H/-ProSmEGY3 plasmid at a 20:1 ratio was infiltrated into the leaves of 5- to 6-week-old tobacco. While the Agrobacterium tumefaciens GV3101 strain harbouring the pEAQ/pEAQ-SmEGY3/pEAQ-SmHSFA8/pEAQ-SmHSFA8+pEAQ-SmEGY3 plasmid mixed with the Agrobacterium tumefaciens GV3101 (psoup) strain harbouring the pGreenII-0800-ProSmCSD1 plasmid at a 20:1 ratio was also infiltrated into the leaves of 5- to 6-week-old tobacco. After 72 h, the tobacco leaves were collected, and the activities of REN and LUC were measured according to the protocol of the Dual-Luciferase Reporter Assay System.
2.17 Yeast Two-Hybrid (Y2H) Assay
The CDSs of SmHSFA8 were recombined into the BD vector, whereas the CDSs of SmEGY3 were recombined into the AD vector. The recombinant plasmids were subsequently transformed into Y2HGold yeast strain. Positive transformants were selected by culturing on SD/-Trp-Leu and SD/-Trp-Leu-Ade-His media. Self-activation of SmHSFA8 was inhibited by 3-amino-1,2,4-triazole (3-AT) (SL0930, Coolaber, Beijing, China). The positive transformants were subsequently stained with X-α-Gal.
2.18 Bimolecular Fluorescence Complementation (BIFC) Assay
The CDSs of SmHSFA8 without the termination codon were recombined into the pSPYNE-35S (YNE) vector, and the CDSs of SmEGY3 without the termination codon were recombined into the pSPYCE-35S (YCE) vector. The recombinant plasmids and nuclear localisation signal (NLS-DsRed) were transformed into Agrobacterium tumefaciens GV3101 strain, which was subsequently mixed at a 1:1:1 ratio and injected into the leaves of 5- to 6-week-old tobacco. After 72 h, the tobacco leaves were collected for fluorescence microscopy observation (BX53F, Carl Zeiss, Jena, Germany).
2.19 Luciferase Complementary Imaging (LCI) Assay
The CDSs of SmHSFA8 without the termination codon were recombined into the 1300-nLUC (nLUC) vector, and the CDSs of SmEGY3 were recombined into the 1300-cLUC (cLUC) vector. The recombinant plasmids and auxiliary plasmid (P19) were transformed into Agrobacterium tumefaciens GV3101 strain, which was subsequently mixed at a 1:1:1 ratio and injected into the leaves of 5- to 6-week-old tobacco. After 72 h, D-luciferin potassium salt D (1 mM) (40902ES01, YEASEN, Shanghai, China) was injected into the tobacco leaves, and the tobacco leaves were collected for fluorescence observation via a fluorescence chemiluminescence gel imaging system (Universal Hood II, Bio-Rad ChemiDOCXRS + , Shanghai, China).
3 Results
3.1 SmHSFA8 Activates SmEGY3 Expression and Enhances the Function of SmEGY3 by Interacting With It
In our previous studies, SmEGY3 played positive roles in the heat tolerance of eggplant by promoting SmCSD1-mediated H2O2 production and H2O2-mediated retrograde signalling pathway (Liu et al. 2024). As a bait protein, SmEGY3 was used to screen the interaction proteins in the cDNA library in eggplants after HTT. SmHSFA8, a heat shock transcription factor, was identified to interact with SmEGY3 (Figure 1A). The interaction result was confirmed by LCI and BiFC assays (Figure 1B,C). Moreover, we also found that SmHSFA8 could bind the promoter of SmEGY3 and activate its expression based on the results of Y1H, EMSA and DLR assays (Figure 1D–F). In the DLR assay, when SmHSFA8 protein was cotransferred with SmEGY3 protein, the expression of SmCSD1 was significantly enhanced (Figure 1G), suggesting that the interaction between SmHSFA8 and SmEGY3 enhanced the activation function of SmEGY3 on SmCSD1. These results suggested that SmHSFA8 might participate in regulating heat tolerance in eggplants. In eggplants, SmHSFA8 contained the typical DNA-binding domain, HR-A/B region, temperature-dependent repression domain, AHA domain and NLS motif (Figure S1). Phylogenetic analysis revealed that HSFA8 genes were relatively conserved in A. thaliana, potato, tomato, pepper and eggplant, and that they were clustered in the same branch as the HSFA1s genes, which functioned in regulating heat tolerance in plants (Yoshida et al. 2011) (Figure S2A). In addition, the homologues of HSFA8 gene were identified in specific Solanaceae species (potato, eggplant and tomato), but they were not found in Capsicum species (Figure S2B), suggesting that the homologues of HSFA8 gene possessed species specificity.
