Variations in ectomycorrhizal exploration types parallel seedling fine root traits of two temperate tree species under extreme drought and contrasting solar radiation treatments
Summary statement
High solar radiation exacerbated the negative effects of extreme drought on plant growth and fine root traits. Ectomycorrhizae did not compensate for fine roots under drought stress. Fine roots biomass determined the role of ectomycorrhizal fungi, supporting the energy limitation hypothesis.
1 INTRODUCTION
Forests are the main body of terrestrial ecosystems and contribute more than 45% of the terrestrial carbon (C) sink (Le Quéré et al., 2018). However, frequent extreme drought caused by climate change severely restricts forest productivity and C uptake: drought can limit tree growth and survival directly by restricting water use and photosynthesis (Hammond et al., 2022; Hartmann et al., 2022), and indirectly by interfering with root nutrient uptake, especially the mycorrhizal symbionts formed with soil fungi (Allen & Ection, 2007; Querejeta et al., 2009). Importantly, extreme drought and high solar radiation often occur together, for example in canopy opening following tree death (Ma et al., 2023). In theory, the exposure of trees to both extreme drought and solar radiation can result in additive, synergistic, or antagonistic effects (Guo et al., 2019). As such, solar radiation can exacerbate the negative effects of drought by allocating more biomass to shoots at the expense of roots, or mitigate drought by reducing water loss, or neutralize the effects of extreme drought (Guo et al., 2019; Holmgren, 2000; Quero et al., 2006). However, previous studies have mainly focused on plant biomass or aboveground response, neglecting roots, especially their associated ectomycorrhizal (ECM) fungi. Clarifying how roots and ECM fungi respond to drought and solar radiation and their relationships is essential for understanding and predicting forest ecosystem functioning in response to future climate changes.
In forests, ECM fungi enhance water and nutrient acquisition of fine roots and increase resistance to stress, and in turn, require carbohydrates from trees (Smith & Read, 2008). ECM fungi are the predominant symbionts in temperate forests (Castaño et al., 2018). They are functionally categorized by their morphology and exploration traits (Agerer, 2001, 2006): contact and short-distance (C-S) exploration types produce only a few emanating hyphae and no rhizomorphs, whereas medium- and long-distance (M-L) types have extensive hyphae and aggregated rhizomorphs. In general, ECM belong to M-L exploration type (ECM fungi with high biomass) exhibited more efficient water transportation and higher nutrient acquisition capacity than the C-S type (low biomass ECM fungi) (Agerer, 2001; Fernandez et al., 2017; Hobbie & Agerer, 2010). Hence, plants subjected to drought may benefit from ECM taxa of M-L type, previously found to improve the efficiency of transport water (Castaño et al., 2023). The activity of ectomycorrhizae in water and nutrient uptake is highly dependent on host C fixation and fine root function (Hobbie & Agerer, 2010; Xie et al., 2021), and trees may rely on ectomycorrhizae when root function is limited by abiotic stress (e.g., drought) (Danielsen & Polle, 2014; Liu et al., 2017). Ectomycorrhizae can alleviate drought stress in plants by increasing access to soil water (Allen & Ection, 2007) and improving host tree nutrition (Smith & Read, 2008) with their external hyphae. Two contrasting hypotheses, functional compensation versus energy-limited, have been proposed to explain the relationship between ectomycorrhizae and roots (Wasyliw & Karst, 2020). The functional compensation hypothesis suggests that M-L type will predominate over C-S type under stress, as M-L type have the capacity to compensate for the limited role of fine roots via efficient water transportation and higher nutrient acquisition capacity (Brownlee et al., 1983; Hobbie et al., 2022; Nickel et al., 2018); whereas the energy-limited hypothesis suggests that C-S type will dominate under stress as they demand less C (Castaño et al., 2018; Fernandez et al., 2017).
Importantly, stress associated with climate change may interfere with fine root function (Freschet et al., 2013) which is likely to affect below-ground resource acquisition and C fixation of trees, and, in turn, could impact ECM exploration type. Extreme drought significantly affects root traits and becomes more frequent and intense due to climate change (Brunner et al., 2015; Ma et al., 2018). In this case, a decline in nutrient acquisition of roots is reflected in the reduction of specific root length (SRL) and specific root surface area (SRA) (Zhou et al., 2018). Similarly, ECM fungal community and function are also affected by drought (Fernandez et al., 2023; Pena & Polle, 2014; Querejeta et al., 2021). For instance, drought can regulate ECM fungal communities by recruiting taxa with mycelial architectures that promote efficient hydraulic functions (Agerer, 2001), or taxa with metabolic adaptations (Treseder et al., 2018), which drives a shift in ECM fungal community composition towards dominance of either C-S or M-L type (Castaño et al., 2023; Gordon & Gehring, 2011). This reflects either the capacity of M-L type for efficient hydraulic function, or low C demand of C-S type, as respectively consistent with the functional compensation or energy-limited hypothesis (Castaño et al., 2023; Fernandez et al., 2017). To date, no consistent conclusion can be drawn from existing studies due to the lack of integration of ECM function with plant growth and carbohydrate allocation. Revealing their relationship can uncover which ECM exploration type plays essential roles under stress, and whether there is greater reliance on mycelial-acquisition capacity or C limitation, will provide a new perspective for exploring the function of ECM fungi in forest ecosystems responding to climate changes.
