Tomato NADPH oxidase SlWfi1 interacts with the effector protein RipBJ of Ralstonia solanacearum to mediate host defence
Guan-Ming Su, Li-Wen Chu and Chih-Cheng Chien contributed equally to this work.
Abstract
Reactive oxygen species (ROS) play a crucial role in regulating numerous functions in organisms. Among the key regulators of ROS production are NADPH oxidases, primarily referred to as respiratory burst oxidase homologues (RBOHs). However, our understanding of whether and how pathogens directly target RBOHs has been limited. In this study, we revealed that the effector protein RipBJ, originating from the phytopathogenic bacterium Ralstonia solanacearum, was present in low- to medium-virulence strains but absent in high-virulence strains. Functional genetic assays demonstrated that the expression of ripBJ led to a reduction in bacterial infection. In the plant, RipBJ expression triggered plant cell death and the accumulation of H2O2, while also enhancing host defence against R. solanacearum by modulating multiple defence signalling pathways. Through protein interaction and functional studies, we demonstrated that RipBJ was associated with the plant's plasma membrane and interacted with the tomato RBOH known as SlWfi1, which contributed positively to RipBJ's effects on plants. Importantly, SlWfi1 expression was induced during the early stages following R. solanacearum infection and played a key role in defence against this bacterium. This research uncovers the plant RBOH as an interacting target of a pathogen's effector, providing valuable insights into the mechanisms of plant defence.
1 INTRODUCTION
There are two tiers of immune responses in plant defence against pathogens (Ngou, Ding, et al., 2022). The first layer of plant immunity involves the detection of certain conserved microbe/pathogen-associated molecular patterns (PAMPs) and the activation of pattern-triggered immunity (PTI). To establish infection in plants, some microbes have evolved to produce effector proteins to suppress PTI by targeting and manipulating various plant components, leading to effector-triggered susceptibility (ETS). However, some plants have further evolved intracellular nucleotide-binding leucine-rich repeat (NLR) receptors to recognize specific effectors via direct or indirect interaction and induce the second layer of defence called effector-triggered immunity (ETI) (Peng et al., 2018). ETI may involve hypersensitive response (HR), a form of programmed cell death in the infected region (Balint-Kurti, 2019; Bentham et al., 2020). Many central components are involved in the complex regulatory network of plant immunity. The accumulation of reactive oxygen species (ROS) occurs at an early stage after the perception of pathogens (Zhang et al., 2020; Zhou & Zhang, 2020). ROS can destroy pathogens as well as act as vital signal molecules to induce mitogen-activated protein kinase (MAPK) and phytohormone signalling pathways, as well as various downstream defence responses (Chen & Yang, 2020).
NADPH oxidases, mostly known as respiratory burst oxidase homologues (RBOHs), are plasma membrane-localized enzymes for the production of ROS (Lee et al., 2020). ROS produced by RBOHs are important messengers for the rapid transmission of local and long-distance signalling, therefore regulating many biological functions (Mittler et al., 2022). In plants, RBOHs generate O2− at the apoplast after perceiving PAMPs and play a central role in plant immunity. In Arabidopsis thaliana, AtRBOHD has a critical role in plant defence against pathogens (Morel et al., 2006; Sagi & Fluhr, 2006). The homologue of AtRBOHD in tomato, SlWfi1 (SlRBOHB), is also involved in the resistance against various pathogens (Li et al., 2015; Song et al., 2018).
Many Gram-negative pathogenic bacteria employ the type III secretion system (T3SS) to inject type III effectors (T3Es) into host cells for their pathogenicity and virulence (Meisrimler et al., 2021; Wang et al., 2022). For phytopathogenic bacteria, T3Es can act as virulence proteins or avirulence (Avr) proteins based on their induced responses in plants. Virulence T3Es are not detected by host plants and can disturb plant physiology, leading to ETS (Ngou, Ding, et al., 2022). The recognition of Avr proteins by specific plant NLRs in resistant plants leads to ETI (Ngou, Jones, et al., 2022). To establish infection in plants, the T3Es of phytopathogenic bacteria target and modulate a wide range of plant components and related proteins (Lang & Colcombet, 2020; Meisrimler et al., 2021; Wang et al., 2022).
Ralstonia solanacearum causes bacterial wilt (BW), a soil-borne vascular deadly wilting disease, on more than 250 plant species from 54 families, leading to severe global losses of many economically important crops (Cho et al., 2019). As a tedious pathogen able to systemically infect an unusually wide range of plants, the pathogenesis of R. solanacearum is complicated and regulated by several systems, including the T3SS (Landry et al., 2020). In R. solanacearum, T3SS constitutively expresses during early and late infection stages (De pedro-Jové et al., 2021), and proper regulation of T3SS by regulators, such as PhcA, HrpG and HrpB, is important for expressing the full-spectrum of its virulence (Hayashi et al., 2019; Leonard et al., 2017). R. solanacearum has a high number of versatile T3Es (Deslandes & Genin, 2014; Hikichi et al., 2022; Peeters et al., 2013), which are predicted to arise via horizontal gene transfer (Gopalan-Nair et al., 2021; Li et al., 2013) and can be grouped into families with functional redundancy (Poueymiro & Genin, 2009; Remigi et al., 2011). These features are proposed to facilitate bacterial adaptation and infection in diverse host species. However, most of the R. solanacearum effectors are not characterized.