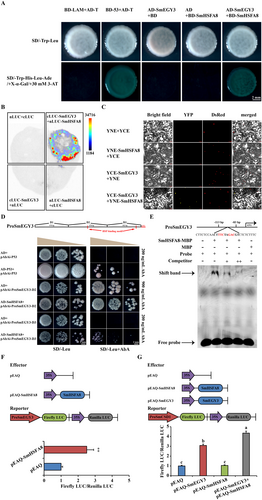
3.2 Expression Pattern and Transcriptional Activity Analyses of SmHSFA8 in Eggplants
To analyse the expression pattern and molecular characteristics of SmHSFA8 in eggplants, qRT‒PCR, GUS staining assay, subcellular localisation assay, transcriptional activation region analysis and transcriptional activity analysis were performed. SmHSFA8 was expressed in the roots, stems and leaves of eggplant (Figure S2C). After HTT, SmHSFA8 was upregulated in the thermotolerant (E390) and thermosensitive (HM) eggplant lines (Figure S2D). SmHSFA8 was induced by exogenous H2O2 treatment (Figure S2E). In addition, the transcript level of SmHSFA8 increased in a dose-dependent manner with temperature (Figure S2F). Several cis-elements, including LTRs, TGA elements, and CGTCA, TCCC and TGACG motifs, were found in the promoter of SmHSFA8 (Figure S3A). The GUS staining assay results indicated that the activity of GUS driven by the SmHSFA8 promoter was strongly induced in the stems and leaves of three independent transgenic A. thaliana plants (Figure S3B). SmHSFA8-Green fluorescent protein (GFP) fusion protein was localised in the cell nucleus (Figure S4A). The transcriptional activation region of SmHSFA8 was located at amino acid residues 193-374 (Figure S4B). The DLR assay results suggested that SmHSFA8 had transcriptional activation activity in planta, suggesting that SmHSFA8 functioned as an activator in response to HT (Figure S4C).
3.3 SmHSFA8 Positively Regulates the Heat Tolerance of Plants
To evaluate the function of SmHSFA8 under HTS, two representative SmHSFA8-silenced (by VIGS) eggplant plants were obtained, and the expression of SmHSFA8 was significantly lower in the SmHSFA8-silenced eggplant plants than in the control plants (pTRV2 empty vector-transformed plants) (Figure 2A). There was no significant difference in phenotypes between the control plants and SmHSFA8-silenced eggplant plants at room temperature (RT) (Figure 2B). The control and SmHSFA8-silenced eggplant plants were subsequently subjected to HTT for 4 days. The control plants presented robust flat leaves, whereas the SmHSFA8-silenced eggplant plants presented dwarfism with shrunken and drooped leaves (Figure 2B). In addition, the MDA content and REL significantly increased in the SmHSFA8-silenced eggplant plants compared with those in the control plants after HTT for 4 days (Figures 2C,D).
