Molecular mechanisms underlying natural deficient and ultraviolet-induced accumulation of anthocyanin in the peel of ‘Jinxiu’ peach
Abstract
Peach varieties that differ in red coloration due to varied anthocyanin accumulation result from transcriptional regulation by PpMYB10s, a group of specific R2R3 MYBs. Here we investigated the mechanisms driving a lack of anthocyanin in yellow-skinned ‘Jinxiu’ peach peel, as well as accumulation induced by UV irradiance. It was found that PpMYB10.1, PpMYB10.2 and PpMYB10.3 were positive regulators of anthocyanin accumulation, but the stimulation by PpMYB10.2 was weak. Low expression of PpMYB10.1 causes natural anthocyanin deficiency in ‘Jinxiu’ peel. However, the promoter sequences of PpMYB10.1 were identical in ‘Jinxiu’ and a naturally red-coloured peach ‘Hujingmilu’. Therefore, potential negative regulator(s) upstream of PpMYB10.1 were explored. A novel R2R3-MYB repressor termed PpMYB80 was identified through comparative transcriptomic analysis and then functionally confirmed via transiently overexpressing and silencing in peach fruit, as well as transformation in tobacco. PpMYB80 directly binds to the promoter of PpMYB10.1 and inhibits its expression, but does not affect PpMYB10.3. In UV-exposed ‘Jinxiu’ fruit, expression of PpMYB10.3 was upregulated, while PpMYB10.1 remained low and PpMYB80 enhanced, which results in accumulation of anthocyanin in peel. This study revealed a transcriptional cascade involving PpMYB activators and repressors in regulating basal and UV-induced anthocyanin accumulation in peach peel.
1 INTRODUCTION
Anthocyanins are a class of important secondary metabolites in plants and contribute to the red coloration for various fruits, including peach, as well as providing various nutritional benefits to consumers (Sun et al., 2023). Anthocyanins are also important for plant life, playing essential roles in protecting against environmental stresses and attracting pollinators (Sun et al., 2023).
The anthocyanin biosynthetic pathway is highly conserved among plant species, involving a series of enzymes, including phenylalanine ammonia lyase, chalcone synthase (CHS), chalcone isomerase (CHI), flavanone 3-hydroxylase, flavonoid 3′-hydroxylase, flavonoid 3′,5′-hydroxylase, dihydroflavonol 4-reductase, anthocyanin synthase (ANS), and UDP-glucose flavonoid 3-O-glucosyltransferase (UFGT) (Winkel-Shirley, 2001). After synthesis in the cytosol, anthocyanins are transported into vacuoles via glutathione S-transferase (GST), as well as other putative transporters (Francisco et al., 2013; Zhao et al., 2020).
The MYB-bHLH-WDR (MBW) complex, comprising R2R3-MYB transcription factors (TFs), basic Helix-Loop-Helix (bHLH) TFs and WD40-repeat proteins, transcriptionally regulates these anthocyanin biosynthetic/transport genes (Ramsay & Glover, 2005). MYB TFs, the core factors of the MBW complex, have been extensively studied in plants, for example, AtPAP1/AtPAP2 in Arabidopsis (Borevitz et al., 2000), MdMYBA/1/10 and MdMYB110a in apple (Ban et al., 2007; Chagné et al., 2013; Espley et al., 2007; Takos et al., 2006) and FaMYB10 in strawberry (Medina-Puche et al., 2014). Some anthocyanin-regulating MYB TFs are encoded by tandemly duplicated gene clusters as reported in grape (Matus et al., 2017; Walker et al., 2007), orange (Huang et al., 2018), Chinese bayberry (Xue et al., 2024) and carrot (Duan et al., 2023). In peach, two gene clusters, that is, PpMYB10.1/PpMYB10.2/PpMYB10.3 located on chromosome (Chr) 3 and PpMYB10.4/PpMYB10.5/PpMYB10.6 on Chr 6, have been reported (Zhou et al., 2014).
In addition to the MYB activators, MYB repressors are also involved in the regulation of anthocyanin accumulation, such as AtMYB4/AtMYBL2 in Arabidopsis (Dubos et al., 2008; Jin et al., 2000), FaMYB1 in strawberry (Aharoni et al., 2001), MdMYB16/MdMYB17 in apple (Lin-Wang et al., 2011) and PpMYB18 in peach (Zhou et al., 2019). These MYB repressors usually exhibit suppressive effect by either directly binding to the promoters of anthocyanin biosynthetic genes or interfering with MBW complex formation (Chen et al., 2019).
Anthocyanin accumulation varies extensively not only in different plants but also between plant varieties. Certain domesticated fruit cultivars gain anthocyanin accumulation ability in fruit or fruit parts because of enhanced expression of MYB activators, such as in red-fleshed apple and blood orange owing to mutations in MdMYB10 and Ruby promoters, respectively (Butelli et al., 2012; Espley et al., 2009). On the other hand, anthocyanin deficiency causing the nonred appearance in fruits often involves either loss-of-function mutations of activators (Castillejo et al., 2020; Kobayashi et al., 2004; Niu et al., 2010), decreased expression of activators (Zhao et al., 2020) or enhanced expression of repressors (Ding et al., 2021; Jiang et al., 2017).
Anthocyanin accumulation is not only genetically regulated but also influenced by various environmental factors, such as temperature, light and stress (Espley & Jaakola, 2023). Light is essential for plants to synthesize anthocyanin (Hu et al., 2021; Yang et al., 2019). Compared with white light, ultraviolet (UV) lights usually result in a more profound effect on anthocyanin accumulation (Fang et al., 2019; Henry-Kirk et al., 2018). In peach, different cultivars showed differential sensitivity to light as well, where both UVA and UVB induced anthocyanin accumulation of cv. ‘Hujingmilu’ (HJML), whereas cv. ‘Yulu’ was only sensitive to UVB (Zhao et al., 2017, 2021).
