Nucleolar actions in plant development and stress responses
Soeui Lee, Ye-Eun Seo, and Jeen Choi contributed equally to this study.
Abstract
The nucleolus is conventionally acknowledged for its role in ribosomal RNA (rRNA) synthesis and ribosome biogenesis. Recent research has revealed its multifaceted involvement in plant biology, encompassing regulation of the cell cycle, development, and responses to environmental stresses. This comprehensive review explores the diverse roles of the nucleolus in plant growth and responses to environmental stresses. The introduction delves into its traditional functions in rRNA synthesis and potential participation in nuclear liquid–liquid phase separation. By examining the multifaceted roles of nucleolar proteins in plant development, we highlight the impacts of various nucleolar mutants on growth, development, and embryogenesis. Additionally, we reviewed the involvement of nucleoli in responses to abiotic and biotic stresses. Under abiotic stress conditions, the nucleolar structure undergoes morphological changes. In the context of biotic stress, the nucleolus emerges as a common target for effectors of pathogens for manipulation of host immunity to enhance pathogenicity. The detailed exploration of how pathogens interact with nucleoli and manipulate host responses provides valuable insights into plant stress responses as well as plant growth and development. Understanding these processes may pave the way for promising strategies to enhance crop resilience and mitigate the impact of biotic and abiotic stresses in agricultural systems.
1 INTRODUCTION
The nucleolus, a multifunctional organelle within the cell nucleus, has long been recognized as the epicentre of ribosome biogenesis, where the intricate dance of RNA and proteins orchestrates the assembly of these cellular workhorses. However, recent advancements have unveiled a more complex narrative, portraying the nucleolus as a dynamic hub involved in an array of cellular processes crucial for plant growth, development, and stress responses. Mutations in nucleolar proteins can lead to an array of phenotypic abnormalities, underscoring the intimate link between ribosome biogenesis and cell growth. Nucleolar proteins regulate key developmental genes, orchestrating processes such as leaf elongation, root development, and flowering. Moreover, certain nucleolar proteins are functionally conserved across organisms, influencing seed development and embryogenesis. In response to abiotic stress, such as temperature extremes, salinity, or heavy metal toxicity, plant nucleoli exhibit dynamic responses, undergoing structural alterations and modulating ribosomal RNA (rRNA) synthesis and processing. Notably, nucleolar proteins play crucial roles in stress adaptation, influencing plant growth and stress tolerance under adverse environmental conditions. In the context of plant-pathogen interactions, the nucleolus emerges as a battleground, where pathogens compete for control by hijacking host nucleolar functions. Viruses and other pathogens exploit nucleolar proteins to circumvent host defence mechanisms, facilitating infection and propagation. Effector proteins from pathogens target nucleolar proteins, disrupting host defences and promoting pathogen proliferation. In this review, we describe the broad involvement of the nucleolus and nucleolar proteins in plant growth, development, and in response to abiotic and biotic stress. As our understanding of the nucleolus continues to expand, fuelled by ongoing research into its diverse functions and interactions, we are poised to unlock new avenues for enhancing crop resilience and improving agricultural sustainability. By elucidating the underlying mechanisms of nucleolar actions, we can leverage this knowledge to develop novel strategies to mitigate the adverse effects of stress conditions on crop productivity, thereby paving the way for the development of more robust and resilient agricultural systems.
2 DYNAMIC STRUCTURE OF RIBOSOMES
Eukaryotic ribosomes consist of two subunits, the small ribosomal subunit (SSU, 40S) containing the 18S rRNA and the large ribosomal subunit (LSU, 60S) containing the 25S, 5.8S, and 5S rRNAs. Ribosome biogenesis is a complex process where hundreds of ribosome biogenesis factors (RBFs) are involved and distributed across three cellular compartments: the nucleolus, nucleoplasm, and cytoplasm. The nucleolus serves as the primary site for transcription by RNA polymerase I (Pol I) of the 45S rDNA which consists of hundreds copy of tandemly repeated genes for three of the four rRNAs (18S, 5.8S, and 25S rRNAs); while the fourth rRNA, 5S, is transcribed by RNA Pol III from tandem repeats elsewhere in the nucleus (Highett et al., 1993; Sáez-Vásquez & Delseny, 2019). These repetitive sequences are referred to as the nucleolar organizer regions (NORs) of the chromosomes (Raska et al., 2006a; 2006b). In Arabidopsis, NORs are located on the left arms of chromosomes 2 and 4 (NOR2 and NOR4, respectively). Notably, NOR4 and the adjacent short arm of chromosome 4 are associated with the nucleolus (Pontvianne et al., 2016), while NOR2 and its neighbouring region in chromosome 2 are excluded from the nucleolus and harbour inactive rRNA genes (Chandrasekhara et al., 2016; Pontvianne et al., 2016). Three key nucleolar activities, encompassing precursor rRNA (pre-rRNA) synthesis, processing, and ribosomal ribonucleoprotein (RNP) assembly, align with the “tripartite” internal structure derived from fibrillar centre (FC), the dense fibrillar component (DFC), and the granular component (GC). FCs are assumed to be the sites of assembly for complexes containing transcription-associated factors which can be in a transcription-ready or inactive state (De Carcer & Medina, 1999; González-Camacho & Medina, 2006). Additionally, FCs contain rDNA, which may become involved in transcription under specific conditions (Mckeown & Shaw, 2009; Shaw, 1996). The DFC constitutes the majority of the nucleolar volume, hosting the simultaneous transcription of pre-rRNAs. Pre-rRNAs produced in the DFC then undergo further processing in the DFC and GC. The GC is responsible for the final assembly of small and large ribosomal subunits from mature rRNAs and ribosomal proteins. Furthermore, it is believed that the GC plays a role in the transit of assembled ribosomes through the nucleoplasm to the cytoplasm (Shaw et al., 1995; Shaw, 1996). The nucleolar structures can be affected when the nucleolar proteins cannot function appropriately. For instance, defective mutations in ROOT INITIATION DEFECTIVE 2 (RID2), RNA HELICASE 10 (RH10), NUCLEOLAR FACTOR 1 (NOF1), ARABIDOPSIS PUMILIO 23 (APUM23), AtLa1, or RESTRICTED TO NUCLEOLUS 1 (REN1) cause enlargement of the nucleolus (Ohbayashi et al., 2011; Reňák et al., 2014). In addition to enlargement, vacuolation also happens in rid2-1 mutant (Ohbayashi et al., 2011). The effects of mutations in these nucleolar proteins in plant growth and development and stress responses are described in the following sections. The nucleolus relies on three major rRNA-associated nucleolar proteins for ribosome biogenesis: fibrillarin, nucleolin, and B23/NPM. Fibrillarin plays a crucial role in box C/D small nucleolar RNP (snoRNP) particles, conducting 2′-O-ribose methylation of rRNA and spliceosomal small nuclear RNAs (snRNAs), thus facilitating pre-rRNA processing and snoRNA splicing (Tollervey et al., 1993; Warner, 1990). Nucleolin contributes to regulating chromatin structure-mediated rDNA transcription and pre-rRNA processing (Ginisty et al., 1998; Pontvianne et al., 2007; Roger et al., 2003). B23, also known as nucleophosmin (NPM), is pivotal in maintaining nucleolar structure, rDNA transcription, rRNA maturation, ribosome assembly and export (Murano et al., 2008). Plant nucleoli also typically feature a nucleolar cavity or vacuole (NoV), often located in the central part of the nucleolus. While the function of the NoV remains unknown, it is speculated that NoV serves as regions for temporary sequestration and storage of biochemical factors, including elements of the ubiquitin-proteasome system (Stępiński, 2012), snoRNAs/snoRNPs and spliceosomal snRNAs/snRNPs (Beven et al., 1996; Lorković & Barta, 2008). These factors may be released into the nucleoplasm as needed during stress responses or specific developmental stages (Mineur et al., 1998).
