Nitrogen nutrition effects on δ13C of plant respired CO2 are mostly caused by concurrent changes in organic acid utilisation and remobilisation
Abstract
Nitrogen (N) nutrition impacts on primary carbon metabolism and can lead to changes in δ13C of respired CO2. However, uncertainty remains as to whether (1) the effect of N nutrition is observed in all species, (2) N source also impacts on respired CO2 in roots and (3) a metabolic model can be constructed to predict δ13C of respired CO2 under different N sources. Here, we carried out isotopic measurements of respired CO2 and various metabolites using two species (spinach, French bean) grown under different NH4+:NO3− ratios. Both species showed a similar pattern, with a progressive 13C-depletion in leaf-respired CO2 as the ammonium proportion increased, while δ13C in root-respired CO2 showed little change. Supervised multivariate analysis showed that δ13C of respired CO2 was mostly determined by organic acid (malate, citrate) metabolism, in both leaves and roots. We then took advantage of nonstationary, two-pool modelling that explained 73% of variance in δ13C in respired CO2. It demonstrates the critical role of the balance between the utilisation of respiratory intermediates and the remobilisation of stored organic acids, regardless of anaplerotic bicarbonate fixation by phosphoenolpyruvate carboxylase and the organ considered.
1 INTRODUCTION
C3 plant photosynthesis discriminates between CO2 isotopologues so that photosynthates are naturally 13C-depleted compared to atmospheric carbon dioxide. Furthermore, the carbon isotope composition (δ13C) in plant organic matter is also the result of CO2 loss by respiration, which may be associated with 12C/13C isotope fractionations. In the past two decades, plant respiration has received increasing attention because of its possible influence on plant and ecosystem 13C budgets. There is now compelling evidence that leaf dark respiration usually generates 13C-enriched CO2 (up to 10‰ compared to total organic matter) while often root respiration produces relatively 13C-depleted CO2 (Ghashghaie & Badeck, 2014). Using plants grown under controlled mesocosm conditions, it has been suggested that at the plant scale, the 13C-enrichment caused by leaf respiratory carbon loss is compensated for by the 13C-depletion caused by root respiration, thus with little overall effect of respiration (Bathellier et al., 2008; Klumpp et al., 2005).
However, δ13C of leaf-respired CO2 varies considerably between species and depends on environmental conditions (e.g. drought, temperature), the availability of respiratory substrates (carbohydrates, lipids or proteins) and the relative activity of metabolic pathways (Bathellier et al., 2017). In the short- or midterm, this has important consequences for δ13C of CO2 in air in terrestrial ecosystems. For example, it has been shown that during the night, the impact of changes in leaf respiratory metabolism influences ecosystem-respired CO2, in a temperature-dependent manner (Knohl et al., 2005; Werner & Gessler, 2011; Werner et al., 2007). Leaf respiration can also have an enormous impact on measured photosynthetic isotope discrimination (Δobs) in particular when respiration uses substrates disconnected from photosynthates (i.e. non-photosynthetic carbon source) and net CO2 assimilation is low (Barbour et al., 2017). In crops like wheat, the respiratory loss has an impact on δ13C of grains, in proportion with carbon use efficiency (i.e. percentage of carbon lost by respiration) and harvest index (Domergue, Abadie, et al., 2022). Taken as a whole, the isotope composition of respired CO2 is of crucial importance for understanding plant and ecosystem carbon balance. However, there is presently no clear empirical relationship or equation predicting δ13C of respired CO2, and this represents a hurdle in plant 13C budget modelling.
In principle, δ13C of respired CO2 should depend on both metabolic pathways (i.e. δ13C of source carbon and metabolic fluxes) and isotope effects associated with enzymes involved therein. Using biochemical degradation or 13C-NMR, it has been shown that within the glucose molecule, δ13C of C-atom positions differ, with C-3 and C-4 positions being relatively 13C-enriched (Gilbert et al., 2012; Rossmann et al., 1991). As a result, pyruvate molecules produced by glycolysis are probably 13C-enriched in C-1 (COOH group) and 13C-depleted in C-2 (C = O) and C-3 (CH3) atom positions. Consequently, CO2 liberated from position C-1 by pyruvate dehydrogenase should be 13C-enriched. This intramolecular effect is believed to be the at the origin of the 13C-enrichment in leaf-respired CO2 (Bathellier et al., 2017; Ghashghaie & Badeck, 2014; Tcherkez et al., 2003). The relative 13C-depletion in glucose (in positions other than C-3 and C-4) probably also leads to a 13C-depletion in CO2 produced by the oxidative pentose phosphate pathway (OPPP) which comes from glucose C-1 atom position. Experiments using positionally 13C-labeled substrates (i.e. glucose and pyruvate) have shown a substantial OPPP activity in roots (22% of CO2 production rate) and thus, the OPPP likely contributes to explaining the natural 13C-depletion in root-respired CO2 (Bathellier et al., 2009). The utilisation of substrates other than glucose by catabolism influences δ13C of respired CO2. For example, lipids are naturally 13C-depleted and their degradation leads to a 13C-depletion in leaf-respired CO2 during prolonged darkness (Tcherkez et al., 2003). Organic acids coming from anaplerotic, phosphoenolpyruvate carboxylase (PEPC)—catalysed fixation are 13C-enriched and thus their decarboxylation generates 13C-enriched CO2. This phenomenon has been shown to occur during light-enhanced dark respiration in leaves (respiratory peak after illumination) (Barbour et al., 2007; Gessler et al., 2009; Lehmann et al., 2016; Priault et al., 2009).