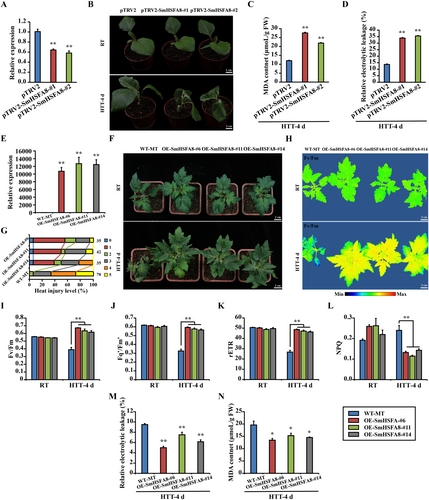
SmHSFA8 was overexpressed in tomato to further determine its function under HTS. Three OE-SmHSFA8 transgenic tomato lines were identified and were found to have more highly SmHSFA8 expression than the WT plants (Figure 2E). The leaf area of the OE-SmHSFA8 transgenic tomato plants was significantly greater than that of the WT plants at RT (Figure 2F and Figure S5). After HTT for 4 days, the leaves of the WT plants were shrunken and drooped, whereas the OE-SmHSFA8 transgenic tomato plants still grew normally (Figure 2F). The heat injury index of the OE-SmHSFA8 transgenic tomato plants was lower than that of the WT plants after HTT for 4 d (Figure 2G). There was no significant difference in the chlorophyll fluorescence, Fv/Fm, Fq'/Fm', rETR and NPQ between the control plants and SmHSFA8-silenced eggplant plants at RT (Figure 2H–L). After HTT for 4 days, the chlorophyll fluorescence, Fv/Fm, Fq'/Fm' and rETR of the OE-SmHSFA8 transgenic tomato plants were significantly greater than those of the WT plants (Figure 2H-K), whereas NPQ was significantly lower than that of the WT plants (Figure 2L). Compared with those of the WT plants, the REL and MDA content significantly decreased in the OE-SmHSFA8 transgenic tomato plants after HTT for 4 d (Figure 2M,N). Moreover, the expression of SlEGY3 and H2O2-mediated retrograde signalling genes (SlAPX2, SlZAT10 and SlWRKY33) and H2O2 content significantly increased in the OE-SmHSFA8 transgenic tomato plants at RT and HTT compared with the WT plants, and the expression of SlERF6 significantly increased in the OE-SmHSFA8 transgenic tomato plants at HTT (Figure S6). These results suggested that SmHSFA8 promoted H2O2 content and H2O2-mediated retrograde signalling pathway by directly activating SlEGY3 expression, thus enhancing the heat tolerance of tomato plants.
SmHSFA8 was also overexpressed in A. thaliana. Three OE-SmHSFA8 transgenic A. thaliana lines were identified (Figure S7A), and were found to have more highly SmHSFA8 expression than the WT plants (Figure S7B). No significant difference was found between the WT and OE-SmHSFA8 transgenic A. thaliana plants at RT. One day after HTT, the leaves of the WT plants were shrunken and drooped, but those of the OE-SmHSFA8 transgenic A. thaliana plants were still flattened. After recovery treatment for 5 days, the WT plants were small and even died, whereas the OE-SmHSFA8 transgenic A. thaliana plants exhibited normal growth (Figure S7C). The seedling survival rate and fresh weight were significantly greater in the OE-SmHSFA8 transgenic A. thaliana plants than in the WT plants after HTT for 1 d following recovery treatment for 5 days (Figure S7D,E). Moreover, the phenotypes of the WT and OE-SmHSFA8 transgenic A. thaliana plants in MS media under HTS were also analysed. After a brief period of HTT and recovery treatment for 7 days, the roots maintained normal growth with abundant lateral roots in the OE-SmHSFA8 transgenic A. thaliana plants but were suppressed in the WT plants (Figure S7F,H). A large proportion of the WT plants died, but the OE-SmHSFA8 transgenic A. thaliana plants maintained normal growth after a brief period of HTT and recovery treatment for 5 days (Figure S7G,I). These results demonstrated that SmHSFA8 played a positive role in regulating the heat tolerance of plants.