Peach varieties differ extensively in red coloration due to anthocyanin accumulation. For example, ‘Jinxiu’, a peach cultivar with superior quality in taste and aroma, is deficient in anthocyanin accumulation in peel, restricting, to some extent, consumer preference. Currently, the underlying mechanism for a lack of anthocyanin in peel is not clear. In this study, we concentrated on the mechanisms behind the natural deficient and UV-regulated accumulation of anthocyanin in ‘Jinxiu’ peel by evaluating the expression patterns and functions of anthocyanin-related PpMYB10 gene cluster members. PpMYB80, a novel R2R3-MYB anthocyanin repressor, was identified and functionally characterized. Our study revealed the molecular mechanisms behind natural and UV-induced anthocyanin accumulation in peach peel, which relied on a TF cascade of PpMYB activators and repressors.
2 MATERIALS AND METHODS
2.1 Plant materials and treatment
Fruit, at commercial harvest maturity, of peach (Prunus persica) cv. ‘Jinxiu’ and cv. ‘HJML’ were obtained from an orchard in Jiaxing. For the comparative experiment involving different fruit tissues of ‘Jinxiu’ and ‘HJML’, nonbagged fruits were divided into three parts for sampling, that is, peel (P), outer flesh near the peel (OF) and inner flesh around the stone (IF). For UV treatment experiment, ‘Jinxiu’ fruit bagged with commercial yellow paper bags at 67 days after full-blossom (DAFB), were harvested at 142 DAFB. The fruit then were irradiated with UVA (320–400 nm, 800 µw/cm2) or UVB (280–320 nm, 40 µw/cm2) or both together for 4 days in a growth chamber at 20°C, and the fruit placed in the dark were used as the control. Five fruit were used in each of the three replicates. Peach fruit of cultivars ‘Zhushahongxuetao’ at colour turn stage and ‘Yanhong’ at commercial harvest maturity, involved in transiently overexpressing and silencing, respectively, were collected from commercial orchards in Nanyang and Lishui.
2.2 Anthocyanin content measurement
The method of anthocyanin extraction from peach fruit and tobacco was as described previously (Xue et al., 2024). High-performance liquid chromatography analysis was performed to quantify anthocyanin using a Waters Alliance 2695 system (Waters Corp.) equipped with a reverse-phase C18 column (4.6 × 250 mm, 5 μm; YMC Co., Ltd). The chromatographic protocol followed a previous study (Cheng et al., 2014). Cyanidin 3-O-glucoside (C3G) chloride was used as a standard and anthocyanins were quantified as C3G equivalents.
2.3 DNA, RNA extraction and reverse-transcription quantitative polymerase chain reaction (RT-qPCR) analysis
The extraction of genomic DNA (gDNA) (Chen et al., 2004) and total RNA (Chang et al., 1993) was according to improved cetyltrimethylammonium bromide protocols. HiScript® II Q RT SuperMix for qPCR (+gDNA wiper) (Vazyme) was used to remove gDNA contamination and synthesize first-strand complementary DNA (cDNA). RT-qPCR was performed with ChamQ Universal SYBR qPCR Master Mix (Vazyme) using a CFX96 equipment (Bio-Rad) and the primers were shown in Supporting Information S1: Table 1. PpTEF2 (GenBank accession No. JQ732180) and NtEF1α (GenBank accession No. NM001326165) were selected as internal controls to normalize gene expression levels of peach and tobacco, respectively.
2.4 RNA sequencing (RNA-seq)
RNA libraries were prepared by staff at Novogene Bioinformatics Technology Co. Ltd. and sequenced using an Illumina NovaSeq. 6000 (Illumina) platform. Raw data filtration and mapping followed the protocol previously described (Zhao et al., 2017). The FPKM (expected number of fragments per kilobase of transcript sequence per millions base pairs sequenced) was used to estimate the gene expression level. The differentially expressed genes (DEGs) were identified using DESeq. 2 with the selection criteria: |log2(FoldChange)| ≥ 1 and padj ≤ 0.05.
2.5 Gene cloning and vector construction
gDNA or cDNA were used as templates for sequence amplification with Phanta Max Super-Fidelity DNA Polymerase (Vazyme). PCR products were inserted into various vectors (Supporting Information S1: Table 2) using ClonExpress II One Step Cloning Kit (Vazyme). Primers for gene cloning and vector construction were listed in Supporting Information S1: Table 2.
2.6 Tobacco (Nicotiana tabacum) stable transformation
The pSAK277 vectors containing individual target genes were introduced into Agrobacterium tumefaciens strain EHA105. Leaf disc cocultivation method (Horsch et al., 1985) was used to perform transformation in tobacco. The petals of full bloom flowers of transgenic lines were sampled for molecular and phenotypic analysis, and wild-type (WT) plant acted as a control.
2.7 Transient colour assay in tobacco
The A. tumefaciens strain EHA105 cultures harbouring the target genes constructed on pSAK277 vectors were suspended in infiltration buffer (10 mM MES, pH 5.6; 10 mM MgCl2; 150 mM acetosyringone) and adjusted to an OD600 of 1.0. The cultures were injected into tobacco leaves, and the infiltrated areas were sampled at 4, 7 or 14 days after infiltration.
