Gβγ dimers mediate low K+ stress-inhibited root growth via modulating auxin redistribution in Arabidopsis
Nuerkaimaier Mulati and Zhong-Qi Li contributed equally to this study.
Abstract
In the investigation of heterotrimeric G protein-mediated signal transduction in planta, their roles in the transmittance of low K+ stimuli remain to be elucidated. Here, we found that the primary root growth of wild-type Arabidopsis was gradually inhibited with the decrease of external K+ concentrations, while the primary root of the mutants for G protein β subunit AGB1 and γ subunits AGG1, AGG2 and AGG3 could still grow under low K+ conditions (LK). Exogenous NAA application attenuated primary root elongation in agb1 and agg1/2/3 but promoted the growth in wild-type seedlings under LK stress. Using ProDR5:GFP, ProPIN1:PIN1-GFP and ProPIN2:PIN2-GFP reporter lines, a diminishment in auxin concentration at the radicle apex and a reduction in PIN1and PIN2 efflux carrier abundance were observed in wild-type roots under LK, a phenomenon not recorded in the agb1 and agg1/2/3. Further proteolytic and transcriptional assessments revealed an enhanced degradation of PIN1 and a suppressed expression of PIN2 in the wild-type background under LK, contrasting with the stability observed in the agb1 and agg1/2/3 mutants. Our results indicate that the G protein β and γ subunits play pivotal roles in suppressing of Arabidopsis root growth under LK by modulating auxin redistribution via alterations in PIN1 degradation and PIN2 biosynthesis.
1 INTRODUCTION
Potassium (K), an indispensable metallic macronutrient, constitutes 2%–10% of a plant's anhydrous biomass (Wang & Wu, 2015; Wang et al., 2021). As a pivotal macronutrient, K+ ions are integral to crop yield and quality, facilitating numerous physiological and metabolic functions including nitrate translocation, organic acid transport, nitrogen assimilation, saccharide deposition, osmoregulation, enzymatic catalysis and ionic homoeostasis (Gruber et al., 2013; Kellermeier et al., 2013). Plants absorption of K+ ions predominantly occurs via root systems from soils. Typical cellular concentrations of K+ in terrestrial flora are approximately 100 mmol/L, juxtaposed with soil concentrations ranging between 0.1 and 1 mmol/L. To manage environmental K+ flux, plants have developed dual absorption modalities: low-affinity and high-affinity K+ uptake pathways. In environments with K+ exceeding 0.3 mmol/L, plants employ low-affinity channels for K+ acquisition. Conversely, at levels below 0.2 mmol/L, high-affinity transporters are utilized for K+ acquisition (Epstein et al., 1963; Kellermeier et al., 2014; Schroeder et al., 1994).
Suboptimal K+ availability manifests in aerial plant structures as osmotic pressure diminution, desiccation and compromised cellular expansion, which collectively result in attenuated foliar surface area, internode elongation inhibition, stem slenderizing and resilience reduction (Mostofa et al., 2022; Osakabe et al., 2013). K+ scarcity precipitates suboptimal cell turgor, leading to inadequate hydration, biomembrane and organelle damage and metabolic disruption. Advanced K+ deficiency stages impair cellular metabolism, evidenced by chlorophyll degradation in senescent foliage and subsequent impairment in new growth, culminating in chlorosis and necrosis at the leaf margins (Xu et al., 2006). In roots, K+ paucity inhibits primary and lateral root development, with a minor negative gravitropic effect and an enhancement in root hair proliferation (Gruber et al., 2013; Kellermeier et al., 2013). Rice rhizogenesis, for instance, is adversely affected by K+ deprivation, altering root architecture and the root-to-shoot biomass ratio (Cai et al., 2012).
Nutrient stress in plants critically influences nutrient absorption through alterations in root architecture. Essential nutrient deficits in edaphic environments, such as nitrogen (N), phosphorus (P), K, calcium (Ca), magnesium (Mg) and micronutrients like iron (Fe), variably impact primary and lateral root growth metrics For instance, K, P and Mg deficits suppress both primary and lateral root development, whereas N and Fe shortages promote such growth. Phytohormonal regulation of root architecture involves auxins, ethylene, cytokinins and others, with auxins being paramount in root structure establishment (Benjamins & Scheres, 2008; Friml et al., 2003; Zhao, 2010).
Auxin exhibits distinctive polarized transport within plant tissue, mediated by plasmalemma auxin transporters—both influx and efflux carriers (Leyser, 2005). Research indicates that the PIN protein family, functioning as auxin efflux facilitators, ensures auxin's polar distribution essential for vegetative growth (Leyser, 2005). The Arabidopsis thaliana genome encodes eight PIN proteins, all present at the root apex. PIN1 localizes at columella cell bases, directing auxin to the quiescent centre (QC), while PIN2 at the cortical cell base mediates upward auxin conveyance from the center (Feraru & Friml, 2008; Vieten et al., 2005; Zažímalová et al., 2007). Under low K+ conditions, the redistribution and homoeostasis of auxin in the roots play a critical role in shaping the root architecture. Research has reported that Arabidopsis Shaker K+ channel AKT1 is essential for regulating K+-dependent root growth and AKT1 responds to external K+ changes by modulating the degradation of PIN1 and the redistribution of auxin in the root (Li, Wu, et al., 2017). The Arabidopsis KUP/HAK/KT K+ transporter protein KUP9 regulates root meristem activity and primary root growth under low K+ stress. KUP9 directly mediates the efflux of K+ and auxin from the endoplasmic reticulum into the cytoplasm to maintain K+ and auxin homoeostasis in QC cells under low K+ conditions (Zhang et al., 2020). Another K+ transporter, KUP4, is also involved in low K+-induced root architecture response mediated by auxin (Templalexis et al., 2022).