Respired CO2 is the net result of several (de)carboxylation reactions (pyruvate dehydrogenase, Krebs cycle decarboxylation, OPPP, PEPC) and thus it depends on the respective contribution of such reactions. This in turn depends on physiological conditions, since the utilisation of carbon skeletons generated by catabolism varies with the physiological status of the plant, environmental cues, nutrients and species. In particular, nitrogen nutrition has a strong effect on respiratory metabolism, since N assimilation abstracts 2-oxoacids (such as 2-oxoglutarate) to form amino acids (such as glutamate). For example, it is well known that nitrate utilisation is associated with an increase in glycolysis, anaplerotic fixation and a decrease in sugar concentration in leaves (Krapp et al., 2014; Stitt et al., 2002). Therefore, N availability should impact on δ13C of respired CO2. In fact, we have previously shown in tobacco (Nicotiana tabacum) that the balance between ammonium and nitrate has an influence on δ13C of leaf-respired CO2, with a progressive 13C-depletion as the proportion of ammonium as a N source increased (Ghiasi et al., 2021). In addition, we suggested that this was partly explained by the contribution of organic acid metabolism. In effect, there was a strong relationship between δ13C of CO2 and that in organic acids (malate, citrate). Since the 13C-enrichment in organic acids likely comes from PEPC activity which fixes naturally 13C-enriched HCO3− (for a review of isotope effects in enzymes, see Tcherkez et al., 2011), δ13C variations in respired CO2 can be viewed as reflecting changes in the metabolic flux associated with PEPC activity. However, nitrate assimilation does not only involve leaves since some proportion of absorbed nitrate is reduced in roots (up to 30%, depending on species) (Andrews, 1986). In addition, when nitrate is balanced against ammonium, a specific effect of ammonium metabolism could be expected. NH4+ is rapidly consumed by the glutamine synthetase/glutamine 2-oxoglutarate aminotransferase (GS-GOGAT) pathway, causes an increase in root respiration rate leading to the biosynthesis of N-rich compounds such as amides, polyamines and/or ureides (González-Moro et al., 2021; Xiao et al., 2023). Therefore, consequences of N nutrition on δ13C of root-respired CO2 can be anticipated.
Here, we looked at changes in δ13C of respired CO2 in both leaves and roots in spinach (Spinacia oleracea) and French bean (Phaseolus vulgaris) plants grown with varying proportions of NH4+ and NO3− fertilizers in the nutrient solution. These two species were selected due to their agronomic importance and their contrasting behaviour with respect to nitrogen use (spinach is nitrophilous, while bean naturally involves ureide metabolism and can have N2-fixing nodules). We first used N isotopes to check whether plants used ammonium and nitrate according to the proportion imposed with the nutrient solution. We then took advantage of compound-specific isotope analyses to compare δ13C of CO2 with that of metabolites (such as sugars and organic acids) and determined the respiration rate and PEPC activity. We integrated the data using multivariate statistics and nonstationary mechanistic modelling to elucidate best candidate drivers and design a predictive model of δ13C in respired CO2. Our working questions were: (1) is there a consistent δ13C pattern in respired CO2 as the ammonium-to-nitrate ratio varies, in leaves and roots? (2) which metabolites influence most δ13C of respired CO2? (3) is it possible to construct a metabolic model of δ13C of respired CO2 that can be applied regardless of N conditions, species, and organs?