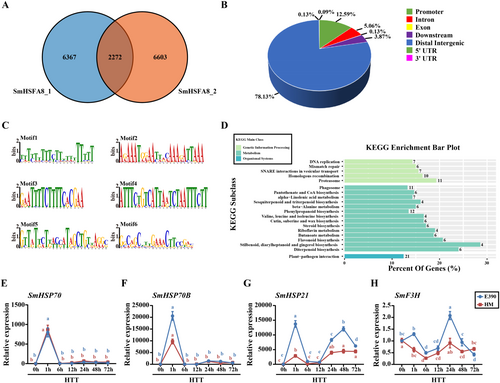
3.4 DAP-Seq Analysis of Candidate Target Genes and Downstream Pathways of SmHSFA8
A DAP-Seq assay was performed to elucidate the molecular mechanism by which SmHSFA8 regulates heat tolerance in eggplants. A total of 2272 SmHSFA8-binding peaks were identified based on two biological replicates (Figure 3A). Among these peaks, 12.59% were located within the promoter regions of the target genes (Figure 3B), which corresponded to 286 genes (Table S2). Six motifs with high confidence were identified using the Meme-ChIP suite 5.0.5 that might be the binding sites of SmHSFA8 (Figure 3C). In particular, the sequences of Motifs 3 and 6 were highly similar to those of HSEs, which are typical motifs of the binding sites of HSFs (Littlefield and Nelson 1999; Andrási, Pettkó-Szandtner, and Szabados 2021). HSFs usually participate in regulating the heat tolerance of plants by activating HSPs expression (Kotak et al. 2007; Hua 2009; Chen, Galli, and Gallavotti 2022). Several SmHSPs containing motifs similar to Motifs 3 and 6 were identified, including SmHSP70, SmHSP70B and SmHSP21. Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis revealed that the target genes of SmHSFA8 were involved in multiple metabolic processes, such as flavonoid biosynthesis (Figure 3D), and that SmF3H was involved in flavonoid biosynthesis. Moreover, SmHSP70, SmHSP70B, SmHSP21 and SmF3H were induced by HTS (Figure 3E–H). SmHSP70B, SmHSP21 and SmF3H were more highly expressed in the ‘E390’ eggplant line than in the ‘HM’ eggplant line after HTT (Figure 3F–H).
3.5 SmHSFA8 Regulates HSPs Expression in Plants
Y1H, EMSA and DLR assays were conducted to determine the effect of SmHSFA8 on SmHSPs. Y1H results revealed that SmHSFA8 bound the promoters of SmHSP70, SmHSP70B and SmHSP21 (Figure 4A). The SmHSFA8-MBP fusion protein was expressed and purified from Escherichia coli. SmHSFA8 could bind to the regulatory regions of SmHSP70, SmHSP70B and SmHSP21 by recognising the predicted binding motif (Figure 4B–D). DLR assay results revealed that SmHSFA8 was able to activate the expression of SmHSP70, SmHSP70B and SmHSP21 (Figure 4E). In addition, compared with the control and WT plants, the expression of HSP70, HSP70B and HSP21 significantly decreased in the SmHSFA8-silenced eggplant plants (Figure S8A–C) but significantly increased in the OE-SmHSFA8 transgenic A. thaliana and tomato plants at RT and HTT (Figure S8D–I).
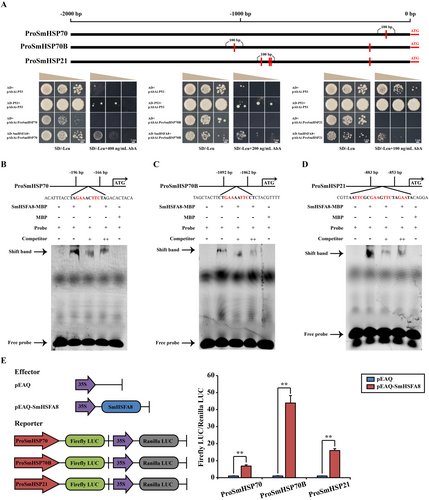
3.6 SmHSFA8 Regulates Flavanone-3-Hydroxylase (F3H) Expression and Flavonoid Content in Plants
Y1H, EMSA and DLR assays were also conducted to determine the effect of SmHSFA8 on SmF3H. The results revealed that SmHSFA8 could bind the promoter of SmF3H and activate its expression (Figure 5A–C). Compared with the control and WT plants, the expression of F3H and flavonoid content significantly decreased in the SmHSFA8-silenced eggplant plants but increased in the OE-SmHSFA8 transgenic A. thaliana and tomato plants at RT and HTT (Figure 5D–I). These results demonstrated that SmHSFA8 regulated the heat tolerance of plants by promoting SmF3H-mediated flavonoid biosynthesis.