Heterotrimeric G protein complexes, often referred to as G proteins, represent an ubiquitous and vital class of signal transduction molecules within eukaryotic cells, orchestrating the transmission of signals from extracellular G protein-coupled receptors (GPCRs) to intracellular effector entities (Oldham & Hamm, 2008). These complexes are molecular conglomerates comprising α, β and γ subunit isoforms. In the human genome, 16 Gα, 5 Gβ and 14 Gγ isoforms have been catalogued, enabling the assembly of a myriad of distinct G protein complexes (Oldham & Hamm, 2008). Contrastingly, Arabidopsis thaliana possesses a more limited G protein repertoire, with a single Gα isoform (GPA1), three extra-large Gαs (XLG1, XLG2, XLG3), one Gβ isoform (AGB1) and a trio of Gγs (AGG1, AGG2, AGG3). Despite their numerical paucity in plant systems, plant G proteins are increasingly recognized for their extensive involvement in modulating plant growth and development. For example, GPA1 and AGB1 have been shown to modulate cell division in roots (Chen et al., 2006; Ullah et al., 2003). Gβγ dimers mediate N-MYC DOWNREGULATED-LIKE1 (NDL1) protein-regulated primary root growth and lateral root formation via modulating basipetal and acropetal auxin transport (Mudgil et al., 2009); G protein complex participates in glucose attenuation of auxin-mediated bimodality in lateral root formation (Booker et al., 2010). Furthermore, G protein complex is also known to play important roles in plant response to various abiotic stresses, such as drought, salt, temperature, heavy metal stress and other environmental factors (Urano & Jones, 2014; Wolfenstetter et al., 2015; Zhang et al., 2008; Zhang, Xie, et al., 2021).
Considering the multifaceted functions of heterotrimeric G proteins in the regulation of plant ontogeny, as well as their mediation of stress-induced signal transduction pathways, we posited that these molecular switches may play a critical role in the vegetative response to K+ deficiency. In the present study, we conducted an in-depth examination of the genotypic and molecular interplay between G protein and low K+ stress. Our findings elucidate that G protein β and γ subunits act as positive modulators of the plant adaptive response to diminished K+ levels, principally through modulating auxin distribution in the apical region of the root.
2 MATERIALS AND METHODS
2.1 Plant materials and growth conditions
In this study, the Arabidopsis thaliana Columbia (Col-0) ecotype was used as wild-type. Seeds of the T-DNA insertion mutants gpa1-3 (SALK_066823), agb1-2 (SALK_061896), agg1-1c (CS16550), agg2-1 (SALK_010956), agg3−3 (SAIL_1209_B01), gpa1-4agb1-2 (CS6535), agg1-1c agg2-1 agg3−1 (CS73483) and nrt1.5 (SALK_043036) were obtained from the Arabidopsis Biological Resource Center. The genotypes of all mutants were confirmed by polymerase chain reaction (PCR) analysis. Other plant materials used in this study were ProDR5:GFP (Friml et al., 2003), ProPIN1:PIN1-GFP (Benková et al., 2003) and ProPIN2:PIN2-GFP (Vieten et al., 2005; Xu & Scheres, 2005). The above transgenic plants were crossed with gpa1-3, agb1-2, agg1-1c, agg2-1 and agg3-3 mutants, respectively, and the homozygous plants with fused GFP were identified by PCR and used for GFP fluorescence observation in the progeny plants.
Seeds were germinated and vertically grown on Murashige and Skoog (MS) medium containing 1.2% (w/v) agar and 3% (w/v) sucrose for 4 days at 22°C under illumination with 60 μmol/m2/s. Then, seedlings were transferred to MS and LK media for 4 days at 22°C under constant illumination with 60 μmol/m2/s, and the root phenotype was observed. To harvest seeds and perform crosses, plants were grown in mixed potting soil (fertile soil:vermiculite = 2:1, v/v) in a growth chamber with 16-h light cycles at 22°C, 120 μmol/m2/s light intensity and approximately 70% relative humidity (Li, Wu, et al., 2017).
2.2 Root growth phenotype analyses
After seedlings were grown on MS medium for 4 days, the seedlings were transferred to MS or the low K+ (LK) medium with or without the chemicals. The LK medium was prepared by modification of MS medium as described previously (Xu et al., 2006). Moreover, 1.25 mM KH2PO4 in MS medium was replaced by 1.25 mM NH4H2PO4 and 18.8 mM KNO3 in MS medium was replaced by 0.05 mM KNO3 and 8.8 mM NH4NO3. So, the LK medium contained 30.65 mM NH4+ and 29.45 mM NO3−, which were higher than 20.6 mM NH4+ and lower than 39.4 mM NO3− in MS medium, respectively. To eliminate the potential influence of high NH4+ concentration in the LK medium on root growth, a low NH4+ LK medium was prepared by directly changing 18.8 mM KNO3 in MS medium to 0.05 mM KNO3. Meanwhile, a NH4+-free LK medium was prepared by replacing NH4NO3 and NH4H2PO4 in the LK medium with NaNO3 and NaH2PO4, respectively. To exclude the potential influence of low NO3− concentration in the LK medium on root growth, 18.8 mM KNO3 in MS medium was replaced by 0.05 mM KNO3 and 18.75 mM NaNO3. NAA (1-naphthlcetic acid, 0.03 µM), TIBA (2,3,5-triiodobenzoic acid, 1 µM) and NPA (N-1-naphthylphthalamic acid, 1 µM) were added until the medium sterilized by autoclaving at 115°C for 15 min and cooled to 60°C. The photographs were taken at the indicated time points. The root growth in the phenotype analyses represents the length of primary root growth after the seedlings were transplanted to MS and LK medium. Data were derived from at least three biological replicates in each experiment.