2.8 Transient overexpression and virus-induced gene silencing in peach fruit
The full-length coding sequence and a cDNA fragment of PpMYB80 (1–300 bp from the start codon) were cloned into pGreenII 62-SK vector (Hellens et al., 2005) and pTRV2 vector, respectively. Each construct was electroporated into the A. tumefaciens strain GV3101. The bacteria were incubated at 28°C until the OD600 reached about 0.75–1.0, and the infiltration buffer was prepared as that described for the transient colour assay. For transient overexpression, the bacteria cultures with PpMYB80-SK were infiltrated into ‘Zhushahongxuetao’ peach fruit and cultures with empty pGreenII 62-SK vector were used as the negative control. For silencing PpMYB80, cultures with pTRV1 and either pTRV2 or PpMYB80-pTRV2 were mixed (1:1, v/v) and injected into ‘Yanhong’ peach fruit. The infiltrated fruits were placed in the dark overnight, then transferred into a growth incubator at 22°C for 4 days. The peel of ‘Zhushahongxuetao’ and the flesh of ‘Yanhong’ around infiltrated sites were sampled at 4 days after infiltration.
2.9 Subcellular localization
PpMYB80 coding sequence was cloned into pCAMBIA2300-eGFP vector and electroporated into the A. tumefaciens strain GV3101, then transiently overexpressing in mCherry-NLS transgenic Nicotiana benthamiana leaves. Infiltration protocol followed as described for the transient colour assay. After 2 days, the infiltrated leaves were detected for eGFP and mCherry fluorescence signals by confocal laser scanning (Nikon A1-SHS).
2.10 Dual-luciferase assay
The coding sequences of each TF and the promoter sequences of target genes were cloned into pGreenII 62-SK vectors and pGreenII 0800-LUC vectors, respectively (Hellens et al., 2005). All constructs were introduced into the A. tumefaciens strain GV3101. The infiltration buffer was described in transient colour assay and the OD600 value was adjusted to 0.75. N. benthamiana leaves were injected with a ratio of 10:1 mixture of TFs and promoters. After 3 days, the ratio of firefly luciferase (LUC) to Renilla luciferase was measured using the dual-luciferase assay kit (Promega) by the GLOMAX 96 Microplate Luminometer (Promega).
2.11 Yeast one-hybrid assay (Y1H)
The promoter sequences of PpMYB10.1/10.2/10.3 and the coding sequence of PpMYB80 were cloned into pAbAi vector and pGADT7 vector, respectively. These constructs were transformed into Y1HGold yeast strain using the MatchmakerTM Gold Yeast One-Hybrid Library Screening System (Clontech). Interaction analysis was screened on the SD/-Leu plates containing aureobasidin A (AbA) and ProPpMYB10.1/10.2/10.3-pAbAi plus empty pGADT7 vector acted as a negative control.
2.12 Electrophoretic mobility shift assay (EMSA)
The coding sequence of PpMYB80 were constructed into pGEX-4T-1 and transformed into Escherichia coli Rosetta (DE3). The recombinant protein was induced by 500 μM β-D-1-thiogalactopyranoside at 16°C for 20 h and purified using the GST-tag Protein Purification Kit (Beyotime). The EMSA was performed using the Chemiluminescent EMSA Kit (Beyotime). The sequences of probes for EMSA were listed in Supporting Information S1: Table 2.
2.13 Yeast two-hybrid assay (Y2H)
The CDS regions of PpMYB10.1, PpMYB10.2 and PpMYB10.3 were individually cloned into pGADT7 vector and PpbHLH3 was cloned into pGBKT7 vector. Each recombinant pGADT7 plus PpbHLH3-pGBKT7 were co-transformed into Y2HGold Chemically Competent Cell (Weidi). pGADT7-T plus pGBKT7-53, as the positive control, as well as pGADT7-T plus pGBKT7-lam and pGADT7-T plus PpbHLH3-pGBKT7, as negative control combinations, were included. The double dropout supplements medium (SD/-Trp/-Leu) was used to test whether the two plasmids were transformed into Y2HGold yeast cell and quadruple dropout supplements (QDO) medium (SD/-Trp-Leu-His-Ade) supplemented with X-α-Gal was used to screen for protein–protein interactions.
2.14 Firefly luciferase complementation imaging assay (LCI)
The CDS regions of PpMYB10.1, PpMYB10.2, PpMYB10.3 and PpbHLH3 were separately cloned into the pCAMBIA1300-nLUC (nLUC) and pCAMBIA1300-cLUC (cLUC) vectors and transformed into A. tumefaciens strain EHA105. Suspensions were prepared as described for transient colour assay, and N. benthamiana leaves were injected with a ratio of 1:1 mixture of nLUC and cLUC. Two days after infiltration, 0.2 mM luciferin was infiltrated into the leaves and the leaves were kept in the dark for 20 min, and then the luciferase activities were detected by NightSHADE LB 985 (Berthold).
2.15 Phylogenetic, cis-element and statistical analysis
Phylogenetic analysis was constructed using MEGA 6 with the neighbour-joining approach and multiple protein sequence alignments were performed by MUSCLE (Hall, 2013), and the information of MYBs was shown in Supporting Information S1: Table 3. The cis-elements analysis was performed using PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/), New PLACE database (https://www.dna.affrc.go.jp/PLACE/?action=newplace) and JASPAR database (http://jaspar.genereg.net/). Data were analysed by one-way analysis of variance and Student's t test using IBM SPSS Statistics 23 (IBM Corp.). The figures were drawn by GraphPad Prism 8.0.2 (GraphPad Software) and TBtools (Chen et al., 2020).