In addition, high solar radiation occurs frequently during drought in forests (Deng et al., 2023; Robson et al., 2008), and the negative effects of extreme drought on root traits and plant growth might be either worsened or partially ameliorated by the combination with solar radiation; often depending on species shade-tolerant or light-demanding strategy (Aranda et al., 2007; Valladares & Niinemets, 2008; Valladares et al., 2016). As important pathways for plant water and nutrient uptake, ectomycorrhizae and fine roots can also be affected indirectly by drought and solar radiation through shifts in plant performance and biomass allocation (Fernandez et al., 2017; Soliman et al., 2022), especially between shade-tolerant and -intolerant species. For instance, high solar radiation could increase plant biomass and biomass fraction of roots under drought for shade-intolerant species (Xu et al., 2015), while not affecting the root/shoot ratio of shade-tolerant species (Zhang et al., 2019). This may be due to the fact that high solar radiation favours shade-intolerant species rather than causing photoinhibition as it does for shade-tolerant species, and therefore, when drought stress is encountered, shade-intolerant species will allocate more biomass to roots to improve root drought resistance or water uptake capacity (van Hees, 1997; Xu et al., 2015). Differences in growth and belowground C allocation are found between species, and appropriate biomass allocation can maximize plant growth, and resistance by meeting efficient resource demand (Bernal et al., 2015; Veresoglou & Penuelas, 2019; Wang et al., 2020). However, little empirical work has directly tested the relationship between ectomycorrhizae and root functions under these dual stresses. Whether shade-intolerant species would allocate more biomass to roots by relying on M-L type for nutrients under high solar radiation and extreme drought needs to be verified by further studies.
The present study investigated the interactive effect of extreme drought and solar radiation on plant growth, root traits, and ECM fungal community composition in a controlled experiment. Tree species with contrasting shade-tolerance were selected: shade-intolerant (Quercus mongolica Fisch. ex Ledeb.) and shade-tolerant (Tilia amurensis Rupr.) (Kitao et al., 2016; Yin et al., 2023). The study addresses two questions: (a) How does plant growth and biomass allocation in shade-tolerant versus intolerant species respond to extreme drought and its interaction with high solar radiation? (b) What is the relationship between ectomycorrhizae and fine roots under these dual stresses? Does it support the energy limitation or functional compensation hypothesis? Does the dominant ECM fungi under drought stress depend on the functional strategy for shade tolerance of particular tree species? Generally, extreme drought can significantly influence the expression of root traits (SRL and SRA) and inhibit belowground biomass accumulation, as compared to moderate drought or well water conditions (Comas et al., 2005; Davies & Bacon, 2003), and the negative effects of extreme drought can be modified by solar radiation in different species (Xu et al., 2015; Zhang et al., 2019). Therefore, we hypothesized that: (1) extreme drought would decrease seedlings' growth and fine roots biomass of both tree species, while shade-intolerant species may mitigate this negative effect when simultaneously growing under high solar radiation. Because they can invest more resources attained from favourable light conditions to improve root growth and/or drought tolerance (Sefcik et al., 2006; Xu et al., 2015); (2) both tree species would display a functional compensation rather than energy-limitation relationship under extreme drought, as M-L type dominates in drought conditions with efficient water transport capacity and their rhizomorphs are able to acquire water efficiently (Agerer, 2001; Castaño et al., 2023); (3) compared to shade-tolerant species, shade-intolerant species would be limited by C assimilation (energy-limitation) under low solar radiation combined with extreme drought, which is exacerbated by the dominance of C-S type and reduced belowground biomass allocation. This occurs because ECM fungal community composition shifts towards less C-demanding taxa, such as C-S type, when plants are C-constrained (Fernandez et al., 2023).
2 MATERIALS AND METHODS
2.1 Plant materials
This study was conducted in a greenhouse used by the Institute of Applied Ecology (123.45° E, 41.76° N), Chinese Academy of Sciences. In March 2023, 1-year-old Q. mongolica and T. amurensis seedlings obtained from a local nursery were selected with uniform height (ca. 18 cm) and root collar diameter (ca. 4 mm), planted in 3-L cylindrical plastic pots filled with well-mixed soil (2.6 kg) and grown under the following environmental conditions (air temperature of 23–25°C, and light intensity of 200 µmol m−2 s−1 for 14 h in each 24-h period). One seedling was grown per pot. Soil was obtained from a temperate mixed deciduous-broadleaved forest (0–20 cm, organic layer + mineral layer without the upper litter) in the Changbai Mountain Nature Reserve (128.28° E, 42.24° N, 736 m above seas level), sieved through a 1-cm mesh and mixed homogeneously with local sand (1/1 ratio). The soil was a dark brown soil (Albic Luvisol) (WRB IUSS Working Group, 2014), with soil pH of 5.9, NO3−–N of 11.76 mg kg−1, and NH4+–N of 9.44 mg kg−1, respectively. We assumed that the pre-existing ECM fungal community was similar for all seedlings, as they grew in the totally same environment and the soil was well mixed before planting. Later changes in the ECM fungal community upon which we mainly focused were caused by the water and light treatments.
