SlWRKY51 regulates proline content to enhance chilling tolerance in tomato
Yixuan Wang and Meihui Zhang contributed equally to this study.
Abstract
Chilling stress is a major environmental factor that significantly reduces crop production. To adapt to chilling stress, plants activate a series of cellular responses and accumulate an array of metabolites, particularly proline. Here, we report that the transcription factor SlWRKY51 increases proline contents in tomato (Solanum lycopersicum) under chilling stress. SlWRKY51 expression is induced under chilling stress. Knockdown or knockout of SlWRKY51 led to chilling-sensitive phenotypes, with lower photosynthetic capacity and more reactive oxygen species (ROS) accumulation than the wild type (WT). The proline contents were significantly reduced in SlWRKY51 knockdown and knockout lines under chilling stress, perhaps explaining the phenotypes of these lines. D-1-pyrroline-5-carboxylate synthetase (P5CS), which catalyses the rate-limiting step of proline biosynthesis, is encoded by two closely related P5CS genes (P5CS1 and P5CS2). We demonstrate that SlWRKY51 directly activates the expression of P5CS1 under chilling stress. In addition, the VQ (a class of plant-specific proteins containing the conserved motif FxxhVQxhTG) family member SlVQ10 physically interacts with SlWRKY51 to enhance its activation of P5CS1. Our study reveals that the chilling-induced transcription factor SlWRKY51 enhances chilling tolerance in tomato by promoting proline accumulation.
1 INTRODUCTION
Chilling stress is a major environmental factor that limits the agricultural productivity of many plant species (Sanghera et al., 2011). Tomato (Solanum lycopersicum) and other warm season vegetable crops are very sensitive to chilling stress (0–12°C), which is common during their growing season. Chilling stress damages cell membrane stability, induces a reactive oxygen species (ROS) burst, influences organic osmolyte content, and causes photoinhibition in these plants (Li et al., 2016; Peng et al., 2015; Wang et al., 2024). Plants have evolved a complex response mechanism to improve chilling tolerance using strategies involving multiple signal transduction pathways, transcription factors, and protein–protein interactions (Hannah et al., 2005; Chinnusamy et al., 2007; Shi et al., 2018). Osmotic regulation, a crucial strategy for plant protection against chilling stress, relies on the uptake and/or intracellular biosynthesis of specific substances (Wang et al., 2024).
The accumulation of proline, an excellent osmolyte, is positively associated with plant chilling tolerance (Fedotova & Dmitrieva, 2016; Yang et al., 2011). A stressful environment results in the overproduction of proline in plants, which imparts stress tolerance by maintaining cell turgor or osmotic balance; stabilising membranes to prevent electrolyte leakage; and bringing ROS concentrations within normal ranges, thus preventing an oxidative burst in plants (Hayat et al., 2012). In addition to serving as an osmoprotectant, proline can act as a potent nonenzymatic antioxidant (Ben Rejeb et al., 2014). For example, the damaging effects of ROS on photosystem II (PSII) were reduced by treating isolated thylakoid membranes with proline (Alia et al., 1997). In addition, the activities of the enzymes ascorbate peroxidase (APX), monohydroascorbate reductase (MDHAR), and dihydroascorbate reductase (DHAR), which are components of the ascorbate-glutathione (ASC-GSH) cycle, and other antioxidant enzymes such as catalase (CAT), peroxidase (POX), and superoxide dismutase (SOD) were significantly enhanced by exogenous proline application in tobacco (Nicotiana tabacum) cultures exposed to salinity stress (Hoque et al., 2007).
In most plant species, two isoforms of pyrroline-5-carboxylate synthetase (P5CS) catalyse the first step in proline biosynthesis from glutamate (Turchetto-Zolet et al., 2009). P5CS1 is a major contributor to stress-induced proline accumulation, whereas P5CS2 is important for embryo development and growth (Funck et al., 2020; Székely et al., 2008). Arabidopsis (Arabidopsis thaliana) knockout mutants of P5CS1 showed reduced proline synthesis and antioxidant enzyme activity under stress conditions. This deficiency was not fully compensated for by overexpressing P5CS2, indicating that P5CS1 played an irreplaceable role in regulating stress-induced proline accumulation (Székely et al., 2008). The level of P5CS1 transcription, which regulates stress-induced proline biosynthesis, appears to be a key determinant of abiotic stress tolerance in plants. However, the mechanism that directly regulates P5CS1 is currently unclear.