The nucleolus, one of the largest membrane-less nuclear bodies, is formed through liquid–liquid phase separation (LLPS) (Lafontaine et al., 2021). It stands out as a prominent organelle within the cell, not only for its central role in ribosome biogenesis but also for its dynamic response to changing cellular conditions by generating and dissolving LLPS-driven condensates. LLPS aids in creating condensates by concentrating intrinsically disordered proteins (IDP) or proteins featuring modular interacting domains or prion-like domains together with RNA (Emenecker et al., 2020). Fibrillarin, primarily localized in the DFC, possesses a disordered arginine/glycine-rich domain at its N-terminus. B23/NPM comprises an N-terminal oligomerization domain, a C-terminal RNA-binding domain (RRM), and an intermediate disordered region. Both proteins can form droplets both in vitro and in vivo. Importantly, the liquid droplets form a core-shell structure, with no merging, attributed to differences in surface tension. The immiscibility of nucleolar subcompartments allows for the coexistence of multiple bodies within the nucleolus (Feric et al., 2016). Moreover, the immiscibility of nucleolar subcompartments facilitates the dynamic process of ribosome biogenesis within it. The components comprising ribosomes undergo various processing steps and interact with other components, leading to changes in their physical characteristics. These changes enable their transition into the next condensate (Riback et al., 2020). Pre-rRNA transcribed in the FC binds to fibrillarin and diffuses into the DFC for rRNA processing (Yao et al., 2019). Subsequently, processed rRNA is pushed into the GC, where it is captured by B23/NPM. Upon combining with ribosomal subunit particles, the changes in its physical properties result in the mature ribosome complex exiting the nucleolus (Lafontaine et al., 2021). The thermodynamic features of each nucleolar compartment need to be maintained well. In mammalian cells, it is suggested that abnormal nucleolar liquidity can lead to severe diseases, including ribosomopathies and cancer. The premature release of immature ribosome particles resulting from increased liquidity, or the inhibition of rRNA processing and component assembly by decrease of liquidity can cause a shortage of functional ribosomes within the cell (Lafontaine et al., 2021). Meanwhile, the physical characteristics of the nucleolus may change in response to various signals. One of the properties of phase-separated condensates in the nucleus is their ability to respond to various signals, including heat and biotic stress, through processes such as generation and destruction or the exchange of components (Huang et al., 2021; Jung et al., 2020; Wang et al., 2023b). Similar to these nuclear condensates, interaction between the components of snoRNP complex located in the DFC changes differently under hormone treatment, suggesting that plants may dynamically alter the nucleolus in response to specific signals (Dai et al., 2023). Although the function of RNA-protein condensates in the plant nucleolus needs to be elucidated, it has been reported that condensates within the nucleolus regulate protein quality control in animals. During heat stress, the GC captures misfolded proteins and sequesters them within liquid-droplet-like structures, thereby preventing their aggregation by reduction of mobility. Additionally, the GC recruits Hsp70 chaperones, facilitating the refolding of misfolded proteins and aiding the cell's response to heat stress (Frottin et al., 2019). Overall, considering the dynamics of LLPS-driven condensates and their role in nucleolar protein quality control, it is apparent that the plant nucleolus not only serves in ribosome biogenesis but also rapidly adapts to various signals, allowing it to engage in various biological processes.
3 NUCLEOLAR ACTION IN PLANT GROWTH AND DEVELOPMENT
The nucleolus is a complex assembly of RNA and proteins that plays a crucial role in various cellular processes that are intricately connected to plant growth and development (Sugiyama & Machida, 2018). Processes that occur within the nucleolus, such as rRNA synthesis and ribosome biogenesis, are essential for various cellular activities in plants. This is because that ribosomes are necessary for protein synthesis in all eukaryotic cells (Jiao et al., 2023). Following the synthesis of pre-60S and pre-40S ribosomal particles in the eukaryotic cell nucleus, subsequent assembly and maturation occur in the cytoplasm. Any irregularities in nucleolar proteins, which are linked to fundamental biological processes, are closely associated with the manifestation of numerous phenotypic abnormalities in plant growth and development (Figure 1, Table 1).