2.2 Experimental design
The experimental treatments commenced in April 2023, after 1 month of growth in the greenhouse. A two-factor (water × light) randomized block experiment with six treatment combinations (three split-plot factor drought treatments × two main-plot factor light treatments). The two light treatments were: (low-control): 200 µmol m−2 s−1, and (high): 400 µmol m−2 s−1. Plants divided into 12 compartments were illuminated with adjustable light-emitting diode panels (Chenghui Equipment Co., Ltd.; 150 cm × 30 cm; white light: 400–700 nm) from 5:00 am to 6:00 pm each day. These treatments simulated the light regimes experienced by seedlings in temperate mixed deciduous-broadleaved forest gaps. The light treatments were regulated using a quantum sensor (LI-190; Li-CorBiosciences Inc.). Three water regimes were applied to plants in each light-intensity treatment. Based on the results from a pre-experiment and records of the local soil water content in the most recent 5 years, three drought treatments were deployed: 60% soil water-holding capacity (W60), as a no drought control (CK); 40% soil water-holding (W40), mild drought stress, and 20% soil water-holding (W20), extreme drought stress, resulting in soil water content (%, w/w) of 23.6%, 15.7%, and 12.3%, respectively. Pots were weighed and watered every 3 days to maintain the expected water regime. A total of 360 seedlings were used for each tree species (six replicate chambers × two light treatments × three drought treatments × 10 pots). All the pots in a given chamber were randomly rearranged every week to ensure the consistency of microclimate (light and temperature [23°C]).
2.3 Sampling and measurements
In August 2023, six seedlings of Q. mongolica and T. amurensis from each treatment were randomly sampled. The chlorophyll fluorescence of fully expanded leaves was measured using a portable fluorometer (MINI-PAM-II, Heinz-Walz Germany) between 9:00 to 11:00 am. The effective photochemical yield of photosystem II (ΦPSII), and the maximum photochemical yield of PSII (Fv/Fm) after a leaf-dark adaptation for 30 min was determined, respectively (Ma et al., 2024; Rosenqvist & van Kooten, 2003). After this, seedlings were divided into roots, stems, and leaves. Plant roots were divided into coarse roots (diameter > 2 mm) and fine roots (diameter < 2 mm). Plant tissues were oven-dried (at 65°C for 3 days), and then weighed to determine biomass of each organ (leaves, stem, coarse roots, and fine roots) and root/shoot ratio (underground part biomass/aboveground part biomass). The leaf-to-total mass ratio (LMR) and fine root-to-total mass ratio (FMR) were also obtained by dividing leaf and fine root biomass by plant biomass, respectively. Another six seedlings of two species per treatment were sampled and divided into three fine-root subsamples. Two of these subsets were stored in the laboratory at 4°C for the analysis of fine root colonization (fine roots were placed in formalin–acetic acid–alcohol fixative [1:1:18]) and morphological traits, respectively. The third subset was immediately placed at −80°C for later analysis of the ECM fungal community.
2.4 Measurements of root morphological traits and ECM colonization
Plant roots were rinsed after overnight immersion in deionized water and live fine roots were then classified according to diameter (diameter < 2 mm) and vitality. In this study, we found that dead roots, distinguished from live roots based on turgescence and colour (Hertel et al., 2013; Schmid, 2002), accounted for less than 1% of the total root tips, as consistent with Coll et al. (2012) and Lima et al. (2010). Thus, dead roots were excluded and ECM colonization of only live roots was monitored. Live fine roots containing the first five orders of branching were washed with deionized water twice, scanned at 300 dpi using a scanner (Epson expression 10000XL), and then the scanned images were processed by the WinRHIZO software (WinRHIZO Pro 2007; Régent Instruments) to quantify root length, surface area, mean diameter, and volume. The scanned root samples were dried at 65°C to a constant weight to determine root dry mass. SRL and SRA were separately calculated by dividing root length and root surface area by root dry mass, and root tissue density (RTD) was calculated as the ratio of root dry mass to volume.
We measured the ECM colonization rate of fine roots by the biometrical method (Bzdyk et al., 2019). For each seedling, 30 subsamples of 1-cm length root segments were selected, and then the number of root tips colonized by ECM fungi and total root tips in each segment were counted under a stereomicroscope. ECM colonization rate (%) was calculated as the number of root tips colonized by ECM fungi divided by the total number of root tips in each root segment (Guo et al., 2008). The average of the 30 segments counted as one replicate.
