Distinct profiles of plant immune resilience revealed by natural variation in warm temperature-modulated disease resistance among Arabidopsis accessions
Abstract
Elevated temperature suppresses the plant defence hormone salicylic acid (SA) by downregulating the expression of master immune regulatory genes CALMODULIN BINDING PROTEIN 60-LIKE G (CBP60g) and SYSTEMIC ACQUIRED RESISTANCE DEFICIENT1 (SARD1). However, previous studies in Arabidopsis thaliana plants have primarily focused on the accession Columbia-0 (Col-0), while the genetic determinants of intraspecific variation in Arabidopsis immunity under elevated temperature remain unknown. Here we show that BASIC HELIX LOOP HELIX 059 (bHLH059), a thermosensitive SA regulator at nonstress temperatures, does not regulate immune suppression under warmer temperatures. In agreement, temperature-resilient and -sensitive Arabidopsis accessions based on disease resistance to the bacterial pathogen Pseudomonas syringae pv. tomato (Pst) DC3000 did not correlate with bHLH059 polymorphisms. Instead, we found that temperature-resilient accessions exhibit varying CBP60g and SARD1 expression profiles, potentially revealing CBP60g/SARD1-dependent and independent mechanisms of immune resilience to warming temperature. We identified thermoresilient accessions that exhibited either temperature-sensitive or -insensitive induction of the SA biosynthetic gene ICS1 (direct target gene of CBP60g and SARD1) and SA hormone levels. Collectively, this study has unveiled the intraspecific diversity of Arabidopsis immune responses under warm temperatures, which could aid in predicting plant responses to climate change and provide foundational knowledge for climate-resilient crop engineering.
1 INTRODUCTION
Plant health is critical to maintaining global food and bioenergy security; however, plant disease outbreaks are only becoming more severe and prevalent with climate change (Chaloner et al., 2021; Deutsch et al., 2018; Savary et al., 2019; Singh et al., 2023). The relationship between host plants and disease-causing pathogens with dynamically changing environmental conditions is well-synthesized by the “plant disease triangle” paradigm (Francl, 2001; Roussin-Léveillée et al., 2024; Stevens, 1960). For example, plants experiencing suboptimal environmental conditions, such as heat or water stress, exhibit weaker immune responses and therefore become more susceptible to pathogen infection (Cohen & Leach, 2020; Desaint et al., 2021; Roussin-Léveillée et al., 2024; Singh et al., 2023; Velásquez et al., 2018).
The cross-kingdom interaction between Arabidopsis thaliana plants and the bacterial pathogen Pseudomonas syringae pv. tomato (Pst) DC3000 has been used extensively as a model system to study host-pathogen interactions and the plant disease triangle (Ausubel et al., 1995; Velásquez et al., 2018; Xin & He, 2013). As shown in Arabidopsis and various other plant species, plants elicit a two-pronged and interlinked immune response during pathogen attack: (1) pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI) and (2) effector-triggered immunity (ETI) (Bigeard et al., 2015; Ngou et al., 2022; Yuan et al., 2021; Zhou & Zhang, 2020). A key plant defence hormone mediating convergent immune signalling is salicylic acid (SA), which is crucial for local and systemic defences against biotrophic and hemibiotrophic pathogens (Delaney et al., 1994; Durrant & Dong, 2004; Gaffney et al., 1993; Malamy et al., 1990; Peng et al., 2021; Rossi et al., 2023; Zhang & Li, 2019). During pathogen challenge, endogenous SA production in plants is important in amplifying both PTI and ETI (Liu et al., 2020; Shields et al., 2022; Tateda et al., 2014; Yang et al., 2021).
Previous studies have demonstrated that elevated temperatures inhibit specific components of the plant immune system, including PTI, ETI, and SA production (Cheng et al., 2013; Huot et al., 2017; De Jong et al., 2002; Kim et al., 2021, 2022; Menna et al., 2015; Shields et al., 2023; Velásquez et al., 2018; Wang, Bao, et al., 2009; Zhu et al., 2010). In particular, ISOCHORISMATE SYNTHASE 1 (ICS1)-mediated SA biosynthesis is downregulated at elevated temperature (Castroverde and Dina, 2021; Huot et al., 2017; Malamy et al., 1992; Shields et al., 2023). Heat suppression of the SA pathway is governed through the thermosensitive, rate-limiting transcription of the master immune regulatory genes CBP60g and SARD1 (Kim et al., 2022). Recently, a study uncovered that warmer temperatures (28°C) reduce the formation of intranuclear GUANYLATE BINDING PROTEIN-LIKE 3 (GBPL3) defence-activated biomolecular condensates (GDACs), thereby abolishing the recruitment of the general transcriptional machinery to the CBP60g and SARD1 genetic loci (Kim et al., 2022). Overall, temperature has a profound impact on the plant immune system through various levels of genetic regulation.
