Stomatal Plasticity Maintains Water Potential Homeostasis in Pinus radiata Needles
ABSTRACT
Vapour pressure deficit (VPD) is a primary determinant of stomatal behaviour and water balance in plants. With increasing global temperature, the accompanying rise in VPD is likely to have a significant impact on the performance of plant species in the future. However, the plasticity of stomatal response to VPD remains largely unexplored. This study examines the plasticity of whole plant stomatal conductance (gc) response to VPD in Pinus radiata plants grown under two temperatures and a water-deficient treatment over a period of 3 months. The soil–stem water potential gradient (ΔΨ), gc and soil−stem hydraulic conductance (Ks-s) were evaluated. The different treatment groups showed significant differences in maximum gc relating to differences in Ks-s, however, gc dynamic response to VPD was very similar in all treatments such that ΔΨ was conserved once VPD increased above an average threshold of 0.64 kPa. The ability to robustly quantify water potential regulation in Pinus presents opportunities to explore variation in this globally important tree genus as well as providing a new approach to characterize the regulation of gas exchange in response to VPD.
1 Introduction
Vapour pressure deficit (VPD) is a key variable influencing the physiological activity and water requirement of plants (Zhong et al. 2023; Yuan et al. 2019). Recent episodes of regional-scale plant water deficit and drought-related mortality are typically related to periods of elevated VPD (Grossiord et al. 2020; Gea-Izquierdo, Sánchez-Gómez, and Aranda 2023; Crockett and Hurteau 2023). In conifers, canopy dieback in the southwest United States exhibited a stronger correlation with VPD than with rainfall and temperature anomalies (Park Williams et al. 2012). The anticipated rise in both the frequency and intensity of droughts (Seneviratne et al. 2021) coupled with the significance of plantation forestry in shaping the hydrological cycle, the response of trees to unprecedented rise in VPD due to a warming climate is likely to become a pivotal concern in the coming decades (Dunningham et al. 2012; Dvorak 2012).
New phenotypic targets are required to improve tree performance under changing water availability and temperature conditions (Matallana-Ramirez et al. 2021). Among the potential target traits, stomatal response to water deficit has been identified as an important candidate, because it influences plant's ability to effectively regulate their water content in the face of increased evaporative demand (Yang et al. 2012). Under low soil moisture or high VPD conditions, stomata close, reducing water loss and restricting the decline in plant water potential beyond a threshold causing hydraulic limitation in the soil (Carminati and Javaux 2020) or xylem damage (Brodribb and Mcadam 2017) but at the cost of reduced carbon gain. Previous research has suggested that plants generally exhibit a range of water potential regulation strategies characterized as iso/anisohydric stomatal behaviours (Tardieu and Simonneau 1998), where isohydric behaviour describes plants in which daytime water potential remains somewhat constant, while anisohydric species exhibit weaker regulation of daytime water potential (Konings and Gentine 2017). This variation in water potential regulation is largely explained by differences in stomatal behaviour (Schultz and Matthews 1997; Medrano et al. 2003), yet the characterization of this VPD response trait has proven challenging, thus restricting the efficiency of identifying breeding targets (Novick et al. 2024).
The sensitivity of stomata to VPD exhibits significant variation at both intra and interspecific levels (Mcnaughton and Jarvis 1991) and has been reported to be plastic in some species (Stojnic et al. 2015; Durand et al. 2020; Marchin et al. 2016). Plasticity in structural traits (Stotz et al. 2022; Alía et al. 2024; Cai et al. 2017) and xylem anatomy (Chin and Sillett 2016; Fonti and Jansen 2012; Férriz et al. 2023; Barnard et al. 2011; S. Li et al. 2024) in response to changing environmental conditions and water stress has shown to be linked to hydraulic adjustments required to enhance water use efficiency and prevent embolism. This acclimation can alter stomatal responses to VPD, further complicating prediction of the response of trees to forthcoming climate change (Vicente-Serrano et al. 2022; Marchin et al. 2016). As a result, determining the suitability of stomatal behaviour as a trait for tree breeding requires investigation of the aspects of stomatal response that remain fixed and those that show plasticity under changing VPD.