Transcription factors play important roles in cold signalling and cold tolerance by modulating the expression of their target genes (Jiang et al., 2020). Members of the WRKY transcription factor family, one of the largest transcription factor families in plants, contain a conserved WRKYGQK motif and a novel zinc-finger-like motif. Arabidopsis and tomato each contain more than 70 WRKY transcription factor family members (Bakshi & Oelmüller, 2014; Chen et al., 2015; Wu et al., 2005). WRKY transcription factors play important roles in the regulation of transcriptional reprogramming during plant stress responses by binding to the core W-box promoter elements (TGACC [A/T]) in their downstream genes (Bakshi & Oelmüller, 2014). Numerous studies have shown that many WRKYs are responsive to pathogens or pathogen elicitors. Studies using overexpression lines or mutants of WRKYs have shown that WRKYs positively or negatively regulate the expression of plant hormone-related genes or genes involved in pathogen defence (Dong et al., 2003; Lai et al., 2008, 2011). WRKY family members are also essential for the cold-stress response. CsWRKY46 of cucumber (Cucumis sativus), OsWRKY71 of rice (Oryza sativa), and VbWRKY32 of Verbena bonariensis all play roles in improving cold tolerance (Kim et al., 2016; Wang et al., 2020; Zhang et al., 2016). AtWRKY34 negatively regulates cold sensitivity in Arabidopsis by regulating the expression of CBF (C-repeat binding factor) genes (Zou et al., 2010).
Valine-glutamine (VQ) motif-containing proteins are a large class of transcriptional regulatory cofactors (Tian et al., 2023). Recently, several groups have reported that proteins containing a short VQ (FxxxVQxLTG) motif interact with WRKY proteins (WRKYs) to act as its coactivators (Cheng et al., 2012). By interacting with WRKYs, VQ proteins (VQs) participate in the multiple signalling pathways to regulate plant growth and development, as well as the defence responses to salt, heat or to plant pathogen (Chen et al., 2022; Dong et al., 2024; Ma et al., 2023; Yang et al., 2024). However, our understanding of the specific roles and molecular mechanisms of WRKYs and VQs under chilling stress remains quite limited.
Tomato is one of the most sensitive crops to chilling stress. Various studies have revealed the crucial roles of proline in chilling tolerance in tomato, but the underlying mechanism is still unclear (Hu et al., 2019, 2021; Li et al., 2018; Wang et al., 2022). Here, we identified the positive role of chilling-induced SlWRKY51 in chilling tolerance in tomato. In this process, SlWRKY51 and the regulatory cofactor SlVQ10 synergistically activate the expression of SlP5CS1 to increase proline accumulation under chilling stress. The increased proline levels reduce chilling-induced ROS accumulation and protect PSII from oxidative damage. These findings shed light on this important chilling-tolerance mechanism in tomato.
2 MATERIALS AND METHODS
2.1 Plant materials, growth conditions, and experimental treatments
Tomato (S. lycopersicum cv. Micro-TOM) plants were used to generate all transgenic materials in this study. The homozygous Slwrky51 mutant line was generated using a pHSE401-based CRISPR/Cas9 system. The SlWRKY51-RNAi lines (W51R-1 and W51R-2) were generated by transforming tomato plants with PC336 vectors containing a specific 226-bp fragment of the SlWRKY51 coding sequence. Tomato plants were transformed using the Agrobacterium strain LBA4404 (Agrobacterium tumefaciens)-mediated leaf disk method (Chetty et al., 2013). The resulting lines were identified by RT-qPCR and DNA sequencing.
Seedlings with approximately four fully developed leaves were exposed to chilling treatment (4°C) in an illuminated incubation chamber (E-41L2, Percival) with a photon flux density (PFD) of approximately 300 μmol m−2 s−1 and a relative humidity of 60%–70% for 10 h. The leaves were harvested for analysis.