2.5 Measurements of ECM fungal community composition
About 50 root segments colonized by ECM fungi of each seedling were selected randomly. Root DNA was extracted using the DNAeasy Plant Mini Kit (Qiagen) according to the manufacturer's protocols. The final concentration and purification of DNA were determined by a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific), and DNA quality was detected by 1% agarose gel electrophoresis. All samples were subjected to MiSeq sequencing of the ITS rDNA. PCR amplification of the ITS1 region was performed using the ITS1F forward primer (5′-CTTGGTCATTTAG-AGGAAGTAA-3′) and the ITS2 reverse primer (5′-GCTGCGTTCTTCATCGATGC-3′) (Adams et al., 2013). The PCR procedure was run with the following reaction conditions: initial denaturation at 94°C for 1 min, 30 cycles (denaturation at 94°C, 30 s; annealing at 52°C, 30 s; extension at 68°C, 30 s) and followed by a 7 min final auto-extension at 68°C. After purification and quantification, amplicons pooled in equal amounts, with paired-end sequenced (2 × 300 bp), were performed by the Illumina MiSeq platform with MiSeq Reagent Kit v3 at Shanghai Personal Biotechnology Co., Ltd. The raw reads were deposited into the NCBI Sequence Read Archive database as BioProject IDPRJNA616138.
The quality of the raw paired-end sequences was assessed using FastQC, and filtered using the QIIME software to remove sequences containing uncertain bases with an average quality of less than Q20 (Caporaso et al., 2010). Sequences were then assigned according to the barcodes of the samples (mismatch = 0) using FASTX-toolkit and quality controlled again in QIIME (parameters: max ambigs = 6; max homop = 6; min length = 150; max length = 400) to exclude low-quality sequences. Usearch (version 7.0) was used to remove chimeric sequences. Subsequently, paired ends were merged using FLASH (Magoc & Salzberg, 2011). The remaining sequences were clustered into amplicon sequence variants (ASVs, equivalent to clustering with 100% similarity). The remaining sequences were matched into the UNITE+INSDC fungal ITS database using the Ribosomal Database Project classifier (Kõljalg et al., 2013), and subsequently normalized by the minimum number of reads (Abarenkov et al., 2010). ASVs were assigned to the following guilds: (a) ECM, (b) saprotroph, (c) animal pathogen, (d) plant pathogen and (e) endophyte, based on FUNGuild (Nguyen et al., 2016) (Supporting Information S1: Figure 4). ECM fungal species or genera were classified into different ECM exploration types (contact [C], short-distance [S], medium-distance [M], and long-distance [L]) comparing the categories described with the online DEEMY database (http://www.deemy.de/) and other studies (Agerer, 2001, 2006; Suz et al., 2014; Tedersoo & Smith, 2013). We further grouped contact and short-distance ECM genus into one category (C-S type), and medium-distance and long-distance ECM genus into one category (M-L type). Detailed ECM exploration-type assignments are given in Table S1.
2.6 Data analysis
In this study, there were a total of 72 pots, 36 per species (three water treatments × two light treatments × six replications). To test the independent and interactive effects of water and light on plant growth, root traits, and ECM exploration types of two species seedlings, linear mixed models (LMM) were fitted with the fixed effect factors (water and light) and block as a random effect factor using the R package ‘lme4’ (Castaño et al., 2023). Tukey's honestly significant difference test was used for post hoc comparison with Benjamini–Hochberg false discovery rate correction of p values (Benjamini & Hochberg, 1995; p < 0.05). The homogeneity of variance (Levene's test) and normality (Shapiro–Wilk test) of all data were checked, and log-transformation of FMR was performed to better conform to these requirements. Differences between Q. mongolica and T. amurensis were also analyzed by LMM using the R package ‘lme4’ with tree species as fixed factors and blocks as random factors. p-Values were Bonferroni-corrected.
Nonmetric multidimensional scaling (NMDS) of Hellinger transformed ECM fungal community data, based on Bray–Curtis dissimilarity (‘vegan’ package) and 999 permutations, was conducted to analyze ECM fungal community dissimilarity among treatments. In this analysis, fine root traits were plotted as explanatory variables, and their effect on the ECM soil fungal community was further tested by ‘envfit’ package. The ‘envfit’ function fits vectors of continuous variables to the permutations, with the length of the arrows being proportional to the correlation of the permutations (Smith et al., 2015). Linear regression analysis was used to assess the correlation between root traits and ECM exploration types. Variance partitioning analysis (VPA) was conducted with the ‘varpart’ function in R package ‘vegan’ to explore the relative proportion of water and light explaining each of root traits, plant biomass, and ECM fungal community. To examine how drought and light affected root traits and the ECM fungal community, partial least squares path modelling (PLS-PM) was applied to reveal the relationships among root traits (SRL and SRA), ECM fungal community (indicated by the relative abundance of M-L type), and plant growth (total biomass and fine root biomass) using the package ‘plspm’ in R (Sanchez et al., 2015). The arrows and the path coefficients represented the direction and strength of relationships between latent variables, and the models were evaluated using the goodness of fit (>0.5). Statistical analyses were conducted in SPSS 26.0 (SPSS Inc.) and R 3.6.3 (R Development Core Team, 2019).