Our study focuses on scrutinizing the dynamics as well as the sensitivity of stomatal responses to VPD in Pinus radiata—which is one of the most widely planted exotic softwood species in Australia, New Zealand, Chile and Spain, and is particularly valued for its role in forest production (Stone, Penman, and Turner 2012). Since the 1950s, successive breeding attempts have significantly improved the growth, structure and wood characteristics of commercially cultivated P. radiata (Mclean et al. 2024). Given the characterization of this species as strongly isohydric (Brodribb and Mcadam 2013; Rodríguez-Gamir et al. 2019), we also consider the stomatal response in terms of its regulatory control of leaf hydration. Here we aim to assess the magnitude of plasticity in the regulation of transpiration and water potential by stomata in P. radiata. To do this, we characterized the VPD response of stomatal conductance and stem water potential in P. radiata plants grown under different temperatures and water availability conditions.
2 Materials and Methods
2.1 Plant Material and Growing Conditions
The study was conducted on fifteen 16-month-old P. radiata plants sourced from Woodlea Nursery (Tasmania). The plants were grown from a single seedlot (‘Seed Energy Blackwood 8’) which was an open-pollinated bulk from a commercial seed orchard, which is part of Tree Breeding Australia's (TBA) national P. radiata breeding programme. The seedlings were directly sown into Lannen 81 trays, in a potting medium of pine bark, along with controlled-release fertilizer, micronutrients and a mycorrhizal inoculation. Seedlings were then repotted in 2 L pots containing potting mix (7:4 of composted fine pine bark and coarse-washed river sand) in glasshouse facilities at the University of Tasmania. Fifteen of the healthiest plants were divided into three random groups of five plants each, which were transferred to a controlled glasshouse and subjected to three different water and daytime temperature regimes: well-watered at 18°C (WW 18°C), well-watered at 30°C (WW 30°C) and water-stressed at 30°C (WS 30°C). Each treatment group was grown under 12 h days, with night temperature of 15°C. The relative humidity (RH) was maintained around c. 40% during the day in all the treatments. The well-watered treatment group plants were watered daily to the maximum field capacity. Seedlings of P. radiata can be acclimatized to a range of daytime temperatures with minimal effect on growth (Rook and Whitehead 1980). The two temperature treatments were selected to evaluate how growth under different VPD ranges may affect the stomatal response, within the extremes of day-time temperatures reported for active growth (Hellmers and Rook 1973). Predawn water potential of −1 MPa was found to be sufficient to induce stomatal closure without inducing excessive damage to the xylem (Brodribb and Mcadam 2013). Therefore, under the third treatment, water-stressed plants were subjected to 9−10 cycles of soil water stress/rehydration as follows: each plant was gradually dried until reaching a predawn stem water potential of −1 MPa, then watered to full capacity. On average, it took 7 days for the plants to reach the targeted water stress level before rewatering. Stem water potential (Ψstem) was monitored continuously in each plant using an optical dendrometer (see below for details). The plants were then given 3 days after rewatering to fully recover their predawn Ψstem before subjecting them to a new cycle of drought. All plants were kept growing under their respective temperature and water stress conditions for 3 months before measurements.
2.2 Experimental Set-Up
Two out of 15 selected plants (one from WS 30°C and WW 18°C treatment group) were excluded from the experiment due to disease. The experiment was carried out in a well-ventilated controlled growth chamber, at 21 ± 1°C with a photoperiod of 10 h and a photosynthetic quantum flux of 800 μmol m⁻² s⁻¹ at the plant canopy level. Plants were kept well-watered throughout the experiment. RH and air temperature inside the chamber were recorded on a datalogger (CR850, Campbell Scientific, Logan, UT, USA) at 1 min intervals using a temperature/humidity probe (HMP45AC; Vaisala Inc., Helsinki, Finland) placed at the plant canopy level. Plants were exposed to a range of VPD (between 0.38 and 2.32 kPa) starting from low VPD in the morning and going to higher levels thereafter. The target VPD levels were achieved by changing RH only while maintaining temperature relatively constant at approximately 21°C. RH was manipulated using commercial humidifiers to maintain high RH and a dehumidifier (SeccoUltra 00563, Olimpia-Splendid) to reduce RH. Each VPD level was maintained relatively constant for atleast 1−2 h until the canopy transpiration (Ec) and needle width achieve stability and remained steady.