2.3 K+ and NO3− content measurements
When the 4-day-old seedlings were transferred to MS or LK medium for 4 days, the root and shoot tissues were harvested and weighed separately. For K+ content measurement, the tissues were placed in an oven drying at 80°C for 48 h. Then, the samples were treated in a muffle furnace at 300°C for 1 h and 575°C for 5 h and then dissolved in 0.1 M HCl. The K+ contents were measured using 5110-ICP OES (Agilent). For NO3− content measurement, 3 mL of milli-Q water per 0.1 g fresh weight (FW) of tissues was added, and the mixture was boiled for 20 min and frozen at −80°C overnight. The material was centrifuged at 20 800×g for 5 min at room temperature, and then, the supernatant was taken up in a 1 mL syringe, passed through a 0.22 µm filter, and its NO3− content was determined by HPLC (Agilent 1200 series) using a PARTISIL 10 strong anion-exchange column (Whatman).
2.4 Fluorescence observation
After the 4-day-old seedlings of GFP crossing lines were transplanted to MS or LK medium for a specified time, observe GFP fluorescence in plant roots using confocal laser scanning microscope (Leica TCSSP8) under 488 nm excitation and 530 nm emission light. To compare different images, capture the corresponding images under the same settings. For relative fluorescence analysis, confocal images were analysed using ImageJ software and the GFP signals of wild-type Col-0 on MS medium represent control. The data comes from three or more biological replicates in each experiment.
2.5 RNA extraction and quantitative real-time polymerase chain reaction (qPCR) analysis
Total RNA was extracted from roots using TRIzol reagent kit (Invitrogen), and then cDNA was synthesized using the HiScript® II Reverse Transcriptase according to the manufacturer's instructions (Vazyme). qPCR was performed using ChamQ™SYBR qPCR Master Mix (Vazyme). Each qPCR result is the average of three independent biological replicates, with ACTIN2 as the internal control (Livak & Schmittgen, 2001). The primers used were PIN2 (5′-TATCAACACTGCCTAACACG-3′, 5′-GAAGAGATCATTGAT
GAGGC-3′) and ACTIN2 (5′-GTTGGGATGAACCAGAAGGA-3′, 5′-CTTACAATTT
CCCGCTCTGC-3′).
2.6 MG132 treatment experiment
Seedlings of proPIN1:PIN1-GFP, proPIN1:PIN1-GFP/gpa1-3, proPIN2:PIN2-GFP and proPIN2:PIN2-GFP/gpa1-3 were grown on MS medium for 4 days and then transferred to MS or LK medium in the presence or absence of 3 µM MG132 for 1 day used to observe the fluorescence signal or for 4 days used to analyse root growth phenotype.
2.7 Statistical analysis
Statistical significance is indicated in the figure legend as *p < 0.05, **p < 0.01, ***p < 0.001 or ****p < 0.0001. All data analyses were performed using GraphPad Prism 8.0. Two-way analysis of variance was used to compare the results of different treatments.
3 RESULTS
3.1 The Gβ and Gγ subunit mutants maintain primary root growth under low K+ stress
Investigating the G protein mutants' reactivity to diminished K+ levels involved transitioning the mutants for Gα, Gβ and Gγ subunits from MS medium to LK medium (with a concentration of 50 μmol/L K+) for a duration of 4 days, followed by phenotypic assessment. Observations indicated that Gβ subunit mutant agb1 and three single Gγ subunit mutants agg1, agg2 and agg3 all exhibited reduced sensitivity to K+ scarcity in terms of root elongation when compared to the wild-type Col-0, whereas the Gα subunit mutant gpa1 manifested an augmented sensitivity to LK (Figure 1). These findings imply that the Gβ and Gγ subunits positively but Gα negatively regulate LK-inhibited root growth. Moreover, compared to three single agg mutants, agg1/2/3 triple mutant showed more insensitivity to LK, indicating that three Gγ subunits function indispensably and somewhat redundantly (Figure 1). The gpa1agb1 double mutant phynocopied agb1 but not gpa1, indicates that loss of AGB1, rather than gain of GPA1 freed from the agb1 mutant, is causative of this agb1 phynotype, and that the phynotype of gpa1 is caused by the gain of Gβγ dimers (Figure 1). These findings, combined with the previous finding that in both animal and plant G-protein signalling, Gβ functions in a dimer with Gγ (Chakravorty et al., 2011; Ma, 1994; Mason & Botella, 2000, 2001; Weiss et al., 1994), further demonstrate that Gβγ dimers but not Gα mediate LK-inhibited root growth. To elucidate the mutants’ responses to K+ sufficiently, we conducted gradient K+ transplantation assays (10 µM, 50 µM, 100 µM) against a control of MS medium, which contains 20 mM K+. It was evident that the phenotypic expressions of the wild-type and G protein mutants differed markedly at the 50 µM K+ level (Supporting Information S1: Figure S1). Consequently, this concentration was employed in subsequent experimental media, designated as the LK medium.