3 RESULTS
3.1 Response of leaf chlorophyll fluorescence to drought and light treatments
The interaction of drought and light treatments significantly affected Fv/Fm of Q. mongolica (Supporting Information S1: Table 2; Figure 1a,b). Compared with control (W60), extreme drought (W20) in high light significantly reduced Fv/Fm, but at low light, drought treatment had no significant effect on Fv/Fm (Figure 1a). While ΦPSII of Q. mongolica, Fv/Fm and ΦPSII of T. amurensis were only affected by water and decreased significantly with increased drought intensity (Figure 1a,b). Results showed that Fv/Fm and ΦPSII of Q. mongolica was significantly higher than that of T. amurensis (Supporting Information S1: Table 3).
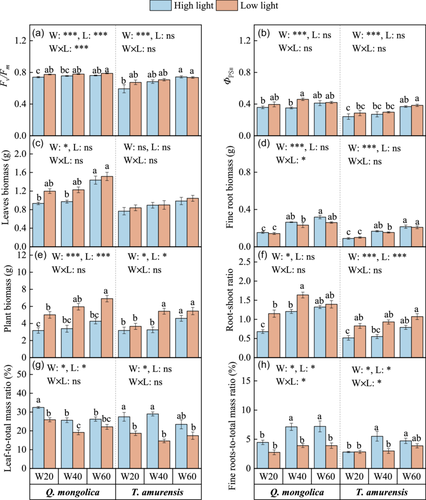
3.2 Response of plant growth and biomass allocation to drought and light treatments
Leaf biomass of Q. mongolica decreased with increasing water stress from control to extreme drought under high light, but that of T. amurensis was unaffected (Figure 1c). Fine root biomass significantly decreased under extreme drought irrespective of species, as compared with control (Figure 1d). Whole plant biomass of both species was significantly suppressed by extreme drought and high light, respectively (Figure 1e). Root/shoot ratio followed a similar trend to plant biomass, across the treatments; values in control and low light were significantly higher than in extreme drought and high light, respectively (Figure 1f). However, variations of LMR and FMR showed the opposite trends when the effect of light was considered, that is, high light promoted biomass allocation to leaves and fine roots of both species (Figure 1g,h). In contrast, drought had a differential and significant effect on allocation, that is, in high light, extreme drought increased biomass allocation to the leaves, but reduced allocation to fine roots (Figure 1g,h). In addition, Q. mongolica had greater leaf biomass, fine root biomass, root/shoot ratio and FMR than T. amurensis (Supporting Information S1: Table 3). VPA showed that the effect of water on plant biomass was higher than that of light, which explained 25.28% of the variance in plant biomass (Supporting Information S1: Figure 1c).
3.3 Response of root traits to drought and light treatments
Root traits, including SRL, SRA and mean diameter were significantly affected by soil water, independent of tree species, while extreme drought significantly decreased SRL, SRA and mean diameter, compared with control (Figure 2a–c). RTD varied with soil water content and light treatments and gradually increased from low to high light, and from control to extreme drought, respectively (Figure 2d). VPA also supported the results, that is, more than 30% of the variation in SRL and SRA was explained by water (Supporting Information S1: Figure 1a,b). Soil water content and light had significant interactive effects on the ECM colonization rate of both species (Supporting Information S1: Table 2), which declined gradually with increasing drought intensity, but increased from low to high light (Figure 1f). ECM colonization rate had no significant differences between high light and low light under extreme drought, but values in high light were significantly higher than low light under moderate drought and control (Figure 1f). Besides, we found that Q. mongolica had a higher SRL and a lower mean diameter than T. amurensis (Supporting Information S1: Table 3).