2.3 Stem Water Potential (Ѱstem) and Canopy Transpiration (Ec) Measurements
An optical dendrometer was connected to a mature needle of each P. radiata plant to continuously track the needle width variation at 1 min intervals from which the temporal variations in Ψstem were derived (Bourbia, Pritzkow, and Brodribb 2021; Bourbia and Brodribb 2023). Needle width was measured in pixels (1 mm² = approximately 134.56 pixels). In each Pinus plant, needle width was calibrated against Ψstem across a range of 4−5 Ψstem values covering the maximum range achieved during VPD treatments (Supporting Information S1: Figure 1). Ψstem was measured with a Scholander chamber (PMS, Albany, OR, USA) on different neighbouring non-transpiring mature needles. These needles were covered with damp tissue paper and aluminium foil for at least 2 h before measurements. One measurement was made in the dark before switching on the lights and the others were made at four different levels of VPD (around 0.4, 0.6, 0.9 and 1.5 kPa) to which the plants were exposed after the width had reached a steady state during daytime. Here, the steady-state condition indicates to a state where both Ec and the associated Ѱstem attain a steady state under constant VPD (< 5% change), with negligible impact from plant capacitance for at least 30 min.
Ec was measured continuously and simultaneously by placing the potted plants with optical dendrometers on computer-interfaced balances which logged at 5 min intervals with an accuracy of ±0.01 g (model VIBRA ALE-6202, 6200 G × 0.01 G). Before placing the plants on the balances, each pot was watered and allowed to drain excess water then covered with aluminium to prevent evaporation from the soil. Ec of each plant was normalized using corresponding calculated whole-plant leaf area (m−2).
Where Ec is canopy transpiration at each steady state VPD, Patm is the atmospheric pressure (101.3 kPa) and VPD is vapour pressure deficit. Given the very narrow width of the pine needles and the circulation of air in the growth chamber, leaf temperature was assumed to be approximately equal to air temperature.
Where RH is the percent relative humidity in the air (in %) and T is air temperature (°C).
2.4 Measurement of Ks-s Dynamics
2.5 Measurement of Morphological Traits
Plant height, total leaf area and leaf mass per area (LMA) were measured once before starting the experiment on all plants to capture the differences and similarities observed among different treatment groups (Supporting Information S1: Table 1; Supporting Information S1: Figures 2−5). Ten randomly sampled needles were scanned and then oven-dried at 60°C to obtain dry weight. The LMA was calculated for each individual using the ratio of the dry mass of 10 randomly sampled needles to the total surface area of needles. Total leaf area (cm2) was measured using needle count and average leaf area measured from 10 randomly sampled needles using ImageJ.
2.6 Statistical Analysis
The analyses were carried out using R version 4.2.2 (R Core Team 2022). Differences in predawn Ψstem, Ec, gc, Ks-s and various morphological traits such as plant height, total leaf area and LMA under different treatments were evaluated using analysis of variance (ANOVA). In case of significant differences, we employed a post hoc Tukey multiple comparison of means test. Differences were considered to be significant when p < 0.05. Results are reported as mean values ± SD.
Various models such as linear, exponential, quadratic and two-piece linear regression were used to study the relationship of ΔѰ and Ec in response to VPD for each treatment. The R2 value and Akaike's information criterion, corrected for small sample size (AICc) were used to find the best fit. The piece-wise linear regression model fitted using segmented package 2.0-3 and a quadratic model yielded the highest R2 and lowest AICs values, thereby proving to be the best-fitted model for the relationship of ΔѰ and Ec in response to VPD.
Leaf temperature was assumed to be equal to air temperature due to the very narrow needle form of leaves and the well-stirred air inside the growth chamber.
