Function of plastid translation in plant temperature acclimation: Retrograde signalling or extraribosomal ‘moonlighting’ functions?
Summary Statement
Specific components of the plastid ribosome could act as pivotal limiting factors in plant temperature acclimation. We endeavour to elucidate the molecular nexus between plastid translation and temperature acclimation by incorporating the concept of extraribosomal ‘moonlighting’ functions of plastid ribosome proteins.
1 INTRODUCTION
The plastid in plants is believed to have resulted from an endosymbiotic event, therefore possessing its own translational apparatus known as the 70S ribosomes. The chloroplast 70S ribosome is intricately assembled from a 50S large subunit and a 30S small subunit. Each subunit encompasses a complement of bacterial-like ribosomal RNAs (rRNAs) and ribosomal proteins (designated as RPL for the 50S and RPS for the 30S, respectively) (Bieri et al., 2017; Yamaguchi & Subramanian, 2003). In recent years, the functions of plastid ribosome proteins (and plastid ribosome-associated proteins) have been investigated through reverse genetics in the model systems of tobacco and Arabidopsis (for instance, Fleischmann et al., 2011; Pesaresi et al., 2001; Romani et al., 2012). There is increasing genetic evidence to suggest that certain component of the plastid ribosome may serve as critical limiting factors in plant acclimation responses, particularly in response to heat and cold stress (Robles & Quesada, 2022). Moreover, several omics analyses have firmly established the critical role of plastid translation in adapting to fluctuating temperature conditions (Gao et al., 2022; Garcia-Molina et al., 2020; Lukoszek et al., 2016; Trösch et al., 2022; Zoschke & Bock, 2018). Some of these functions in acclimation responses can be attributed to the central role of chloroplasts in plant primary metabolism and protein synthesis in certain cases (Garcia-Molina et al., 2020; Zoschke & Bock, 2018). However, other studies suggest that the involvement of the plastid translation machinery in acclimation responses may be linked to specific signalling pathways associated with plastid translation, such as the well-known retrograde signalling pathway (Li et al., 2018; Yu et al., 2012). However, there are considerable challenges in identifying the nature of the signalling molecule(s) and reconstructing the complete signalling network. Here, we attempt to provide another comprehensive interpretation of this molecular connection by considering the concept of extraribosomal ‘moonlight’ functions of plastid ribosome proteins, drawing upon genetic, biochemical and bioinformatic knowledge.
2 FUNCTION OF PLASTID TRANSLATION IN TEMPERATURE ACCLIMATION
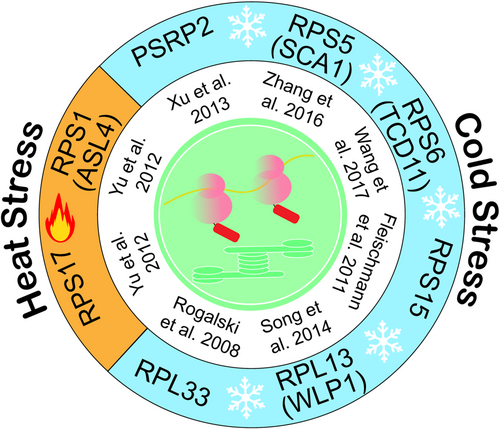
Temperature stress stands as a significant form of abiotic stress that plants must contend with (Ding & Yang, 2022; Kerbler & Wigge, 2023). Accumulating evidence indicates that various abiotic stresses are intricately linked to the functions of plant cell organelles such as chloroplast and mitochondrional (Zhu, 2016). The signals generated in response to stress within these organelles are orchestrated to modulate the expression of stress-responsive genes and other cellular activities to restore cellular homoeostasis (Zhu, 2016). The biogenesis and function of plastid translation apparatus are highly sensitive to temperature. For a long time, it has been observed that several ribosome-associated mutants, such as the ery-M2 mutant in Chlamydomonas reinhardtii (Hanson & Bogorad, 1978), the tigrina-O34 mutant in barley (Hoyerhansen & Casadoro, 1982), and the virescent mutants v16 and hcf7 in maize (Barkan, 1993; Hopkins & Elfman, 1984), exhibit temperature-dependent ribosome deficiencies (Barkan, 1993). Unlike maize, Arabidopsis is a chilling-tolerant plant; however, it becomes chilling-sensitive when there