In addition to changing environmental conditions, SA production is also influenced by the profound intraspecific diversity of the Arabidopsis pangenome (Ahmad et al., 2011; Alcázar et al., 2009; Bechtold et al., 2010; Bruessow et al., 2021; Van Leeuwen et al., 2007; Velásquez et al., 2017; Yang et al., 2015). At nonstress temperatures (16–22°C), Bruessow et al. (2021) discovered remarkable natural variation in basal (uninduced) SA accumulation among numerous Arabidopsis accessions. Through a genome-wide association study (GWAS) of >100 natural A. thaliana accessions, thermoresponsive basal SA production was found to be mediated by the transcription factor BASIC HELIX-LOOP-HELIX 059 (bHLH059; Bruessow et al., 2021). Bruessow et al. (2021) found two significant SNPs that distinguish Arabidopsis accessions into thermosensitive or thermoresilient phenogroups based on SA levels. Furthermore, the authors also identified that bHLH059 transcripts increased at 22°C compared to 16°C in temperature-sensitive accessions (e.g., Col-0); however, bHLH059 gene expression remained unchanged in temperature-resilient accessions (Bruessow et al., 2021).
A critical gap in the current literature is that the Arabidopsis immune variation at elevated temperature and its underlying mechanisms have not been fully dissected. Here we show extensive intraspecific diversity among Arabidopsis accessions in terms of disease susceptibility to the bacterial pathogen Pst DC3000 between ambient (23°C) and moderately elevated temperatures (28°C). Unlike at the nonstress temperature range, we further demonstrate that this natural variation is independent of bHLH059 but exhibits accession-specific dependence on the expression of the immune regulatory genes CBP60g and SARD1. Collectively, leveraging the naturally occurring variation within the A. thaliana species may prove to be an effective strategy to better understand how plant immune systems respond to our changing climate.
2 MATERIALS AND METHODS
2.1 Plant materials and growth conditions
Arabidopsis mutants and accessions used in this study were obtained from the Arabidopsis Biological Resource Centre, The Ohio State University (see Supporting Information S1: Table S1). Seeds were surface-sterilized with 70% ethanol, rinsed three times with autoclaved water and then stratified in 0.1% agarose at 4°C for 3 days to promote uniform germination (Rivero et al., 2014). Seeds were sown onto autoclaved soil comprised of equal parts ProMix PGX or BX (Plant Products), Turface (Turface Athletics), and Vermiculite (Therm-O-Rock East), supplemented with MiracleGro (The Scotts Company). Plants were incubated in environmentally controlled growth chambers with the following conditions: 23°C temperature, 60% relative humidity, 12 h day/12 h night cycle, and 80–100 µmol m−2 s−1 light conditions.
2.2 Plant infection and disease assays
Three leaves of 4- to 5-week-old Arabidopsis plants were syringe-infiltrated with the Pst DC3000 suspension (OD600 = 0.001), which was prepared as previously described (Huot et al., 2017; Kim et al., 2022; Shields et al., 2023). Plants were then incubated at 23°C (normal) or 28°C (elevated) with the same relative humidity and light cycling conditions. At 3 days postinoculation (dpi), in planta bacterial quantification was performed as described previously (Huot et al., 2017). Briefly, circular leaf punches were collected from the infiltrated leaves and homogenized in 0.25 mM MgCl2 with a TissueLyser II (Qiagen). Samples were then serially plated onto LM media containing rifampicin and incubated at 21–24°C overnight. Colony-forming units (CFUs) were counted 2 days later, and bacterial levels were reported as log CFU/cm2.