2 MATERIAL AND METHODS
2.1 Plant material
French bean (Phaseolus vulgaris L.) cv. Contender, and spinach (Spinacia oleracea L.) cv. Géant d'Hiver were purchased from Vilmorin (France). Seeds were germinated in vermiculite with tap water in darkness. Seven days old seedlings were transplanted to pots (8.3 cm diameter, 11 cm height; one plant per pot) filled with sand (previously washed with tap water and sterilised in an autoclave) and grown in the greenhouse. Whole culture duration was 33 and 38 d for bean and spinach, respectively. Under our conditions (sufficient total N availability), bean plants did not form nodules. Bean plants were grown in January 2017 under natural light, with supplementary light supplied by lamps (Metal Halide Lamps, HSI-THX, 400 W, Sylvania) 10 h per day, providing a photosynthetic photon flux density (PPFD) of 140–160 μmol photons m–2 s–1 at plant height. Air temperature was 23 ± 2°C during the day and 17 ± 2°C at night. Humidity was 42% ± 8% during the day and 55% ± 15% at night. Spinach plants were grown in July and August 2017, with an artificial light source (200–210 μmol photons m–2 s–1 PPFD at plant height) only 16 h per day. Temperature and humidity were the same as for bean cultivation. Maize plants were grown at the same time to have an indirect measurement of ambient CO2 using maize leaf organic matter. The carbon isotope composition of the ambient CO2 in the culture rooms was about –9.5‰ (measured, Vienna-Pee Dee Belemnite as standard) and –14.1‰ (estimated, considering δ13C of maize) for bean and spinach, respectively. In figures below, the difference between maize leaf and bean or spinach organic matter (weighted average of root and leaf δ13C) is referred to as MaizeΔδTOM.
2.2 Nutrient conditions
Plants were supplied with nutrient solutions with 6 different proportions of ammonium (NH4+) and nitrate (NO3–) as N source as follows (NH4+/NO3– ratios): 0/100, 25/75, 50/50, 75/25, 90/10 and 100/0. In the following, N-treatments are referred to proportions of NH4+ (i.e. 0%, 25%, 50%, 75%, 90% and 100%, respectively). Nutrient solutions contained 6 mmol L−1 of N (total) formed with a combination of KNO3, Ca(NO3)2, NH4NO3 or (NH4)2SO4 to obtain different N-treatments. Additional components were: 1 mM CaCl2, 0.25 mM KH2PO4, 1 mM MgSO4, and trace elements (2 μM MnSO4, 2 μM ZnSO4, 0.5 μM CuSO4, 25 μM B(OH)3, 0.5 μM Na2MoO4, 40 μM Fe-EDTA). The pH of solutions was kept constant at 5.5. In 100% NH4+ and 90% NH4+ treatments, 1 mM K2SO4 was added to keep K concentration constant. Although the sand was sterilised by autoclaving, 1 ppm nitrification inhibitor (2-chloro-6-trichloromethylpyridine) was added to nutrient solutions for spinach, to avoid contamination by nitrifying bacteria from the environment. Nutrient solutions were used for watering the plants only after appearance of cotyledons (750 mL solution plate−1 d−1, with 8 pots in each plate). The amount of the solution was so that pots reached full capacity and allowed to drain to plates. During the last week before harvesting, the amount of the solution was increased to 1000–1500 mL plate−1 d−1 depending on plant growth rate. Chlorophyll content and Nitrogen Balance Index were measured every 3 days with a DUALEX instrument (DUALEX SCIENTIFIC + TM, ForceA, France, Orsay) to check plants were not N deficient.
2.3 Leaf gas exchange parameters
One month after planting, gas exchange and chlorophyll fluorescence were measured using a Licor 6400 with LCF chamber (LI-COR, Inc.) in the morning (9–12 h) during 3 successive days. One plant with mature leaves was selected from each treatment each day. Therefore, there were 3 replications for gas exchange measurement. Plants used for measurement were selected randomly. Before photosynthetic measurements, plants were dark-adapted for 30 min, then one mature leaf was used. Gas exchange conditions were set as follows: flow rate 300 μmol air s–1, CO2 mole fraction 390 μmol mol–1 and PPFD 400 μmol m–2 s–1 (with 10% blue). Leaf temperature was fixed at 22°C. Leaf net CO2 assimilation rate (An), stomatal conductance (gs) and the ratio of intercellular to ambient CO2 concentrations (Ci/Ca) data were automatically recorded every 3 min, reaching steady values after about 30 min. Three values in the steady state were recorded to get a mean value for each plant.