R. solanacearum is a highly complex species composed of four phylotypes (Hayes et al., 2017). The virulence of R. solanacearum, which is usually defined by quantitative assessment on host plants, varies among strains (Chesneau et al., 2017). However, information on the virulence and the related factors of this bacterium is limited. A comparative virulence study carried out on tomato, pepper and eggplant revealed no correlation between virulence and Phylotypes of R. solanacearum strains (Lebeau et al., 2011). The genotypes and virulence of R. solanacearum Phylotype I strains on tomato are highly diverse (Horita et al., 2014; Jaunet & Wang, 1999). Among the 40 Phylotype I strains tested on six tomato cultivars with varied degrees of BW-resistance, strain Pss4 confers medium virulence on tomato plants, while Pss190 confers reliable high-virulence and can overcome the resistance of the currently most reliable tomato BW-resistance source Hawaii 7996 (H7996) (Jaunet & Wang, 1999). In Phylotype II R. solanacearum strains, metE and metR, two genes that are present in the higher-virulence strain UW551 but absent in the lower-virulence strain IPO1609, have a positive role in the bacterial virulence on tomato (González et al., 2011).
By comparative secretome analysis in R. solanacearum, RipBJ is discovered to secrete through T3SS and translocate into plant cells and thus is characterized as a T3E (Lonjon et al., 2016). It has no obvious effect on the infection of R. solanacearum on A. thaliana and Medicoga truncatula (Lonjon et al., 2016). However, in this study, we found that the expression of RipBJ, which was present in the low/medium-virulence Phylotype I strains but absent in the high-virulence strains, caused decreased bacterial virulence in tomato. Ectopic expression of RipBJ in tomato induced plant cell death and activated multiple defence signalling pathways. RipBJ was associated with plant plasma membrane and its proper localization was required for its effects on plants. SlWfi1 was identified as the interacting target of RipBJ and played a positive role in RipBJ-induced effects on plants. Furthermore, SlWfi1 promoted tomato tolerance to R. solanacearum. Our study demonstrates that a host RBOH is the target of a pathogen effector and provides important information on the interplay between the pathogen and its host plant.
2 MATERIALS AND METHODS
2.1 Plant materials, microorganisms and growth conditions
Tomato (Solanum lycopersicum) cv. Hawaii 7996 (H7996, BW-resistant), L390 (BW-susceptible), Solanum pimpinellifolium cultivar West Virginia 700 (WVa700, BW-susceptible) and Nicotiana benthamiana were grown in growth chambers at 25°C under a 12 h light/12 h dark cycle. R. solanacearum strains used for this study belong to phylotype I, biovar 3. The R. solanacearum phcA::Tn and hrpG::Tn mutants in strain Pss4 background were generated previously (Lin et al., 2008). The ∆ripBJ mutant in strain Pss4 background was generated by homologous recombination using primers listed in Supporting Information S2: Table S1 and the complemented strain of the mutant was generated using the low-copy plasmid pUFR047 (De Feyter et al., 1993). Additional microorganisms used in this study included: Escherichia coli DH5α and BL21(DE3), and Agrobacterium tumefaciens GV3101. All of the R. solanacearum strains used in this study were prepared using the plasmids listed in Supporting Information S2: Table S2 and described in Supporting Information S2: Table S3.
2.2 Southern blot and genomic DNA polymerase chain reaction (PCR) analyses
The genomic DNA samples of R. solanacearum were prepared using a cetyltrimethylammonium bromide method (Saghai-Maroof et al., 1984) were and used to determine the presence of ripBJ. For Southern blot analysis, the bacterial genomic DNA samples were digested with SalI and the digoxigenin (DIG)-labelled ripBJ probe was prepared by PCR (Roche) using the genomic DNA of strain GMI1000 as the template. Southern blot analysis was carried out under a low-stringency hybridization condition (2× saline sodium citrate, 0.1% sodium dodecyl sulfate [SDS]). The luminescence was detected by LAS-3000 (Fujifilm) after an incubation in anti-DIG antibody solution (anti-DIG-alkaline phosphatase (anti-DIG-AP), Roche). For PCR analysis, the amplification of the full-length ripBJ was performed with an annealing temperature of 45°C for at least 30 cycles.
2.3 β-galactosidase assay for bacterial promoter activity analysis
The putative promoter sequence of ripBJ was amplified from the genomic DNA of strain Pss4 using primers listed in Supporting Information S2: Table S1. The PCR product was cloned into the vector pCZ962, which carries a lacZ reporter gene, and then introduced into the wild type, the phcA::Tn mutant and the hrpG::Tn mutant of R. solanacearum strain Pss4. The β-galactosidase activity was measured as described previously (Yang et al., 2013). Briefly, the ripBJ promoter-lacZ fusion carried by the pCZ962 plasmid was introduced into the wild-type Pss4 strain, a phcA::Tn mutant, or a hrpG::Tn mutant. The bacteria were grown in a rich medium (Bacto-agar and glucose medium) or T3SS-inducing minimal medium (MP medium + glutamate). Three colonies of each strain were analysed independently with two technical replicates in each experiment and the assay was repeated three times.