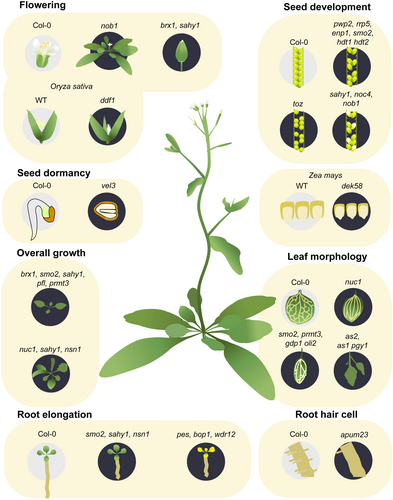
| Protein | Nucleolar process | Phenotype/Function | Reference | |
|---|---|---|---|---|
| AtNUC1 | Processing of 45S pre-rRNA | Mutated AtNUC1: stunting of above-ground growth; deceleration of root development; elongated stalks and multiple stem branching | Kojima et al. (2007) | |
| AtBRX1 | Maturation of 90S and 60S preribosomal complexes | Mutated AtBRX1: impaired leaf development and delayed flowering | Weis et al. (2015) | |
| AtSMO2 | A nucleolar protein maintains the abundance of RID2 (methyltransferase-like protein) to regulate ribosome biogenesis | Mutated AtSMO2: pointed leaves, abnormal leaf veins, unfertilized ovules, and reduced normal seed production | Guo et al. (2023) | |
| PeBoW complex | Pre-rRNA processing and 60 S ribosomal subunit maturation | PeBoW complex or components mutant: morphological defects in leaf, root growth inhibition, and premature flowering | Ahn et al. (2016); Cho et al. (2013); Holzel et al. (2007); Wang et al. (2022); Zografidis et al. (2014) | |
| AtSAHY1 | Involved in 18 S pre-rRNA processing and ribosome subunit assembly | Mutated AtSAHY1: delayed overall growth with short roots and pointed leaves, Altered stamens and petals, defective pollens, aborted seeds, and shorter root tips due to fertility and auxin issues | Hsu et al. (2021) | |
| AtPFL | SSU protein encoding genes | Mutated AtPFL1: pointed and narrow shaped leaves | Van Lijsebettens et al. (1994) | |
| AtPRMT3 | a cytoplasmic protein maintains balance between different pathways of pre-rRNA processing | Mutated AtPRMT3: smaller and narrower first true leaves and abnormal vascular patterns | Hang et al. (2014) | |
| AtAS2 | Regulate kn1 and related knotted-like homeobox (KNOX) gene expression | Leaf development and establish polarity | Machida et al. (2022) | |
| PGY1/2/3 | Nucleolar protein | pgy shows dorsal lamina outgrowth in as1 backgrounds | Pinon et al. (2008); Yao et al. (2008) | |
| GDP1 and OLI2 | Nucleolar proteins highly expressed in cell proliferating organs, involved in rRNA processing | Double mutants: Smaller shoots, narrower leaves, altered leaf palisade cell sizes. | Kojima et al. (2017) | |
| AtNSN1 | A nucleolar GTP-binding protein, function in maturation of 25 S rRNA and biogenesis of the 60 S ribosome subunit | Mutated AtNSN1: growth retardation, dwarfism and slower primary root growth | Jeon et al. (2015); Wang et al. (2018) | |
| APUM23/SAHY9 | Nucleoli-localizing protein-encoding gene controlling germination, growth, and root-hair cell differentiation | APUM23/SAHY9 mutants: Delayed germination, growth, and abnormal root-hair cell differentiation. | Abbasi et al. (2010); Huang et al. (2014); Wang et al. (2020) | |
| RID2 and RID3 | Involved in pre rRNA processing | Mutants: temperature-sensitive developmental defects including pointed leaves, simplified leaf veins, delayed root growth, and uncontrolled expression of CUC1 and STM | Konishi and Sugiyama (2003); Ohbayashi et al. (2011); Ohbayashi et al. (2017); Shinohara et al. (2014) | |
| OsDDF1 | A nucleolar anchored F-box protein regulate B-class floral homeotic genes | Mutated OsDDF: abnormal development of floral organs | Duan et al. (2012) | |
| AtHDT1/AtHDT2 | Histone deacetylases involved in the regulating of rRNA transcription and ovule development | Mutated AtHDT1/AtHDT2: a reduced seed setting rate when used as the maternal parent in crosses | Luo et al. (2022) | |
AtRrp5/AtPwp2/ AtEnp1/AtNob1/AtNoc4 |
Homologs of ribosome biogenesis cofactors | Mutated AtRrp5/AtPwp2/AtEnp1: small, underdeveloped, or early aborted seeds Mutated AtNob1/AtNoc4: lower germination rates and pale seeds |
Missbach et al. (2013) | |
| AtTOZ | 18 S rRNA biosynthesis | Mutated AtTOZ: severe effects on embryo and arresting embryogenesis | Griffith et al. (2007) | |
| ZmDEK58 | A nucleolar protein containing Rrp15p domain, regulating LSU biogenesis. | Mutated ZmDEK58: Smaller maize kernels due to disrupted 35 S rRNA processing | Ma et al. (2024) | |
| AtVEL3 | Epigenetic regulation of rRNA transcription | Regulate maternal control of seed dormancy | Chen et al. (2023) | |
3.1 Broad impacts of nucleolar proteins in plant development
Mutations in nucleolar proteins can have a significant impact on growth and morphology due to the close synchronization between ribosome biogenesis and cell growth. Nucleolar proteins, including NUCELOLIN1 (NUC1), Biogenesis of Ribosomes in Xenopus (BRX1), Small organ 2 (SMO2), and the PeBoW complex, play a significant role in plant development by processing rRNA. A mutation in AtNUC1, which is essential for the processing of 45S pre-rRNA, results in stunted above-ground growth, delayed root development, elongated stalks, and multiple stem branching (Kojima et al., 2007). Similarly, a mutation in the ortholog of yeast BRX1, which is essential for the maturation of 60S and 90S preribosomal complexes, leads to impaired leaf development and delayed flowering in Arabidopsis (Weis et al., 2015). The Arabidopsis mutant smo2 results in morphological defects, such as pointed leaves with abnormal leaf veins and reduced seed production. The lack of SMO2 results in inadequate ribosome biogenesis because of the insufficient amount of pre-rRNA processing participating protein RID2, which is necessary for proper ribosome biogenesis (Guo et al., 2023). Furthermore, the PeBoW complex, which is composed of Pescadillo (PES), Block of Proliferation 1 (BOP1), and WD Repeat Domain 12 (WDR12), inhibits cell division and expansion, leading to retarded root growth and leaf defects as well as inducing premature flowering (Ahn et al., 2016; Cho et al., 2013; Holzel et al., 2007; Wang et al., 2022; Zografidis et al., 2014).