2.4 Carbon isotope composition of respired CO2
To ensure that plants had enough photosynthates as (potential) respiratory substrates, they were taken from the greenhouse after at least 4 h light. They were then placed in the dark for 30 min to avoid light enhanced dark respiration (LEDR). One intact mature leaf was cut off and put into a flask (50 mL) completely covered by aluminium foil. The flask was flushed with CO2-free air for 5 min, then sealed with a septum and left for respired CO2 accumulation. CO2 concentration was analysed by micro-GC (490 Micro GC, Agilent Technologies), with an injection volume of 5 μL every 3 min. When CO2 mole fraction was above 1000 µmol mol−1 (approximately after 12 min), air samples were manually collected from the flask with a syringe (0.5 to 1 mL depending on CO2 concentration) and injected into a GC-IRMS, made of a gas chromatograph (GC HP 5890) coupled to a stable isotope ratio mass spectrometer (Optima Isochrom-µG, Fisons Instruments) to measure the carbon isotope composition of respired CO2 (denoted as δ13CR). Syringes were flushed with helium five times and then with sample air inside a given flask 10 times before each injection. Each flask was sampled and measured 3 times to ensure reproducibility of measurements. For root-respired CO2, roots were first washed with tap water and dried with absorbing paper before incubation in flasks. Since the respiration rate and the size of roots were greater than that of leaves, bigger flasks (120 mL) were used. Measurements were made between 10:00 and 16:00 each day, and with a random selection of plants.
2.5 Sampling
Plants used for measurements of δ13C in respired CO2 were harvested for biomass determination. Plants were divided into leaves, stems and roots. Samples were plunged into liquid nitrogen and then freeze-dried. Samples were then ground into a fine powder (using a Retsch MM200 mill ball, Bioblock Scientific) used for isotope measurements and extractions.
2.6 Extraction of soluble fraction
Plant powder (50 mg) was suspended in 1 mL cold distilled water and maintained on ice for 60 min with agitation with vortex every 10 min. After centrifugation (14,000 g, 5°C, 15 min), the supernatant (containing soluble sugars, organic and amino acids and soluble proteins) was separated from the pellet. The supernatant was heated at 100°C for 5 min and then kept on ice for 30 min to precipitate heat-denatured proteins. After centrifugation (14,000 g, 5°C, 15 min), the soluble fraction was collected, kept at –20°C for subsequent utilisation. Samples were referred to as water soluble organic matter (WSOM). Aliquots of 200 μL were poured into tin capsules and oven-dried at 50°C for isotope analysis.
2.7 Carbon and nitrogen isotope composition of organic material
2.8 Compound-specific isotope analysis
The δ13C of common organic acids (malate and citrate) and main sugars (sucrose, glucose and fructose) were determined using liquid chromatography coupled to isotope ratio mass spectrometry (IRMS) as in (Ghiasi et al., 2021). Briefly, WSOM extracts were diluted 500 times, and the pH was adjusted to 7. Then 4 mL were passed through cation exchangers (DionexTM OnGuard II H 1.0 cc cartridges, Thermo Scientific) and anion exchangers (DionexTM OnGuard II A 1.0 cc cartridges) to retain amino acids and organic acids, respectively, and collect soluble sugars. Purified soluble sugars were collected in amber silanised glass vials (Vial short tread, VWR International). Organic acids were collected in amber glass vials. The Thermo Finnigan LC-IsoLink system (Ultimate 3000, Dionex/Thermo Scientific) coupled to Delta V Advantage isotope ratio mass spectrometer (Thermo Scientific) was used for δ13C of individual soluble sugars and organic acids (malate and citrate). Amounts (denoted as [C]) and δ13C of individual sugars were used to calculate δ13C of total soluble sugars (δ13CSug) using mass balance, as δ13CSug (‰) = (δ13CGlc · [C]Glc + δ13CSuc · [C]Suc + δ13CFru · [C]Fru)/([C]Glc + [C]Suc + [C]Fru). Similarly, using amounts and δ13C of individual sugars and organic acids, we calculated the predicted δ13C of total water-soluble fraction to compare with measured values (δ13CWSOM).
The δ13C of the most abundant free fatty acid (palmitic or palmiteaidic acid depending on species; simply referred to as “palmitate” thereafter), maltose and amino acids was determined on derivatised samples (methoxyamine and MSTFA in pyridine) using gas chromatography coupled to IRMS using a GC 7693 A coupled to a combustion module GC5 (filled with the GCN reactor) and precisION IRMS (Elementar), after (Domergue, Lalande, et al., 2022). Isotope standards were caffeine (IAEA-600) and nicotine (home standard, from Sigma-Aldrich/Merck) and retention time indices were obtained using an alkane mix (injected separately; Connecticut n-Hydrocarbon Mix, Supelco). Specific standards of known δ13C were used to determine the δ13C of trimethylsilyl (TMS) groups (–33.32‰) and thus correct the δ13C of analytes (palmitate 1 TMS, maltose 8 TMS, and TMS derivatives of amino acids) for the contribution of TMS carbons to raw isotope composition. δ13C was corrected for linearity to account for the fact that some metabolites were more abundant than others (in practice this correction was small, generally less than 0.2‰). The weighted-average δ13C of amino acids was calculated using the observed signal (mass-44) and δ13C of individual amino acids.