To ascertain whether the insensitive phenotype observed in Gβ and Gγ mutants cultivated in LK medium is attributable to differential K+ uptake, we quantified the K+ concentrations in the shoots and roots of these plants. The data revealed no significant variation in K+ content between the mutants and the wild-type plants in both MS and LK medium (Supporting Information S1: Figure S2). This implies that the insensitivity in primary root elongation exhibited by Gβ and Gγ mutants is not a consequence of altered K+ levels of plants.
Furthermore, considering that the LK medium used here contained lower concentration of NO3− than that in MS medium, and that the elongation of Arabidopsis primary root depends on NO3− concentration and G protein regulates root elongation in a NO3− dependent manner (Boonyaves et al., 2022), we eliminate the possibility that reduced NO3− content might influence the observed phenotype by augmenting the NO3− level in LK medium with NaNO3 to replenish nitrate levels. The subsequent analysis demonstrated that the mutants maintained a phenotype consistent with previous LK medium experiments when grown in the low-K+ medium supplemented with NaNO3 (Supporting Information S1: Figure S3). This denotes that the altered phenotype under the LK medium is independent of NO3− reduction. Considering that G proteins might affect NO3− uptake under the LK conditions, we also measured NO3− concentrations in both wild-type and G protein mutants under MS and LK medium and observed no significant differences (Supporting Information S1: Figure S4). This indicates that NO3− is not responsible for the LK-insensitive phenotype in the G protein mutants.
The presence of high ammonium (NH4+) concentrations in LK medium could potentially influence AKT1-mediated apical root development (Xu et al., 2006). Given the initial LK medium's NH4+ concentration was 30.65 mM, we utilized both the LK medium with a reduced NH4+ concentration (20.6 mM) and the LK medium with NH4+-free (replacing NH4NO3 and NH4H2PO4 in the LK medium with NaNO3 and NaH2PO4, respectively) to confirm the G protein mutants' sensitivity to K+ deprivation. Phenotypic consistency between the G protein subunit mutants in the LK mediums with high NH4+ (Figure 1), low NH4+ (Supporting Information S1: Figure S5) and NH4+-free (Supporting Information S1: Figure S6) confirmed that the mutants' insensitivity to LK stress was independent of the NH4+ concentration, allowing for the use of high NH4+ medium in the following experiments.
Previous studies have shown that LK-induced leaf senescence (Xu et al., 2006), and that nrt1.5 mutant displayed faster leaf senescence and root growth than wild-type under LK (50 µM) medium due to a remarkable defect in both K+ and NO3− translocation from root to shoot (Li, Yu, et al., 2017). To clarify whether G proteins also participate in LK-induced leaf senescence, the nrt1.5 mutant was employed as a control, given the congruence of the LK mediums used (Li, Yu, et al., 2017). Contrary to the nrt1.5 mutant, G protein mutants displayed the wild-type leaf phenotype but did not display analogous chlorotic leaves of nrt1.5 under LK conditions (Supporting Information S1: Figure S7), which were consistent with the results that G protein mutants did not affect K+ and NO3− contents in both roots and shoots under LK conditions (Supporting Information S1: Figures S2 and S4). Together, these results suggest a specific involvement of G proteins in mediating the primary root growth response to LK availability.
3.2 The root growth phenotype of G protein mutants under low K+ stress is controlled by auxin signalling
Auxin, the inaugural phytohormone identified, is pivotal in the architectural development of roots. We posited that the observed retardation in primary root elongation in both wild-type and Gα subunit mutant under K+ deprivation might be auxin-linked. To substantiate this postulate, we administered an auxinic compound, namely NAA, along with auxin efflux blockers TIBA and NPA, monitoring root morphogenesis outcomes. The data indicated that NAA augmentation in MS medium curtailed primary root growth in both wild-type and G protein mutants (Figure 2a,b). Nonetheless, NAA markedly enhanced primary root extension in wild-type and gpa1-3 mutant under LK, while suppressing it in Gβ and Gγ subunit mutants under LK medium (Figure 2c,d). These findings infer a potential decrement in apical auxin concentration within the primary roots of the wild type and gpa1-3 under LK stress, with exogenous NAA ameliorating growth. Conversely, an excess of NAA impeded Gβ and Gγ mutants primary root extension, implying an unchanged or elevated auxin concentration at their root apices.
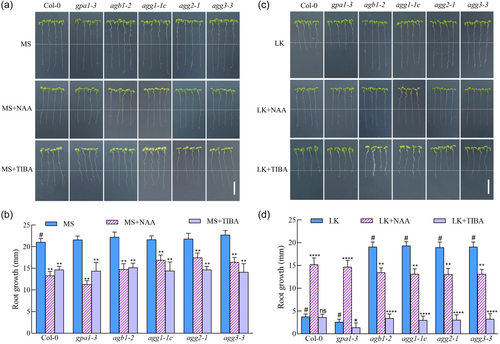
In MS media with TIBA and NPA, both TIBA and NPA repressed primary root extension across all plants tested (Figure 2a,b; Supporting Information S1: Figure S8a,b). In LK milieu, both TIBA and NPA markedly repressed Gβ and Gγ mutant primary root extension. However, the innate short primary roots of wild-type and gpa1-3 mutants rendered the suppressive influence of TIBA and NPA less discernible (Figure 2c,d; Supporting Information S1: Figure S8c,d). These results suggest that K+ scarcity may impinge on primary root elongation of wild-type and gpa1 mutant via diminishing auxin polar transport to the apex, while Gβ and Gγ mutants exhibit insensitivity to this decreased auxin polar transport induced by K+ insufficiency. In sum, G protein's role in the suppression of Arabidopsis root growth under LK conditions appears to be modulated through auxin-dependent signalling pathways.