3 RESULTS
3.1 Expression patterns of PpMYB10 cluster members in different fruit tissues of two peach cultivars
Anthocyanin accumulation differed extensively between varieties and fruit tissues. In ‘Jinxiu’, the peel and the OF showed an absence of red coloration, while the IF was deeply coloured (Figure 1a). In 'HJML', red pigmentation was observed in all three fruit tissues (Figure 1a). Consistently, the anthocyanin content in ‘Jinxiu’ peel was low, only 3% and 5% of ‘Jinxiu’ IF and ‘HJML’ peel, respectively. Anthocyanin was not detectable in OF of ‘Jinxiu’, whereas OF of ‘HJML’ accumulated moderate anthocyanin, 0.32 mg/100 g FW (Figure 1b).
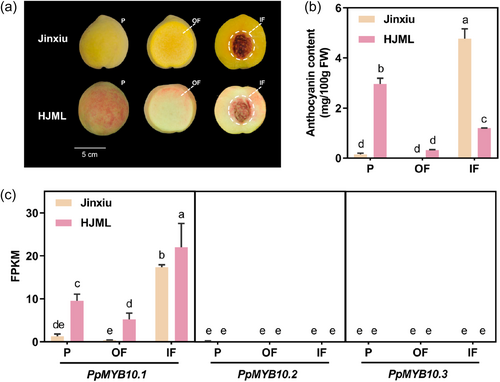
The peel, OF and IF of two peach cultivars were taken for RNA-seq and the data were available at NCBI PRJNA1103001. The RNA-seq data were of high quality (Supporting Information S1: Table 4), with data further validated using RT-qPCR analysis (Supporting Information S1: Figure 1). PpMYB10.1 was highly expressed in IF of ‘Jinxiu’ and ‘HJML’, as well as the peel of ‘HJML’, whereas it was lowly expressed in ‘Jinxiu’ peel, accounting for only 7%, 5%, and 13% of the former, respectively (Figure 1c). The expression of PpMYB10.2 and PpMYB10.3 in all three fruit tissues of ‘Jinxiu’ and ‘HJML’ was either undetectable or very weak (Figure 1c). Besides, the transcript of PpMYB10.4/10.5/10.6 was not detected in any fruit tissue. Therefore, the lack of anthocyanin in ‘Jinxiu’ peel was owing to the significantly low expression of PpMYB10.1.
3.2 Identification of candidate regulator(s) upstream of PpMYB10.1
The sequence of PpMYB10.1 promoter, 2074 bp upstream of the start codon ATG, was consistent between ‘Jinxiu’ and ‘HJML’. Various cis-acting elements in PpMYB10.1 promoter, such as light-responsive elements, hormone-responsive elements and TF-binding sites, were predicted (Supporting Information S1: Figure 2). To explore whether negative regulator(s) were involved in the inhibition of PpMYB10.1 expression in ‘Jinxiu’, we performed a comparative transcriptome analysis between different fruit tissues of ‘Jinxiu’ and between the peel of ‘Jinxiu’ and ‘HJML’. A total of 6500 genes were differentially expressed in the four comparative combinations (Figure 2a). To screen the candidate genes associated with anthocyanin accumulation, the intersection of four comparative combinations was analysed. A total of 252 DEGs were harboured in this intersection, including several anthocyanin biosynthetic genes and the core regulatory gene PpMYB10.1, as well as 14 potential TFs (Figure 2b).
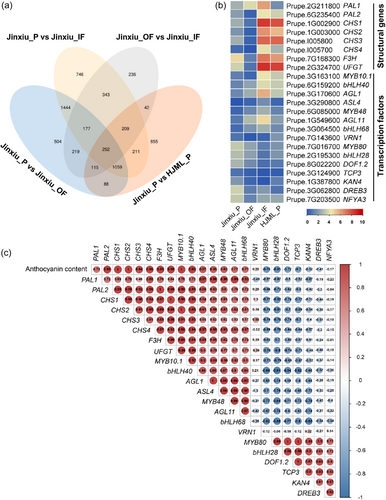
To further explore whether these potential TFs could regulate anthocyanin accumulation, we analysed the correlation between anthocyanin content and the expression levels of structural and regulatory genes. As expected, the expression levels of structural genes and PpMYB10.1 were highly positively correlated with anthocyanin content. As for the 14 potential TFs, six of them were positively correlated with anthocyanin content and PpMYB10.1 expression, such as PpbHLH40 and PpAGL1, whereas another six were negatively correlated, for example, PpMYB80 and PpbHLH28 (Figure 2c).
3.3 The effect and regulatory mechanism of PpMYB80 on PpMYB10.1
To analyse the effects of the above-mentioned candidate upstream regulators on the PpMYB10.1 promoter, dual-luciferase assays were performed. One TF was excluded because of its low FPKM < 1. PpMYB80 (Prupe.7G016700) significantly repressed the activity of PpMYB10.1 promoter, with only 21% activity remaining (Figure 3a). In Y1H assay, proPpMYB10.1-pAbAi plus PpMYB80-pGADT7 could grow on the SD/−Leu/AbA50 plate, whereas the negative control proPpMYB10.1-pAbAi plus pGADT7 could not, indicating that PpMYB80 could directly bind to PpMYB10.1 promoter (Figure 3b). Motifs for MYB TFs, [TCA]AACG[GT][TCA]NNTNNN, were predicted cis-regulatory sites (O'Malley et al., 2016). This core cis-element was present in PpMYB10.1 promoter located in the region from −669 to −649 bp. The EMSA results showed that PpMYB80 could specifically bind the biotin probe from PpMYB10.1 promoter. With the increase of the cold probe as competitor, the binding signal weakened. When the cold probe was mutated, it did not compete for binding (Figure 3c).