2.2 RT-qPCR analysis
Total RNA was isolated from tomato leaves using an RNAprep Plant plus Kit (DP441) from Tiangen and reverse-transcribed into first-strand cDNA using a FastKing RT Kit (KR116) from Tiangen. qPCR was conducted using a SuperReal PreMix Plus (SYBR Green) Kit (FP205, Tiangen) in an Applied Biosystems quantitative PCR Q6 instrument (Thermo Fisher Scientific).
Gene expression levels were normalised to that of the housekeeping gene SlACTIN, and the values in the wild type (WT) were set to 1. For each assay, at least three biological replicates were performed. All primers used in this study are listed in Supporting Information S1: Table S1.
2.3 Measurement of physiological parameters
The photochemical efficiency of PSII (Fv/Fm) was determined with a Handy Plant Efficiency Analyser system (Hansatech Instruments) using dark-adapted leaves (20 min) under ambient CO2 conditions. Exactly 0.1 g of mature leaf tissue from each tomato genotype before and after chilling treatment was used to measure O2•− and H2O2 concentrations, together with malondialdehyde (MDA) content as described by Kong et al. (2014). Ten fresh leaf discs from different tomato lines before and after chilling treatment were used to measure relative electrical conductivity (REC) (Kong et al., 2014). Hydrogen peroxide (H2O2) and superoxide radical (O2•−) were stained with 3,3-diaminobenzidine (DAB) and nitroblue tetrazolium (NBT), respectively, as described in Kong et al. (2014). To observe ROS using a fluorescent probe, leaf discs were collected from transgenic and WT plants grown at 25°C and 4°C. The leaf discs were soaked in PBS (0.01 mM) for 15–30 min, and the liquid was removed and replaced with 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA, 10 µM, Invitrogen). The leaf discs were placed under vacuum for 30 min, shaken, and placed on slides. ROS fluorescence was observed under a confocal microscope (LSM 880 NLO). Four-week-old seedlings were used to harvest the used mature leaves in above detections.
To measure proline content, fresh leaf material (500 mg) was extracted with 5 mL of 3% sulfosalicylic acid at 100°C for 10 min. Proline was detected as described previously (Yang et al., 2011). Briefly, 2 mL of the aqueous extract was mixed with 2 mL of glacial acetic acid and 2 mL of acid ninhydrin reagent (1.25 g ninhydrin, 30 mL glacial acetic acid, and 20 mL 6 M orthophosphoric acid) and heated at 100°C for 30 min. After cooling, the reaction mix was partitioned against toluene (4 mL), and the absorbance of the organic phase was determined at 520 nm. The resulting values were compared with a standard curve constructed using known concentrations of proline (Sigma).
2.4 GUS staining assay
The 1.686-kb sequence upstream of the SlWRKY51 translation initiation site was amplified and inserted into the KpnI and SalI sites of the pZP211-GUS vector. Tomato plants were transformed with the resulting plasmid (proSlWRKY51::GUS) as described above. After transformation, T1 transgenic plants were selected based on growth on medium containing kanamycin. Leaves before and after 4°C treatment were fixed in 90% (v/v) acetone, incubated on ice for 20 min, rinsed in staining solution without X-GLUC, and transferred to staining solution (0.5 M Na2HPO4, 0.5 M NaH2PO4, 10% TritonX-100, 100 mM K3Fe(CN)6, 100 M K₄Fe(CN)₆, 0.5 M EDTA, and 2 mM X-GLUC). The leaves were vacuum-infiltrated for 30 min, followed by incubation at 37°C overnight.
2.5 Yeast one-hybrid (Y1H) and two-hybrid (Y2H) assays
The Y1H assay was performed essentially as described by Zhuang et al. (2019). The promoter regions (W1–W4) of SlP5CS1 and the promoter region (W5) of SlP5CS2 were cloned into the KpnI and SalI sites of the pLacZi2u vector. The full-length coding sequence of SlWRKY51 was cloned into the EcoRI and XhoI sites of the pGAD vector. The appropriate pairs of plasmids (according to Figure 5c) were co-transformed into yeast strain EGY48. Positive colonies were selected for growth on synthetic defined (SD) medium lacking Ura and Trp, and positive clones were grown on SD medium lacking Ura and Trp but containing 20% (w/v) galactose, 20% (w/v) raffinose, BU salts (0.27 M Na2HPO4 and 0.23 M NaH2PO4.2H2O), and 0.05 M X-Gal.