3 Results
3.1 Dynamics of Ψstem and Ec Under Changing Diurnal VPD
In all plants, the increase in VPD led to a rapid decline in Ψstem as the Ec increased. The kinetics of Ψstem matched closely to those of Ec indicating a minimum capacitance effect after step changes in VPD. Steady states were achieved approximately 1 h after VPD was modified (Figure 1b). No impact of temperature and water stress treatments was observed on the predawn stem water potential (Ψstempd) (Supporting Information S1: Figure 6). However, WS 30°C exhibited a slightly more negative Ψstem compared to WW18°C and WW 30°C at 1.5 kPa (Supporting Information S1: Figure 7a) and throughout the day under the different VPD levels. Furthermore, the magnitude of Ec displayed at different steady-state VPD levels also varied between the three treatment groups, with WS 30°C having the lowest Ec compared with WW 30°C and WW18°C (Figure 1a).

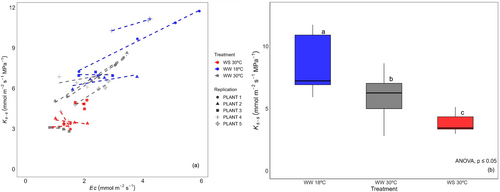
3.2 Response of Ks-s to Changes in Ec
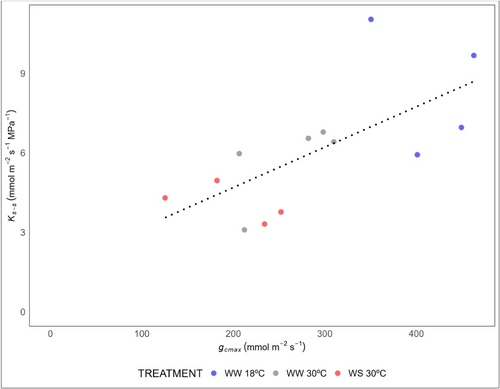
Ks-s appeared to change with Ec in some plants within treatment groups (Figure 2a). Comparisons of Ks-s between the treatment groups were thus made as means across the whole VPD range. The mean Ks-s significantly differed among the three treatment groups (ANOVA, p < 0.001; Figure 2b) ranging from a maximum of 8.6 ± 2.1 mmol m−2 s−1 MPa−1 in WW 18°C to a minimum of 3.8 ± 0.7 mmol m−2 s−1 MPa−1 in WS 30°C.
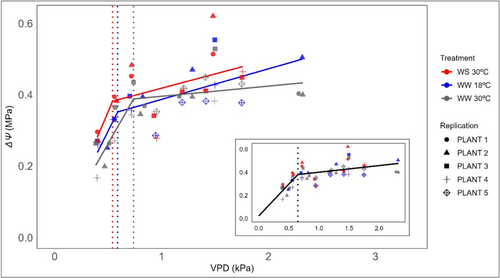
3.3 Response of ΔѰ to VPD
All plants showed similar behaviour in terms of plant water potential gradient response over a range of VPD among the three treatment groups (p > 0.05) (Figure 3). The overall response of plants from each treatment was best described by two-piece segmented ΔΨ response to changing VPD with a VPD breakpoint. In all treatment groups, ΔѰ increased proportionately with VPD (in the absence of stomatal closure) until a given threshold above which further increases in VPD resulted in second shallow linear relationship (due to the effect of stomatal closure) between ΔΨ and VPD. The VPD breakpoints of each treatment occurred along a range from 0.74 ± 0.06 kPa in WW 30°C to 0.54 ± 0.12 kPa in WS 30°C (Figure 3). Although the breakpoint obtained from WW 30°C treatment group was significantly different from WW 18°C and WS 30°C, the magnitude of these differences was small (~0.2 kPa). Considering the small variation in VPD breakpoints among all three treatment groups, we pooled data together to create a single breakpoint function (Figure 3 insert picture). This function provided the best fit to our data with R2 value of 0.97 to model the gc/VPD response.