are defects in chloroplast ribosomal biogenesis and rRNA processing. In the Arabidopsis seedlings with impaired 16S rRNA methylase PFC1, normal growth is observed at 22°C, but chilling-induced chlorosis occurs at 5°C, indicating the involvement of PFC1 in chilling tolerance (Tokuhisa et al., 1998). The Arabidopsis mutant SVR3, which encodes a putative TypA-like translation elongation factor localized in the chloroplast, exhibits uniformly pale green leaves at 22°C and normal levels of plastid rRNA accumulation (Liu et al., 2010). However, when exposed to chilling temperatures (8°C), svr3 plants develop pronounced chlorosis accompanied by abnormal chloroplast rRNA processing and chloroplast protein accumulation (Liu et al., 2010). Additionally, mutations in plastid ribosome subunits themselves affect the response to cold stress. For example, disruption of a nonessential plastid ribosomal protein RPL33 in tobacco does not impact efficient translation under standard conditions, but it reduces the plants' ability to recover from prolonged chilling periods (Rogalski et al., 2008). Intriguingly, overexpression of the plastid RPS5 (referred to SCA1 in that study) improves plant cold tolerance; however, the underlying mechanism is yet to be determined (Zhang et al., 2016).
A genome-wide screen was conducted to identify genes that play a crucial role in chilling tolerance in Arabidopsis thaliana, using their hypothetical knockout mutants (Wang et al., 2016). It was observed that mutants in 16 genes, which encode proteins located within chloroplasts, had a significantly sensitive response to chilling (Wang et al., 2016). Notably, five of these mutants exhibited defects in the processing of the 23S rRNA, indicating the importance of normal chloroplast ribosome in chilling tolerance. Among these mutants, the rbd1 mutant displayed the most severe defect in the processing of 23S rRNA. RBD1 is a protein that contains an RNA-binding domain, which directly binds to 23S rRNA (Wang et al., 2016). Interestingly, this binding interaction is stronger under chilling conditions compared to normal growth temperatures. In Arabidopsis, the process of cold acclimation is partially mediated by the C repeat binding factors (CBFs), which regulate the expression of a large number of Cold Responsive (COR) genes (Park et al., 2015; Wang et al., 2016). Some of these COR genes are believed to confer freezing tolerance. However, the loss of RBD1 does not impact the expression of CBF or COR genes, suggesting that the chilling sensitivity observed in the rbd1 mutant is likely independent of the CBF pathway (Wang et al., 2016).
Chloroplasts are the primary targets of both cold and heat stress in plants. The heat stress response (HSR) in plants is highly conserved, involving various signalling and sensor molecules (Ohama et al., 2017; Rai et al., 2020). One crucial component of the HSR is the heat-shock protein (HSP)/chaperone. The expression of HSP genes is mainly regulated at the transcriptional level by heat shock transcription factors (HSFs), which recognize conserved cis-elements in HSP gene promoters. The heat-responsive protein, chloroplast ribosomal protein RPS1 (named as ASL4 in that study), has been identified in Arabidopsis. Knockdown of RPS1 expression in the rps1 mutant results in the loss of heat tolerance due to the elimination of the heat stress-activated expression of HsfA2 and its target genes (Yu et al., 2012). Overexpression of HsfA2 using a viral promoter in the rps1 mutant reestablishes lost heat tolerance, indicating that the participation of RPS1 in heat tolerance is HSFA2-dependent (Yu et al., 2012). Knockdown of RPS17 also reduces the heat-responsive expression of HsfA2, leading to a heat-sensitive phenotype in the rps17 mutant plants (Yu et al., 2012). However, the genetic relationship between RPS17 and HsfA2 has yet to be explored.