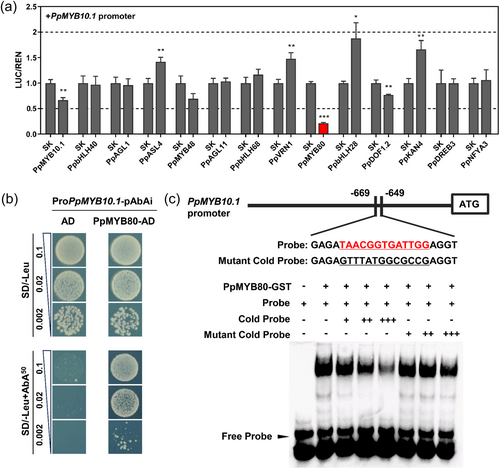
PpMYB80 is a R2R3-MYB with 412 deduced amino acid residues (Supporting Information S1: Figure 3a). PpMYB80 differs in structure with other reported MYB repressors, especially as the protein has an extended N-terminus, presenting in its homologues as well, of around 120 amino acid residues containing an ethylene-responsive element binding factor-associated amphiphilic repression (EAR) motif (DLNxxP) (Supporting Information S1: Figure 3b). Phylogenetic analysis showed that PpMYB80 did not cluster with canonical MYB repressors (Supporting Information S1: Figure 4). It was determined that PpMYB80 was localized in the nucleus (Supporting Information S1: Figure 5).
Possible additional mechanisms, besides directly inhibiting PpMYB10.1 expression, for PpMYB80 were evaluated. To analyse whether PpMYB80 can interfere MBW activation complex formation, LCI assay was performed, and it was found that PpMYB80 cannot interact with PpMYB10.1 or PpbHLH3 (Supporting Information S1: Figure 6a). In addition, PpMYB80 did not exert an effect on anthocyanin biosynthetic/transport gene promoters and the promoter of PpMYB80 was not regulated by PpMYB10.1 (Supporting Information S1: Figure 6b). These results suggested that PpMYB80 regulates anthocyanin biosynthesis through repressing PpMYB10.1 expression via direct binding to its promoter.
3.4 Function characterization of PpMYB80
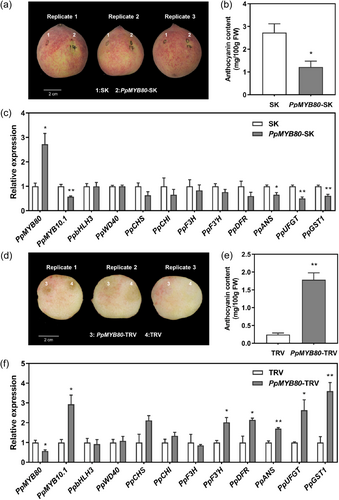
To further validate the function of PpMYB80 in negatively regulating anthocyanin accumulation, transient overexpression and silencing tests were conducted in peach fruit tissues. Transiently overexpressing PpMYB80 significantly repressed anthocyanin accumulation near the injection site (Figure 4a,b) and, conversely, the silencing of PpMYB80 induced peach anthocyanin accumulation (Figure 4d,e). Furthermore, the tissues around the injection site in PpMYB80-SK fruit showed a significantly lower level of PpMYB10.1 expression (Figure 4c), whereas the PpMYB10.1 expression was significantly higher in PpMYB80-TRV fruit (Figure 4f). The expression levels of anthocyanin biosynthetic/transport genes decreased in the PpMYB80-SK fruit but increased in the PpMYB80-TRV fruit (Figure 4c,f).
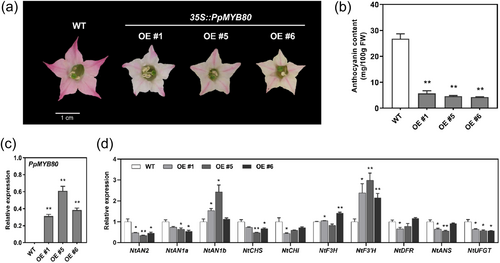
The function of PpMYB80 was further evaluated via stable transformation in tobacco. A significant loss of petal pigmentation was observed in transgenic tobacco overexpressing PpMYB80 (Figure 5a). The petal anthocyanin content in the transgenic lines #1, #5 and #6 was 78%, 82% and 84% lower than that in WT plant, respectively (Figure 5b). Expression of PpMYB80 was confirmed in the transgenic tobacco (Figure 5c) and the expression of NtCHS, NtCHI, NtANS and NtUFGT, as well as the TFs NtAN2 and NtAN1a, was downregulated in transgenic tobacco petals (Figure 5d). These results validated that PpMYB80 negatively regulated anthocyanin accumulation.
3.5 Expression patterns of PpMYB10 cluster members and PpMYB80 in the ‘Jinxiu’ peel following UV irradiance
To further investigate the mechanisms for anthocyanin accumulation in ‘Jinxiu’ peel, the fruit was exposed to UV irradiance, because the dark-red-coloured IF indicated that the anthocyanin biosynthetic genes were functional. At 4 days following UVB or UVA + UVB irradiance, the peel appeared red, whereas UVA-exposed fruit were not red (Figure 6a). These observations matched well with anthocyanin content. Anthocyanin accumulated with irradiance of UVA + UVB and to a lesser extent with UVB (Figure 6b).