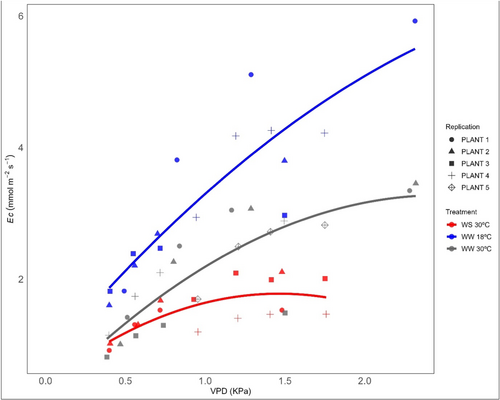
3.4 Response of Ec to VPD
The overall response of Ec to changing VPD was best described by quadratic fit to each treatment group. Ec increased with increasing VPD between 0 and approximately 1−1.3 kPa but the slope of the relationship decreased thereafter. We observed a significantly higher transpiration rate in the WW 18°C treatment group as compared to WS at 30°C (p < 0.05) at 1.5 kPa (Supporting Information S1: Figure 7b) and over the entire range of VPD (Figure 4).
3.5 Response of gc to VPD
In all plants, gc showed very little sensitivity to VPD between 0.38 kPa and the respective VPD breakpoints identified for ΔΨ in each treatment group (Figure 5) but declined thereafter, representing the two-phase stomatal response to VPD. All the treatment groups, therefore, reflect low stomatal sensitivity before the breakpoint and increase after that. Linear, quadratic, exponential and polynomial models provided acceptable fits to the gc data, however, the best-fitted model (with the highest r2 and lowest AICc value) was found to be the one using the modelled gs predicted from ΔΨ dynamics using Equation 4 (refer to Materials and Methods).
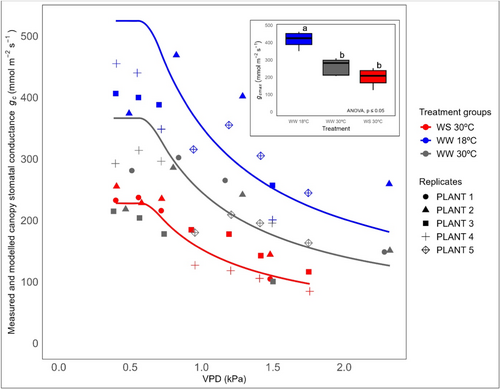
The mean maximum gc (gcmax) measured in the range 0.4−0.8 kPa VPD was found to be significantly higher in WW 18°C (415 mmol m−2 s−1 ± 51) as compared to WS 30°C (198 mmol m−2 s−1 ± 56) and WW 30°C (261 mmol m−2 s−1 ± 48) (p < 0.05). However, no significant difference in gcmax was found between WS 30°C and WW 30°C (p > 0.05) (refer to insert boxplot in Figure 5) which shows a more pronounced effect of growth temperature compared to the water stress treatment. The gcmax was strongly positively correlated with Ks-s across all treatment groups (Figure 6).
4 Discussion
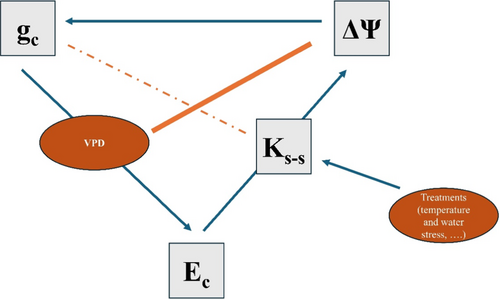
The exchange of carbon and water vapour between the plants and atmosphere is regulated by stomatal control (Meinzer et al. 2009; Buckley 2005). Stomata exhibit strong responsiveness to fluctuations in VPD (Klein 2014). However, according to (Monteith 1995), this response is not direct; rather the underlying response mechanism is associated with shifts in the water potential of the leaf, driven by variations in Ec (Mcadam and Brodribb 2015). Our study examined plasticity in this behaviour in P. radiata showing that despite the significant treatment effects on Ks-s, ΔΨ remained highly conserved over a range of VPD through strong modification of gcmax and a conserved gc response to VPD (Figure 7). This highlights the fact that some aspects of gc appear plastic while others remain fixed in P. radiata.