2.9 PEPC activity
Samples used for PEPC activity were periodically harvested during the daytime and conserved at –80°C. 150 mg frozen roots or leaves (without petioles and midribs) were ground in a mortar with liquid nitrogen with 100 mg sand. Then 1 mL extraction buffer (HEPES 50 mM; MgCl2 10 mM; EDTA 1 mM; EGTA 1 mM; BSA 0.025% (w/v); glycerol 10% (w/v); DTT 5 mM and protease inhibitor) was added (4°C). After agitation with a vortex for 2 s, the sample was thawed on ice and agitated again with a vortex for 5 s and centrifuged at 10,000 g, 4°C for 10 min. PEPC activity was measured using a coupled reaction assay (NADH-oxidation via malate dehydrogenase). The assay medium contained 2 mM phosphoenolpyruvate (PEP), 100 mM Tricine (pH 8.0), 10 mM NaHCO3, 20 mM MgCl2, 0.05% Triton, 0.5 mM NADH, 1 U mL–1 malate dehydrogenase, 20 μL enzyme extraction solution, with 200 μL as the final volume. Two technical replicates for each sample were measured. Measurements used a 96-well plate placed in a microplate spectrophotometer (PowerWave HT, BioTek) to record NADH oxidation at 340 nm averaged across 20 min. A blank without PEP was also recorded for subtraction. An assay where PEP was replaced by H2O was checked as a control. PEPC activity was calculated as the blank-corrected difference in NADH oxidation rate before and after the addition of PEP.
2.10 Statistics
Omics analyses were carried out on 3 to 4 samples per condition and species (i.e., 4 independent replicate). Univariate statistical analysis was conducted using an analysis of variance (one-way ANOVA) to examine differences between NH4+:NO3− conditions (Figures 1-4). The data were also analysed via multivariate statistics to identify features that correlate significantly with the δ13C in respired CO2 conditions, using orthogonal partial least squares-discriminant analysis (OPLS) with Simca®, version 17.0.2 (Sartorius). In the OPLS, the response variable (Y variable) was δ13C of respired CO2 expressed relative to δ13C in total organic matter (i.e., δ13CR – δ13COM denoted as OMΔδ13CR). We used OMΔδ13CR to account for the fact that spinach and bean did not have the same range of δ13C due to photosynthetic fixation. Also, for statistics, it was better to express δ13C relative to organic matter rather than sugars (as in the model described below) to assess whether the Δδ13C of sugars could be a driver of respired CO2. The performance of the OPLS was assessed with the correlation between observed and predicted Y (R²), the cross validated correlation coefficient (Q²) and the P-value of the statistical model quantifying the probability that the OPLS model was not different from a random model mean±random error (this P-value is referred to as PCV-ANOVA). Univariate analysis was also conducted via regression and calculation of the associated P-value. Volcano plots combine results from univariate (regression) and multivariate (OPLS) analyses, showing —log(P) versus OPLS loadings (illustrated in Figure 5).
2.11 Nitrogen isotope mass balance
2.12 Modelling
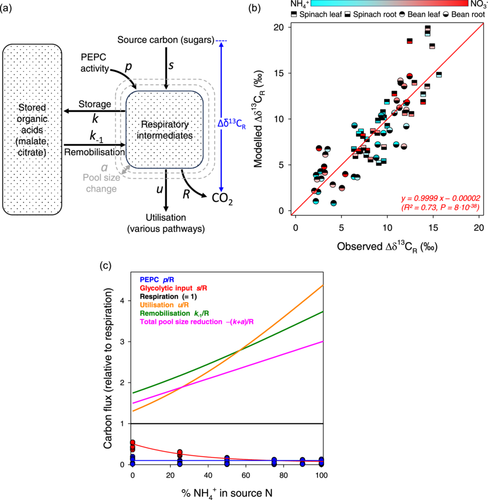
The δ13C of night-time respired CO2 was modelled using a metabolic mechanistic approach illustrated in Figure 6. It was assumed that the metabolically active pool of respiratory intermediates (organic acids) was fed by both stored organic acids (remobilisation, flux k−1), glycolysis (s), and PEPC activity (p). This active pool is then used to produce CO2 (respiration R), feed storage (flux k), and sustain amino acid synthesis and other metabolic pathways (utilisation u). Variation in pool size was assumed to be possible, i.e. the pool could be nonstationary. It was assumed that organic acids purified from organs mostly reflected vacuolar material and thus their δ13C were associated with stored organic acids. The change in pool size with time was denoted as a. Since the absolute δ13C is dissimilar in spinach and bean due to different CO2 source and photosynthetic isotope fractionation, δ13C was expressed relative to source carbon, that is, the weighted average δ13C of sugars (fructose, sucrose, glucose) (i.e., δ13CR – δ13Csugars denoted as Δδ13CR in blue in Figure 6a).