3.3 Auxin content was maintained in the primary root tips of Gβ and Gγ subunit mutants under low K⁺ stress
In an experimental assay to quantify auxin levels post-LK stimulus, DR5::GFP reporter constructs were introgressed into the genetic backgrounds of gpa1-3, agb1-2, agg1-1c, agg2-1 and agg3-3 mutants (Friml et al., 2003; Ulmasov et al., 1997). Plant samples were subjected to LK medium for 3 days, succeeded by 2 days of recuperation on MS medium. Continuous monitoring of auxin via DR5::GFP luminescence in radicular tissues revealed that, while LK induced a diminution of signal intensity at the root apex of both wild-type and Gα mutant rootlets, which was subsequently ameliorated upon K+ restoration, the luminescence in the root apices of agb1-2, agg1-1c, agg2-1 and agg3-3 remained unaltered irrespective of the K+ concentration (Figure 3a,b). This constancy in auxin levels within the Gβ and Gγ mutants' root apices might underpin their resistance to LK-induced attenuation of primary root elongation. Further scrutiny of DR5::GFP luminescence in the proximal maturation zone of the extension region evidenced LK-induced an amplification in the wild-type and Gα mutant specimens but no changes in the Gβ and Gγ mutants (Figure 3c,d). These findings imply that LK stress reconfigures auxin distribution in the apices of Col-0 and gpa1-3 primary roots, potentially impeding auxin translocation to the calyptra through both the extension and meristematic zones, thereby diminishing apical auxin concentrations and provoking anomalous accumulation in the maturation zone adjacent to the elongation region. Conversely, the agb1-2, agg1-1c, agg2-1 and agg3-3 mutants mitigate this effect, permitting auxin conveyance to the root apex, thereby facilitating primary root extension. In summation, the perturbation of primary root elongation in response to LK stress in wild-type and Gα mutant is predominantly mediated via modulation of auxin gradients. However, the Gβ and Gγ mutants maintain unaltered auxin distribution under similar stress conditions, implicating the Gβ and Gγ subunits in the regulatory circuitry, conferring an adaptive response to LK stress. These observations suggest that the Gβ and Gγ subunits modulate primary root growth under LK conditions through the regulation of apical auxin distribution.
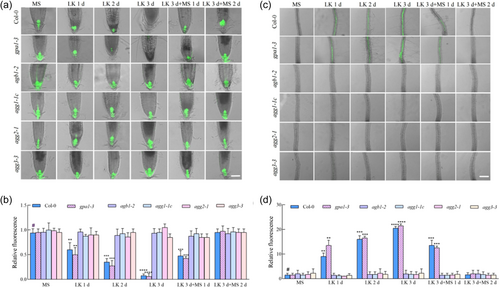
3.4 G protein is involved in the reduction of PIN1 and PIN2 protein levels under low K+ condition
Among the eight PIN proteins encoded by the Arabidopsis genome, PIN1-PIN4 and PIN7 are categorized as typical PIN proteins, whereas PIN5, PIN6, and PIN8 are classified as atypical class of PIN proteins. The canonical PINs exhibit considerable spatial colocalization within plant tissues and possess functional redundancy to maintain the robustness of directional auxin transport (Vieten et al., 2005). In the investigation of auxin homoeostasis in G protein-deficient root apices, the green fluorescent protein (GFP) tagged canonical PINs (PIN1-GFP to PIN4-GFP and PIN7-GFP) served as proxies for assessing the spatial dynamics of auxin efflux facilitators in wild-type root tips subjected to K+ deficiency. Observations indicated that the GFP signal alterations for PIN1 and PIN2 were markedly pronounced under K+-deficient conditions, with PIN1 exhibiting the most rapid response (Supporting Information S1: Figure S9).
PIN1 is localized to the central stele of the root, mediates the downward transport of shoot-derived auxin to the root apex and contributes to processes including meristematic activity, organogenesis, initiation of leaves and floral buds, tropistic responses to gravity in stems and vascular tissue patterning (Blilou et al., 2005; Gälweiler et al., 1998). PIN2 expression is confined predominantly to the cortical cells of the root elongation zone and during embryonic development, where it facilitates basal auxin flow and modulates root gravitropism (Chen et al., 1998). Employing transgenic lines expressing PIN1-GFP and PIN2-GFP under their respective promoters, crossed with the G protein mutants gpa1-3, agb1-2, agg1-1c, agg2-1 and agg3-3, enabled the analysis of PIN1 and PIN2 abundance under K+-deprived conditions across various genetic backgrounds. In the homozygous progeny of hybrids carrying the PIN1-GFP construct and grown on LK medium, PIN1 was undetectable in both Col-0 and gpa1-3 mutant after 24 h, but restoration of PIN1 was observed upon transferring LK-stressed Col-0 and gpa1-3 back to MS medium (Figure 4a,b). In contrast, the presence of PIN1 persisted in agb1-2, agg1-1c, agg2-1 and agg3-3 mutants, with no discernible alteration in protein levels throughout the exposure period (Figure 4a,b).