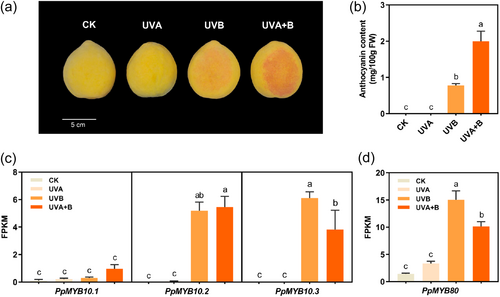
The expression of PpMYB10.1 remained low in UV-treated fruit and was not significantly affected by UV irradiance due to high expression of PpMYB80 (Figure 6c,d). However, PpMYB80 did not repress the promoter activity of PpMYB10.2 and PpMYB10.3, as the repression degree was below 50% in dual-luciferase assay (Supporting Information S1: Figure 7a). Moreover, Y1H assay showed that PpMYB80 did not bind to the promoters of PpMYB10.2 and PpMYB10.3 (Supporting Information S1: Figure 7b). The expression of PpMYB10.2 and PpMYB10.3 was induced significantly in ‘Jinxiu’ peel by UVB or UVA + UVB (Figure 6c), resulting in accumulation of anthocyanin. Transcript of PpMYB10.4/10.5/10.6 was not detected in UV-treated fruit.
3.6 Function characterization of PpMYB10.1/10.2/10.3
PpMYB10.1, PpMYB10.2 and PpMYB10.3 are highly homologous, over 88.4% and 81.4% at nucleotide and deduced amino acid level, respectively, and are of high similarity in structure (Supporting Information S1: Figure 8a) with highly conserved domains and motifs (Supporting Information S1: Figure 8b). Function of these three PpMYB10s in regulation of anthocyanin accumulation was characterized by transient overexpression in tobacco. When PpMYB10.1 and PpMYB10.3 were co-infiltrated with PpbHLH3, anthocyanin accumulation was induced in tobacco leaves within 4 days, whereas PpMYB10.2 along with PpbHLH3 had little effect (Figure 7a,b). After 7 days or 2 weeks, PpMYB10.2 co-infiltrated with PpbHLH3 induced anthocyanin accumulation, but was weakly, with accumulated anthocyanin 85% and 76% less, respectively, as compared with that of PpMYB10.3 plus PpbHLH3 at 4 days (Supporting Information S1: Figure 9).
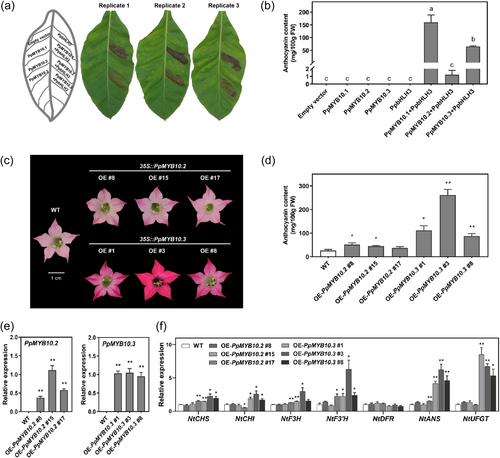
PpMYB10.1 has already been verified to be an activator of anthocyanin accumulation previously via its overexpression in tobacco (Tuan et al., 2015). Transgenic tobacco overexpressing PpMYB10.2 displayed redder petal colour than WT plants, whereas 35S::PpMYB10.3 transgenic tobacco flowers were darker (Figure 7c). Consistent with the appearance, the anthocyanin content of 35S::PpMYB10.2 and 35S::PpMYB10.3 transgenic lines was 0.71-fold and 4.88-fold, on average, higher than that in WT, respectively (Figure 7d). RT-qPCR results confirmed the expression of introduced genes (Figure 7e) and upregulated expression of structural genes in transgenic lines (Figure 7f). These results demonstrated that all three PpMYB10s are positive regulators of anthocyanin accumulation, but PpMYB10.2 is not as a strong activator.
3.7 Interaction between PpMYB10.1/10.2/10.3 and PpbHLH3, and the effects of PpMYB10.1/10.2/10.3 on the promoters of anthocyanin biosynthetic/transport genes
Interactions between PpMYB10.1/10.2/10.3 and PpbHLH3 were evaluated with Y2H and LCI assays. Y2H assay showed that yeast cells carrying PpMYB10.1/10.2/10.3 and PpbHLH3 could grow on QDO plate containing X-α-Gal (Figure 8a). In LCI assay, N. benthamiana leaves co-expressing each PpMYB10 plus PpbHLH3 exhibited strong fluorescence intensity (cps), whereas no fluorescence was observed in any negative control combinations (Figure 8b). These results suggested that PpMYB10.1/10.2/10.3 could physically interact with PpbHLH3.
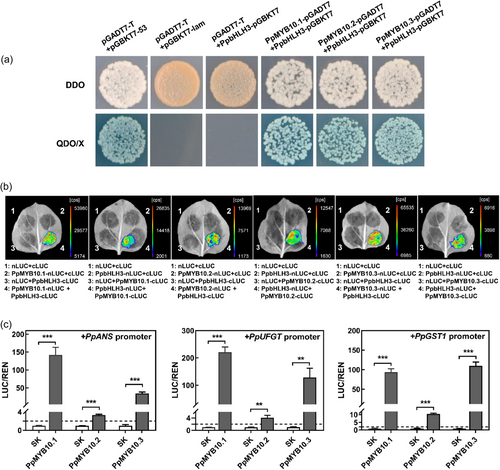
The activation effects of PpMYB10.1/10.2/10.3 on anthocyanin biosynthetic and transport genes were tested by dual-luciferase assay. The activities of PpANS, PpUFGT and PpGST1 promoters were significantly induced by PpMYB10.1 or PpMYB10.3, by 93 to 220-fold for the former and 34 to 128-fold for the latter (Figure 8c). Although the activation effect of PpMYB10.2 was not strong, by 3 to 10-fold (Figure 8c), which was consistent with the data obtained in transient colour and stable transformation assays in tobacco as shown in Figure 7.