2.4 Transient gene manipulation in plants
The full-length or fragments of ripBJ were amplified from R. solanacearum strain Pss4 by PCR using primers listed in Supporting Information S2: Table S1. The full-length or fragments of the plant genes were amplified from complementary DNA of tomato cv. H7996 by PCR using primers listed in Supporting Information S2: Table S1. The bacterial and plant genes were then cloned into the indicated vectors (Supporting Information S2: Table S2). Potato virus X-based virus-mediated gene overexpression and Tobacco rattle virus-based virus-induced gene silencing in tomato, and the transient gene overexpression in N. benthamiana were performed as described previously (Liu & Cheng, 2017). After transiently manipulating the expression of the test genes, validation assays for the levels of transcript and protein accumulation in plants were carried out in all experiments before further analyses to ensure the reliability of the test materials. For transcript analyses, plant samples were randomly combined from at least four leaflets of two leaves collected from at least two plants. The plant samples were used for RNA extraction using HiYieldTM Total RNA Extraction Kit (ARROWTEC). Reverse-transcription PCR (RT-PCR) assays were performed as described previously (Lin et al., 2014; Liu & Cheng, 2017) using the Bio-Rad CFX RT-PCR Detection System (Bio-Rad Laboratories). Tomato ELONGATION FACTOR 1α (EF1α) genes, whose expressions were not modulated by pathogen infection or altered expression of ripBJ based on the instructions and criteria of the manufacturer, were used as internal controls for the normalization of gene expression. Immunoblot analyses of plant total proteins were performed as described previously (Liu & Cheng, 2017). Preparation of plant membrane proteins was performed as described by Avila et al. (2015).
2.5 Measurement of H2O2 and ion leakage levels
H2O2 accumulation was detected using the 3,3-diaminobenzidine (DAB) staining method by following the procedure described previously (Jambunathan, 2010). Leaves were harvested and incubated in DAB solution (1 mg mL−1) at room temperature in the dark for 16 h. The samples were then incubated in 95% (v v−1) ethanol at 70°C to remove chlorophyll. The ion leakage assay was performed as described previously (Liu & Cheng, 2017). Briefly, leaves of 4-week-old plants were infiltrated with A. tumefaciens GV3101carrying the indicated 35 S::RipBJ-yellow fluorescent protein (YFP) constructs. Ion leakage assay was performed 2 days after agro-infiltration using a conductivity meter (Suntex).
2.6 Assessment of plant disease responses
The plant wilting symptom caused by R. solanacearum and the in planta bacterial proliferation were evaluated as described previously (Chen et al., 2009). Briefly, the plants were inoculated with R. solanacearum strains (OD600 = 0.003, 25 mL per pot) by soil soak and the wilting progress was monitored as indicated. The disease severity index (wilting score) with a rating from 0 to 5 was used in the evaluation of disease symptoms; 0 represents no infection; 1 is symptoms with <10% of wilted leaves; 2 indicates 11%–25% of wilted leaves; 3 is 26%–50% of infected leaves; 4 is 51%–75% of wilted leaves; 5 represents symptoms more than 76% (Winstead & Kelman, 1952).
2.7 Protein localization, bimolecular fluorescence complementation (BiFC), luciferase complementation imaging (LCI), co-immunoprecipitation (Co-IP) and pull-down assays
Protein localization was performed as described previously (Liu & Cheng, 2017). Briefly, A. tumefaciens GV3101 containing indicated constructs (Supporting Information S2: Table S2) from a single colony was used. The A. tumefaciens was first cultured overnight in the YEP medium with appropriate antibiotics and acetosyringone. The overnight culture was diluted to OD600 = 0.1 and transfected/infiltrated into protoplasts or leaves of N. benthamiana. Confocal microscopic photographs were taken 16–24 h after transfection of protoplasts or 48–60 h after agro-infiltration of leaves. The PIP2A-mCherry was used as a marker for the plasma membrane localization. For BiFC assay, the test genes were cloned into the vectors expressing nYFP (pEarleygate201) and cYFP (pEarleygate202) fusion proteins. The LCI assay was carried out as described previously (Zhou et al., 2018) using the pCAMBIA1300 vectors. Briefly, the indicated NLuc constructs were co-expressed with CLuc constructs in N. benthamiana leaves for 48 h (Supporting Information S2: Table S2). Then, 1 mM of d-luciferin potassium salt solution (Abcam, ab143655, CAS No. #115144-35-9) was then infiltrated into the areas of N. benthamiana leaves expressing NLuc/CLuc-fused proteins. For the LCI qualitative assay, the leaves were cut off from the plants and kept in the dark for 7 min, followed by signal detection using the KETA CL Chemiluminescence Gel Imaging System (Wealtec). For LCI quantitative assay, the 6 mm leaf discs prepared after luciferin-infiltration were put into a 96-well white plate (with 200 μL of ice ddH2O) and signals were detected immediately by an enzyme-linked immunosorbent assay reader (Tecan Infinite® 200 PRO). The protein localization and BiFC samples were examined using Zeiss LSM 780 confocal microscope, and LCI samples were examined by KETA C image system (WEALTEC). Validation assays for the levels of protein accumulation in plants were carried out in all experiments to ensure the reliability of the experiments. The Co-IP assay was performed by following the protocol provided by TOOLs Mag-Bead-Protein A, and the input and immunoprecipitated (IP) proteins were analysed by immunoblot analysis using anti-luciferase (SI-L0159, Sigma) and anti-green fluorescent protein (α-GFP) (ab290, Abcam) antibodies. For the pull-down assay, glutathione agarose resin was used by following the manufacturer's instructions. The input and pull-downed samples were analysed using anti-GST (α-GST) (GST001, Bioman) and anti-MBP (α-MBP) (A00190-49, GenScript) antibodies.
