Manipulating hormones to mitigate trade-offs in crops
Summary statement
Addressing trait coupling due to gene pleiotropy presents challenges in conventional breeding system. However, targeted hormonal manipulation and precise genetic engineering designs hold promise to alleviate trade-offs and unlock the potential of crops for multiple desirable traits.
1 INTRODUCTION
Over the past half-century, hundreds of genes regulating tillering, grain weight and quality and immunity have been cloned and utilized to potentially improve crop production. However, when integrating certain excellent alleles in breeding, it was found that they have struggled to effectively contribute to desired high-yield, high-quality, and stress-tolerant varieties (Lu et al., 2024). This is primarily due to the intricate trade-offs among these agronomic traits and their trait components. Enhancing crop genetics necessitates managing intricate trade-offs stemming from linkage drags and gene pleiotropy (Laitinen and Nikoloski, 2022). The molecular marker-assited conventional breeding can alleviate the trade-offs resulting from linkage drags through precise manipulating the localization and recombination of closely linked genes. However, resolving the trade-off resulting from pleiotropism is challenging with traditional breeding methods, particularly when aiming for the simultaneous control of multiple complex quantitative traits.
In this context, most of pleiotropic genes are positioned at critical crossroads in plant hormone biosynthesis and signaling pathways. The well-known Green Revolution genes, rice sd1 (semi-dwarf 1) and wheat Rht1-B1c, which encode the GA biosynthetic enzyme GA20ox and growth-repressing DELLA proteins, respectively, and confer gibberellic acid (GA)-deficient and insensitive semi-dwarfism, have substantially boosted crop yields by reducing lodging risk. Yet they have led to adverse effects, including decreased biomass, low nitrogen use efficiency and increased seed dormancy (Li et al., 2018; Van De Velde et al., 2021). Grain size and quality are regulated by a complex network of multiple developmental and environmental signals, encompassing GA, cytokinin (CK), auxin, abscisic acid (ABA) and brassinosteroid (BR), which typically prioritize high yield over taste and nutrition (Li et al., 2014). Immune responses, regulated by the jasmonic acid (JA), counteract growth induction governed by GA, through the antagonistic interplay between JA-dependent CORONATINE INSENSITIVE 1 (COI1) and GA-dependent DELLA signaling (Huang et al., 2017). NONEXPRESSOR OF PATHOGENESIS-RELATED GENES 1 (NPR1) and WRKY transcription factors-mediated salicylic acid (SA) signals also play a pivotal role in the growth-immunity trade-off and are influenced by ABA and auxin signaling pathways (van Butselaar and Van den Ackerveken, 2020). These inter-pathway hormone crosstalks pose challenges for harnessing the potential of pleiotropic genes to address additional adverse effects on performance.
2 TISSUE-SPECIFIC INHIBITION OF BR DISRUPTS THE TRADE-OFF IN YIELD COMPONENTS
The negative correlation between grain yield-related traits poses one of the most challenging hurdles and is a significant factor contributing to the bottleneck in high-yield breeding processes (Guo et al., 2018). Breeders have also focused on enhancing grain yield through genetic modification aimed at endogenous hormone levels in crops, such as CK, auxin and BR. However, it has been observed that yield trade-offs persist due to pleiotropic genes involved in hormone manipulation (Dash and Rai, 2022). Reducing gene pleiotropy could aid in crop breeding to alleviate trade-off effects, but the development of effective strategies for achieving this goal has been limited. In a recent study published in Science, Zhang et al. dissected the tissue-specific regulation of BR in mitigating trade-offs between grain number and grain size in a clustered-spikelet (CL) variety, thus dramatically unlocking the yield potential in rice (Zhang et al., 2024) (Figure 1).