For the Y2H assay, the full-length coding sequence of SlWRKY51 was cloned into the EcoRI and XhoI sites of the pGADT7 vector. The full-length coding sequence of SlVQ10 was cloned into the EcoRI and SalI sites of the pGBKT7 vector. Protein–protein interactions were detected using the Matchmaker Gold Yeast Two-Hybrid System according to the manufacturer's instructions (Clontech).
2.6 LUC reporter assay
For the luciferase reporter assay, the promoter sequence of SlP5CS1 (W2) was inserted into the pGreen0800 vector (a double luciferase reporter vector) via the KpnI and SalI sites. Both the SlWRKY51 promoter (1600 bp upstream of the ATG translation initiation codon) and its coding sequence were ligated into the pZP211-FLAG vector without the 35S promoter after EcoRI/SalI digestion. The resulting constructs were introduced into Agrobacterium strain GV3101. For transient expression experiments, Agrobacterium cultures harbouring the appropriate pairs of constructs were infiltrated into Nicotiana benthamiana leaves, using each promoter:LUC reporter construct and empty vector as controls. Infiltration of N. benthamiana and LUC reporter assays were conducted as described by Zhuang et al. (2019). To analyse the response of the SlWRKY51 promoter to 4°C treatment, the SlWRKY51 promoter (1600 bp) was inserted into pGreen0800 via the KpnI and SalI sites. The resulting vector was introduced in Agrobacterium strain GV3101, which was infiltrated into N. benthamiana leaves. After 2 days, the infiltrated plants were exposed to chilling for 40 min. A dual-luciferase reporter assay kit (FR201-01, TransGen Biotech) was used to measure LUC signals. For the fluorescence images, the D-luciferin solution (Gold Biotechnology Inc.) was used to inject into the leaves before or after chilling treatment, and the in vivo imaging system (Xenogen) was then used to observe the fluorescence of these leaves.
2.7 Bimolecular fluorescence complementation (BiFC) assay and luciferase complementation assay
BiFC assays were performed as described by Walter et al. (2004). The full-length coding sequence of SlWRKY51 was subcloned into the pUC-SPYNE vector. The full-length SlVQ10 coding sequence was cloned into the pUC-SPYCE vector. These constructs were introduced into Agrobacterium strain GV3101, and Agrobacterium cultures harbouring the appropriate pairs of constructs were infiltrated into N. benthamiana leaves. YFP fluorescence was imaged under a confocal laser-scanning microscope (LSCM 880; Carl Zeiss AG).
For the luciferase complementation assay in N. benthamiana leaves, the SlWRKY51 coding sequence was inserted into the KpnI and SalI sites of the pCAMBIA1300-nLUC vector. The full-length SlVQ10 sequence were inserted into the KpnI and SalI sites of the pCAMBIA1305-cLUC vector. All resulting constructs were transformed into Agrobacterium strain GV3101 by the freeze-thaw method. SlWHY2-nLUC and SlRECA-cLUC in Meng et al. (2020) were used as control. LUC signals were observed as described by Zhang et al. (2023).
2.8 Electrophoretic mobility shift assay (EMSA)
The EMSAs were performed as described by Zhang et al. (2023). Probes containing the W-box in the W2 region (as shown in Figure 4c) were synthesised by Sangon Biotech. Recombinant purified SlWRKY51-HIS was produced using a His-Tag Protein Purification kit according to the manufacturer's protocol (P2249S, Beyotime). The purified protein was incubated with 0.5 μM of the corresponding biotin-labelled double-stranded DNA (ssDNA) probe in a total volume of 10 μL for 20 min at 25°C. Unlabelled probes were added for the competition experiments. A LightShift Chemiluminescent EMSA Kit (Thermo Fisher Scientific) was used to detect chemiluminescence.
2.9 Statistical analysis
The data represent the means ± standard deviation (SD) of three biological replicates. One-way analysis of variance (ANOVA) with Fisher's least significant difference (LSD) test was used to examine the differences among genotypes. ANOVA was conducted using the ‘aov’ function in the R package ‘stats’. The LSD test was used to assess differences in measured parameters among genotypes using the ‘LSD.test’ in the R package ‘agricolae’. These analyses were performed using R software v.4.3.1 (http://cran.r-project.org).