2.3 Gene expression analyses
Leaves of 4- to 5-week-old Arabidopsis plants were syringe-infiltrated with mock solution (0.25 mM MgCl2) or Pst DC3000 suspension (OD600 = 0.001) as described above. Plants were then incubated at 23°C (normal) or 28°C (elevated) with the same relative humidity and light cycling conditions. Gene expression levels were quantified based on a previously published protocol (Kim et al., 2022) with slight modifications. At 1 dpi, total RNA was extracted from flash-frozen plant tissues using the Qiagen Plant RNeasy Mini Kit (Qiagen) and cDNA was synthesized using qScript cDNA super mix (Quantabio) based on manufacturers' recommendations. Real-time quantitative polymerase chain reaction (qPCR) was performed using PowerTrack SYBR Green master mix (Applied Biosystems) or ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech). qPCR amplification was performed using the Applied Biosystems QuantStudio3 platform (Life Technologies), and individual Ct values were determined for target genes (ICS1, CBP60g, SARD1) and the internal control gene (PP2AA3) (Huot et al., 2017; Kim et al., 2022). Gene expression values were reported as 2−ΔCt, where ΔCt is Cttarget gene–CtPP2AA3. qPCR was carried out with three technical replicates for each biological sample. Primers used for qPCR are shown in Supporting Information S1: Table S2.
2.4 Quantification of SA hormone levels
Leaves of 4- to 5-week-old Arabidopsis plants were syringe-infiltrated with mock solution (0.25 mM MgCl2) or Pst DC3000 suspension (OD600 = 0.001) as described above. Plants were then incubated at 23°C (normal) or 28°C (elevated) with the same relative humidity and light cycling conditions. SA levels were quantified using an Acinetobacter sp. ADPWH_lux SA biosensor strain (Huang et al., 2005), based on an optimized protocol by Defraia et al. (2008). This biosensor strain contains the chromosomally located fusion of the salA (SA hydroxylase) gene from the Acinetobacter operon with the promoterless bioluminescence gene cassette luxCDABE from Photorhabdus luminescens (Huang et al., 2006). SA standards and plant extracts were loaded into a white luminescence-compatible 96-well plate and combined with a 4-h culture of the biosensor (OD = 0.4). The plate was incubated at 37°C for 1 h before reading the luminescence with a BioTek Synergy HT plate reader pre-warmed to 37°C. The plate reader was set to read luminescence with the following settings: read type was endpoint, sensitivity at 135, integration time of 5.0 s, and filter set 1. All samples and standards were plated in triplicate. SA levels were calculated based on the SA-luminescence standard curve and normalized to the leaf fresh weight.
2.5 Statistical analyses
Statistical analyses of bacterial quantification and gene expression values were carried out using R (R Core Team, 2023) or Prism (GraphPad). Specific statistical tests for each experiment are detailed in the respective figure captions.
3 RESULTS
3.1 The transcription factor bHLH059 is not involved in temperature-sensitive Arabidopsis immunity under warm temperatures
Because bHLH059 acts as a thermoresponsive SA regulator at nonstress temperatures between 16°C and 22°C (Bruessow et al., 2021), we tested whether bHLH059 also controls the natural variation of temperature-modulated immunity at warm temperatures in the 23–28°C range. As shown in Figure 1a,b, the reference accession Col-0 expectedly exhibited temperature-sensitive disease resistance in terms of pathogen levels and disease symptoms, consistent with previous studies (Huot et al., 2017; Kim et al., 2022; Mang et al., 2012; Wang, Bao, et al., 2009). Both blhh059 mutant alleles similarly showed thermosensitive disease susceptibility to Pst DC3000, with higher bacterial levels and disease symptoms at 28°C than at 23°C (Figure 1a,b). The two bhlh059 mutants were also evaluated for gene expression levels of CBP60g and SARD1, which were recently reported to be the rate-limiting nodes in warm temperature suppression of the plant immune system (Kim et al., 2022). As shown in Figure 1c,d, the reference accession Col-0 showed temperature-sensitive CBP60g and SARD1 gene expression as expected (Kim et al., 2022). Similar to Col-0, both bhlh059 mutants also showed temperature-sensitive CBP60g and SARD1 gene expression, with lower transcript levels at 28°C compared to 23°C after pathogen infection. Taken together, these results indicate that the transcription factor bHLH059 does not play an essential role in controlling warm temperature-modulated immunity in Arabidopsis plants.