RPS15 and RPL33 are nonessential ribosomal proteins for plastid translation (Fleischmann et al., 2011; Rogalski et al., 2008). However, their deletion from the tobacco plastid genome together surprisingly leads to synthetic lethality under autotrophic conditions (Ehrnthaler et al., 2014; Robles & Quesada, 2022). Interestingly, this lethality can be rescued by growing the double mutants at elevated temperatures, which enhances ribosome biogenesis efficiency (Ehrnthaler et al., 2014). This suggests a complex relationship between temperature and ribosome biogenesis. It appears that higher temperatures may assist in the proper folding of rRNAs in the absence of RPS15 and RPL33 by melting incorrectly folded secondary structures. These structures would typically require the help of rRNA binding ribosomal proteins under normal temperatures (Ehrnthaler et al., 2014). However, the compensation of higher temperatures for the loss of ribosomal protein function has not been observed in other plastid ribosome subunits. In addition to the Arabidopsis and tobacco, plastid ribosome proteins and ribosome biogenesis factors involved in temperature acclimatation have been uncovered in crop plants such as rice (Song et al., 2014; Wang et al., 2017, 2022). All plastid ribosome proteins involved in cold and heat stress were summarized in Figure 1 while ribosome-associated factors including ribosome biogenesis and translation factors involved in temperature acclimation are listed in Table 1.
| Protein | Biochemical function | References | |
|---|---|---|---|
| Cold acclimation | PFC1 | 16S rRNA methylase | Tokuhisa et al. (1998) |
| SVR3 | TypA-like translation elongation | Liu et al. (2010) | |
| RBD1 | Protein bound to 23S rRNA | Wang et al. (2016) | |
| OsPUS1 | rRNA pseudouridine synthase | Wang et al. (2022) | |
| Heat acclimation | RABE1b | Translation elongation factor Tu | Li et al. (2018) |
3 THE RETROGRADE-SIGNALLING MODEL
The majority of components in the plastid translation machinery are crucial for chloroplast biogenesis, and consequently, for the survival of plants (Tiller & Bock, 2014). In numerous species, plastid translation is indispensable even under heterotrophic growth conditions, likely attributed to the necessity to express several vital plastid genes such as accD, clpP, ycf1 and ycf2 (Zoschke & Bock, 2018). Temperature serves as a significant abiotic signal influencing chloroplast translational regulation, with both heat and cold conditions leading to the downregulation of chloroplast translation activity by creating more nonfunctional or less functional secondary structures of rRNA (Wang et al., 2016) or inducing ribosome pausing (Grennan & Ort, 2007). Therefore, one could argue that the deficiencies in temperature acclimation observed in these mutants may be generally attributed to an impairment of chloroplast translation activity under stressful conditions.
However, upon closer examination of the behaviours of these mutants during temperature acclimation, the situations appear to be more intricate than initially interpreted. First, the chilling tolerance varies among different chloroplast ribosome-associated mutants, but this variance does not align well with changes in translation activities (Wang et al., 2016). Second, the constitutive overexpression of HsfA2 partially restored the heat tolerance of the rabe1b mutant, which exhibits a defect in the chloroplast translation elongation factor Tu (EF-Tu) (Li et al., 2018). Nevertheless, the rabe1b mutant's reduced growth and pale green leaves were not remedied (Li et al., 2018), indicating an uncouple between plastid translation capacity and heat tolerance to some extent. With reports of chloroplast ribosome dysfunction leading to changes in nuclear gene expression in various instances (Wang et al., 2022; Zou et al., 2020), it appears that, in addition to chloroplast translation itself, retrograde signalling derived from chloroplast translation may play a role in this process by regulating the expression of stress-responsive genes encoded by nuclear genomes.
Retrograde signalling refers to the process by which organelles utilize specific signalling molecules to communicate their status to the nucleus and, in turn, adjust nuclear gene expression (Chi et al., 2013; Richter et al., 2023; Wu & Bock, 2021). Research on two barley mutants, the albostrians and Saskatoon mutants of Hordeum vulgare L. cv. Haisa, which contain undifferentiated plastids lacking ribosomes, initiated research on retrograde signalling in higher plants (Bradbeer et al., 1979). These mutants exhibit reduced accumulation of a set of proteins encoded by photosynthesis-associated nuclear genes, including light-harvesting chlorophyll a/b-binding protein, the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase, and Calvin-Benson-cycle enzymes, indicating that a signal originating from plastids is involved in regulating nuclear gene expression (Bradbeer et al., 1979). Further studies revealed that this retrograde signal also initiates in plastids when plants are treated with chloramphenicol, which specifically blocks plastid gene translation (Oelmuller et al., 1986). Several studies have broadened the biological significance of this signalling pathway beyond chloroplast biogenesis to various plant stress response processes (Calderon & Strand, 2021; de Souza et al., 2017; Fernández & Strand, 2008; Richter et al., 2023).