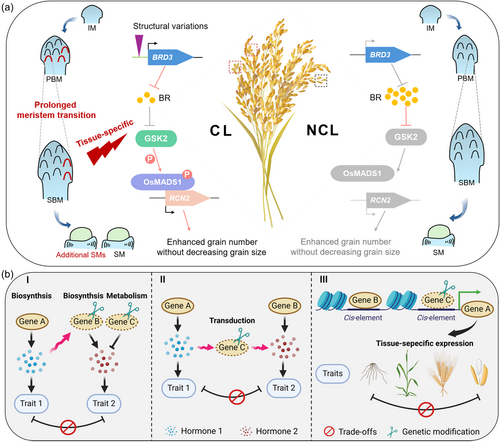
CL represents a distinctive rice variety characterized by the clustering of three spikelets sited together at the terminus of secondary branch. Along with the shortening of the pedicels, this compound-spikelet morphology resembles the multi-floret spikelet of wheat and the triplet spikelet of barley (Xie et al., 2023). With its intriguing panicle structure and increased grain number but without affecting grain weight, CL has been under the spotlight since the 1930s, breaking through breeding predicaments and offering novel varieties for crop yield improvement. Due to the potential complex structural variations located at the causal locus of the CL line, obtaining the precise region using traditional positional cloning methods has always been challenging. Through screening CL suppressor mutants instead of crossing with non-CL varieties, a functional BR-degrading enzyme encoded by BRD3 was identified. Notably, creating simple mutations artificially is an effective method for fine-mapping crucial genes, avoiding issues caused by structural genomic variations that hinder precise localization. Natural variation analysis indicated that BRD3 underwent chromosomal structural variations such as inversions, deletions and insertions upstream of its promoter, activating its expression and reducing BR levels, resulting in the appearance of clustering. We consider the structural variations occurring in the gene promoter of BRD3 to serve as an example of driving forces in plant evolution, regulating gene expression to achieve desirable phenotypes for adaptation to changing environments without necessarily altering the gene itself. In addition, large-scale structural variations may alter the chromatin accessibility of promoter regions, thereby causing changes in gene expression, such as a reduction in repressive methylation modifications (Wu et al., 2023; Zhou et al., 2021). This might be a potential avenue for future research on enhancing BRD3 gene expression.
BRs constitute a class of steroid hormones that play crucial roles in regulating various physiological processes in plants, encompassing growth, development and stress responses (Kim and Russinova, 2020). Research findings elucidate the underlying signaling pathway behind the CL-phenotype likening its components to intricately synchronized parts of a clock. The research utilizes transgenic rice lines with tissue-specific inhibition of BR signaling, achieved via the overexpression of BR signaling components within the reproductive organs. A tissue-specific BR pathway (BRD3-BR-GSK2-OsMADS1-RCN2) has been described, wherein loss-of-function mutants of any component have consequences for the transition of CL or NCL phenotypes. BRD3 was specifically expressed in the secondary branch meristem (SBM), leading to a decrease in BR content within its region. This activates the core inhibitor of the BR signaling pathway, GSK2. GSK2 phosphorylates the transcription factor OsMADS1, stabilizing it further. OsMADS1 directly binds to RCN2-an important factor in regulating spikelet meristem (SM) identity and promotes its expression. Activation of RCN2 delays the transition from SBM to SM, allowing CL plants more time for producing the increased secondary branching and grain number per panicle. Panicle branching is a complex and orchestrated process, accompanied by a series of events involving meristematic tissue transformation. This study represents the remarkable identification of tissue-specific BR in regulating secondary branching development in rice panicles.
The introduction of BRD3 into different varieties has confirmed the significant yield-increasing potential of CL. Due to differing mechanisms in promoting panicle branching, CL can be combined with the well-known panicle number control gene Gn1a (Tu et al., 2022) to dramatically boost grain yield by simultaneously manipulating BR and CK. Through hybrid breeding, combining BRD3, which reduces BR levels, with the null allele of Gn1a, associated with increased CK levels, can lead to rice panicles with over a 40% increase in grain number. The consistent changes in BR content were also observed in pepper and rose, which share clustered growth patterns similar to rice, suggesting that the mechanism governing clustering through BR may be conserved among plants. Field trials conducted over several years and locations have revealed that the yield-increasing effect of CL is closely related to BR levels in different varieties and planting conditions, as CL functions by controlling hormone levels, which are inherently sensitive to environmental factors. This finding also serves as an example of employing molecular design to integrate two independent hormone regulatory pathways, leading to tangible increases in crop yield.
Panicle number, grain number per panicle and grain weight are three major determinants of crop grain yield. BR is typically considered to positively regulate grain size and weight while negatively affecting grain number (Yang et al., 2024b), suggesting the existence of a natural yield compromise between the two. This study found that in CL rice, there was a tissue-specific decrease in BR expression, mainly in secondary branches and pedicels, but not in spikelets. It resulted in an increase in grain number per panicle without changes in grain size and quality, representing a novel mechanism that balances this yield component trade-off. It is noteworthy that the regulatory factors associated with the BR cascade signaling pathways, including BRD3, GSK2, OsMADS1 and RCN2, are all selectively activated in the SBM. Therefore, the tissue-specific inhibition of BR in CL rice avoids the negative impact of BR defects on grain size and yield. In conclusion, these findings reveal the pivotal role of BRs in coordinating panicle branching and grain number through precise meristem modulation, highlighting the efficacy of tissue-specific hormonal manipulation in alleviating trade-offs among different traits and unleashing the crop potential for increased yield. This strategy also holds reference value for other hormones as well, especially for hormones such as cytokinin and auxin that undergo dynamic transport within plants. It is essential to further strengthen basic research on the spatial regulation of plant hormone distribution to provide more targets in crop breeding enhancement.