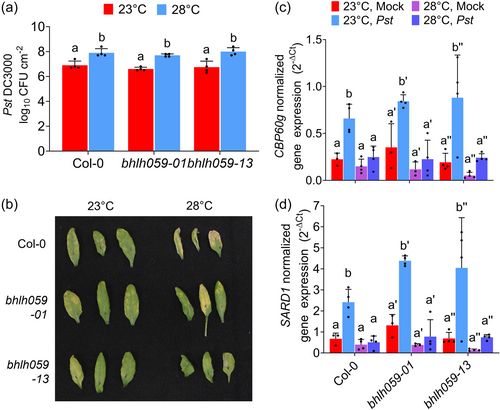
3.2 Impact of elevated temperature on disease resistance in various natural accessions of A. thaliana
As shown above and in previous studies, the reference accession A. thaliana Col-0 exhibits temperature-sensitive immune responses at nonstress temperatures (Bruessow et al., 2021; Li et al., 2020) and at warm temperatures (Huot et al., 2017; Kim et al., 2022). To investigate the intra-species diversity of Arabidopsis immunity at elevated temperature, disease resistance assays were performed by inoculating 4-week-old A. thaliana natural accessions (including Col-0) with virulent Pst DC3000. As shown in Figure 2a, certain accessions (Col-0, Ler, Fei-0, and Est-1) had significantly higher bacterial levels at 28°C than at 23°C (i.e., temperature-sensitive), while other accessions (Cvi-0, NW-1, Se-0, Ei-2, NFA-8, Sf-2, Bay-0, and Mz-0) did not have significantly different Pst DC3000 pathogen levels between the two temperatures (i.e., temperature-resilient). It is important to note that there was considerable natural variation in basal disease resistance within each group. Among the temperature-sensitive accessions, Col-0 had the overall highest bacterial levels, while Est-1 had the lowest bacterial levels (Figure 2a). On the other hand, among the temperature-resilient accessions, Cvi-0 had the highest overall Pst DC3000 levels, while Mz-0 had the lowest (Figure 2a). Similar to the in planta bacterial levels, there was also considerable variation in disease symptom development among these accessions as shown in Figure 2b. Overall, these results indicate that elevated temperature differentially impacts the defence responses of various A. thaliana accessions to Pst DC3000.
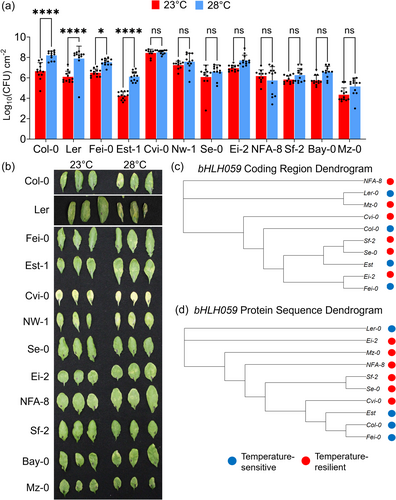
Under the nonstress temperature range from 16°C to 22°C, distinct polymorphisms in the SA regulator bHLH059 controls natural variation in temperature-modulated basal SA levels (Bruessow et al., 2021). To better understand if the intraspecific variation under the warmer temperature (23–28°C) also correlates with bHLH059 sequence polymorphisms, we clustered different accessions based on the bHLH059 coding region and protein amino acid sequences using Clustal Omega (Sievers and Higgins, 2021; Supplemental Data 1). As shown in Figure 2c,d, hierarchical clustering of bHLH059 protein-coding DNA sequences, as well as the bHLH059 protein amino acid sequences, did not strictly separate the different accessions based on their disease resistance phenotypes determined in Figure 1a,b. Collectively, this suggests that bHLH059-independent mechanisms govern differential immune resilience or sensitivity to elevated temperatures in Arabidopsis plants.