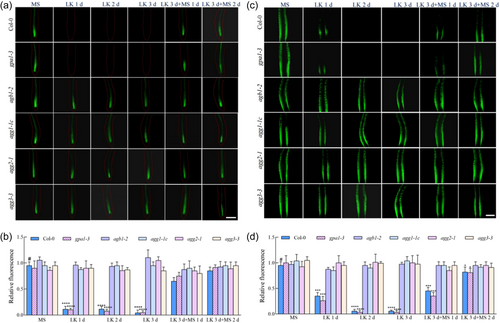
Analogously, PIN2-GFP-tagged gpa1-3, agb1-2, agg1-1c, agg2-1 and agg3-3 mutants were analysed. Following transplanting to LK medium, a progressive decline in PIN2 was observed in Col-0 and gpa1-3 mutants until fluorescence became undetectable after 2 days, but restoration of PIN2 was observed upon transferring LK-stressed Col-0 and gpa1-3 back to MS medium (Figure 4c,d). Conversely, PIN2 levels remained relatively stable in agb1-2, agg1-1c, agg2-1 and agg3-3 mutants (Figure 4c,d). These data suggest that the hyporesponsiveness of Gβ and Gγ subunit mutants to LK stress may be attributable to the differential modulation of PIN1 and PIN2 protein abundance.
3.5 G protein regulates the degradation of PIN1 in response to low K+
Utilizing confocal microscopy, the temporal dynamics of ProPIN1: PIN1-GFP were monitored. Observations revealed that before LK stress, all tested plant specimens exhibited pronounced polar localization of PIN1 at the plasma membrane. Upon exposure to LK conditions for a duration of 6 h, the incipience of cytoplasmic agglomerations, consistent with endocytic vesicle formation, was noted in both Col-0 and the gpa1-3 mutant phenotype. This vesicular accumulation was augmented both in size and quantity after a 12-h continuation of K+ deficiency, concomitant with a discernible attenuation of ProPIN1: PIN1-GFP signal at the plasma membrane. Subsequent intervals (16 and 20 h) witnessed a regression of these endosomal structures, culminating in the complete absence of fluorescence at the 24-h mark. In stark contrast, the agb1-2, agg1-1c, agg2-1 and agg3-3 mutants did not exhibit significant alterations in PIN1 protein levels or its localization under analogous conditions (Figure 5a). These findings suggest a K+ deficit-induced promotion of PIN1 degradation, leading to a diminished presence within the plasma membrane, thereby modulating auxin gradients and impeding primary root elongation, which are positively regulated by AGB1 and AGGs.
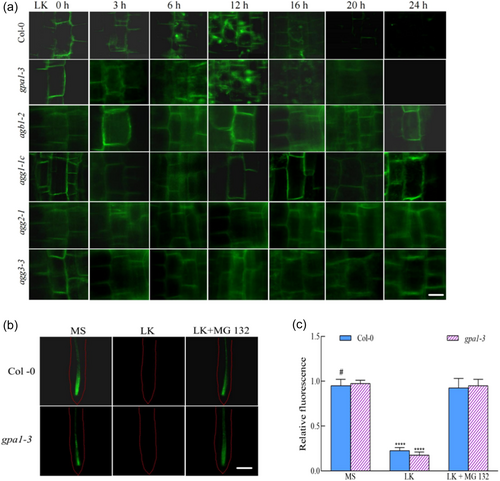
To elucidate the mechanism by which K+ scarcity precipitates a decrease in PIN1 protein levels, seedlings were subjected to the 26 S proteasome inhibitor MG132. Treatment with MG132 partially alleviated the inhibition of primary root growth under LK stress in both wild-type and gpa1-3 mutant (Supporting Information S1: Figure S10). Meanwhile, the fluorescence signal of PIN1 was retained (Figure 5b,c), and MG132 treatment partially inhibited the primary root growth of agb1-2, agg1-1c, agg2-1 and agg3-3 (Supporting Information S1: Figure S10). These observations infer that the G protein-regulated diminishment of PIN1 is partially reliant on the 26S proteasome pathway in the context of K+ deprivation.
3.6 G protein positively modulates low K+ stress inhibition of the PIN2 gene expression
During the assay of luminescence emanating from the PIN2 protein under protracted LK stress intervals, we discerned that PIN2 was initially localized on the plasma membrane lipid bilayer in MS medium (0 h). As the LK stress duration augmented, a pronounced decrement in the fluorescence of the PIN2-GFP fusion was observed in both the wild-type and gpa1-3 mutant phenotypes, yet no subcellular vesicular formations were detected within the root cells of wild-type and gpa1-3 mutant (Figure 6a). In contrast, the agb1-2, agg1-1c, agg2-1 and agg3-3 mutants did not exhibit significant decrements in PIN2 protein levels under LK conditions (Figure 6a). This observation diverges from the behaviour exhibited by the PIN1 protein (Figure 5a), insinuating the possible involvement of alternate pathways regulated by AGB1 and AGGs in the diminution of PIN2 protein abundance. Subsequent investigation utilizing the 26 S proteasome inhibitor MG132 did not ameliorate the luminescence of PIN2 in both the wild-type and the gpa1-3 mutant under LK stress (Figure 6b,c), in stark contrast to its effect on PIN1 fluorescence restoration (Figure 5b,c), substantiating the proposition that the molecular pathways by which AGB1 and AGGs govern the reduction of PIN1 and PIN2 under LK stress are distinct.