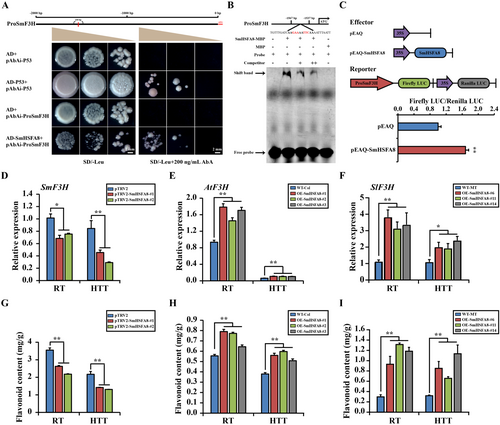
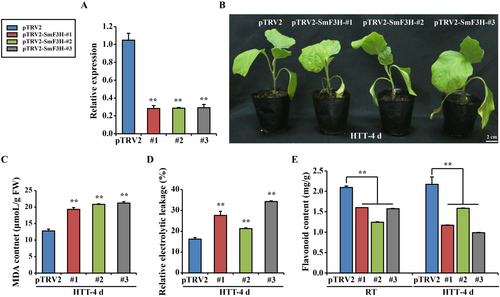
Three SmF3H-silenced lines generated via VIGS were produced from eggplants (Figure 6A). When exposed to HTT for 4 days, the control plants presented normal growth with relatively flat leaves; however, the SmF3H-silenced eggplant plants presented inhibited growth with shrunken, drooped, or even dried-up leaves (Figure 6B). Compared with those of the control plants, the MDA content and REL significantly increased in the SmF3H-silenced eggplant plants after HTT for 4 days (Figure 6C,D). Under RT condition and 4 days of HTT, the flavonoid content significantly decreased in the SmF3H-silenced eggplant plants compared with the control plants (Figure 6E). These results revealed that SmF3H affected the flavonoid biosynthesis and heat tolerance in eggplants.
3.7 Flavonoids are Involved in Regulating the Heat Tolerance of Eggplant
To investigate the effect of flavonoids on the heat tolerance of eggplant, the determination of flavonoid content and exogenous flavonoid treatment assays were performed. The flavonoid content in the ‘HM’ eggplant line significantly decreased, whereas it gradually increased in the ‘E390’ eggplant line under HTS (Figure S9). No significant difference in phenotypes between the 0.5, 1.5 and 5 mg/mL exogenous flavonoid-treated eggplant plants and the control plants was found at RT (Figure S10A). After exposure to HTT for 4 days, the leaves of the control plants presented wilting symptoms, whereas the leaves of the 5 mg/mL exogenous flavonoid-treated eggplant plants remained robust (Figure S10A). The chlorophyll fluorescence of the control plants was similar to that of the exogenous flavonoid-treated eggplant plants at RT. However, the chlorophyll fluorescence of the 5 mg/mL exogenous flavonoid-treated eggplant plants remained stable after HTT for 4 days compared with other eggplants plants (Figure S10B). The Fv/Fm, Fq'/Fm' and rETR of the exogenous flavonoid-treated eggplant plants were significantly greater than those of the control plants after HTT for 4 days (Figure S10C–E), while the NPQ of the 5 mg/mL exogenous flavonoid-treated eggplant plants was lower than that of the control plants after HTT for 4 days (Figure S10F). In addition, the heat injury index of the exogenous flavonoid-treated eggplant plants was lower than that of the control plants (Figure S10G) after HTT for 4 days, and the MDA content and REL of the exogenous flavonoid-treated eggplant plants were significantly lower than those of the control plants (Figure S10H and S10I).