4 DISCUSSION
4.1 Expression characteristics and functional divergence of six anthocyanin-related MYB gene cluster members in Prunus
Duplicated genes derived from a common ancestor usually form gene clusters and perform complementary functions (Sandve et al., 2018). Anthocyanin-related MYB TFs are tandemly duplicated in some plants, such as grape (Walker et al., 2007), carrot (Duan et al., 2023), orange (Huang et al., 2018) and Chinese bayberry (Xue et al., 2024). In Prunus, anthocyanin-related MYB gene clusters were discovered, including peach, plum, almond and sweet cherry (Alioto et al., 2020; Fiol et al., 2021; Shirasawa et al., 2017; Verde et al., 2017). In peach, two MYB gene clusters were identified, the one was located on a 72 kb region of Chr 3, including PpMYB10.1, PpMYB10.2 and PpMYB10.3, and the other one consisted of PpMYB10.4, PpMYB10.5 and PpMYB10.6 on a 63 kb region of Chr 6 (Zhou et al., 2014).
MYB10.1 genes, the commonly recognized anthocyanin activators, were responsible for the anthocyanin accumulation in various Prunus fruits (Fang et al., 2021a; Jin et al., 2016; Rahim et al., 2014; Tuan et al., 2015). PpMYB10.2 was involved in coloration of peach flower and nectarine peel (Ravaglia et al., 2013; Zhou et al., 2016), and PsMYB10.2 caused anthocyanin accumulation in red-fleshed plum (Fang et al., 2021b). The expression of PpMYB10.3 was detectable in OF of cv. ‘Redhaven’, ‘Roza’ and ‘Fantasia’, though being over 10 times weaker than that of PpMYB10.1 (Rahim et al., 2014). MYB10.4 and MYB10.6 regulate leaf red coloration in ‘Hongyetao’ peach (Zhou et al., 2014) and purple-foliage plum (Gu et al., 2015), respectively. Expression of MYB10.5 has not been reported.
In this study, in three fruit tissues of ‘Jinxiu’ and ‘HJML’, the expression of PpMYB10.1 matched well with anthocyanin accumulation, whereas PpMYB10.2 and PpMYB10.3 showed quite weak expression (Figure 1). In peel of UV-exposed ‘Jinxiu’ fruit, PpMYB10.1 expression was not significantly affected and remained low, whereas the expression of PpMYB10.2 and PpMYB10.3 was induced to be fourfold to 20-fold higher than that of PpMYB10.1 (Figure 6). Transcript of PpMYB10.4/10.5/10.6 was not detected in any fruit tissue in this study. Therefore, PpMYB10 cluster members exhibited expression differentiation in different varieties and fruit tissues, as well as in response to different environments.
Functional divergence of tandem-duplicated genes has been observed in a number of plants (Huang et al., 2018; Matus et al., 2017; Walker et al., 2007). PpMYB10.1 as a positive regulator of anthocyanin accumulation is well established previously (Tuan et al., 2015; Zhou et al., 2019), whereas the functions of PpMYB10.2 and PpMYB10.3 are not certain. By using transient expression in peach fruit and tobacco, Rahim et al. (2014) found that PpMYB10.2 and PpMYB10.3 were functional, while through stable tobacco transformation, Tuan et al. (2015) found that the flower of PpMYB10.2/10.3 overexpressing lines showed no coloration differences with WT. The difference might be related to peach variety. Here, in ‘Jinxiu’ and ‘HJML’ peaches, sharing identical nucleotide sequence between cultivars for each of PpMYB10.1/10.2/10.3, all three PpMYB10s were activators of anthocyanin accumulation, but the stimulation by PpMYB10.2 was quite weak (Figures 7 and 8). Functional divergence was owing to amino acid changes in the R3 repeat of PpMYB10.2 (Zhou et al., 2018), while whether the 18-amino acid deletion in C-terminus (Supporting Information S1: Figure 8b) severely impaired the function of inducing anthocyanin accumulation needs further investigation. In conclusion, PpMYB10 cluster members coregulated anthocyanin accumulation and exhibited functional diversity in different varieties.
4.2 PpMYB80 acts as a novel R2R3-MYB repressor for anthocyanin accumulation via transcriptional repression of PpMYB10.1
MYB repressors have been extensively studied and discovered to exert a negative regulation of anthocyanin accumulation. In some plants, the loss of colour in certain cultivars was due to MYB repressors, for instance, FcMYB1 and MdMYB28 negatively regulated anthocyanin accumulation in white strawberry and yellow-skinned apple, respectively (Ding et al., 2021; Salvatierra et al., 2013). MYB repressors include two distinct classes: R2R3-MYB with two repeats in the DNA-binding domain and R3-MYB with only the R3 repeat in the region (Chen et al., 2019). Most R2R3-MYB repressors belong to the Subgroup 4 (SG4) MYB TFs with two C-terminal conserved motifs required for repressive effects, the C1 and the C2/EAR motif (Kagale & Rozwadowski, 2011; Kranz et al., 1998). However, SG1 R2R3-MYB TFs, AtMYB60 and MdMYB306-like, also function as anthocyanin repressors though lack of the C1 and C2 motifs (Wang et al., 2022b). Our study identified and functionally verified (Figures 4 and 5) a novel R2R3-MYB anthocyanin repressor, termed PpMYB80, and according to phylogenetic tree of R2R3 MYBs from peach and Arabidopsis (Zhang et al., 2018), this MYB member, with ID Prupe.7G016700, belongs to SG21 instead of SG1 or SG4. PpMYB80 is also structurally different from and does not cluster with canonical MYB suppressors (Supporting Information S1: Figures 3 and 4), especially owing to an extended N-terminus containing around 120 amino acid residues, including an EAR motif (Supporting Information S1: Figure 3b). The structure–function relationship is worthy of further investigation.