4.1 Influence of Temperature and Water Stress Treatments on Ks-s
Strong stomatal regulation of ΔΨ means that the plant's ability to transpire in well-watered conditions is largely dictated by Ks-s (Sperry et al. 2002; Brodribb and Jordan 2008). Plant hydraulic conductance depends on various factors like leaf anatomy (Aasamaa, Sõber, and Rahi 2001), sapwood area to leaf area ratio (Mencuccini et al. 2019) and soil drought (Oren et al. 2001). Interestingly, we found no significant differences in the total leaf area (Supporting Information S1: Figure 3) among the different treatment groups, indicating that the reduction in Ks-s observed here in plants exposed to recurrent mild soil drought was probably attributed to changes in the root hydraulic conductance caused by permanent suberization of the roots, downregulation of aquaporins or root damage caused by cavitation and cortical lacunae (Cuneo et al. 2016; Lo Gullo et al. 1998). This downregulation in Ks-s in response to mild drought stress has also been found to be linked with changes in the root hydraulic conductance in P. radiata (Rodríguez-Gamir et al. 2019) and Pinus sylvestris (Poyatos, Aguadé, and Martínez-Vilalta 2018).
Plant hydraulic conductance is also affected by changes in temperature. The temperature treatment effect was much bigger on Ks-s than on plant size and growth. Changes in shoot and root hydraulic conductance in response to change in temperature between 20°C and 30°C have been reported due to alterations in viscosity of water (Tyree and Zimmermann 2002). Various studies have reported decline in Ks-s at lower temperatures, primarily due to decrease in root hydraulic conductance as a result of increasing viscosity of water (Wan et al. 2001) and decreasing permeability of root membranes (Carvajal, Cooke, and Clarkson 1996). However, we found xylem in WW 18°C treatment group to be more conductive leading to higher Ks-s as compared to WW 30°C at 20°C. This may be attributed to the fact that the higher Ks-s in plants grown under lower temperature has compensated for the viscosity effect which would have otherwise resulted in lower Ks-s.
4.2 Influence of Temperature and Water Stress Treatments on gc
Ec increased with increasing VPD until reaching a threshold beyond which it remained relatively constant in all three treatment groups. Our findings are consistent with several studies that reported saturated Ec under high VPD in various tree species (Chen Lixin et al. 2012; Chen et al. 2019; Wieser, Gruber, and Oberhuber 2018; Tor-Ngern et al. 2017; Wang et al. 2020) including pine species and can be attributed to stomatal closure at higher VPD (Tor-Ngern et al. 2017; Wieser, Gruber, and Oberhuber 2018). The stomatal closure in response to VPD in some conifers has been shown to be governed by passive-hydraulic regulation where stomata act as pressure-sensitive valves, closing and opening in response to leaf turgor (Mcadam and Brodribb 2015). The restriction of Ec by stomatal closure at high VPD resulted in conserved water potential, which could otherwise trigger xylem cavitation (Brodribb et al. 2021; Bourbia, Lucani, et al. 2023) and has been observed in various pine species like Pinus sylvestris, cembra and halepensis (Dulamsuren et al. 2009; Wieser, Leo, and Oberhuber 2014; Wagner et al. 2022).
Prior studies have highlighted that the variations in gc are largely influenced by environmental factors like VPD (Yoshida et al. 2016). The difference in the magnitude of gc observed among the three treatment groups was highly correlated with Ks-s. Similar associations between Ks-s and gc have been shown between species (Brodribb 2009; Addington et al. 2004), but here we found coordination within the same species subjected to different growth treatments. Significantly lower Ec observed in WS 30°C treatment group might be due to the imposed water stress cycles which led to permanent reduction in Ks-s of these plants (X. Li, Sinclair, and Bagherzadi 2016). This permanent loss in Ks-s did not allow the plants to recover their Ec rates even under non-limiting soil water conditions (Brodribb and Cochard 2009).