Although the concept of the retrograde signalling has been conceived neatly 40 years before, many aspects of signalling pathways still seem to be obscure and lack concrete, mechanistic hypotheses. An open question remains regarding the components of the signalling pathway that function downstream of plastid translation and that control nuclear target genes, which generate the ultimate output of this signalling pathway. GUN1, a pentatricopeptide-repeat protein localized in the plastid, appears to integrate multiple retrograde signals, including the signal from the chloroplast translation apparatus, and regulate the expression of PhANGs (Chi et al., 2013; Koussevitzky et al., 2007; Wu & Bock, 2021). However, the mechanism through which GUN1 transmits the information from the plastid to the nucleus remains elusive, and there are ongoing debates surrounding the subcellar localization and biochemical feather underlying this process (Pesaresi & Kim, 2019). Conversely, the downstream target of this retrograde signalling pathway involved in heat stress tolerance appears to be not well defined. Overexpression of HsfA2 re-establishes the lost heat tolerance of rps1, indicating that HsfA2 is the downstream target of this retrograde signalling pathway (Yu et al., 2012). However, the mechanism connection between RPS1 and HsfA2 remains unclear.
There are several challenges to constructing a signalling transduction chain based on the canonical signalling pathway concept. One of the biggest obstacles is understanding the actual nature of the signals produced by plastid translation. For many years, there was little knowledge in this area. However, mounting evidence suggests that retrograde signals are metabolites such as tetrapyrroles, carotenoids, nucleotides, and isoprene precursors (Chan et al., 2016; Chi et al., 2015; Wu & Bock, 2021). If this is the case, what is the nature of the chemical molecules functioning as signals derived from the plastid translation apparatus? Keep in mind that no metabolites directly and specifically related to plastid translation have been uncovered yet. Currently, based on different approaches and experimental models, diverse retrograde signalling pathways derived from plastid translation are proposed (Calderon & Strand, 2021; Chan et al., 2016; Chi et al., 2015; Kleine & Leister, 2016; Richter et al., 2023). However, it appears to be challenging to reconstruct the signalling networks to match the distinct and specific defects of plastid translation.
In general, the retrograde-signalling model provides an appealing hypothesis for explaining the role of plastid translation in acclimation. However, while some transduction components have been identified, their connection is not yet well-established based on the current evidence. In the following section, we will explore the mechanism of plastid translation in plant acclimation from the perspective of the extraribosomal moonlight functions of ribosomal proteins.
4 EXTRARIBOSOMAL FUNCTION OF RIBOSOMAL PROTEINS
The concept of extraribosomal function of ribosomal proteins was first proposed in 1974 when the interaction between a bacteriophage protein Qβ and three bacterial host proteins, namely translation factors EFTu and EFTs, as well as 30S ribosomal subunit S1, was discovered (Wahba et al., 1974). This interaction allowed the protein S1 to function as an RNA replicase (Wahba et al., 1974). This hypothesis gained further support from the study of S10, a component of the NUS complex, which was found to be involved in transcript elongation in phage-infected Escherichia coli (Mason & Greenblatt, 1991; Warner & McIntosh, 2009). It is now widely accepted that numerous ribosomal proteins possess additional ‘moonlighting’ functions beyond their roles in translation (Aseev & Boni, 2011; Weisberg, 2008). To evaluate whether a ribosomal protein functions in an extraribosomal capacity, three criteria are typically examined: (1) the specific interaction of the ribosomal protein with nonribosomal components of the cell, such as RNA, DNA, proteins, or metabolites; (2) the physiological impact of this interaction on the living cell; and (3) the independence of the interaction and its physiological effects from the ribosome machinery (Warner & McIntosh, 2009; Xiong et al., 2021).