R2R3-MYB TFs inhibit anthocyanin accumulation by two mechanisms, the one is active suppression by inhibiting the expression of target genes, for example, MdMYB17 directly binds to and represses the promoters of anthocyanin biosynthetic genes (Wang et al., 2022a), and the other one mechanism is interfering with MBW complex formation as passive repressors (Chen et al., 2019), for example, FaMYB1 and PpMYB18 interacts with corresponding bHLHs to compete with MYB activators, repressing anthocyanin biosynthesis in strawberry and peach, respectively (Aharoni et al., 2001; Zhou et al., 2019). Here in this study, PpMYB80 was highly expressed in ‘Jinxiu’ peel and transcriptionally repressed PpMYB10.1 via direct binding to its promoter, causing the natural anthocyanin deficiency in ‘Jinxiu’ peel (Figure 3). Besides, the expression of PpMYB80 was enhanced following UV irradiance (Figure 6), resulting in PpMYB10.1 remaining lowly expressed. The promoter sequence of PpMYB80, 2721 bp upstream of the start codon ATG, were identical in ‘Jinxiu’ and ‘HJML’, and the reasons for the strong expression of PpMYB80 in peel during ‘Jinxiu’ peach ripening need further investigation.
4.3 UV irradiance induces anthocyanin accumulation via upregulating the expression of different PpMYB gene cluster members
Environmental factors, such as light, temperature and stress, can affect anthocyanin biosynthesis (Espley & Jaakola, 2023). Among these factors, light is indispensable for anthocyanin accumulation in most plants, and different wavelengths of light have different regulatory effects on anthocyanin accumulation. Light increased the expression of MdMYB1, promoting anthocyanin accumulation in apple peel (Li et al., 2012). PpMYB114 participated in blue-light-induced anthocyanin accumulation in ‘Red Zaosu’ pear (Ni et al., 2018). Compared to visible light, UV lights, including UVA and UVB, usually present a deeper impact on anthocyanin accumulation. UVA irradiance induced anthocyanin accumulation in the swollen hypocotyls of turnip, with the upregulated expression of structural genes (Zhou et al., 2007). UVB positively regulated anthocyanin accumulation in several plants such as apple (Henry-Kirk et al., 2018), grape (Matus et al., 2017) and blueberry (Li et al., 2021).
In peach, the regulatory effects of UV light on anthocyanin accumulation have been determined in specific varieties. The naturally deeply-coloured peach ‘HJML’ was shown to have induced anthocyanin accumulation by UVA or UVB (Zhao et al., 2021). Another cultivar ‘Yulu’, with low pigmentation, was only sensitive to UVB (Zhao et al., 2017). In this study ‘Jinxiu’ fruit accumulated anthocyanin following UVB or UVA + UVB irradiance (Figure 6). The underlying molecular mechanisms were further investigated, the expression of PpMYB10.1 was not significantly induced by UV irradiance probably due to the enhanced expression of PpMYB80 (Figure 6). However, because PpMYB10.3 expression is not inhibited by PpMYB80 (Supporting Information S1: Figure 7), PpMYB10.3 expression was upregulated by UV irradiance, resulting in anthocyanin accumulation (Figure 6). Further work is needed to determine the causation of upregulation of PpMYB10.3 following UV irradiance.
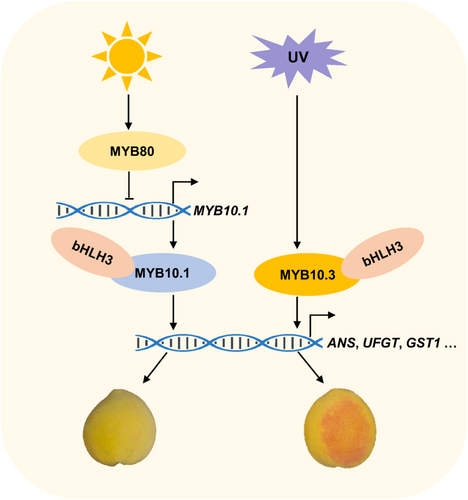
In conclusion, PpMYB10.1, PpMYB10.2 and PpMYB10.3 were all found to be positive regulators of anthocyanin accumulation, although the stimulation by PpMYB10.2 was relatively weak. A model for a TF cascade involving two PpMYB activators and one repressor in co-regulating anthocyanin accumulation in peach peel (Figure 9) was proposed. In this model, PpMYB80, a R2R3 MYB repressor newly identified in this study, represses the expression of PpMYB10.1 but not PpMYB10.3 by direct binding to its promoter, leading to the natural anthocyanin deficiency in ‘Jinxiu’ peel. Following UV irradiance, the expression of PpMYB10.3 is upregulated, although transcript of PpMYB10.1 remains low due to enhanced PpMYB80 expression, resulting in accumulation of anthocyanin.
ACKNOWLEDGEMENTS
We would like to thank colleagues Mr. Changqing Zhu for his assistance on anthocyanin measurement and Ms. Rong Jin for her help on growing tobacco plants. This work was financially supported by the National Natural Science Foundation of China (32072542), the 111 project (B17039), Zhejiang Provincial Cooperative Extension Project of Agricultural Key Technology (2022XTTGGP01), Ningbo Key Research and Development Program (2022Z179), and Pao Yu-Kong International Fund, Zhejiang University.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data are available within the manuscript and its Supporting Information S1 materials.