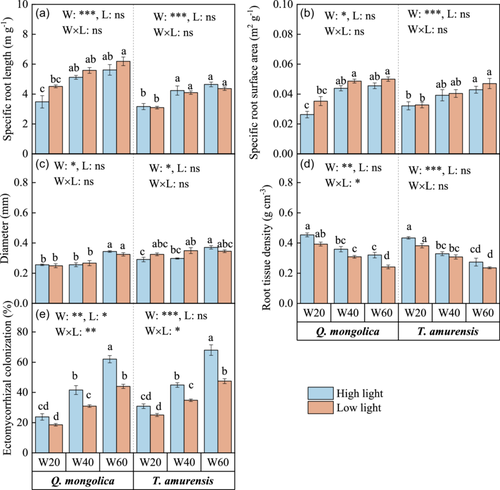
3.4 Response of ECM fungal community to drought and light treatments
Rarefaction analysis showed that the obtained sequence data were sufficient to allow for root fungal community analysis (Supporting Information S1: Figure 2). A total of 2 416 892 (ranging from 10 114 to 6617 fungal sequences were obtained from all samples, and identified into 2239 root fungal ASVs, and then into 265 ECM fungal ASV by FUNGUILD function (Supporting Information S1: Figure 3). Across all treatments, ECM fungal communities were primarily composed of ascomycota and basidiomycota, belonging to five classes, nine orders, 16 families and 23 genera. The dominant ECM fungal genera were: Hebeloma, Tomentella, Thelephora, Scleroderma, Laccaria, Hymenogaster, Astraeus, Chloridium, Sphaerosporella and Tuber across all samples (Supporting Information S1: Table 4; Figure 3). ECM community composition of both species varied with water and light treatments (Figure 3a,b), and ECM fungi defined as M-L exploration type, such as, Tomentella, Laccaria, Sphaerosporella and Astraeus for Q. mongolica, or Scleroderma and Laccaria for T. amurensis, showed a higher relative abundance under control compared to extreme drought (Supporting Information S1: Table 4; Figure 3a,b).
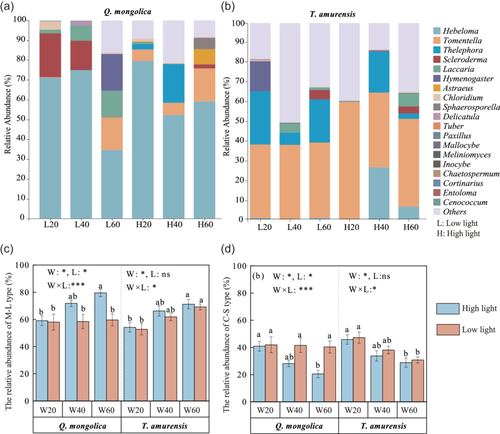
The results showed that, at the level of ECM exploration type, the relative abundance of M-L type (60%–80%) and C-S type (20%–40%) of both tree species was significantly affected by water–light interaction (Figure 3c,d). Extreme drought under high light significantly decreased the relative abundance of M-L type, but increased the relative abundance of C-S type, as compared with control (Figure 3c,d). There was no significant difference in the relative abundance of M-L type and C-S type among soil water regimes under low light, and similarly, their relative abundance did not significantly vary between moderate drought and control under the same light conditions (Figure 3c,d). VPA analysis also found that water displayed a higher effect on the variations of M-L type than light (Supporting Information S1: Figure 1d).
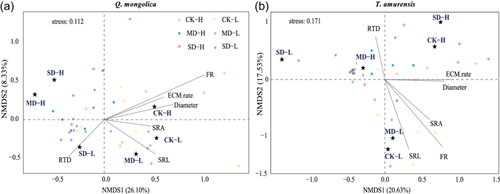
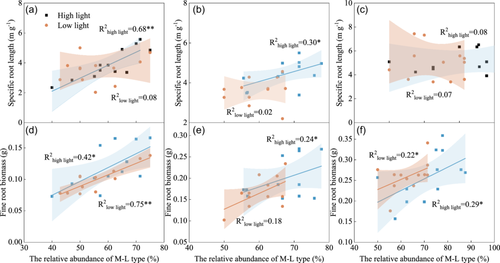
3.5 Relationship between root traits and ECM exploration types
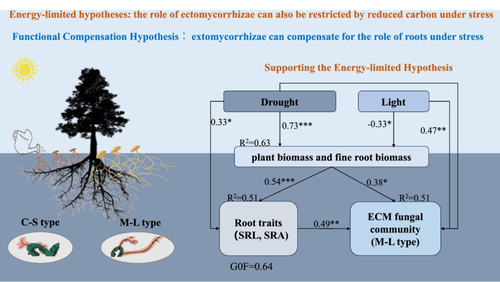
Results of NMDS combined with vector analysis (envfit) revealed that fine root traits were significantly related to ECM fungal communities of both species across the water and light treatments (Figure 4). Within the studied factors, fine root biomass and SRL could explain the variations of ECM community composition (Supporting Information S1: Table 5; Figure 4). At the level of ECM exploration type, SRL was positively correlated with the relative abundance of M-L type in extreme drought and medium drought under high light, and there was no significant correlation between SRL and the relative abundance of M-L type for low light across water treatments (Figure 5a–c). Likewise, we observed that fine root biomass was positively correlated with the relative abundance of M-L type across all water and light treatments (Figure 5b–d). In the PLS-PM analysis (Figure 6), the results suggested that water and light directly, or indirectly significantly affected both fine root traits and ECM fungal community by regulating plant growth and biomass allocation, and there were positive correlations between root traits (SRL and SRA) and ECM fungal community (M-L type), which supported the energy-limited hypothesis.