Increasing evidence indicates that extraribosomal functions of ribosomal proteins are widespread and involved in distinct biological processes. By utilizing the RNA/DNA binding characteristics of ribosomal proteins, they can maintain a balance between the synthesis of individual ribosomal proteins, rRNA, and other proteins (Warner & McIntosh, 2009). This autoregulation of ribosomal proteins is commonly observed in eubacterial cells and has also been documented in several cases of eukaryotic cells. Although this represents an extraribosomal function, it is still considered part of the ribosome system. In fact, ribosomal proteins can act as sentinels, alerting the cell to defects in ribosomal assembly, which is essential for the cell cycle and apoptosis (Lessard et al., 2019; Xu et al., 2016). This extraribosomal function is known as the ribosomal (nucleolar) stress response and is considered a genuine extraribosomal function that has evolved in vertebrates (Lessard et al., 2019; Xu et al., 2016). On the other hand, increasing evidence suggests that the extraribosomal capacity of ribosomal proteins is utilized in many biological processes that are unrelated to the ribosome or its synthesis (Aseev & Boni, 2011; Weisberg, 2008). These extraribosomal functions may explain why deficiencies in ribosomal proteins result in complex phenotypes during development, ranging from lethality and growth retardation to alterations in cell development and morphological abnormalities. These diverse outcomes are not only caused by insufficient ribosome production, which affects protein production specifically or nonspecifically, but also by the independent regulation of diverse cellular processes through distinct extraribosomal functions.
5 DO PLASTID RIBOSOMAL PROTEINS HAVE EXTRARIBOSOMAL FUNCTIONS?
Less attention has been given to whether plastid ribosomal proteins truly have extraribosomal functions, as has been demonstrated for cytoplasmic ribosomal proteins (Xiong et al., 2021). However, a few studies suggest that several plastid ribosomal proteins are capable of forming complexes independent of the ribosomal machine within chloroplasts, thus meeting one of the three criteria for extraribosomal functions. The earliest evidence came from the discovery by Zhou and Mache (1989) of a substantial pool of plastid ribosomal protein RPS5 in the stroma of chloroplasts. This pool of RPS5 is not associated with plastid ribosome fractions and is synthesized very early in the development of spinach seeds, before the synthesis of other ribosomal proteins. However, the significance of this pool of RPS5 remains to be determined.
The study of Trifa et al. (1998) found that RPL4 protein co-purified with the transcription factor CDF2 which plays a role in the regulation of plastid rDNA transcription. Given that RPL4 inhibits the transcription of the S10 operon by transcriptional interference via an attenuation mechanism in prokaryotes, it seems to be expected that plastid RPL4 has the similar extraribosomal function. Indeed, RPL4 protein can replace the extraribosomal function of E. coli L4 protein, that is the NusA-dependent attenuation control of the E. coli S10 operon. In addition, the RPL4 inhibits transcription of the plastid rrn operon in vitro (Trifa et al., 1998). Although it has not been confirmed in vivo, this result suggests extra-ribosomal function(s) for RPL4 in the expression of ribosomal components.