Additionally, salt hypersensitive mutant 1 (sahy1) of Arabidopsis demonstrates overall growth delay with short roots and pointed leaves. SAHY1 is involved in 18S pre-rRNA processing and ribosome subunit assembly through interacting with ribosome proteins and RBFs. The number of stamens and petals are changed in sahy1, and pollens are defective and deformed with lower viability and germination rates. Seeds are often aborted in a white or transparent colour, due to the failure of fertilization. It also fails to translate auxin transport carrier proteins and expression of auxin responsive marker in the root tips, causing shorter root tips (Hsu et al., 2021).
3.2 Nucleolar proteins in plant organ development
Aberrant leaf formation is a common phenotype observed in many ribosome-related protein mutants, such as POINTED FIRST LEAF (PFL) and PROTEIN ARGININE METHYLTRANSFERASE 3 (PRMT3). For example, mutation in one of SSU protein encoding genes PFL results in leaves of pointed and narrow shapes (Van Lijsebettens et al., 1994). Similarly, mutation in Arabidopsis PRMT3, a cytoplasmic protein that acts in maintaining the balance between alternate pre-rRNA processing pathways, produces smaller and narrower first true leaves and abnormal vascular patterns similar to smo2 (Hang et al., 2014).
Mutations in nucleolar protein-encoding genes such as ASYMMETRIC LEAVES2 (AS2) and PIGGYBACK (PGY) alter the leaf polarity establishment. AS2 that localizes on perinucleolar AS2 bodies, epigenetically represses kn1 and related knotted-like homeobox (KNOX) genes, establishing leaf elongation and polarity (Machida et al., 2022). AS genes are also known to collaborate with other nucleolar proteins like NUC1 and RH10 to co-regulate the transcription levels of target genes (Machida et al., 2022). PGY genes (PGY1/2/3) are essential to leaf patterning, and pgy shows dorsal lamina outgrowth in as1 backgrounds (Pinon et al., 2008; Yao et al., 2008).
G-patch domain protein1 (GDP1) and OLIGOCELLULA2 (OLI2) are nucleolar proteins highly expressed in cell proliferating organs like leaf primordia, root apical meristem and flower buds. gdp1 oli2 double mutants have smaller shoots and narrower leaves with reduced number but enlarged sizes of leaf palisade cells. Although involved in progressing rRNA processing, these mutations do not enhance or alleviate leaf polarity defects of as2, suggesting that leaf adaxial-abaxial patterning is not a general growth defect caused by ribosome-related mutations (Kojima et al., 2017). Moreover, a nucleolar GTP-binding protein that positively regulates key cell cycle transition genes is involved in root development. Depletion of the nucleolar GTP-binding protein such as NUCLEOSTEMIN-LIKE1 (NSN1) results in dwarfism and slower primary root growth (Jeon et al., 2015; Wang et al., 2018). Mutations in APUM23/SAHY9, a nucleoli-localizing protein-encoding gene, not only cause delayed germination and growth (Abbasi et al., 2010; Huang et al., 2014) but also abnormal root-hair cell differentiation. In apum23/sahy9, transcription factor MYB23 induces GLABRA2, which directly represses multiple root hair transcription factor genes, producing nonhair cell (Wang et al., 2020).
Mutants impaired in rRNA processing show temperature-sensitive developmental defects, such as rid2 and rid3 (Konishi & Sugiyama, 2003; Ohbayashi et al., 2011). These mutants both display pointed leaves with simpler leaf veins and delayed root growth when grown at high temperature (28°C) and are impaired in proper shoot regeneration due to the uncontrolled expression of CUP-SHAPED COTYLEDON (CUC1)1 and SHOOT MERISTEMLESS (STM) (Ohbayashi et al., 2017; Shinohara et al., 2014). The loss of function of ANAC082 by the sriw1 mutation did not alter the abnormal accumulation of rRNA intermediates in rid2 and rid3, but markedly alleviated their developmental phenotypes including defective shoot regeneration at high temperature (Ohbayashi et al., 2017). ANAC082 did not alter the abnormal accumulation of rRNA intermediates in rid2 and rid3, but changed expression levels of CUC1 and STM (Shinohara et al., 2014). This implies that ANAC082 acts downstream of hindered ribosome biogenesis in response to ribosomal stress conditions.
Nucleolar proteins also regulate several genes involved in the cell cycle and floral organ formation. For instance, DDF1, a nucleolar anchored F-box protein, positively regulates genes that are involved in cell division and expansion, as well as B-class floral homeotic genes such as MADS4 and MADS16. Mutations in DDF1 in rice (Oryza sativa) result in dwarfism with decreased cell numbers and size, as well as abnormal development in floral organs due to negative regulation of the carpel specification gene DROOPING LEAF expression. Whorls 2 and whorls 3, where lodicules and stamens typically develop, are replaced with glume- and pistil-like organs in the mutated rice, resulting in male-sterile flowers (Duan et al., 2012). Additionally, nucleolar protein, histone deacetylases HDT1 and HDT2 are involved in the regulating of rRNA transcription and ovule development through histone acetylation in Arabidopsis (Luo et al., 2022). HDT1/HDT2 mutants exhibit a reduced seed setting rate when used as the maternal parent in crosses with the wild type, indicating their involvement in the development of the female gametophyte.
3.3 Functionally conserved nucleolar proteins in plant development
As ribosomes are essential components in all eukaryotic cells, certain nucleolar proteins are functionally conserved across different organisms, even across kingdoms. Homologs of ribosome biogenesis cofactors in yeast (S. cerevisiae), such as rRNA processing 5 (Rrp5), periodic tryptophan (W) protein 2 (Pwp2), Nin1 binding protein 1 (Nob1), essential nuclear protein 1 (Enp1), and nucleolar complex associated 4 (Noc4), influence seed development and embryogenesis by affecting ribosome biogenesis. Mutations in Rrp5, Pwp2, and Enp1 result in small, underdeveloped, or early aborted seeds, which reduces the number of seeds per silique due to the asynchronous embryo development. Furthermore, mutations in Nob1 and Noc4 lead to lower germination rates and pale seeds due to embryo development arrest caused by the inhibition of asymmetrical cell division at the globular stage (Missbach et al., 2013). Mutation in TORMOZ (TOZ), which is involved in 18s rRNA biosynthesis, can cause changes in cell plate orientation, from longitudinal to transverse. This change can result in embryonic arrest due to different expression pattern signals caused by altered positional cues (Griffith et al., 2007).