3 RESULTS
3.1 SlWRKY51 is induced by chilling stress in tomato
Chilling stress induces a complex response mechanism involving transcription factors and protein–protein interactions that improve plant chilling tolerance. The expression of SlWRKY51 was significantly enhanced after chilling treatment (4°C), suggesting that it may play important roles in the response to chilling stress (Figure 1). SlWRKY51 transcript levels increased gradually during the chilling treatment and reached a maximum level at 9 h (Figure 1a). In a β-glucuronidase (GUS) reporter assay of transgenic tomato plants carrying the SlWRKY51pro::GUS construct, GUS expression in mature leaves greatly increased in response to chilling stress (4°C) (Figure 1b). In tomato seedlings, the GUS signal was mainly detected in stems, which is consistent with the RT-qPCR results (Supporting Information S1: Figure S1a,b), and we detected strong GUS staining in leaves following treatment at 4°C (Figure 1b).

To further explore the regulation of SlWRK51 expression, we divided its promoter into three fragments (P1[+1- −473 bp], P2[+1- −1084 bp] and P3[+1- −1686 bp]; the initiation codon ATG was defined as +1; Figure 1c), which we cloned individually upstream of the luciferase (LUC) gene for transient expression in N. benthamiana leaves via Agrobacterium-mediated infiltration. Following chilling treatment, we detected higher LUC activity driven by the P3 promoter fragment compared to the P1 and P2 fragments (Figures 1c and 1d). These results indicate that SlWRKY51 is a chilling induced gene.
3.2 Decreased SlWRKY51 expression results in reduced chilling tolerance in tomato
A transient expression assay in N. benthamiana indicated that SlWRKY51-GFP localised to the nucleus (Supporting Information S1: Figure S1c). To analyse the physiological function of SlWRKY51, we produced SlWRKY51 RNA interference (RNAi) lines (W51R1 and W51R2) and generated a wrky51 mutant via CRISPR (clustered regularly interspaced short palindromic repeat)/Cas9 (CRISPR-associated nuclease 9)-mediated gene editing. We identified the wrky51 mutant by DNA sequencing and measured SlWRKY51 transcript levels in RNAi lines W51R1 and W51R2 by reverse-transcription quantitative PCR (RT-qPCR) (Figure 2a).
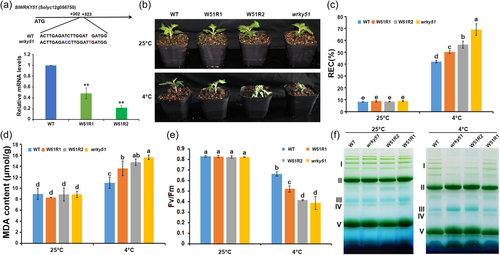
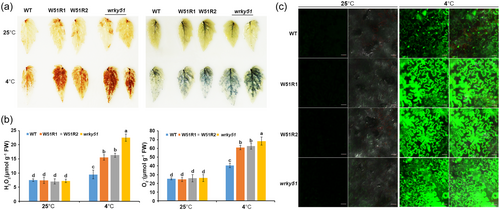
No significant differences among the phenotypes of the wrky51 mutant, SlWRKY51 RNAi lines, and WT (4-week-old seedlings) were observed when grown at 25°C. By contrast, the growth of the wrky51 mutant and SlWRKY51 RNAi lines was impaired by treatment at 4°C for 10 h compared to the WT (Figure 2b). In agreement with this observation, REC and MDA accumulation, two indicators of membrane damage, increased to a greater extent in wrky51 and SlWRKY51 RNAi leaves than in the WT in response to chilling treatment (Figure 2c,d). In addition, the maximal photochemical efficiency of PSII in the dark (Fv/Fm), which decreases when PSII is photodamaged, was significantly lower in wrky51 and SlWRKY51 RNAi plants than the WT after chilling stress (Figure 2e). Blue native-PAGE (BN-PAGE) analysis revealed that the stability of thylakoid protein complexes, especially PSII, was greater in WT than in the wrky51 and SlWRKY51 RNAi plants after chilling treatment (Figure 2f). Photodamage to PSII in plants under chilling stress typically occurs due to excess ROS accumulation. Indeed, significantly more H2O2 and O2.– accumulated in the leaves of wrky51 and SlWRKY51 RNAi plants than the WT under chilling stress (Figure 3a,b). The fluorescence-based measurement of ROS accumulation confirmed that ROS levels were higher in wrky51 and SlWRKY51 RNAi plants than in WT under chilling stress (Figure 3c). These results suggest that SlWRKY51 positively regulates plant tolerance to chilling stress.