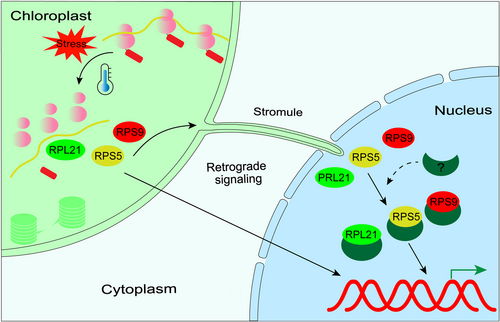
If the mentioned plastid ribosome proteins indeed possess extraribosomal functions as proposed, it is likely that these functions are limited to within the chloroplasts. Could it be possible that plastid ribosome proteins also have extraribosomal functions outside of the plastid compartment? If so, it would anticipate that these plastids ribosomal proteins accumulate in other subcellular compartments. It is interesting to note that a GFP assay revealed the localization of the RPS9/LEM1 protein of maize in both the nucleus and plastids (Ma & Dooner, 2004). The loss of RPS9 results in early embryo aborts. However, not all mutations in plastid ribosomal proteins exhibit the same phenotype. Moreover, a decreased translation rate in plastids does not always lead to an embryo-lethal phenotype. Therefore, the embryo lethality in lem1 has been attributed to an extra-ribosomal function necessary for embryogenesis rather than a general compromise in plastid protein synthesis caused by the absence of ribosome subunits (Ma & Dooner, 2004). It is the first reported instance suggesting the presence of extra-ribosomal functions of plastid ribosome proteins outside of plastids but no subsequent research follow these finding, nevertheless, an independent study using proteomics assays identified the chloroplast RPS9 protein from Arabidopsis in the nucleus (Sakamoto & Takagi, 2013), further verifying those observations. As well as RPS9, other PRPs have also been detected in the nucleus in published nuclear proteomes. For instance, Bae et al. (2003) reported the detection of RPS5 and a protein identified as a homologue of RPL21 (named as ALS2 in that study) within a whole leaf tissue nuclear protein extract. Goto et al. (2019) reported the presence of RPS5, RPS17 and RPL21 in the proteome extracted from cultured cell nuclei, while Sakamoto and Takagi (2013) reported the presence of 24 plastid ribosome proteins in the proteome of protoplast nucleus. Although the dual-localization of these ribosomal proteins needs further verification by other experimental approaches, this raises the possibility that these plastid ribosome proteins are able to fulfil their extraribosomal roles in the nucleus. Interestingly, the presence of RPS5 and RPL21 in the nucleus was only detected after the application of cold stress (Bae et al., 2003), which is consistent with the findings that mutations of some plastid ribosomal proteins affect plant development only under stressful conditions.
How are plastid ribosome proteins targeted to nuclear compartments? Dual distribution between subcellular compartments can be achieved through three general strategies. The first involves a single gene producing two protein variants with different subcellular targeting signals due to alternative transcription or translation initiation sites (Thatcher et al., 2007). The second mechanism involves a single polypeptide harbouring two targeting signals: a nuclear localization signal and an organellar targeting signal (Vongsamphanh et al., 2001). The third mechanism entails plastid-to-nucleus retrograde protein translocation, in which the dual-localization protein is first localized and proteolytically processed in the plastids before being relocated to the nucleus (Krause & Krupinska, 2009; Krause et al., 2012). It is worth noting that the third strategy has been validated for several chloroplast proteins, including Whirly, HEMERA, and RCB (Chen et al., 2010; Grabowski et al., 2008; Nevarez et al., 2017; Yoo et al., 2019). This strategy may represent the primary mechanism for the dual distribution of chloroplast proteins (Krause & Krupinska, 2009; Krause et al., 2012).
Utilizing RPS9 and RPS5 as illustrative cases of putative dual-targeted chloroplast ribosomal proteins-as previously discussed, our investigation of the public databases, including Arabidopsis.org and the Protein Prediction Database at Cornell University (PPDB), did not uncover any evidence of alternative transcription start sites or nuclear localization signals. This absence of identifiable signals implies that RPS9 and RPS5 may not be utilizing the first or secondary mechanisms associated with dual-targeting. Additionally, the nuclear localization of RPS5 was detected in the absence of the N-terminal signal sequence (Dupouy et al., 2022). Consequently, it is very likely that the nuclear localization of plastid ribosome proteins may predominantly employ the third strategy, though other mechanisms cannot be entirely ruled out.
The mechanism of retrograde translocation is still largely unknown but several possible pathways have been proposed in the past: stromule tip shedding, membrane contacts and vesicle budding, and channels or retrograde protein transporters (Krause et al., 2012). Additionally, it is plausible that the initiation of reactive oxygen species (ROS) production under stress conditions could result in the permeabilization of chloroplast membranes (Kim et al., 2012), facilitating the release of chloroplast proteins-a process analogous to what is observed in mitochondria (Joza et al., 2001). If ribosome-free ribosome proteins can indeed be transported from plastids to the nucleus, they represent promising candidates for plastid-to-nucleus communication. In contrast to other components involved in retrograde signalling (such as intermediates of plastid metabolic pathways), proteins involved in gene regulation could directly mediate changes in gene expression. The release of proteins from plastids and their subsequent translocation to the nucleus could enable a rapid response to changes in plastids triggered by specific factors.