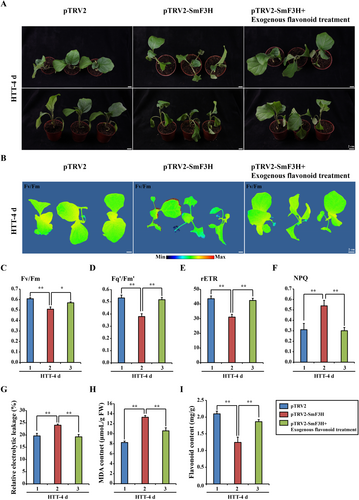
Furthermore, the SmF3H-silenced eggplant plants were treated with 5 mg/mL exogenous flavonoids. Compared with those of SmF3H-silenced eggplant plants, the leaves of the control plants and the SmF3H-silenced eggplant plants treated with exogenous flavonoids remained robust after HTT for 4 d (Figure 7A). The chlorophyll fluorescence, Fv/Fm, Fq'/Fm' and rETR of the control plants and the SmF3H-silenced eggplant plants treated with exogenous flavonoids were significantly greater than those of the SmF3H-silenced eggplant plants after HTT for 4 days (Figure 7B–E), whereas thier NPQ was lower than that of the SmF3H-silenced eggplant plants (Figure 7F). The MDA content and REL of the control plants and the SmF3H-silenced eggplant plants treated with exogenous flavonoids were significantly lower than those of the SmF3H-silenced eggplant plants after HTT for 4 days (Figures 7G,H). In addition, the flavonoid content was significantly lower in the SmF3H-silenced eggplant plants than in the control plants and the SmF3H-silenced eggplant plants treated with exogenous flavonoids (Figure 7I). These results indicated that flavonoids are involved in regulating the heat tolerance of eggplant.
4 Discussion
HTS restricts the growth, commodity quality and yield of eggplant (Zhang and Huang 2011; Wu et al. 2020). HSFs are important factors for plant response to various abiotic stresses, especially HTS (von Koskull-Döring, Scharf, and Nover 2007). The HSF gene family has been identified in eggplants (Gong et al. 2021). However, studies on the regulation of heat tolerance in eggplants by HSFs have not been reported. In our previous studies, SmHSFA8 was identified as a candidate gene in response to HTS via transcriptome analysis and by yeast assay (Liu et al. 2023). In this study, we elucidated the molecular mechanism by which SmHSFA8 regulated the heat tolerance of eggplant, which provids a basis for adaptation to climate change and eggplant production under HTS.
In plants, protein interaction is a complex process. Plant proteins usually perform their biological functions by interacting with other proteins (Sun et al. 2020). In pepper, RING-type E3 ligase CaASRF1 interacted with CaJAZ1-03 and ubiquitinated it (Koh et al. 2023). In tomato, SlIAA23 reduced the transcriptional inhibition ability of SlARF6 by interacting with it (Li et al. 2023), while SlVQ11 enhanced the activation of SlWRKY55 on SlHsfA2 expression by interacting with it (Ma et al. 2024). In eggplant, SmHSFA8 interacted with SmEGY3 protein, activated SmEGY3 transcription, and enhanced the activation function of SmEGY3 on SmCSD1 expression (Figure 1). SmEGY3 played positive roles in the regulation of heat tolerance in eggplants (Liu et al. 2024). These results revealed that SmHSFA8 might be involved in regulating the heat tolerance of eggplant.
The HSF gene family has been identified in many species, such as A. thaliana (Guo et al. 2008), Oryza sativa (Guo et al. 2008), Solanum lycopersicum (Yang et al. 2016), Brassica rapa (Song et al. 2014; Huang et al. 2015) and Capsicum annuum (Guo et al. 2015). Most HSFAs contain one or more AHA motifs, which function as transactivators (Kotak et al. 2007; Zhang et al. 2009; Liu, Liao, and Charng 2011; Nishizawa-Yokoi et al. 2011). In eggplants, SmHSFA8 possessed an AHA domain (Figure S1). SmHSFA8 was localised in the cell nucleus and functioned as an activator. The transcriptional activation region of SmHSFA8 was identified at amino acid residues 193-374 (Figure S4). Compared with HSFBs and HSFCs, more studies have focused on the regulation of plant thermotolerance by HSFAs (Ohama et al. 2017). In this study, SmHSFA8 was induced by HTS and showed obvious transcript accumulation with increasing temperature (Figure S2D,F). Silencing SmHSFA8 reduced the heat tolerance of eggplant plants, whereas overexpressing SmHSFA8 increased the heat tolerance of A. thaliana and tomato plants (Figure 2 and Figure S7). These results suggested that SmHSFA8 played a positive role in regulating the heat tolerance of eggplant. In addition, SmHSFA8 directly activated SlEGY3 expression and promoted H2O2 content and H2O2-mediated retrograde signalling pathway, thus enhancing the heat tolerance of tomato plants (Figure S6). As a central signalling molecule, H₂O₂ activates the antioxidant defence mechanism and retrograde signalling pathway at the cellular level, and it also interacts with the hormone signalling pathway and promotes stomatal regulation and water retention, thereby enhancing plant thermotolerance (Hasanuzzaman et al. 2013). Under HTS, HSFs typically activate HSPs expression, which helps maintain normal cellular function and mitigate heat damage in plants (Mittler, Finka, and Goloubinoff 2012). HSFs also maintain ROS homoeostasis and activate the expression of downstream antioxidant enzyme genes through retrograde signalling, thereby reducing oxidative damage induced by HTS (Scharf et al. 2012). The mechanism underlying heat toleance mediated by SmHSFA8 exhibits both similarity and complementarity with other known HSF-mediated heat tolerance mechanisms.