4 DISCUSSION
In this study, the relationship between fine roots and ectomycorrhizae across extreme drought and solar radiation has been explored, and results suggested that the functions of ectomycorrhizae are highly associated with fine root biomass, supporting the energy-limited hypothesis (Figures 5 and 6). Extreme drought significantly reduced plant biomass, especially under high light, and the response was consistent between shade-tolerant and shade-intolerant species, opposing the first hypothesis. Besides, SRL, SRA and the relative abundance of M-L type reduced significantly with the increased drought intensity suggesting that ectomycorrhizae did not compensate for the role of fine roots, opposing the functional compensation hypothesis. High light increased the relative abundance of M-L under nondrought treatments, and this may be related to higher fine root biomass, supporting the energy limitation hypothesis.
4.1 Consistent patterns of plant growth and biomass allocation in response to drought and light
In this study, extreme drought significantly reduced plant biomass and fine root biomass of two species of seedlings, and this negative role was intensified by high light, especially for plant biomass (Figure 1d,e), thus partially supporting H1. These findings were consistent with previous studies that extreme drought can decrease plant growth due to reduced photosynthesis and limited efficiency of conversion of light energy to biomass (Armas & Pugnaire, 2009; Xu et al., 2015). Whereas, low light under extreme drought increased plant biomass for both species, possibly due to the protective effect of low light on seedlings under extreme drought, as well as the reduced evaporative demand (Du et al., 2013; Quero et al., 2006). Specifically, although the two species have different shade tolerance, their growth responses to light are consistent reflecting universal functional trade-offs in ecophysiology and the need for seedlings to exploit canopy gaps (Valladares & Niinemets, 2008). In addition, the declined fine root biomass may be because drought can reduce new root production and increase root mortality by depleting starch and sugar reserves (Eldhuset et al., 2013; Maguire & Kobe, 2015), which is aggravated by high light (Guo et al., 2019). As such, the role of ectomycorrhizae would be restricted by the reduction in fine root biomass, which may be a consequence of the reduction in carbohydrates allocated to roots under drought stress (Castaño et al., 2017; Fuchslueger et al., 2014; Hagenbo et al., 2020). Moreover, a positive correlation between ECM biomass and fine root biomass suggests that the growth of ECM fungi highly depended on fine root biomass (Majdi et al., 2008; Nilsson et al., 2005; Xie et al., 2024). Therefore, fine root biomass could be considered as an indirect proxy of the energy source for ECM fungi. These differences in biomass allocation belowground can, therefore, affect ECM fungal symbionts alike (Fernandez et al., 2017).
4.2 Shifts in root traits and ECM exploration types under drought and high light
Another aim was to weigh support for the two competing hypotheses regarding the relationship between root traits (SRL and SRA) and ECM fungal community (M-L type) across drought and light treatments. We assumed that the decrease in SRL and SRA under extreme drought and high light may lead to a higher reliance on ectomycorrhizae (functional compensation hypothesis) (Castaño et al., 2023); alternatively, the ECM fungal community may be regulated by host C supply (i.e., fine root biomass), and M-L type abundance should therefore decline (energy-limitation hypothesis) (Fernandez et al., 2023; Wasyliw & Karst, 2020). The results were consistent with energy limitation rather than the functional compensation hypothesis (Figure 6).
Contrary to the functional compensation hypothesis, both root traits (SRL and SRA) and the relative abundance of M-L type declined from control to extreme drought. These results suggested that ectomycorrhizae were unable to compensate for the role of fine roots because reductions in SRL and SRA did not correspond to an increase in M-L type abundance. Actually, there was a significant positive relationship between fine root biomass and the relative abundance of M-L type (Figure 5d–f), which supported the energy-limitation hypothesis. Evidence suggested that the response of ECM fungi was coherent with that of root biomass, and their peak growth coincided with that of fine roots (Comas et al., 2013; Smith & Read, 2008; Wallander et al., 2001). In fact, the exploration distance of ECM hyphae is considered as an important feature affecting the ECM fungal community in response to environmental change (Fernandez et al., 2017; Morgado et al., 2015). Exploring greater distances and larger soil volumes requires a high C cost that must paid by the host (Weigt et al., 2012). The reduced plant growth and fine root biomass under drier conditions are expected to alter ECM fungal community composition from fungi with exploration strategies to taxa with shorter distance exploration types, as demonstrated in previous studies (Fernandez et al., 2017; Fransson, 2012). Given their relatively thin mantles and short extra-mycelial distances (Agerer, 2001), C-S type may have a lower C cost to their host, which could be beneficial in drought conditions (Gordon & Gehring, 2011). Other studies also reported that drought significantly altered ECM fungal community composition, leading to an increase in the relative abundance of C-S exploration types (Fernandez et al., 2023).