If some ribosome proteins can really be relocated from plastids to nucleus, what is the intrinsic trigger for the release of ribosomal proteins from ribosome machinery in plastids? For ribosome synthesis, stoichiometry is essential, requiring the equimolar production of rRNA and ribosomal proteins (Emmott et al., 2019). As mentioned above, the processing of plastid rRNA is very sensitive to environmental stress, especially temperature stress, which often hampers the assembly of plastid ribosomal proteins with rRNA molecules (Zhang et al., 2016). Additionally, a defect in plastid rRNA processing appears to be the most significant secondary effect when normal plastid gene expression and/or chloroplast development is impaired, which also hampers plastid ribosome assembly to varying degrees (Ma et al., 2015). Under such conditions, an imbalance between ribosomal proteins and rRNAs leads to the accumulation of ribosome-free ribosomal proteins, which might subsequently trigger the movement of plastid ribosome proteins. We propose that this operational mechanism of plastid ribosomal proteins might be conceptually similar to nucleolar stress or ribosomal stress response, a quality control surveillance mechanism that monitors the synthesis and assembly of the rRNA and protein components of ribosomes via ribosomal proteins translocated from the nucleolus to the nucleoplasm in animal cells (Lessard et al., 2019).
6 CONCLUSION AND PERSPECTIVES
The findings presented in this review emphasize the significance of plastid translation in various aspects of the response to unfavourable environmental conditions. Despite significant advancements in recent years, our understanding of the molecular connections between each plastid subunit or plastid ribosome-associated protein and its specific biological processes remains limited. While retrograde signalling has long been considered the key explanation for the specificity of those mutants, it is conceivable that the specific properties of plastid ribosome proteins may also arise from their presence in locations beyond chloroplasts and/or their involvement in extraribosomal functions (Robles & Quesada, 2022). This parallel can be drawn from the discoveries concerning cytoplasmic ribosomes. Regrettably, this aspect has received less attention, although some evidence supporting this hypothesis has come to light.
We here propose an extraribosomal function model for plastid ribosomal proteins, as illustrated in Figure 2. When the assembly of plastid ribosomes is disrupted due to environmental stress certain ribosomal proteins may detach from the ribosome machinery and relocate from plastids to the nucleus. Once in the nucleus, these ribosomal proteins directly regulate gene expression by interacting with DNA-binding and/or transcription factor partners. In this capacity, the free plastid ribosomal proteins serve as sentinels, detecting changes within the chloroplasts. Different relocalized ribosome proteins have specific targets in the nucleus, thereby explaining the diverse phenotypes observed in plastid-ribosome mutants during environmental acclimation. Additionally, apart from ribosomal proteins and rRNA processing factors directly involved in ribosome biogenesis, translation elongation factors have been discovered to aid in ribosome assembly (Hang et al., 2023; Li et al., 2010). Hence, the model centred on ribosome stress response also aligns with the involvement of translation elongation factors in temperature acclimation. The extraribosomal function model does not exclude the possibility of its coexistence with the retrograde signalling model, as free ribosome proteins can potentially serve as signalling components in retrograde signalling pathways. To date, no metabolites that are directly and specifically derived from plastid translation and capable of migrating to the nucleus have been identified. However, within the extraribosomal model, free ribosome proteins are posited to fulfil this role effectively. One of the major challenges for the extraribosomal function model lies in understanding the mechanism by which proteins move from organelles to the nucleus. Another challenge is to identify the complex(es) associated with plastid ribosome proteins in the nucleus and elucidate their direct link to plant acclimation.
ACKNOWLEDGEMENTS
This study was supported by the National Key Research and Development Program of China (grant number: 2020YFA0907600), the Nature Science Foundation of China (grant number: 32070263) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). We would like to thank Reimo Zoschke of Max Planck Institute of Molecular Plant Physiology and Guozhang Wu of Shanghai Jiaotong University for critical reading of the early version of our manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