2.8 Statistics analyses
All of the quantitative assays were carried out in at least three independent experiments. The assays with bi-group comparisons were further analysed by Student's t test for significance (p < 0.05). The assays with multigroup comparisons were analysed by one-way analysis of variance with Turkey's honest significance test (p < 0.05). The sample number and SDs in each analysis were indicated in the figure legends.
3 RESULTS
3.1 Expression of ripBJ reduces infection of R. solanacearum
Phylotype I strains of R. solanacearum display differential virulence on tomato plants (Horita et al., 2014; Jaunet & Wang, 1999). As effector proteins have crucial roles in plant–pathogen interactions, we hypothesized that R. solanacearum Phylotype I strains might have certain T3Es that are crucial for differential virulence. In a pilot DNA microarray assay, ripBJ (RSp0213) was detected in four low-virulence strains (Pss991, Pss216, Pss79, Pss965) but not in four high-virulence strains (Pss190, Pss365, Pss1308, Pss749). Southern blot and PCR analyses showed consistent results (Supporting Information S1: Figure S1a,b). ripBJ was also detected in the medium-virulence strains GMI1000 and Pss4 (Supporting Information S1: Figure S1a,b). The deduced amino acid (aa) sequences of ripBJ protein products of the four low-virulence strains and the two medium-virulence strains are identical. To validate the T3SS-mediated expression of ripBJ, we examined its promoter region and found the presence of the HrpB-binding site, hrpBII box (TTCG-N16-TTCG). The promoter activity assays showed that ripBJ expression was regulated by PhcA and HrpG (Supporting Information S1: Figure S1c,d), which further elucidated the secreting mechanism of RipBJ.
To investigate the existence of RipBJ in R. solanacearum strains and survey whether the presence of RipBJ could be correlated with the low/medium virulence of R. solanacearum, we first searched for orthologs (e value < 10−5) in the available genome sequences of 291 R. solanacearum strains by BLASTX analysis. The results showed that RipBJ was found in some Phylotype I and IV strains (Supporting Information S2: Table S4). In addition, among the eight R. solanacearum Phylotype I, III and IV strains with a defined virulence on tomato plants, RipBJ was found in the four low/medium-virulence strains (GMI1000, Pss4, Pss216, PSI07) but not found in the four high-virulence strains (Pss190, Pss1308, CFBP3059, CMR15) (Supporting Information S2: Table S4). Furthermore, none of the four R. solanacearum Phylotype II strains with defined high- or low-virulence (CFBP2957, CIP120, CFBP6783, IPO1609) contains RipBJ. These together suggest that the presence of RipBJ might be involved in the low/medium virulence of R. solanacearum Phylotype I, III and IV strains.
Loss of ripBJ in R. solanacearum strain GMI1000 did not cause obvious defects in the bacterial virulence on Arabidopsis and Medicago plants, and in the capacity of causing HR on tobacco (Lonjon et al., 2016). To determine whether RipBJ plays a determinant role in the virulence of R. solanacearum on tomato, we generated a ripBJ-deleted (∆ripBJ) mutant in the background of the medium-virulence strain Pss4. The ∆ripBJ mutant grew similarly to the wild-type strain in vitro and in planta (Supporting Information S1: Figure S1e,f) but displayed enhanced virulence on the BW-susceptible tomato cultivar L390 (Figure 1a). Complementation of the ∆ripBJ mutant with a functional ripBJ (∆ripBJ (ripBJ)) restored the low virulence of Pss4 (Supporting Information S1: Figure S2), verifying that the existence of ripBJ decreases the virulence of R. solanacearum on tomato. Consistently, the introduction of ripBJ into the high-virulence strain Pss190 (Pss190 (ripBJ-HA)) (Figure 1b) or in planta expression of RipBJ (Figure 1c) reduced the virulence of Pss190 on the BW-resistant tomato cultivar H7996. These results together indicate that expression of ripBJ lessens infection of R. solanacearum on tomato.
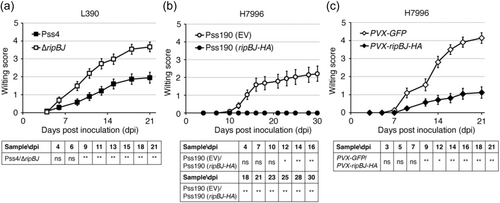
3.2 RipBJ induces plant cell death, ROS accumulation and defence signalling
We noticed that in planta expression of ripBJ in tomato led to retarded plant growth and the development of spontaneous lesions on leaves (Figure 2a,b and Supporting Information S1: Figures S3 and S4a,b). Transient overexpression of ripBJ caused similar spontaneous lesions on Nicotiana tabacum and N. benthamiana leaves (Supporting Information S1: Figure S4c,d), and increased ion leakage in N. benthamiana leaves (Supporting Information S1: Figure S4e). A β-estradiol-inducible expression system was further used to validate the link between ripBJ expression and the development of spontaneous lesions on plants. The β-estradiol specifically drives the expression of ripBJ through the oestrogen receptor and its inducible promoter, and this system has been used to study the effect of effectors in plants (Xin et al., 2015). The results showed that spontaneous lesions appeared on N. benthamiana leaves after the production of RipBJ was induced (Supporting Information S1: Figure S4f,g). As ROS burst is involved in plant cell death, we further measured the RipBJ-induced changes of the H2O2 level before the appearance of lesions. The results showed that ripBJ expression caused increased H2O2 accumulation in tomato and N. benthamiana (Figure 2c and Supporting Information S1: Figure S5). These results indicate that RipBJ induces plant cell death and ROS accumulation.