The mechanism underlying this may be driven by a direct ECM fungal response, as different ECM taxa respond differently to drought (Hobbie & Agerer, 2010). Although the ECM fungal community of the two species were dominated by Hebeloma and Tomentella, the presence of specific taxa (M-L type, i.e., Scleroderma, Laccaria and Sphaerosporella) appeared only in no-drought condition may contribute to the results. Species belong to the M-L type with extensive hyphae, which efficiently transport water and nutrients due to the differentiated rhizomorphs, but is also costly (Agerer, 2001; Raidl, 1997). The trade-off between traits related to stress resistance (e.g., water/nutrient transport) and energy consumption (e.g., structures) has been documented in the ECM fungal community, where specific fungi dominants tend to have a narrower niche breadth (Maynard et al., 2019). Together, the amount and differentiation of ECM hyphae is an important ecological factors for tree performance and adaptation to climate change.
Additionally, light contributed less than water to the variation in root traits (Supporting Information S1: Figure 1a,b), suggesting drought may obscure the effects of light, as supported by Guo et al. (2019). Although root traits respond more to water than light, high light promoted ECM colonization and the relative abundance of M-L type in control (Figures 2e and 3c). Because light can influence ECM fungi colonization and community by impacting host C assimilation (Trocha et al., 2016), and light limitation can drive reductions in ECM colonization or changes in ECM fungal community composition (Clark & Clair, 2011; Markkola et al., 2016). In this study, high light under control increased fine root biomass compared with low light, suggesting that high light promotes C allocation to ECM fungi and favours the colonization of M-L type, supporting the energy limitation hypothesis. Favourable light conditions that allow ample C assimilation should facilitate ECM growth (Markkola et al., 2016). In addition, in this study, we focused ECM fungi on live roots but acknowledged that root mortality may increase with drought intensity. Nevertheless, we found that dead roots accounted for <1% of the total root tips. Similar results can be found in Lima et al. (2010) and Coll et al. (2012). Specifically, Lima et al. (2010) found that root mass in the dry season was higher than in the wet season for both live (dry = 18.71 ± 1.58, wet = 11.00 ± 0.75 gm−2 month−1) and dead (dry = 0.51 ± 0.11, wet = 0.20 ± 0.03 gm−2 month−1) roots over 2 years, and that the average dead root biomass as a proportion of total fine root biomass remained similar irrespectively of dry or wet season at ∼3%. Likewise, Coll et al. (2012) also found that the percentage of dead root tips for ECM colonization in fine roots was lower 0.5% over the whole year. Although root tip mortality was not recorded in our experiment, the results would not change significantly even if dead roots were considered.
4.3 The interaction of water and light on ECM traits
Inconsistent with our expectation for a light-water interaction, there was no significant effect on root traits, but a significant effect on ECM traits (colonization and fungal community) (Figures 2e and 3). Why were ectomycorrhizae affected by the drought-and-light interaction but not fine root traits? First, the effect of light on root traits is mediated through direct effects on the leaves, through stomatal conductance and gas exchange, which then indirectly influence root traits and regulation of plant growth and biomass allocation (Parmenter & Littlejohn, 2000; Tani et al., 2003). Conversely, extreme drought directly limits soil moisture availability and causes root mortality (Brunner et al., 2015). Second, root traits within the first five-order of branching (complete root order with diameter <2 mm) were measured in the study, whereas most symbiotic associations with ECM fungi are formed within the first two root orders (Comas & Eissenstat, 2009; Guo et al., 2008). These root orders are expected to be the most sensitive to environmental stresses associated with climate change (Hishi, 2007; McCormack & Iversen, 2019).
5 CONCLUSION
Our experiment, testing the interactive effects of drought and light treatments, revealed that seedlings of both shade-tolerant and light-demanding species exhibited consistent responses in plant growth and biomass allocation: that is, extreme drought reduced plant biomass and fine root biomass. High light exacerbated the negative effects of extreme drought, but low light mitigated these effects. Extreme drought significantly decreased SRL, SRA and the relative abundance of M-L type, and ectomycorrhizae did not compensate for the loss of fine roots, contrary to the functional compensation hypothesis. Fine root biomass was positively correlated with M-L type abundance, suggesting ectomycorrhizae can respond to changes in host C supply, which aligns with the energy limitation hypothesis. Overall, the relationship between root traits and ectomycorrhizae regarding biomass allocation and belowground water and nutrient acquisition provides a theoretical basis for how trees acclimate to extreme drought and high light under the context of climate change.
ACKNOWLEDGEMENTS
This research was supported by the National Natural Science Foundation of China (32122059), the Chinese Academy of Sciences Young Talents Program, and LiaoNing Revitalization Talents Program (XLYC2007016) to Qing-Wei Wang, by the National Natural Science Foundation of China (32201331) to Jiaojiao Deng, and the Postdoctoral Fellowship Program of CPSF (GZC20232881) and the China Postdoctoral Science Foundation (2024M753411) to Lulu Xie.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. The RNA-seq data are freely available from https://www.ncbi.nlm.nih.gov/sra with the BioProject ID: PRJNA1031078.