The HSP gene family has been identified in many species, such as A. thaliana (Siddique et al. 2008), pepper (Guo et al. 2015), soybean (Lopes-Caitar et al. 2013) and Chinese cabbage (Tao et al. 2015). HSPs can effectively increase plant thermotolerance by binding denaturing proteins and preventing them from irreversible aggregation (Park et al. 2012). HSPs are divided into five subgroups on the basis of their molecular weights and sequence homologies: small HSPs (sHSPs), HSP60s, HSP70s, HSP90s and HSP100s (Lindquist and Craig 1988). sHSPs played a major role in improving plant thermotolerance by forming molecular chaperones and cell membrane stabilising factors (Li et al. 2012), and the expression of sHSPs was positively correlated with thermostability (Lopez-Matas et al. 2004). HSP70s and HSP90s were induced by HTS and enhanced heat tolerance in plants (McLellan et al. 2007). In this study, three SmHSPs, SmHSP70, SmHSP70B and SmHSP21, were identified as candidate target genes of SmHSFA8 according to the DAP-Seq analysis. The expression of these genes was upregulated by HTS in eggplants (Figure 3E). SmHSFA8 directly bound the promoters of SmHSP70, SmHSP70B and SmHSP21, and activated their expression (Figure 4). Moreover, compared with the control and WT plants, the expression of HSP70, HSP70B and HSP21 significantly decreased in the SmHSFA8-silenced eggplant plants but increased in the OE-SmHSFA8 transgenic A. thaliana and tomato plants at RT and HTT (Figure S8). These results confirmed that SmHSFA8 regulated the heat tolerance of plants by activating HSPs expression. SmF3H was also identified as a candidate target gene of SmHSFA8 via DAP-Seq and KEGG analyses. The expression of SmF3H was induced by HTS (Figure 3H), and SmHSFA8 directly bound the promoter of SmF3H and activated its expression (Figure 5A–C). Compared with the control and WT plants, the expression of F3H significantly decreased in the SmHSFA8-silenced eggplant plants but increased in the OE-SmHSFA8 transgenic A. thaliana and tomato plants at RT and HTT (Figure 5D–F). In particular, SmHSP70B, SmHSP21 and SmF3H were more highly expressed in the ‘E390’ eggplant line than in the ‘HM’ eggplant line after HTT (Figure 3F–H). The differential expression of SmHSP70B, SmHSP21 and SmF3H might be caused by the difference of their promoters in the ‘E390’ and ‘HM’ eggplant lines or elevated protein levels of upstream regulators (include SmHSFA8) in the ‘E390’ eggplant line. In pepper, the specific variations of MYB31 promoter in Capsicum chinense probably accounted for the higher expression of MYB31 in this species than in the other four species (Zhu et al. 2019). The SNP differences of OsNPF8.9a promoter might be responsible for the difference of OsNPF8.9a expression in indica and japonica rice (Luo et al. 2023). However, there was no difference in the promoter sequences of SmHSP70B, SmHSP21 and SmF3H between the ‘E390’ and ‘HM’ eggplant lines (Figure S11–S13). Hereto, increased protein level of upstream regulators (include SmHSFA8) of SmHSP70B, SmHSP21 and SmF3H in the ‘E390’ eggplant line might be the main reason of high expression of them in the ‘E390’ eggplant line, and it needed further study to confirm that. Hence, the difference in protein levels of upstream regulators (including SmHSFA8) in the E390 and HM eggplant lines is one of our future research directions.