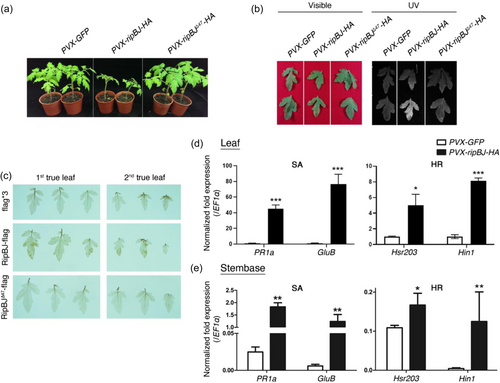
As ripBJ expression reduced bacterial infection, we further asked whether plant defence hormone pathways are involved in the RipBJ's effects on plants by analysing the transcriptional expression levels of representative defence marker genes. The results showed that RipBJ expression led to increased transcript levels of salicylic acid (SA) and HR marker genes in tomato leaves and stembases (Figure 2d,e). These results indicate that RipBJ expression activates multiple defence signalling pathways, leading to plant cell death and H2O2 accumulation.
3.3 Proper localization of RipBJ at the plant plasma membrane is required for its effects
RipBJ neither shares obvious sequence similarity with proteins of known functions nor contains defined functional motifs. The N-terminal 1-47 aa (RipBJ1-47aa) is predicted as a bacterial translocation motif (TM) by Softberry (http://www.softberry.com/berry.phtml) and shares sequence homology with RipBJ of R. solanacearum strains and other bacterial effectors (Supporting Information S1: Figure S6). Subcellular localization analysis demonstrated that GFP/YFP recombinant proteins of RipBJ predominantly exhibited a distribution pattern consistent with the plant plasma membrane and possibly nuclear localization (Figure 3a,b and Supporting Information S1: Figures S7a,b and S8). To discern the role of the N-terminal motifs of RipBJ in its subcellular localization and impacts on plants, recombinant GFP/YFP proteins fused with different forms of RipBJ were examined. In planta expression of truncated RipBJ forms, specifically those lacking 1–47 aa (RipBJ∆47), failed to induce effects similar to the full-length RipBJ. These effects encompassed plant growth retardation (Figure 2a and Supporting Information S1: Figure S4a,b), spontaneous lesions, cell death (Figure 2b) and elevated H2O2 accumulation (Figure 2c and Supporting Information S1: Figure S5). In planta expression of RipBJ∆47 also restored wilting following Pss190 infection (Figure 3c,d). Moreover, in planta expression of the truncated form with only 1–47 aa of RipBJ (RipBJ1-47aa) produced a cell death effect akin to RipBJ in N. benthamiana (Figure 3e,f). Consequently, it was inferred that RipBJ1-47aa was crucial for stable expression and played a pivotal role in its effects on plants. Subsequent examination of the importance of the subcellular localization of RipBJ1-47aa revealed co-localization with the plant plasma membrane, mirroring the expression pattern of full-length RipBJ-YFP/GFP (Figure 3a and Supporting Information S1: Figures S7b and S8c). In contrast, RipBJ∆47 did not co-localize with the plant plasma membrane, displaying a distribution pattern similar to free YFP/GFP (Figure 3b and Supporting Information S1: Figure S8d,e). Collectively, these findings indicate that proper localization of RipBJ at the plant plasma membrane is imperative for its effects on plants.
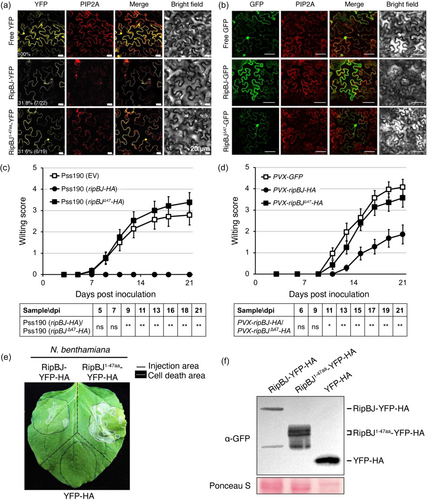
3.4 RipBJ interacts with tomato RBOH SlWfi1
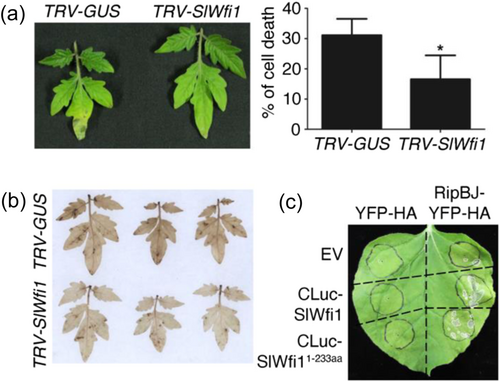
To investigate how RipBJ causes the effects on plants, we further searched for its interacting plant proteins. RBOHs, which localize at the plant plasma membrane, are the key enzymes responsible for ROS bursts involved in various plant defence responses (Kadota et al., 2015; Marino et al., 2012). Similar to these properties of RBOHs, RipBJ induced H2O2 accumulation and HR cell death (Figure 2a–c), and it colocalized with the plant plasma membrane (Figure 3a,b). We postulated that plant RBOHs could be candidate interacting targets of RipBJ. The Arabidopsis RBOHs AtRBOHD plays a crucial role in the plant defence response (Morales et al., 2016). The tomato ortholog of AtRBOHD is SlWfi1 (SlRBOHB). Consistent with AtRBOHD, SlWfi1 localized at the plant plasma membrane (Supporting Information S1: Figure S9). BiFC assay showed that RipBJ could interact with SlWfi1 (Figure 4a). Therefore, the interacting domains of SlWfi1 and RipBJ were further determined. SlWfi1 contains several known or predicted functional domains, including a NADPH oxidase catalytic domain, an EF-hand motif, a ferric oxidoreductase domain, an FAD-binding site and an NAD-binding site (Figure 4a). The truncations of SlWfi1 were designed based on its predicted functional domains. The results showed that the BiFC signal was observed when the full-length (SlWfi1) or the 1–233 aa fragment (SlWfi11-233aa) of SlWfi1 was co-expressed with RipBJ (Figure 4a).