In Equation 5, p/R is known (since PEPC activity and respiration were measured) thus unknown relative metabolic fluxes are u/R, k−1/R and (k + a*)/R. These metabolic fluxes were not assumed to be constant and rather, supposed to vary with NH4+:NO3− nutrition, using a sigmoid (u/R, k−1/R) or linear response (k + a*/R; a linear response was chosen in this case since a* contains a logarithm and thus an exponential component should transform to linear). Δδfix and Δδstock were known since they are the isotope difference between sugars and fixed bicarbonate, and between sugars and weighted average organic acids, respectively. Δδfix and Δδstock could thus be calculated for each sample. Solving (fitting) was performed using the Excel® solver, to optimise the match between predicted and observed Δδ13CR via the minimisation of the sum of squares. The value of embedded coefficients (particular, of sigmoid and linear responses) obtained by optimisation are listed in Supporting Information S1: Table S2.
3 RESULTS
3.1 N assimilation
The utilisation of nitrogen from source N of the nutrient solution was assessed using the nitrogen isotope composition (δ15N). The δ15N of source ammonium, nitrate and total organic matter of leaves and roots is shown in Figure 1a. In both spinach and bean, leaves were slightly 15N-enriched by 1‰–2‰ compared to roots. Spinach organic matter (in both leaves and roots) tended to be more 15N-depleted than that of bean, suggesting that there was higher isotope fractionation during N assimilation in spinach. In fact, using 100% ammonium and 100% nitrate, the apparent isotope fractionation was calculated using the weighted average (biomass-weighted) of leaf and root δ15N. In bean, there was a fractionation in favour of 15N by about 4‰ during ammonium utilisation (while there was almost no fractionation in spinach) and there was a small fractionation during nitrate utilisation, i.e., about 2‰ in spinach (against 15N) and −0.5‰ (in favour of 15N) in bean (Figure 1b, inset). Accounting for these isotope fractionations, we calculated the proportion of effective ammonium utilisation (Figure 1b). Effective ammonium utilisation closely followed the proportion of ammonium in source N, although it was slightly lower in bean than in spinach, suggesting that the two species were differentially selective for nitrate and ammonium. N conditions also influenced biomass and photosynthesis (summary of results in Supporting Information S1: Figures S1 and S2). As anticipated, ammonium caused a small decline in total plant biomass and at 100% ammonium, led to a significant drop in net photosynthesis. However, there was a concurrent change in stomatal conductance so that the intercellular-to-atmospheric CO2 ratio (Ci/Ca) did not change dramatically.

3.2 δ13C of organic matter and respired CO2
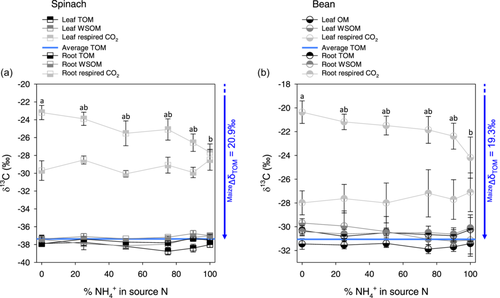
In both spinach (Figure 2a) and bean (Figure 2b), there was little change in δ13C of total organic matter, suggesting that the photosynthetic isotope fractionation did not vary much. The comparison with maize organic matter cultivated under the same conditions (blue arrows in Figure 2) suggests that photosynthetic fractionation was slightly higher (about 21‰) in spinach than in bean (about 19‰). The δ13C of water-soluble organic matter (WSOM) was relatively close to that in total organic matter and did not change considerably with NH4+:NO3− nutrition. There was a relatively good agreement between the estimated isotope composition calculated with the photosynthetic isotope fractionation (Δi) and observed WSOM (Supporting Information S1: Figure S3). δ13C of leaf-respired CO2 decreased when the proportion of ammonium increased in source N (Figure 2a,c), with a depletion of about 4‰ in 100% ammonium compared to 100% nitrate. Such a decline was likely not due to a decline in photosynthetic isotope fractionation at least in spinach, since Ci/Ca decreased under high ammonium conditions, and thus photosynthetically fixed carbon was probably slightly 13C-enriched (Supporting Information S1: Figures S2 and S3). The δ13C of root-respired CO2 did not change much, although it tended to be higher at 100% ammonium compared to 100% nitrate, by about 1‰ (Figure 2b,d).