3 STRATEGIES FOR BREAKING TRAIT COUPLING BY MANIPULATING HORMONE SIGNALING PATHWAYS
Plant hormones are vital for orchestrating interactions between plant growth and their multifaceted biotic and abiotic environments. Each hormone activates a unique molecular pathway, and these pathways are intricately interwoven in a complex network of synergistic, antagonistic and additive interactions. Drawing from recent successful advancements in weakening or lifting the crosstalk of plant hormone pathways, we have formulated three effective approaches to simultaneously enhance multiple desirable traits by overcoming constraints imposed by pleiotropy. This fundamentally enables the balanced optimization of trait trade-offs in hormone regulation during the molecular design-aggregated breeding process (Figure 1b). The first is targeting the modification of a node in independent hormone synthesis and metabolism pathways to prevent the emergence of unfavourable traits. OsNPR1, a SA receptor, serves as a crucial regulator of innate immunity but inhibits plant growth and development through repression of the auxin synthesis pathway by activating OsGH3.8 (an indole-3-acetic acid (IAA)-amido synthetase responsible for degrading IAA) (Zhong et al., 2021). A potential way for utilizing OsNPR1 in defense breeding without compromising yield is to block the altered downstream auxin metabolism in plant growth by knocking down or knocking out of OsGH3.8 using gene-editing technology.
Secondly, directly truncating crucial signal transduction genes connect two hormonal pathways, rendering them unable to interfere with each other and allowing them to act separately. Here are two typical examples: Tomato CYP94C1, activated by ethylene-mediated ripening, suppresses JA-mediated defense by converting JA-Ile to its inactive form. Mutation of this gene using CRISPR/Cas9 can disrupt this trade-off, resulting in increased resistance to B. cinerea infection without impacting the ripening process (Yang et al., 2024a). A common trade-off between plant responses to abiotic and biotic stresses involves the crosstalk between the ABA and SA signaling pathways, facilitated by protein tyrosine phosphatase (PTP) (Gao et al., 2024; Ueno et al., 2015). OsWRKY45, as the crucial transcription factor in the SA defense signaling pathway, is phosphorylated and activated by OsMPK6 in response to benzothiadiazole, a chemical defense inducer. However, OsMPK6 is suppressed following tyrosine dephosphorylation by OsPTP1/2, which is mediated by ABA in response to cold and salinity stress. Consequently, this disrupts WRKY45 activity and reduces defense against pathogens. Silencing PTP effectively disrupts the interaction between ABA and SA signals, thereby preventing abiotic stresses from diminishing pathogen-induced resistance.
Additionally, modulating cis-regulatory regions to alter gene expression in a tissue-specific manner is a promising strategy to overcome gene pleiotropy and yield trade-offs. Rice IDEAL PLANT ARCHITECTURE (IPA1) affects various agronomic traits related to plant development and stress responses. IPA1 enhances disease resistance by stimulating the expression of defense-related genes, maintaining a balance between growth and defense. However, in terms of yield components, IPA1 also functions as a typical pleiotropic gene, making it challenging to balance the grain number per panicle and the number of tillers. Similar to this study, a constructive tiling-deletion-based CRISPR–Cas9 screen resolves this tradeoff, leading to substantially enhanced both total grain yield and immunity (Song et al., 2022). An excellent allele with 54-bp deletion located in the promoter of IPA1, IPA1-Pro10, was found to enhance the spatial-temporal expression in panicle and root by removing a target site for the transcription factor An-1, thereby simultaneously facilitating both panicle size and tiller number in rice. In summary, we believe these practical design strategies offer a wealth of targets for refining breeding programmes aimed at developing complementary beneficial traits, particularly manipulating the pleiotropic genes in a tissue-specific manner to address the adverse effects in crops.
ACKNOWLEDGEMENTS
This work was funded by the National Science Foundation for Young Scientists of China (32201780), the Fundamental Research Fund for the Central Universities (77000-12240011), Shenzhen Postdoctoral Funding Project (szbo202410) and the National Natural Science Foundation of China (32241045 and 32241038).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