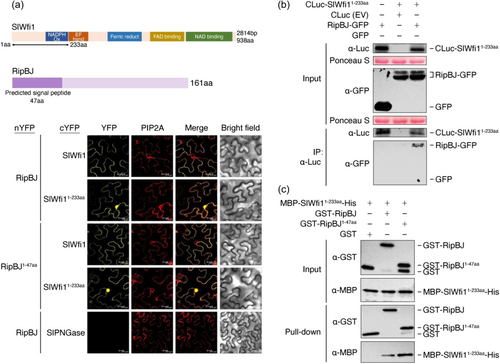
As RipBJ1-47aa was required for its localization at the plant plasma membrane and this localization was required for RipBJ's effects on plants (Figures 2, 3 and 3), we also determined whether RipBJ1-47aa could sufficiently interact with SlWfi11-233aa. The results showed that SlWfi11-233aa could form fluorescent signals with the full-length RipBJ or RipBJ1-47aa (Figure 4a). To validate the interaction between these two protein domains, we further conducted Co-IP, pull-down and LCI assays. All these assays showed that RipBJ/RipBJ1-47aa could interact with SlWfi11-233aa (Figure 4b,c and Supporting Information S1: Figure S10). These results reveal that the N-terminal domain of RipBJ1-47aa is responsible for RipBJ's interaction with SlWfi11-233aa.
3.5 Plant RBOHs play a key role in RipBJ-induced plant cell death and H2O2 burst
The role of SlWfi1 in RipBJ-induced plant cell death was further investigated by manipulating its expression in plants. The results demonstrated that silencing SlWfi1 in tomato (Supporting Information S1: Figure S11a) reduced RipBJ-induced plant cell death (Figure 5a) and H2O2 accumulation (Figure 5b). Overexpression of SlWfi1/SlWfi11-233aa alone did not induce significant changes in N. benthamiana (Figure 5c). However, SlWfi1/SlWfi11-233aa overexpression enhanced RipBJ-induced plant cell death (Figure 5c). To confirm the importance of SlWfi1 in RipBJ-induced plant cell death and rule out any potential nonspecific effects resulting from the simultaneous constitutive overexpression of these proteins in the plant, we employed the β-estradiol-inducible expression system for in planta RipBJ expression. Consistently, the results indicated that SlWfi1 overexpression significantly enhanced RipBJ-induced plant cell death (Supporting Information S1: Figure S11b). Moreover, NbRBOHB and AtRBOHD are identified as the close orthologs of SlWfi1 in N. benthamiana and A. thaliana, respectively (Supporting Information S1: Figure S12). The N-terminal regions of these RBOHs also shared medium to high similarity with SlWfi1 (58.8%–96.8%) (Supporting Information S1: Figure S13). The LCI assay demonstrated that the N-terminal region of NbRBOHB (NbRBOHBN-OX) and the full-length AtRBOHD also interacted with RipBJ (Supporting Information S1: Figure S14). Additionally, gene-specific silencing of the NbRBOHB gene (Supporting Information S1: Figure S15a) resulted in a discernible reduction in RipBJ-induced plant cell death (Supporting Information S1: Figure S15b), underscoring the pivotal role of these plant RBOHs in RipBJ-induced plant cell death.
To assess the impact of plant RBOHs on RipBJ accumulation, we scrutinized the levels of RipBJ in NbRBOHB-RNAi plants (Supporting Information S1: Figure S16a). Notably, NbRBOHB-RNAi did not elicit substantial alterations in the fluorescence signal intensity or YFP accumulation (Supporting Information S1: Figure S16b). In N. benthamiana leaves pre-infiltrated with the RNAi empty vector (RNAi-EV), robust RipBJ-YFP-HA fluorescence was evident. In contrast, NbRBOHB-RNAi leaves exhibited a lack of RipBJ-YFP-HA fluorescence (Supporting Information S1: Figure S16b). This observation was corroborated by immunoblot analysis, in which RipBJ-YFP-HA was detectable in RNAi-EV leaves but remained undetectable in NbRBOHB-RNAi leaves (Supporting Information S1: Figure S16c). Collectively, these findings suggest that NbRBOHB positively influences the in planta accumulation of RipBJ.