3.3 δ13C of metabolites
The δ13C varied considerably between metabolites (Figure 3), ranging between –40 and –25‰. There were considerable differences between sugar species, maltose being 13C-enriched and glucose being 13C-depleted. Malate was always relatively 13C-enriched and was isotopically close to citrate (except in spinach leaves where it was about 2‰–3‰ 13C-enriched compared to citrate). This suggests that malate carbon isotope composition was influenced by PEPC activity, which fixes 13C-enriched bicarbonate to form C4 acids. Amino acids tended to be 13C-enriched with considerable variation. As expected for lipids, palmitate was generally 13C-depleted, in particular compared to citrate and malate.
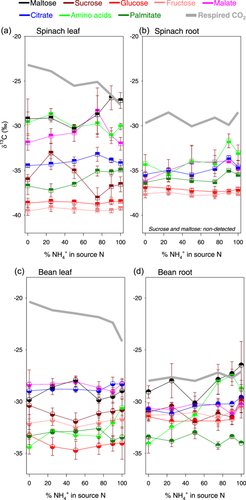
Taken as a whole, there was no consistent metabolic pattern of variation with respect to ammonium and nitrate proportions across organs and species. For example, amino acids were progressively 13C-enriched as the proportion of ammonium increased in bean, but it was not the case in spinach. Interestingly, there were important changes in δ13C of malate in spinach, with a maximum value (about −28‰) observed under 75% ammonium in leaves. Citrate tended to be enriched in 13C by about 1‰ as ammonium proportion increased to 100%, except in spinach leaves where it reached a maximum at 75% ammonium, like malate. It is worth noting that except for maltose at high ammonium, no metabolite was more 13C-enriched than respired CO2. It suggests that CO2 generation by respiration was either (1) fed by a 13C-enriched, small metabolic pool that was not well reflected by the isotope analysis of metabolites (i.e., total pools extracted from leaves and roots; Figure 3); or (2) associated with an isotope fractionation in favour of 13C.
3.4 Respiration, enzymatic activities and pool sizes
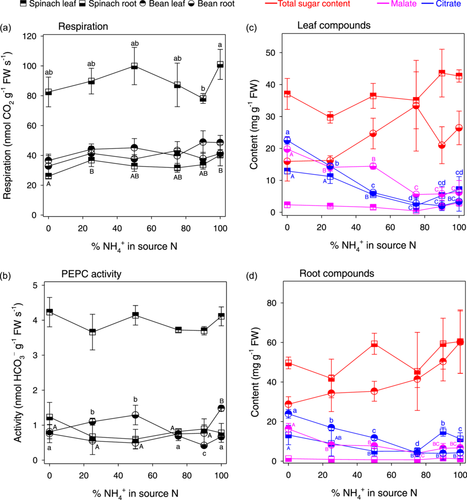
The respiration rate did not change considerably with NH4+:NO3− ratio and was in the same order of magnitude (20–40 nmol g−1 s−1) in bean organs and spinach leaves (Figure 4a). The respiration rate of spinach roots was comparatively high (80–100 nmol g−1 s−1). PEPC activity tended to be higher in bean leaves when ammonium was less than 50% of source N but it was not affected by N nutrition in spinach leaves. PEPC activity in roots remained in the same order of magnitude and was only significantly affected by N nutrition in bean roots with an increase under 100% ammonium (Figure 4b). Total sugar content was quite variable in leaves with no clear pattern when N nutrition changed (Figure 4c). Leaf malate decreased significantly as ammonium proportion increased; the citrate content decreased in bean leaves but was always very low in spinach leaves. A rather similar pattern in sugars, malate and citrate was observed in roots (Figure 4d), despite a progressive (insignificant) increase on average sugar content and an increase in spinach root malate content under 90% and 100% ammonium.