3.3 Differential CBP60g and SARD1 gene expression in Arabidopsis accessions with temperature-resilient disease resistance
To investigate the underlying mechanisms of thermoregulated plant immune variation, we examined gene expression levels of two master immune regulatory genes CBP60g and SARD1 (Wang et al., 2011; Wang, Tsuda, et al., 2009; Zhang et al., 2010) in three representative temperature-resilient accessions (Se-0, Sf-2, NFA-8) and reference accession Col-0. This aimed to establish if these two genes are the driving force behind temperature-resilient immunity in naturally occurring Arabidopsis accessions. As shown in Figure 3a,b, Col-0 expectedly showed temperature-sensitive CBP60g and SARD1 gene expression, with significantly lower transcript levels at 28°C compared to 23°C after pathogen infection (Kim et al., 2022; Shields et al., 2023). Interestingly, the temperature-resilient accessions Se-0 and NFA-8 behaved similarly as Col-0 in terms of defence gene expression, showing significantly lower levels of pathogen-induced CBP60g and SARD1 at 28°C compared to 23°C (Figure 3a,b). In contrast, the temperature-resilient accession Sf-2 showed temperature-resilient CBP60g and SARD1 gene expression profiles, with similar pathogen induction of transcript levels between 23°C and 28°C (Figure 3a,b).
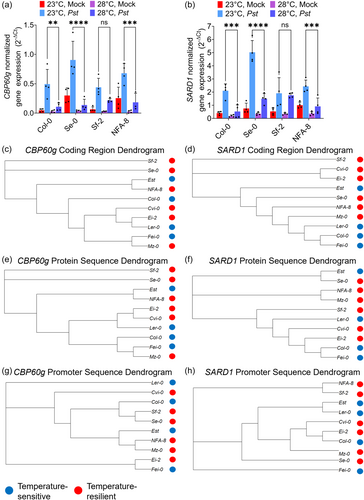
To clarify whether genetic polymorphisms in these master immune regulators also contribute to temperature-resilient immunity, we clustered the CBP60g and SARD1 coding sequences (Supplemental Data 2 and 3) of the full accession panel in Figure 3c,d. However, there was no association between coding region sequence polymorphisms in the different accessions and their level of disease thermosensitivity (Figure 3c,d). Additionally, no sequence correlations with the varying immune phenotypes were revealed by analyzing polymorphisms in protein amino acid sequences (Figure 3e,f) or promoter sequences (Figure 3g,h). Overall, these results suggest that Sf-2 may require sustained CBP60g/SARD1 expression at elevated temperature for its immune resilience, while Se-0 and NFA-8 have additional mechanisms to compensate for downregulated CBP60g and SARD1 gene expression at warm temperatures.
3.4 Naturally occurring plant immune resilience in Arabidopsis accessions exhibit thermoresilient or thermosensitive ICS1-SA pathway
A primary target gene of the master transcription factors CBP60g and SARD1 is ICS1, which encodes a rate-limiting enzyme for SA biosynthesis in response to pathogen infection (Wildermuth et al., 2001). Temperature-regulated levels of the central plant defence hormone SA has been associated with thermoregulated disease resistance in Arabidopsis Col-0 plants at both low (Kim et al., 2017; Li et al., 2020) and high temperatures (Huot et al., 2017; Kim et al., 2022; Mang et al., 2012). To investigate whether the SA biosynthesis via the ICS1 pathway (Wildermuth et al., 2001) mediates naturally occurring plant immune resilience, we measured ICS1 transcript levels in three representative accessions (Se-0, Sf-2, and NFA-8) that showed a temperature-resilient disease resistance phenotype. As shown in Figure 4a, the thermosensitive Arabidopsis accession Col-0 showed the expected trend of temperature-sensitive ICS1 gene expression levels, where ICS1 was induced by Pst pathogen infection at 23°C but not at 28°C. As for the accessions exhibiting thermoresilient immunity, both Se-0 and NFA-8 demonstrated temperature-sensitive induction of ICS1 gene expression profiles (Figure 4a). In contrast, Sf-2 showed constitutive expression of the SA biosynthetic gene ICS1 regardless of treatment (Figure 4a).

To further explore the underpinning mechanisms, we measured SA levels in Col-0, Sf-2 and NFA-8. We found that SA accumulation in these representative accessions mirrored the trends observed with ICS1 transcript levels (Figure 4c,d). Both Col-0 and NFA-8 showed temperature-sensitivity in the induction of SA by pathogen infection, wherein pathogen-induced SA levels were significant at 23°C but not at 28°C (Figure 4b,d). In contrast, the Sf-2 accession showed low constitutive levels of SA that was insensitive to changing temperature conditions (Figure 4c). Taken together, our findings show intraspecific variation in the thermosensitivity of the ICS1-SA pathway and that ICS1-independent pathways may robustly buffer disease resistance under warm temperatures in certain accessions.