3.3 SlWRKY51 increases proline contents under chilling stress by regulating the expression of SlP5CS1
In addition to serving as an osmoprotectant, proline is also a component of the antioxidative network involved in mitigating the effects of stress-induced oxidative damage (Ben Rejeb et al., 2014). We measured the proline contents and transcript levels of the major proline biosynthesis-related genes (SlP5CSs) in wrky51, SlWRKY51 RNAi, and WT plants. Proline levels were significantly higher in WT versus wrky51 and SlWRKY51 RNAi plants after chilling treatment (Figure 4a). Accordingly, the SlP5CS genes, especially SlP5CS1, were expressed at higher levels in the WT than in wrky51 and SlWRKY51 RNAi plants (Figure 4b).
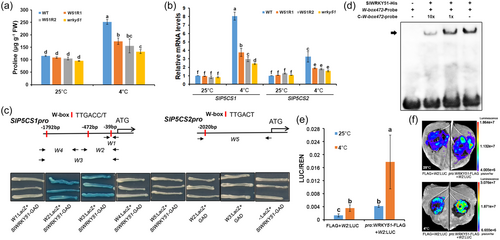
To explore the mechanism by which SlWRKY51 regulates SlP5CS1 and SlP5CS2 expression, we searched for W-box elements (TTGACC/T) in the promoter regions of SlP5CS1 and SlP5CS2 (Figure 4c). A Y1H assay (Figure 4c) and EMSA (Figure 4d) confirmed that SlWRKY51 binds to the single W-box element in the W2 region of the SlP5CS1 promoter. We then performed a transient expression assay in which we introduced a LUC reporter construct driven by the SlP5CS1 promoter into N. benthamiana leaves. When we co-infiltrated leaves with the SlWRKY51 effector construct driven by its own promoter, we observed significant activation of proSlP5CS1::LUC expression, as revealed by changes in relative LUC activity, especially under chilling stress (Figure 4e,f). These results suggest that SlWRKY51 increases proline contents and thereby chilling tolerance in tomato by directly activating SlP5CS1 expression.
3.4 SlVQ10 interacts with SlWRKY51 to enhance the regulation of SlP5CS1
Exploring the interacting proteins of chilling-responsive SlWRKYs is important for understanding their regulatory mechanisms under chilling stress. Chi et al. (2013) determined that WRKY–WRKY, WRKY–VQ, and WRKY–MAPK are the most common interactions of WRKY transcription factors. SlVQ10 is a chilling-induced member of the tomato VQ protein family (Supporting Information S1: Figure S2). We confirmed the interaction of SlWRKY51 with tomato SlVQ10 by Y2H (Figure 5a), bimolecular fluorescence complementation (BiFC) (Figure 5b), and LUC complementation assays (Figure 5c). To explore the role of SlVQ10 in the activation of SlP5CS1 expression by SlWRKY51, we performed LUC reporter assays. SlVQ10 enhanced the SlWRKY51-induced activation of SlP5CS1 expression (Figure 5d,e). These results indicate that SlVQ10 plays a synergistic role with SlWRKY51 to positively regulate SlP5CS1 expression.

4 DISCUSSION
Proline is an excellent osmolyte and potential ROS scavenger. Therefore, proline accumulation is a widespread response of plants to cold stress as well as drought and salt stress (Hayat et al., 2012). D-1-pyrroline-5-carboxylate synthetase enzymes, which catalyse the rate-limiting step of proline biosynthesis, are encoded by two closely related P5CS genes in Arabidopsis (Sekhar et al., 2007). The transcription of P5CS genes is differentially regulated by drought, salinity, and abscisic acid, suggesting that these genes play specific roles in regulating proline biosynthesis (Székely et al., 2008). In Arabidopsis, P5CS1 has been identified as the major contributor to stress-induced proline accumulation, whereas P5CS2 is important for embryo development and growth (Mattioli et al., 2009; Székely et al., 2008).