F3H plays a key role in flavonoid metabolism and can catalyse the production of dihydrokaempferol (DHK) or dihydroquercetin (DHQ) using naringenin (NAR) or eriodictyol (ERI) as a substrate (De Carolis and De Luca 1994; Prescott and John 1996; Schaart et al. 2013). The functions of F3H have been identified in many species, such as rice (Kim et al. 2021), A. thaliana (Aguadé 2001), Longan (Jiang et al. 2016) and tomato (Maloney, DiNapoli, and Muday 2014), but the function of SmF3H has not yet been reported in eggplants. In this study, the silencing of SmF3H reduced the flavonoid content and heat tolerance of eggplant (Figure 6). The results indicated that SmF3H was involved in regulating heat tolerance in eggplants by affecting flavonoid biosynthesis.
As an ancient and specialised group of secondary metabolites in plants, the accumulation of flavonoids is considered to be a hallmark of plant stress (Winkel-Shirley 2002). Under environmental stress, the production of flavonoids can effectively regulate stress-induced ROS accumulation and enhance plant resistance (Li et al. 1993; Tattini et al. 2004; Walia et al. 2005; Korn et al. 2008). Exogenous flavonoids alleviated the stress damage caused from Cd by altering the permeability of cell membranes (Kang et al. 2022). In this study, the flavonoid content of the thermosensitive eggplant line ‘HM’ significantly decreased, whereas that of the thermotolerant eggplant line ‘E390’ gradually increased under HTS (Figure S9). Exogenous flavonoid treatment reduced HTS damage to eggplant by maintaining photosynthesis and membrane system (Figure S10). In addition, exogenous flavonoid treatment restored the heat tolerance of the SmF3H-silenced eggplant plants (Figure 7). These results suggested that flavonoids are involved in regulating the heat tolerance of eggplant. In rice, OsRLCK160 and OsbZIP48 synergistically regulated flavonoid biosynthesis to increase tolerance to UV-B (Zhang et al. 2022). In Populus tomentosa, the IAA17.1-HSFA5a module enhanced salt tolerance by regulating flavonoid biosynthesis (Song et al. 2024). In apple, MdHSFA8a regulated drought tolerance by modulating flavonoid biosynthesis (Wang et al. 2020). In this study, compared with the control and WT plants, the flavonoid content significantly decreased in the SmHSFA8-silenced eggplant plants but increased in the OE-SmHSFA8 transgenic A. thaliana and tomato plants at RT and HTT (Figure 5G–I). The results confirmed that SmHSFA8 regulated the heat tolerance of eggplant by affecting flavonoid biosynthesis.
5 Conclusion
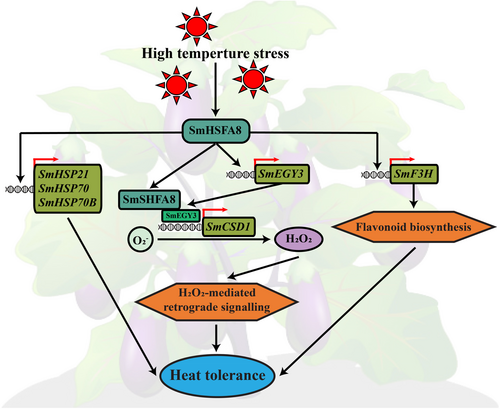
Taken together, SmHSFA8 is upregulated by HTS. SmHSFA8 subsequently activates SmHSPs (SmHSP21, SmHSP70 and SmHSP70B) expression, mediates the SmEGY3-SmCSD1 module and promotes flavonoid biosynthesis by activating SmF3H expression. Consequently, the heat tolerance of eggplant increases (Figure 8). The molecular mechanism by which SmHSFA8 regulates heat tolerance in eggplants is highly important for adaptation to climate change and eggplant production under HTS.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32472734), Key R&D Projects of Guangdong Province (2022B0202080003), Key R&D Projects of Hainan (ZDYF2023XDNY041), Seed Industry Revitalisation Project of Guangdong (2022-NPY-00–026) and Fruit and Vegetable Industry System Innovation Team Project of Guangdong (2022KJ110).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).