4 DISCUSSION
Rising global temperatures associated with climate change have a profound impact on plant health, including effective immune responses to infectious diseases (Chaloner et al., 2021; Deutsch et al., 2018; Savary et al., 2019; Singh et al., 2023; Velásquez et al., 2018; Roussin-Léveillée et al., 2024). However, the extent of intraspecific variation in plant immunity at warm temperatures has remained unexplored. In this study, we demonstrated extensive variation in temperature-modulated disease susceptibility among natural A. thaliana accessions at elevated temperatures (23–28°C). In striking contrast to the nonstress temperature range (16–22°C), this natural variation is independent of the recently identified SA regulator bHLH059 (Bruessow et al., 2021). Instead, temperature-resilient Arabidopsis immunity can be mediated by distinct regulatory mechanisms that are either dependent or independent on sustained expression of CBP60g and SARD1, which encode master transcription factors of SA biosynthesis and other drivers of the plant immune system (Kim et al., 2022; Sun et al., 2015; Wang et al., 2011; Wang, Tsuda, et al., 2009; Zhang et al., 2010).
Using a panel of Arabidopsis accessions based on previous studies (Bruessow et al., 2021; Sanchez-Bermejo et al., 2015), disease resistance assays to the bacterial pathogen Pst DC3000 revealed two key groups: (1) temperature-sensitive accessions, with greater bacterial growth in planta at 28°C compared to 23°C, and (2) temperature-resilient accessions, with similar levels of in planta bacterial growth at 28°C and 23°C. Temperature-sensitive accessions included Col-0, Ler, Fei-0, and Est-1, while temperature-resilient accessions included Se-0, Ei-2, NFA-8, Sf-2, Cvi-0, Bay-0, and Mz-0. A previous study has found that certain accessions experience temperature-sensitive or -resilient immune responses at nonstress temperatures between 16°C and 22°C (Bruessow et al., 2021). However, Bruessow et al. (2021) did not evaluate how these various accessions respond to warmer temperatures (e.g., 28°C), which had remained a major knowledge gap until our study. Comparing the nonstress (16–22°C) and elevated temperature ranges (23–28°C), certain differences in thermoregulated immunity are apparent. For example, while Bruessow et al. (2021) found that Bay-0 exhibited thermosensitive disease resistance between 16°C and 22°C, we found that this accession is thermoresilient at warmer temperatures. In contrast, Ler was determined to have better immunity at 22°C compared to 16°C (Bruessow et al., 2021), but this accession had higher disease vulnerability at 28°C than at 23°C as shown in our present study. Collectively, our findings (together with those in previous work) reveal the naturally occurring intra-species diversity of disease phenotypes conferred by the Arabidopsis pangenome. Future research could expand this study by examining a broader panel of natural accessions.
Having established the natural variation in temperature-sensitivity or -resilience of Arabidopsis disease resistance to the pathogen Pst DC3000, we then sought to determine the underlying mechanism regulating this intraspecific diversity. We investigated the role of the transcription factor bHLH059, since it has been implicated in thermoregulation of immunity at nonstress temperatures (16°C vs. 22°C; Bruessow et al., 2021). As shown by Bruessow et al. (2021), bhlh059 mutants exhibit temperature-insensitive disease resistance and basal SA levels between 16°C and 22°C. Surprisingly, our results showed that bhlh059 mutants are temperature-sensitive in terms of Pst DC3000 disease susceptibility and SA-related defence gene expression in the 23°C to 28°C temperature range like the reference accession Col-0 (Kim et al., 2022). Furthermore, thermoresilient and thermosensitive Arabidopsis accessions did not correlate with bHLH059 DNA or protein sequence polymorphisms. Taken together, these findings underscore that bHLH059 is not involved in thermoregulated immunity under warmer temperatures, suggesting distinct genetic determinants depending on the temperature range.
Because we eliminated bHLH059 as a potential regulator of Arabidopsis plant immunity in the elevated temperature range (23–28°C), we sought out to determine if the master immune regulators CBP60g and SARD1 potentially govern immune resilience in accessions exhibiting sustained disease resistance under changing temperature. CBP60g and SARD1 are key thermoresponsive genes that encode master transcription factors that are rate-limiting for Arabidopsis Col-0 immune resilience to warming conditions (Kim et al., 2022). We specifically focused on three representative thermoresilient accessions (Se-0, Sf-2, NFA-8) since they all have been shown to exhibit better disease resistance to Pst DC3000 at normal or ambient temperatures (Bruessow et al., 2021; Kover & Cheverud, 2007; Saleem et al., 2017; Todesco et al., 2014; Whalen et al., 1991). Specifically, we examined the expression of CBP60g and SARD1 in these temperature-resilient accessions, with Col-0 as the temperature-sensitive control accession.