Maize Defective Kernel 58 (DEK58) is an Rrp15p domain containing protein, which is exemplified by yeast Rrp15 known as a nucleolar protein that regulates LSU biogenesis. The dek58 mutation impairs 35S rRNA processing due to the lack of interaction with ribosome processome homologous proteins, resulting in smaller kernels (Ma et al., 2024). Additionally, nucleolar proteins often regulate developmental genes through epigenetic mechanisms. It has been discovered that VERNALIZATION5/VIN3-LIKE 3 (VEL3) associates with a histone deacetylase complex (HDAC) to accumulate H3K27me3 at the pericentromeric heterochromatin in the central cell, thereby regulating maternal control of seed dormancy (Chen et al., 2023). These findings suggest that seed development and embryogenesis may be affected by asynchronous division or abnormal preferences in cell plate orientation caused by mutated nucleolar proteins.
4 NUCLEOLAR ACTION IN PLANT ABIOTIC STRESS RESPONSES
Plant nucleoli react dynamically to various abiotic stresses to survive in diverse environments. Recent research has shed light on the responses and functions of plant nucleoli following exposure to environmental stimuli, including chilling, heat, drought, salinity, and heavy metal stresses. These stress conditions induce changes in the morphology and function of plant nucleoli (Figure 2a, Table 2). For example, chilling stress leads to the disappearance of major ultrastructural components of FC and DFC, aligning with reduced characteristic domains corresponding to nucleolar proteins like fibrillarin and B23/NPM in the nucleoli of soybean root meristematic cells (Stępiński, 2009). Similar disruptions occur under heat stress, where Arabidopsis Fibrillarin2 undergoes dispersion, indicating the disruption of DFC (Picart-Picolo et al., 2020). In Arabidopsis, as the duration of heat stress increases, nucleoli grow more scattered and eventually collapse (Darriere et al., 2022). Similar phenotypes are detected with heavy metal toxicity. In the root cells of Allium cepa, the aluminium treatment causes the leaching of nucleolar materials to accumulate in the cytoplasm, and this phenomenon is boosted with prolonged duration and concentration of treatment (Qin et al., 2010). A high concentration of aluminium (e.g., 50 µM) disturbs the normal organization of nucleolus during mitosis (Qin et al., 2010). Likewise, reduction of the size and the number of nucleoli in plant cells are observed under copper stress (Castro et al., 2021) as well as salt stress (Teerarak et al., 2009), suggesting decreased nucleolar activity. Additionally, NoVs, plant nucleoli-specific structures, respond differently to various abiotic stresses showing distinct morphologies. Cold shock or prolonged chilling stress results in a large round structure of NoV (Hayashi & Matsunaga, 2019; Stępiński, 2009). Under severe heat stress, NoVs appear as speckled structures but no significant changes in the morphology of NoVs are observed under osmotic stress (Hayashi & Matsunaga, 2019). However, when stressed plants return to normal growth conditions, the disrupted nucleolar structure is re-established, often with even more distinct domains, implying more improved nucleolar activity (Darriere et al., 2022; Picart-Picolo et al., 2020; Stępiński, 2009). The underlying mechanism of the dynamics of plant nucleolar structure is yet elusive, however, it may result from changes in nucleolar liquidity. In animal cells, appropriate alteration of nucleolar liquidity is important for ribosome biogenesis and nucleolar protein quality control. The disruption of nucleolar structure is also observed in mammalian cells exposed to heat shock (Rubbi & Milner, 2003; Welch & Suhan, 1985). As nucleolar disruption leads to the stabilization of p53, a mammalian tumour suppressor, and thereby induces cell cycle arrest or apoptosis (Rubbi & Milner, 2003), alteration of nucleolar structure is essential for stress responses. Although there are no p53 homologs in plants, recent studies revealed that NAC082 transcription factor functions as a ribosomal stress response mediator (Ohbayashi & Sugiyama, 2018; Ohbayashi et al., 2017). As in animals, dynamic changes in the nucleolar structure would be a pivotal process for stress responses, with nucleolar activity increasing upon the return to normal growth conditions.