3.6 SlWfi1 promotes tomato tolerance to R. solanacearum
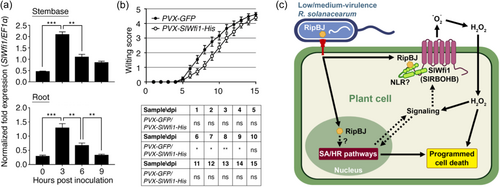
We further investigated the role of SlWfi1 in the response to R. solanacearum infection. Transcription analysis revealed that SlWfi1 expression was induced in the stembases and roots of tomato plants during the early stages following soil-soak inoculation with R. solanacearum strain Pss4, a medium-virulent strain that carries ripBJ (Figure 6a). Although overexpression of SlWfi1 did not produce noticeable changes in tomato plants, it did delay the development of wilting symptoms after infection with R. solanacearum (Figure 6b). These findings suggest a positive role for SlWfi1 in enhancing tomato tolerance to R. solanacearum.
4 DISCUSSION
4.1 Expression of ripBJ reduces R. solanacearum infection and triggers plant defence
RipBJ is regulated by PhcA and HrpG (Supporting Information S1: Figure S1c,d), and is previously shown to be a T3E and translocated into plant cells; nevertheless, loss of ripBJ in R. solanacearum strain GMI1000 does not cause obvious defects in virulence on Arabidopsis and Medicago and in HR on tobacco (Lonjon et al., 2016). We found that, among the R. solanacearum strains in Phylotype I, III and IV, whose virulence has been determined on tomato plants, RipBJ was present in the low- or medium-virulence strains but absent in the high-virulence strains (Supporting Information S2: Table S4). Consistently, our functional genetic analyses revealed that ripBJ was not involved in bacterial proliferation (Supporting Information S1: Figure S1e,f), but its expression led to reduced infection of R. solanacearum on tomato plants (Figure 1). Consistent with the involvement of SA- and HR-related pathways in tomato resistance against R. solanacearum (Chen et al., 2009), ripBJ expression enhanced the expression of marker genes involved in these pathways (Figure 2d,e). The ripBJ-caused plant growth defects, such as a dwarf symptom and spontaneous cell death (Figure 2a,b and Supporting Information S1: Figure S4), echoes the constitutive expression of the plant defence responses (Monson et al., 2022). The ripBJ-mediated enhancement of plant defence responses was correlated with its negative effect on R. solanacearum infection.
In general, the co-evolution concept presupposes the coexistence of pathogen and host; however, naïve host plants might possess specific resistance to newly encountered pathogens (Tobias et al., 2016) and vice versa. R. solanacearum is in fact a species complex that has originated and evolved in diverse regions with native vegetation as well as introduced plants (Buddenhagen, 1985). Given the functions of microbial effectors in aiding infection in hosts (Büttner & Filloux, 2016; Wagner et al., 2018; Xie et al., 2019), it is rational that RipBJ is a virulence factor assisting the infection of R. solanacearum on native host plants with or without the development of wilt symptom. Tomato, however, is not native in Asia where the Phylotype I strains of R. solanacearum originated from, and therefore is a new encounter host for most native Phylotype I strains. Since plants have evolved a large number of diverse NLRs, it is postulated that tomato might have an unidentified NLR that recognizes RipBJ, triggering plant defence and resulting in a reduced infection of the ripBJ-containing R. solanacearum strains. The absence of RipBJ in certain R. solanacearum strains enables the bacteria to escape from plant defence systems and thus yield a high virulence on tomato plants. Since the expression of RipBJ in tomato, N. tabacum and N. benthamiana triggers similar spontaneous cell death, it is possible that these plants have a shared mechanism to detect RipBJ and activate defence responses.
4.2 Association of RipBJ at the plant plasma membrane is required for its effects on plants and interaction with SlWfi1
The TMs of bacterial T3Es are important motifs for T3Es’ secretion from the bacterial cell (Büttner & Bonas, 2002; Samudrala et al., 2009). In some cases, after translocation into the plant cell, the TM of T3Es are removed and modified by plant enzymes, resulting in proper localization of T3Es in the plant cell (Nimchuk et al., 2000). Yet, the predicted TM of RipBJ (RipBJ1-47aa) is present in the secretome of R. solanacearum (Lonjon et al., 2016) and is required to induce HR response as shown in this study (Figure 2). Furthermore, many effector proteins of phytopathogenic bacteria can undergo myristoylation, a fatty acylation modification of an N-terminal glycine residue, which provides sufficient hydrophobicity and therefore affinity for membranes in plant cell (Popa et al., 2016). Myristoylation is essential for the targeting of effectors to the plant plasma membrane, pathogenicity and recognition by host immune system (Lewis et al., 2008; Thieme et al., 2007). Here we showed that RipBJ was mainly associated with the plant plasma membrane and its 1–47 aa motif was required and sufficient for this association (Figure 3a,b and Supporting Information S1: Figure S8). This plasma membrane association was required for its effects on plants (Figures 2 and 3c,d, and Supporting Information S1: Figure S4). RipBJ does not have a known signal peptide for the localization at plant plasma membrane; however, RipBJ1-47aa contains a putative myristoylation residue (glycine as the second amino acid) (Supporting Information S1: Figure S6) (Lewis et al., 2008; Thieme et al., 2007). Future mutation tests could be performed to determine whether the glycine residue is required for RipBJ's association with the plant plasma membrane and its functionality.