The temperature-resilient accession Sf-2 showed sustained CBP60g and SARD1 at both normal and elevated temperatures, which is consistent with our previous study showing that plants with constitutive CBP60g or SARD1 expression exhibit immune resilience to changing temperatures (Kim et al., 2022). Surprisingly, the other two temperature-resilient accessions Se-0 and NFA-8 showed temperature-sensitive CBP60g and SARD1 levels (i.e., still suppressed at warm temperature). These thermosensitive defence gene expression profiles diverge from their disease susceptibility phenotypes and in planta pathogen levels. In addition to defence gene expression profiling, our sequence analyses found no distinguishing nucleotide or amino acid polymorphisms in CBP60g and SARD1 (coding or promoter sequences) to delineate the naturally occurring temperature-resilient or sensitive immunity in representative Arabidopsis accessions. This suggests CBP60g/SARD1-expression-independent immune resilience in Se-0 and NFA-8, which will be important directions to pursue in future studies.
CBP60g and SARD1 directly target the ICS1 gene promoter to regulate its expression (Sun et al., 2015; Wang et al., 2011; Wang, Tsuda, et al., 2009; Zhang et al., 2010). ICS1 encodes an important biosynthetic enzyme that primarily contributes to pathogen-induced SA biosynthesis in Arabidopsis (Wildermuth et al., 2001). Expectedly, the temperature-regulated trends in CBP60g and SARD1 transcript levels were largely reflected by our analyses of ICS1 gene expression and SA production in representative accessions. Col-0, Se-0 and NFA-8 showed significant induction of ICS1 and/or SA at normal temperatures but not at warm temperatures, while Sf-2 exhibited temperature-insensitivity in both ICS1 or SA levels. Overall, these results indicate that diverse immune resilient accessions may be dependent (as shown in Sf-2) or independent (as shown in Se-0 and NFA-8) of sustained CBP60g, SARD1 and ICS1 gene expression. The CBP60g/SARD1 and ICS1-independent mechanisms may play a role in providing robust disease resistance of certain A. thaliana accessions to Pst DC3000. For example, it is possible that immune-resilient accessions like Se-0 and NFA-8 may have sustained MAP kinase activation, which has been shown to compensate for loss of SA induction (Tsuda et al., 2013). Another potential mechanism could be sustained EDS1 activation in Se-0 and NFA-8, since EDS1 has been previously demonstrated to function redundantly with the SA defence network to provide robustness to the plant immune system (Venugopal et al., 2009). In the future, GWAS could be a powerful tool to identify and characterize novel genetic factors involved in the temperature modulation of plant immunity across the entire Arabidopsis pangenomic landscape.
Taken together, our study has started to shed light on current knowledge gaps regarding plant immune variation under climate warming conditions. The identification of Arabidopsis accessions with temperature-resilient and -sensitive defences will help further uncover molecular components underpinning temperature-sensitive plant immunity. Our findings could aid in predicting how plants respond to our changing climate, potentially laying the groundwork for engineering disease-resistant and climate-resilient plants, which will have broad impacts on agricultural and natural ecosystems.
ACKNOWLEDGEMENTS
We thank Castroverde lab members and Dr. Sheng Yang He (Duke University) for meaningful discussion and feedback. We are also grateful to Max Pottier and Gena Braun for their instrumentation support. Christian Danve M. Castroverde is supported by research funding from the NSERC Discovery Grant, NSERC Research Tools and Instrumentation, Canada Foundation for Innovation, Ontario Research Fund and Wilfrid Laurier University start-up and equipment funds. Christina A. M. Rossi is supported by the Ontario Graduate Scholarship and NSERC Canada Graduate Scholarship. We would like to acknowledge that Wilfrid Laurier University is located on the shared traditional territory of the Neutral, Anishinaabe and Haudenosaunee peoples. This land is part of the Dish with One Spoon Treaty between the Haudenosaunee and Anishinaabe peoples.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