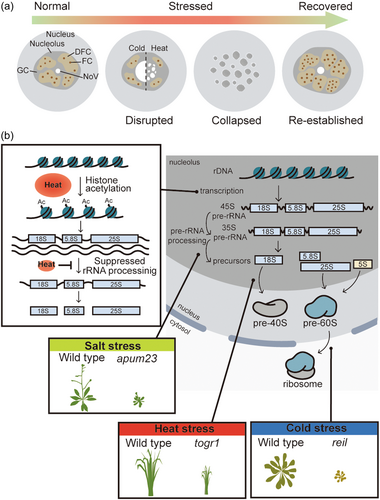
| Abiotic Stress | Impact on plant nucleoli/vital factor | Changes in nucleolar structure | Reference |
|---|---|---|---|
| Chilling | Disappearance of major ultrastructural components; reduced characteristic domains corresponding to nucleolar proteins. AtREIL involved in the cytosolic pre-60S LSU maturation step; crucial for cold-induced accumulation of cytosolic ribosome subunits and rRNA; vital for growth in cold conditions |
Cold shock or prolonged chilling stress: large round structure of NoVs, improved nucleolar activity upon return to normal growth conditions | Hayashi and Matsunaga (2019); Stępiński (2009) |
| Heat | Heat stress: dispersion of nucleolar proteins, indicating disruption of DFC; the transcription of 45S rRNA increased due to increased acetylation levels of H3K9 and H4K5 in euchromatin, 35S transcripts (precursors of 18S, 5.8S, and 25S RNAs) decreased. OsTOGR1 mediate normal plant growth at high temperature. Increasing duration of heat stress: nucleoli become scattered and eventually collapse. Prolonged heat stress: nucleolar disruption and increased acetylation levels of H3K9 and H4K5 in 45 S rDNA |
Speckled structures of NoVs, disrupted nucleolar structure re-established with more distinct domains upon return to normal growth conditions | Coccia et al. (2017); Darriere et al. (2022); Ghoshal and Jacob (1996); Picart-Picolo et al. (2020); Yue et al. (2021); Zhao et al. (2016) |
| Heavy metal (e.g., Aluminium) | Aluminium exposure: Leaching of nucleolar materials into the cytoplasm. Prolonged and concentrated aluminium treatment: Heightened leaching of nucleolar materials in cytoplasm High aluminium concentration (e.g., 50 µM): Disruption of normal nucleolus organization during mitosis |
Accumulation of leached materials in the cytoplasm, disrupted nucleolar structure re-established upon return to normal growth conditions | Qin et al. (2010) |
| Heavy metal (e.g., Copper) | Reduction in size and number of nucleoli, decreased nucleolar activity | N/A | Castro et al. (2021) |
| Salt stress | Reduction in size and number of nucleoli, decreased nucleolar activity | N/A | Teerarak et al. (2009) |
| Osmotic stress | N/A | No significant changes in NoV morphology | Hayashi andMatsunaga (2019) |
| Salinity | AtAPUM23/AtSAHY9 required for salt-stress response in Arabidopsis | N/A | Abbasi et al. (2010); Huang et al. (2018) |
In addition to the morphological changes in nucleolar structure, abiotic stresses affect rRNA synthesis and processing. In plant cells, there is an increase in rRNA transcript levels under stress conditions, which may be a result of epigenetic regulations. In a heat-sensitive maize line, prolonged heat stress induces nucleolar disruption and increased acetylation levels of H3K9 and H4K5 in 45S rDNA (Yue et al., 2021). As acetylation of H3K9 and H4K5 is the mark of euchromatin, this will lead to the increased transcription of 45S rRNA. This differs from animal cells where heat stress inhibits rRNA transcription through changes in chromatin structure by histone deacetylation and the inactivation of RNA Pol I transcription machinery (Coccia et al., 2017; Ghoshal & Jacob, 1996; Zhao et al., 2016). In contrast to the increase in the 45S transcript level, the level of the 35S transcripts, precursors of 18S, 5.8S, and 25S RNAs are decreased under heat stress (Darriere et al., 2022), which indicates a failure in pre-rRNA processing (Darriere et al., 2022; Picart-Picolo et al., 2020). Structural disruption of nucleoli under stress conditions would lead to loss of ability to perform rRNA processing. Interestingly, the type of rRNA gene variation expressed from NOR2 or NOR4 remains unaltered. Regardless of the stress environments, NOR4-derived rRNA variants 2 and 3 are actively expressed, whereas NOR2-derived rRNA variants 1 and 4 are silenced (Darriere et al., 2022; Picart-Picolo et al., 2020).
Appropriate pre-rRNA processing is essential for plants to adapt to various environmental conditions. Several nucleolar proteins are involved in various steps of these processes for normal growth and development under stress conditions (Figure 2b, Table 2). For example, Arabidopsis REIL proteins, which are required for normal growth in the cold, are involved in the cytosolic pre-60S LSU maturation step (Beine-Golovchuk et al., 2018; Cheong et al., 2021; Firmino et al., 2020; Schmidt et al., 2013; Yu et al., 2020). REIL proteins are responsible for cold-induced accumulation of cytosolic ribosome subunits and cytosolic rRNA. The accumulated nontranslating ribosome complexes may buffer fluctuating demands on translation under rapidly changing environmental conditions. Additionally, these proteins boost the expression of genes related to structural components of cytosolic ribosomes, RBFs, and initiating or elongating cytosolic translation, such as eIF3C2 or NUC2. Therefore, REIL-deficient plants cannot grow normally in the cold, for example, at 4°C or at 10°C, which suggests that REIL-mediated control in ribosome biogenesis is required for cold acclimation (Cheong et al., 2021; Schmidt et al., 2013). Another protein TOGR1, a nucleolar located DEAD-box RNA helicase, mediates normal plant growth at high temperature. TOGR1 associates with pre-rRNA processosome and maintains normal rRNA levels under heat stress via increased helicase activity (Wang, Hu, et al., 2016). Overexpressing TOGR1 in rice confers improved heat tolerance with enhanced rice growth and yield under high-temperature conditions (Wang, Qin, et al., 2016). Likewise, Chinese cabbage plants that express OsTOGR1 exhibit considerably enhanced growth and higher chlorophyll content than wild-type plants when exposed to high-temperature stress. Moreover, heat stress-responsive genes, such as NAC069, HSP70, and HSP27B, are highly upregulated upon heat stress treatment (Yarra & Xue, 2020). As TOGR1 functions as a heat-sensitive RNA chaperone in the nucleolus and potentially initiates other HSPs/chaperones pathways in response to high-temperature, the concerted function of TOGR1 and other HSPs/chaperones in nucleolus may protect pre-rRNA processing during ribosomal biogenesis at high temperatures (Yarra & Xue, 2020). Furthermore, APUM23/SAHY9, a nucleolar protein involved in pre-rRNA processing and ribosomal biogenesis (Abbasi et al., 2010), is required for salt-stress response in Arabidopsis (Huang et al., 2018). Under high salinity conditions, mutation of APUM23/SAHY9 changes primarily the expression of genes involved in abscisic acid (ABA) biosynthesis and signalling as well as ribosome biogenesis compared with wild type. Indeed, apum23/sahy9 mutant has a lower ABA level and its salt hypersensitivity phenotype is rescued by exogenous ABA treatment. However, exogenous ABA treatment does not restore other mutant phenotypes such as small plant size, short roots, and serrated and scrunched leaves, indicating that altered composition and abundance of ribosome profiles in apum23/sahy9 have effects on various physiological signalling as well as abiotic stress responses in plants (Huang et al., 2018). These show the importance of nucleolus and its role in abiotic stress responses in concert with other biological components.