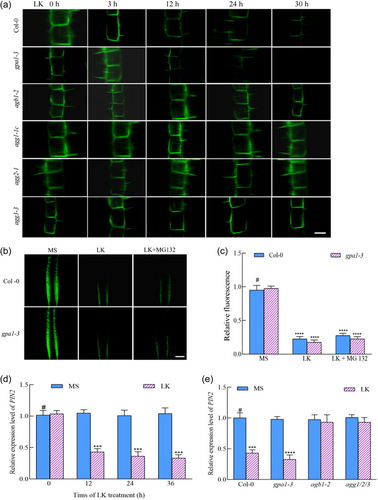
Inquiring into whether the attenuation of PIN2 protein was a consequence of transcriptional regulation, we employed qPCR to quantify PIN2 mRNA levels in wild-type roots at temporal milestones subsequent to LK treatment (T0, T12, T24, T36). The data demonstrated a significant transcriptional suppression of the PIN2 gene in wild-type plants post 12 h of LK exposure (Figure 6d). Further analysis of PIN2 mRNA levels in G protein mutants revealed a downregulation in both Col-0 and gpa1-3 mutant, a trend not observed in the agb1-2 and agg1/2/3 mutants post LK intervention (Figure 6e). These findings corroborate the hypothesis that the decrement in PIN2 protein under LK duress correlates with the transcriptional repression of its gene. The modulation of PIN2 protein expression on the plasma membrane via transcriptional downregulation orchestrates the auxin flux, thereby modulating its distribution and culminating in the inhibition of primary root elongation.
4 DISCUSSION
K+ is an indispensable macronutrient critical for the physiological and developmental processes of plants, exerting a pivotal function in the proper proliferation and maturation of root systems (Li, Yu, et al., 2017; Li, Wu, et al., 2017; Xu et al., 2006). Insufficient K+ concentrations can precipitate a cascade of deleterious phenotypic manifestations, including compromised photosynthetic efficiency, suppression of primary root elongation and chlorosis (Xu et al., 2006). G proteins are integral to cellular signal transduction and are known to mediate a spectrum of biotic and abiotic stress responses, including the modulation of root architecture (Booker et al., 2010; Boonyaves et al., 2022; Chen et al., 2006; Mudgil et al., 2009; Ullah et al., 2003; Urano & Jones, 2014; Wolfenstetter et al., 2015; Zhang, Xie, et al., 2021). The prevalence of K+-deficient substrates in terrestrial environments is a notable factor in the attenuation of both foliar senescence and root system development (Li, Yu, et al., 2017; Xu et al., 2006). Nonetheless, the involvement of G proteins in these phenomena remains to be elucidated. The present study definitively demonstrates the engagement of G proteins in the attenuation of radicular extension in the context of K+ paucity, while discounting their participation in the K+-deficiency-induced senescence of foliage. Within the framework of G protein signalling, signal perception by GPCRs or receptor-like kinases results in the dissociation of Gα from Gβγ subunits, and then Gα or Gβγ dimers can activate downstream signalling (Oldham & Hamm, 2008). The functionality of the Gγ subunit is closely interconnected with Gβ, as the Gβγ dimer operates as a unified signalling entity (Chakravorty et al., 2011; Ma, 1994; Mason & Botella, 2000, 2001; Weiss et al., 1994). Under LK conditions, gpa1 and agb1 mutants exhibit contrasting phenotypes in primary root growth, characterized by increased and decreased sensitivity, respectively, whereas the double mutant gpa1 agb1 exhibits a phenotype analogous to that of agb1 (Figure 1). In the gpa1 mutant, the absence of GPA1 results in a more active free Gβγ dimer, while in the agb1 mutant, the Gβγ dimer is lacking. Thus, our observation implies that AGB1, rather than GPA1, mediates the suppression of primary root growth under LK conditions. All three Gγ subunit mutants display insensitivity to primary root growth under LK, suggesting their roles in the LK response (Figure 1). Consequently, we infer that Gβγ dimers, rather than Gα, are involved in the integration or transmission of LK signal.
Extant literature on LK influence indicates a repressive effect on root elongation, correlating with diminished concentrations of the phytohormone auxin at the apical meristem (Li, Wu, et al., 2017). This reduction in auxin is attributable to the catabolism of the auxin transporter protein PIN1, thereby obstructing PIN1-dependent auxin efflux (Li, Wu, et al., 2017). Our investigation corroborates these observations (Figures 2-5) and further validates, with the application of the 26S proteasome pathway inhibitor MG132, that the proteolysis of PIN1 under LK conditions is mediated via the 26S proteasome route (Figure 5b,c). Additionally, we present evidence that the G protein βγ complex plays a role in the LK-induced degradation of PIN1 (Figures 4 and 5). Furthermore, the partially mitigated effect of MG132 on LK-suppressed primary root elongation in both wild-type and gpa1-3 mutant plants (Supporting Information S1: Figure S10) underscores the critical role of PIN1-mediated auxin efflux in primary root development. However, MG132 treatment not only partially enhanced the primary root growth in wild-type and gpa1-3 mutants but also partially inhibited root growth in agb1-2, agg1-1c, agg2-1 and agg3-3 mutants under LK conditions (Supporting Information S1: Figure S10). Given that MG132 inhibits the degradation of both auxin transporter proteins (Abas et al., 2006) and the auxin signalling components Aux/IAA within the TIR1/AFB-Aux/IAA coreceptor complex (Gray et al., 2001), these findings possibly suggest that the TIR1/AFB-Aux/IAA signalling pathway is indispensable for primary root growth in both wild-type and G protein mutants under LK conditions.