Despite the function of plant RBOHs in initiating ROS bursts in various defence responses (Kadota et al., 2015; Marino et al., 2012), whether plant RBOHs are potential targets that physically interact with microbial components remained unknown. Our results revealed that RipBJ directly interacts with SlWfi1 (Figure 4). Notably, RipBJ1-47aa was sufficient for the interaction with SlWfi11-233aa and influenced its localization (Figure 4). The potential myristoylation of RipBJ might enable it to target the plant plasma membrane where its interaction with SlWfil1 could be achieved. Since protein myristoylation is inadequate to keep a stable association with the plasma membrane (Resh, 1999; Udenwobele et al., 2017), the observed RipBJ's association with the plant plasma membrane might be caused by the interaction with SlWfi1. It will be interesting to further investigate if the glycine residue is essential for RipBJ's interaction with SlWfi1. In addition, myristoylation is an irreversible co-translational modification, whereas RipBJ-YFP also localizes in the nuclei of some plant cells. Hence, the mechanisms underlying such modification and regulation remain to be determined.
4.3 SlWfi1 and NbRBOHB have a positive role in RipBJ-induced defence cell death and ROS burst
RBOHs are crucial players in plant immunity (Hu et al., 2020). In Arabidopsis, AtRBOHD and AtRBOHF are involved in many defence responses (Marino et al., 2012), and their transcriptional expression can be induced by pathogens and their components (Morales et al., 2016). In tomato, SlWfi1 is involved in the resistance against Botrytis cinerea (Li et al., 2015) and root-knot nematode (Song et al., 2018), and the wound responsiveness (Sagi et al., 2004). In N. benthamiana, NbRBOHB participates in the ROS burst in INF1-triggered PTI, AVR3a-triggered ETI and the resistance against Tobacco mosaic virus (Deng et al., 2016). In addition to the interactions of SlWfi1 and NbRBOHB with RipBJ, our results also showed a key role of SlWfi1 and NbRBOHB in RipBJ-induced plant cell death and H2O2 accumulation (Figure 5 and Supporting Information S1: Figures S11 and S15), and a positive effect of NbRBOHB on the in planta accumulation of RipBJ (Supporting Information S1: Figure S16c). Furthermore, SlWfi1 expression was induced in tomato stembases and roots at early stages after soil-soak inoculation with R. solanacearum strain Pss4, a medium-virulent strain containing ripBJ and SlWfi1 played a positive role in tomato tolerance to Pss4 (Figure 6a,b). Therefore, RipBJ could be a newly discovered effector to interact with plant RBOHs. Also, the plant defence enhanced by RipBJ-SlWfi1 interaction might be mediated by the recognition of an unknown NLR through direct, guardee, decoy, or integrated decoy models (Bentham et al., 2020). Accordingly, RipBJ could be an Avr factor in tomato, which induced ETI through SlWfi1.
4.4 Role of RipBJ in the activation of SlWfi1-mediated defence pathway
In this study, we showed that SlWfi1 had a positive role in RipBJ-induced HR response and it directly interacts with RipBJ. ROS production is known to be mediated by the phosphorylation and activation of RBOHs (Wang et al., 2020). To search for possible posttranslational modifications in SlWfi1, three programmes (Netphos 3.1, GPS 5.0, PhosphoSVM) were used and eight predicted phosphorylation sites (70Y, 129S, 137S, 153S, 225T, 285T, 336S, 468Y) were identified in the 1–605 aa region of SlWfi1. Interestingly, five of these predicted phosphorylation sites were located in the N-terminal 1–233 aa of SlWfi1, which was the key domain for the interaction with RipBJ. It will be interesting to further determine whether SlWfi1 undergoes phosphorylation, whether such modification influences the enzyme activity and whether RipBJ has a role in this modification. If so, manipulating the enzyme activity of the interacting plant RBOHs might account for the RipBJ-mediated induction of ROS burst and activation of multiple defence signalling pathways. It is postulated that the ROS burst induced by the activated defence complex, SlWfi1-RipBJ-unknown NLR, might activate MAPK cascades to transduce the defence signalling (Gechev & Hille, 2005; Lang & Colcombet, 2020), leading to downstream events including activation of SA and HR signalling pathways and defence cell death. In addition, one might consider a possible role of the occasionally nucleus-localized RipBJ (Figure 3a,b and Supporting Information S1: Figure S8) in the transcriptional regulation of the defence pathways by a yet-to-identified mechanism. However, although the nuclear localization of RipBJ∆47 increased compared with that of RipBJ (Figure 3b), these truncated proteins lost their effects on plants. We thus reason that the RipBJ-induced defence responses are mainly mediated by its interactions with the plant RBOH.
5 CONCLUSIONS
BW caused by R. solanacearum causes globally severe crop losses; however, information about the functions of the virulence-related factors is very limited. As shown in Figure 6c, our study reveals that, after delivery into the plant cell, R. solanacearum T3E RipBJ interacts with the plasma membrane-localized SlWfi1, leading to increased H2O2 accumulation, plant cell death, activation of multiple defence pathways and the subsequent defence against R. solanacearum. Our study demonstrates that a pathogen effector interacts with a plant RBOH to modulate plant immunity. These findings gain new insights into plant defence mechanisms against pathogens.
ACKNOWLEDGEMENTS
This study was supported by grants from the Ministry of Science and Technology of Taiwan (MOST 106-2313-B-002−012-MY3, MOST 109-2313-B-002-044-MY3), National Taiwan University (NTU-CC-108L893104, NTU-CC-110L893606, NTU-CC-111L893006, NTU-CC-112L891806, NTU-CC-113L895006), and National Science and Technology Council (NSTC 113-2313-B-002-040) to Chiu-Ping Cheng.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