5 NUCLEOLAR ACTION IN PLANT BIOTIC STRESS RESPONSES
Upon pathogen infection, the nucleolus plays a crucial role in plant immunity by regulating defence-related gene expression and ribosome biogenesis. Pathogens utilize the host nucleolar functions for their own production and transport within cells. Recent studies have shown that the nucleolus is a common target of viruses and other pathogens such as fungus and oomycetes. They hijack nucleolar proteins to counteract the plant's defence responses, as demonstrated in various studies (Hiscox, 2007; Taliansky et al., 2010) (Figure 3, Table 3).
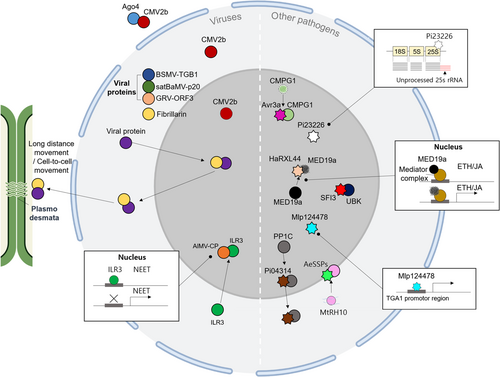
| Pathogen protein | Target | Function | Reference |
|---|---|---|---|
| Groundnut rosette virus ORF3 | Fibrillarin | Virus target fibrillarin for long-distance movement and cell-to-cell movement to complete systemic viral infections | Canetta et al. (2008); Chang et al. (2016); Kim, Macfarlane, et al. (2007); Kim, Ryabov, et al. (2007) |
| Satellite virus of bamboo mosaic virus (satBaMV) p20 | |||
| Barley stripe mosaic virus (BSMV) TGB1 | Jiang et al. (2020); Li et al. (2018) | ||
| Alfalfa mosaic virus (AMV) Cilevirus movement protein p32 | Leastro et al. (2021) | ||
| Cucumber mosaic virus CMV 2b protein | Argonaute 4 | Control target protein distribution to enhance virulence | Du et al. (2014) |
| Alfalfa mosaic virus coat protein (AlMV CP) | ILR3 | Regulate plant hormone responses through translocation of ILR3 from the nucleus to the nucleolus | Aparicio and Pallas (2017) |
| Phytophthora infestans AVR3a | NbCMPG1 | Nucleolus serves as a target to promote pathogen infection | Bos et al. (2010) |
| Phytophthora infestans Pi04314 | AtPP1c | Reduce host defence by relocation of PP1c from the nucleolus to the nucleoplasm | Boevink et al. (2016) |
| Hyaloperonospora arabidopsidis HaRxL44 | AtMED19a | Localizes to plant cell nucleoli, Regulate plant defence by MED19a degradation | Caillaud et al. (2013) |
| Phytophthora infestans PiSFI3 | NbUBK | The unique trans-homodimer formation of PiSFI3 is crucial for its nucleolar localization, StUBK interaction, and enhancing infection | He et al. (2019) |
| Phytophthora infestans Pi23226 | 3’ end of 25S rRNA precursors | Disrupt pre-rRNA processing and induce nucleolar stress | Lee et al. (2023) |
| Melampsora laricis-populina effector Mlp124478 | TGA1a promoter | Inhibit the expression of genes in response to pathogen infection | Ahmed et al. (2018) |
| Aphanomyces euteiches small secreted protein effectors AeSSP1256 | MtRH10 | AeSSP1256 relocates MtRH10 from nucleocytoplasm to the nucleolus rim, inhibiting its binding to plant RNA | Camborde et al. (2022) |
| Ralstonia solanacearum type III effector RipAS | StTOPP6 | RipAS reduces the nucleolar accumulation of StTOPP6 to compromise host defence | Wang et al. (2023a) |
Among pathogens, viruses are extensively studied for their specific targeting of the nucleolus (Taliansky et al., 2010; Xu et al., 2020). Various viral proteins, such as those associated with the major nucleolar component fibrillarin, play crucial roles in the long-distance and cell-to-cell movement, essential for systemic viral infections (Decle-Carrasco et al., 2021). Examples include the groundnut rosette virus (GRV) ORF3 and p20 proteins encoded by the satellite virus of Bamboo mosaic virus (satBaMV), both of which recruit fibrillarin for replication and long-distance movement (Canetta et al., 2008; Chang et al., 2016; Kim, Macfarlane, et al., 2007; Kim, Ryabov, et al., 2007). The interactions between fibrillarin and viral movement protein, such as Triple Gene Block1 (TGB1), facilitate the cell-to-cell movement of the virus (Jiang et al., 2020; Li et al., 2018). Additionally, some viral proteins, such as the Cilevirus (CILV) movement protein p32, enable cell-to-cell and long-distance transport of movement-defective Alfalfa mosaic virus (AMV) (Leastro et al., 2021). Importantly, the interaction between viral proteins and fibrillarin is not limited to specific viral taxa, indicating that fibrillarin is a general target among various plant virus proteins (Rajamaki & Valkonen, 2009).
The nucleolus is also suggested to sequester molecules that usually function outside of the nucleolus including the nucleoplasm and cytoplasm (Sirri et al., 2008). Viruses hijack these molecules for their localizing and storing their proteins and exploit the nucleolus function to regulate the balance between their proliferation and plant viability. For example, the RNA silencing suppressor Cucumber mosaic virus (CMV) 2b protein is found in the nucleus/nucleolus and cytoplasm. Its RNA silencing suppression activity primarily occurs in the cytoplasm, where it interacts with Argonaute 4. By controlling its distribution between these compartments, CMV achieves a balance between virus accumulation and plant damage, enhancing its virulence (Du et al., 2014). The coat protein of alfalfa mosaic virus (AlMV CP) plays multiple roles in virion assembly, translation, and movement. It localizes in the nucleus/nucleolus and cytoplasm; balance in the localizations of CP regulates the life cycle of the virus (Bol, 2005). Moreover, AlMV CP also interacts with basic helix-loop-helix (bHLH) transcription factor (ILR3), a transcription factor regulating a class of genes encoding iron-sulfur proteins that contain Asn-Glu-Glu-Thr sequence in their C-terminal domain (NEET), which is involved in the regulation of iron and ROS homeostasis (Nechushtai et al., 2012). In a recent study, the interaction between AlMV CP and ILR3 causes the translocation of ILR3 from the nucleus to the nucleolus. This relocation leads to the downregulation of NEET, resulting in the activation of plant hormone responses (Aparicio & Pallas, 2017). Together, these data suggest that the viruses utilize the nucleolar function to regulate the balance between their proliferation and plant viability for successful infection.