In the current study, both SlP5CS1 and SlP5CS2 were induced by chilling treatment in tomato, but SlP5CS1, which is directly activated by SlWRKY51, was expressed at a higher level than SlP5CS2 under chilling stress, supporting the dominant role of SlP5CS1 in chilling-induced proline production (Figure 4). Although there is also a W-box in the promoter of SlP5CS2, our Y1H assay indicated that SlWRKY51 could not bind to this region, suggesting that SlWRKY51 directly regulates the expression of SlP5CS1 but not SlP5CS2 (Figure 4c).
VQ family members interact with WRKY transcription factors, playing essential roles in enhancing their DNA binding and transcriptional regulatory activities, and thereby downstream gene expression (Chi et al., 2013). Less studies indicated the specific role of VQ proteins under chilling stress, only VQ5 was found to interact with WRKY26 and attenuate its induced activation of JA biosynthetic genes under cold stress in banana (Ye et al., 2016). Here we showed that SlVQ10 is induced by chilling stress and that SlVQ10 interacts with SlWRKY51 to enhance the activation of SlP5CS1 expression (Figure 5). These results reveal a SlWRKY51-mediated mechanism for regulating of SlP5CS1 in response to chilling stress. In addition, there were no significant differences in SlP5CS1 expression among WT, wrky51, and SlWRKY51 RNAi plants at 25°C (Figure 4b). In a GUS reporter assay of transgenic tomato plants carrying the SlWRKY51pro::GUS construct, SlWRKY51 was expressed at low levels in leaves at 25°C but was strongly induced in leaves after 4°C treatment (Figure 1b,c). This might be the reason for proline accumulation under chilling stress but not normal growth conditions.
Chilling stress accelerates the production of ROS, which attack the photosynthetic machinery and biological membranes (Allen & Ort, 2001). The damaging effects of ROS on PSII were reduced by treating isolated thylakoid membranes with proline (Alia et al., 1997). Exogenous proline acts as an active oxygen scavenger, thereby overcoming the oxidative stress induced by chilling (Posmyk & Janas, 2007). Hong et al. (2000) concluded that the role of proline as a free radical scavenger in alleviating stress is more important than its role as a simple osmolyte. APX, CAT, and SOD activities were significantly enhanced by exogenous proline treatment (Hoque et al., 2007). In the current study, ROS accumulated more strongly and PSII was damaged more severely in wrky51 and SlWRKY51 RNAi plants versus the WT under chilling stress (Figures 2 and 3), suggesting that the photoinhibition caused by reduced SlWRKY51 expression was due to the lack of activation of SlP5CS1 expression.
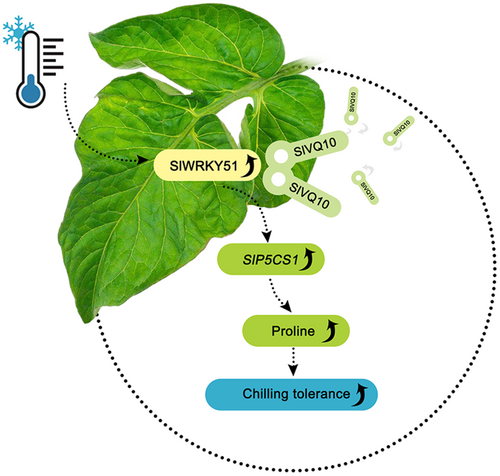
In summary, we identified a regulatory mechanism for proline accumulation under chilling stress in tomato. The chilling-induced transcription factor SlWRKY51 plays a positive role in this process. In tomato leaves, SlWRKY51 interacts with SlVQ10 and directly activates the expression of SlP5CS1 to regulate proline accumulation, thus enhancing chilling tolerance in tomato (Figure 6).
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (grant number 32100203, 32201737), the China Postdoctoral Foundation (grant number 2021M691974), and the Natural Science Foundation of Shandong Province (grant no. ZR2021QC149, ZR2021QC197).
Open Research
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available from the corresponding author upon request.