Upon the initiation of LK treatment, PIN1 exhibits an immediate response, with the fluorescence of ProPIN1:PIN1-GFP constructs vanishing within a 24-h period (Figures 4a,b and 5a). Although the fluorescence intensity of PIN2 is also diminished on the first day of treatment, its decline is not as precipitous as that of PIN1 (Figures 4c,d and 6a), suggesting a more gradual response to K+ scarcity. Nevertheless, this occurs before the observable reduction in auxin concentration at the root apex (Figure 3) and the ensuing inhibition of apical growth (Figure 1). Thus, we postulate that PIN2 contributes to the decrease in apical auxin concentration as well. Subsequent analysis revealed that akin to PIN1, PIN2 protein levels are reduced under LK (Figure 4c,d), albeit without intracellular accumulation (Figure 6a) and unresponsive to MG132 treatment (Figure 6b,c). However, transcriptional analyses of the PIN2 gene indicated that LK conditions suppress PIN2 mRNA expression (Figure 6d,e). Consequently, we surmise that G proteins modulate root development under LK primarily through the regulation of PIN1 and PIN2 at both the proteic and transcriptional tiers, thereby influencing auxin dynamics and radicular proliferation.
Precedent literature identifies the CBL-CIPK-AKT1 cascade as a critical regulator in both LK-induced root growth inhibition and leaf senescence (Li, Wu, et al., 2017; Luan et al., 2009; Xu et al., 2006). Here, our study indicates G protein βγ dimers specific participation in the LK-inhibited root growth but not in the LK-induced senescence of foliage, suggesting the same and different roles between G proteins and the CBL-CIPK-AKT1 cascade in response to LK stress. Similar to the role of G proteins in LK-inhibited root growth, AKT1 mediates LK-induced root growth inhibition also via promoting PIN1 degradation and subsequently modulating auxin redistribution in root tip (Li, Wu, et al., 2017). Although the involvements of both G protein and CBL-CIPK-AKT1 signalling modules in LK-inhibited root growth are clear, the nexus between them still remains unclear. In mammalian cells, it has been firmly established that G protein βγ dimers can directly interact with and activate inwardly rectifying K+ channels (K+in) (Dascal, 2001; Huang et al., 1995). In plant cells, preliminary pharmacological data reveal that G proteins participate in the inhibition of K+in current via membrane-delimited pathway in guard cells (Wu & Assmann, 1994). Later accumulated genetic evidence demonstrate that G proteins mediate multiple stimuli-inhibited K+in currents in guard cells (Chakravorty et al., 2011; Coursol et al., 2003; Fan et al., 2008; Wang et al., 2001; Zhang et al., 2008, 2012). Although the direct interaction between G proteins and K+in channels in plant cells remains speculative, we postulate two scenarios. First is the hypothesis that within the AKT1-CBL-CIPK cascade, AKT1 functions as a K+ deficiency sensor, possibly modulating the G protein-K+ channel interaction upon detecting low K+, thereby initiating G protein activation and consequent modulation of PIN protein concentration, ultimately influencing root growth. The mechanism by which AKT1 triggers G protein activation warrants additional inquiry. The alternative hypothesis posits that G proteins, along with their cognate receptors, detect low K+, instigating G protein activation. Subsequently, active G proteins may modulate K+ channel activity, which in turn impacts PIN1 protein abundance. The intricate regulatory mechanisms of this proposed pathway are yet to be elucidated.
Our investigation has elucidated a novel function of the Gβγ subunit in modulating the homoeostasis of PIN efflux carriers, specifically PIN1 and PIN2, during conditions of K+ deficiency. An outstanding query remains regarding the precise mechanisms by which Gβγ influences PIN1 proteolysis and PIN2 gene expression. Prior research has posited that heterotrimeric G proteins may exert a regulatory effect on the mitogen-activated protein kinase (MAPK) cascade, either through direct interaction or via intermediary pathways (Su et al., 2015; Xu et al., 2015). The activation of MAPK enzymes is known to catalyze the biosynthesis of ethylene and the generation of reactive oxygen species (ROS) under assorted stress stimuli (Han et al., 2010; Li et al., 2012; Zhang, Li, et al., 2021). Ethylene, in turn, enhances the synthesis of auxin and alters its spatial distribution by acting on auxin influx and efflux transporters (Růžička et al., 2007). Moreover, recent discoveries have indicated that MPK6 can directly bind to PIN1, facilitating its phosphorylation and subsequent proteolytic turnover (Dory et al., 2018; Jia et al., 2016), a phenomenon corroborated by the observed degradation of PIN1 protein in our study.
Advancements in the realm of plant resilience to K+ scarcity have been substantial, propelling forward our comprehension of the molecular underpinnings for K+ deficiency tolerance in model organisms. Our research contributes to this body of knowledge by demonstrating the involvement of G protein complexes in the modulation of auxin dynamics under K+-limited conditions. Such insights lay the groundwork for the development of crop varieties with improved K+ deficiency tolerance through precise manipulation of G protein pathways, potentially diminishing the agricultural reliance on K+-based fertilisers. Nevertheless, significant lacunae persist within the molecular framework of K+ deficit signal perception, transduction and the intricate signal network crosstalk in plants, designating these as pivotal research vectors in plant adaptive responses to K+ paucity.
ACKNOWLEDGEMENTS
The authors thank Drs. Yu-Zhou Zhang, Guo-Dong Wang and Guang-Hui Xiao for sharing materials. This study is supported by the National Natural Science Foundation of China (31870375 to J.-M. H.) and the Natural Science Basic Research Program Project of Shaanxi Province of China (2023-JC-QN-0179 and 2024JC-ZDXM-11 to N. M., J.-F. G., X. L. and J.-M. H.).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the supplementary material of this article.