Despite the plasticity in gcmax, the response of gc to VPD was relatively consistent in all treatments. An insensitivity of gc to low VPD followed by high sensitivity at VPD > 0.64 kPa was consistent in all the treatment groups. This type of biphasic response has been observed in several species demonstrating minimal stomatal sensitivity under low VPD and variable responsiveness beyond a specific threshold (Choudhary et al. 2014; Bourbia, Lucani, et al. 2023). The observed negative correlation between gc and VPD beyond the VPD threshold point can be owed to the induction of stomata closure as a response to escalating Ec and declining leaf water potential (Barron-Gafford, Grieve, and Murthy 2007; Oren et al. 1999).
There is some evidence to suggest that species with higher gcmax are more sensitive to VPD than the ones with lower gcmax (Oren et al. 1999). However, our results reported similar stomatal response among all the treatment groups despite significant variations in the gcmax.
4.3 Water Potential Regulation in P. radiata
We observed a small effect of VPD on midday Ѱstem at 1.5 kPa. Ѱstem was found to be slightly more negative in WS 30°C treatment group possibly due to a small amount of osmotic adjustment (p < 0.05). This is consistent with the findings in Pinus pinaster and Pinus canariensis where both species showed a capacity of osmotic adjustment in needles in response to gradually induced drought stress (López, Aranda García, and Gil 2009). We, therefore, expected variations in the overall ΔѰ response to VPD among the treatment groups. However, our results showed nonsignificant variation between different treatments over the entire range of VPD, despite significant differences in Ec and Ks-s. This consistent relationship between ΔѰ and VPD among the different treatment groups was attributed to difference in the magnitude of gc due to acclimation and conserved gc response to VPD. The conserved isohydric response observed in this species is an evolutionary mechanism to maintain water status of the plant at the expense of carbon gain over long-term dry periods (Mcdowell et al. 2008).
4.4 Correlation Between gc and Ks-s
Several studies have reported strong coordination between gcmax and Ks-s (Brodribb and Jordan 2008; Boyce et al. 2009). This coordination was effectively maintained by all our treatment groups. Our results showed that lower Ks-s in the WS 30°C treatment group resulted in low gcmax which can be owed to the developmental differences under different growth treatments (Aasamaa, Sõber, and Rahi 2001). This harmonious equilibrium established between gcmax and Ks-s together with conserved response of gc to VPD among the treatment groups lead to conserved water potential (Figure 7). However, since stomata serve as a common pathway for both water and CO2, therefore it is crucial to understand that stomatal response to VPD is also directly correlated to the carbon supply or the maximum photosynthetic capacity (Amax). This strong regulation of water potential must come at the cost of limited carbon assimilation.
5 Conclusion
Our study highlights the importance of evaluating the interplay between stomatal behaviour and Ks-s which significantly influenced the Ec regulation of our treatment groups. The differences in the magnitude of Ks-s led to changes in the gcmax. However, no significant differences in ΔΨ among all the treatment groups over a range of VPD, despite the presence of random genetic variation in our sample, illustrated a very conservative regulation of water potential in P. radiata plants. Overall, we found significant plasticity in gcmax but no plasticity in the overall gc response and water potential regulation. Therefore, our study suggests that although the stomatal response to VPD is fixed, the gcmax can be plastic and highlights the need to account for such plasticity for precisely predicting plant responses to future climatic conditions. Further study employing increased sample size and replicated genotypes will help to determine whether or not the stomatal response to VPD is conserved across more diverse germplasm in P. radiata and other pines.
Acknowledgements
This research was supported by the NZ Ministry of Business, Innovation and Employment (MBIE) programme entitled ‘Seeing the forest for the trees: transforming tree phenotyping for future forests’ (programme grant number C04X2101). We thank the ARC Centre of Excellence for Plant Success in Nature and Agriculture. We also thank Christopher Lucani for technical support, Kate Johnson for guidance with Cavicam set-up and Faustino Rubio Perez and Greg Jordon for assistance with statistical analysis.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.