Other than viral components, such as RXLR effectors from hemi-biotropic oomycete pathogen Phytophthora infestans also target the nucleolus to enhance infection (Chaudhari et al., 2014). These effectors interact with host proteins within the nucleolus, promoting and facilitating pathogen infection. For instance, AVR3a from P. infestans associates with and stabilizes plant E3 ligase (CMPG1) in the nucleolus to promote pathogen infection (Bos et al., 2010). Another Phytophthora RXLR effector, Pi04314, is localized in the nucleolus and interacts with host protein phosphatase 1 catalytic (PP1c), causing the relocation of PP1c from the nucleolus to the nucleoplasm, thereby reducing the transcriptional responses of host plant defence genes (Boevink et al., 2016). Similarly, RXLR effector HaRxL44, produced by the biotrophic oomycete pathogen Hyaloperonospora arabidopsidis, localizes to plant cell nucleoli. It interacts with host mediator subunit 19a (MED19a), leading to MED19a degradation and a shift in plant defence gene transcription from salicylic acid-responsive to jasmonic acid and ethylene-responsive pathways, enhancing the plant's susceptibility to infection (Caillaud et al., 2013). In the recent study, PiSFI3 targets the host nucleus and nucleolus, where it interacts with U-box-kinase protein (UBK), a positive regulator of PTI pathways in potato and Nicotiana benthamiana. The unique trans-homodimer formation of PiSFI3 is crucial for its nucleolar localization, interaction with UBK of Solanum tuberosum, and its role in enhancing P. infestans infection (He et al., 2019). Moreover, Pi23226 has recently been shown to directly bind to the 3’ end of 25S rRNA precursors, disrupting pre-rRNA processing and inducing nucleolar stress. In planta expression of Pi23226 results in nucleolar inflation and triggers necrotic cell death, positively affecting pathogen growth (Lee et al., 2023).
In other case of pathogen, the rust fungal effector Mlp124478 mainly localizes within the nucleolus and interacts with the TGA1a promoter in the host, leading to transcriptional modifications that inhibit the expression of genes in response to pathogen infection (Ahmed et al., 2018). One of the small secreted protein effectors (AeSSPs) of soilborne legume pathogen Aphanomyces euteiches, AeSSP1256 localizes to the nucleolar rim. AeSSP1256 interacts with RNA helicase of Medicago truncatula (MtRH10), which is linked to ribosome-related genes, root development and defence response. By interacting with MtRH10, AeSSP1256 relocates MtRH10 from nucleocytoplasm to the nucleolus rim, inhibiting its binding to plant RNA. This redistribution of MtRH10 causes nucleolar stress by interfering with the ribosome biogenesis pathway and enhances susceptibility to A. euteiches infection (Camborde et al., 2022). More recently, the bacterial type III effector RipAS reduces the nucleolar accumulation of a type one protein phosphatase (PP1) StTOPP6, a susceptibility factor to promote bacterial wilt, compromising host defence against R. solanacearum when expressed in potato (Wang et al., 2023a). These findings suggest that plant pathogens employ effectors to manipulate various host processes, with a particular focus on activities occurring within the host nucleolus.
In conclusion, a more in-depth exploration of how plant pathogens target and manipulate host proteins, along with elucidating the role of nucleolus in these processes, will yield new insights into stress responses, growth, and development.
6 FUTURE PERSPECTIVE
For many years, the nucleolus was primarily understood as having a limited function in ribosome biogenesis. However, recent research has illuminated a broad spectrum of biological activities localized within this nucleolar region. What unifies these activities is the essential role of RNA, often involving RNA in the biogenesis or assembly of RNA/protein complexes, similar to other (LLPS). Consequently, it is becoming increasingly appropriate to view the nucleolus as an RNA processing centre rather than merely a ribosome factory. These recent discoveries have opened up exciting possibilities for comprehending how the nucleolus participates in various cellular processes.
Despite significant advancements, several key questions remain unresolved. For example, the precise molecular mechanisms through which nucleolar proteins interact with other cellular components to regulate plant growth and development under stress conditions remain unclear. Moreover, the specific roles of numerous nucleolar proteins in plant immunity and stress responses must be elucidated. To address these gaps, it will be necessary to employ advanced techniques such as high-resolution imaging, single-cell RNA sequencing, and proteomics. Another area of interest for future research is the exploration of nucleolar dynamics under varying environmental conditions. A deeper understanding of how the nucleolus responds to and recovers from stress at a molecular level could reveal novel targets for genetic or chemical interventions aimed at enhancing plant resilience. Furthermore, the potential cross-talk between nucleolar functions and other cellular organelles, such as mitochondria and chloroplasts, remains largely unexplored and could provide a more integrated view of cellular stress responses.
In the context of biotic stress, a more thorough examination of the tactics employed by pathogens to expoit nucleolar functions could potentially lead to the development of novel plant protection strategies. The identification and characterization of pathogen effectors that target nucleolar components will be essential in the development of crops with enhanced resistance to diseases. Finally, the application of synthetic biology to manipulate nucleolar functions represents a promising frontier. By engineering nucleolar components, it may be possible to develop plants with optimized growth and stress response traits, tailored to thrive in specific environmental conditions. This approach could revolutionize agricultural practices, leading to more sustainable and productive crop systems.
In summary, while substantial progress has been made in understanding the multifaceted roles of the nucleolus, numerous exciting avenues for research remain. By addressing these unresolved issues, we can fully realize the potential of nucleolar biology to enhance crop resilience and mitigate the impact of biotic and abiotic stresses, ultimately contributing to global food security and agricultural sustainability.
ACKNOWLEDGEMENTS
This work was supported by National Research Foundation of Korea (NRF) grants funded by the Korean government (MSIT) to Joo Hyun Lee (2019R1C1C1008698) and to Doil Choi (No. 2018R1A5A1023599 [SRC] and RS-2024-00333777) and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education to Ye-Eun Seo (RS-2023-00272323).
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.