3.5 Multivariate analysis of respired CO2
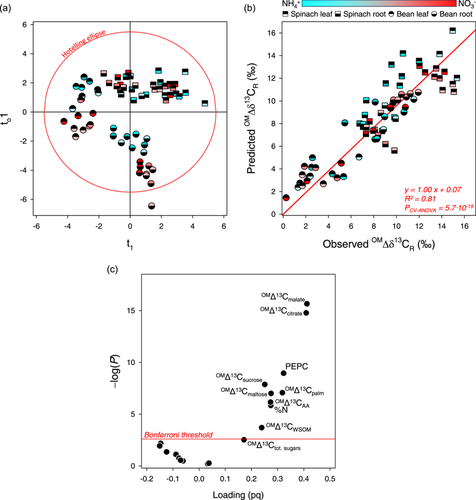
We explored best drivers of δ13C of respired CO2 using a supervised multivariate method (OPLS), with OMΔδ13CR as the response variable to be modelled. We used the full data set (i.e., all individual measurements, not average values) to assess whether it was possible to design a statistical model that was valid regardless of organs and species. Datapoints could be placed in the multivariate space according to OMΔδ13CR along axis 1, with residual variance along axis 2 (Figure 5a). We obtained a very good statistical performance with R² of 0.81 (Figure 5b), high cross-validated coefficient Q² (0.77) and very high significance (PCV-ANOVA = 5.7 ∙ 10−19). Best drivers are shown as a volcano plot combining results of univariate and multivariate statistics (Figure 5c). The isotope composition in malate and citrate were the strongest drivers, followed by PEPC activity and various other parameters. It demonstrates that respired CO2 was determined by a combination of metabolic parameters (multivariate component) in which the isotope signature of organic acids had a relatively high weight. In other words, several metabolic fluxes and source metabolites are involved in the mechanism behind δ13C of respired CO2.
3.6 Modelling
A two-pool model was designed to compute the Δδ13CR as a function of source carbon, δ13C of stored organic acids and metabolic fluxes (Figure 6a). Although simplistic, it should in principle capture the most important metabolic features that may explain the isotope composition of respired CO2. Two flux parameters were known: respiration (R), PEPC activity (p). The glycolytic input (s) could be deduced from other parameters. Three input parameters, namely storage/build-up (k + a*), remobilisation (k−1) and utilisation (u) were unknown and their response function to ammonium was determined using least squares optimisation. Taken as a whole, the model explained quite well Δδ13C (isotope difference between CO2 and sugars) of respired CO2 with a correlation coefficient (R²) of 0.73 and a very close alignment to the 1:1 line (Figure 6b). Modelled storage, remobilisation and utilisation increased with ammonium proportion while the glycolytic input decreased (Figure 6c). Interestingly, remobilisation and utilisation fluxes represented 1.5 to 4 times the respiration rate, showing that the respiratory metabolic pool was highly dynamic (high turn-over). Comparatively, the input of carbon by PEPC was small (a few percent of respiration) (see also Figure 4). There was also a predicted change in total pool size, with a more pronounced reduction in total organic acid pool as ammonium proportion increased (pink line, Figure 6c). It is worth noting that it is effectively what was observed with direct organic acid content measurements (Figure 4c,d).
4 DISCUSSION
4.1 Overall response of δ13C in respired CO2 to N nutrition
We found that unlike in roots, the carbon isotope composition of respired CO2 in leaves decreased as the proportion of ammonium increased in the N source (Figure 2). This pattern was remarkably similar in both species.
This effect was unlikely to be driven by N nutrition on photosynthetic isotope fractionation and thus on δ13C of fixed carbon. First, no similar decrease in root-respired CO2 was found (Figure 2), suggesting that metabolic, post-photosynthetic effects are involved. Second, variations (or the lack thereof) in Ci/Ca would lead to an increase (not a decrease) in δ13C (Supporting Information S1: Figure S2). Similarly, (Høgh-Jensen & Schjoerring, 1997) reported that white clover plants supplied with nitrate had a lower apparent photosynthetic fractionation Δ than those supplied with ammonium. Conversely, Raven & Farquhar (1990) suggested that in nitrate-fed plants, there could be a lower Ci/Ca due to the combined effect of increased PEPC activity and higher photosynthetic capacity. Here, we found little change in δ13C of total organic matter (Figure 2), also suggesting minimal effect of N conditions on photosynthetic fractionation. In results obtained so far, the impact of N nutrition on δ13C of plant organic matter is variable, see for example (Brück & Guo, 2006; Guo et al., 2002) in bean. That said, we found here a slight 13C-depletion in WSOM as the proportion of ammonium increased (Figure 2). This is also visible when WSOM is compared to predicted δ13C of photosynthates (Supporting Information S1: Figure S3, points inside ellipses). It is more likely that under our conditions, WSOM was not properly representative of photosynthates, since the proportion of sugars and organic acids varied considerably (Figure 4) while their δ13C differed considerably (Figure 3). In other words, WSOM was impacted by post-photosynthetic isotope fractionations in metabolism and its metabolic composition had an impact on its δ13C. In fact, when we calculated the weighted average of major compounds (organic acids and sugars), we could reconstruct the isotope signature of WSOM satisfactorily (R² = 0.84; Supporting Information S1: Figure S4).
We thus argue that the change in δ13C of respired CO2 as N nutrition varied was due to modifications in metabolism, leading to changes in respiratory substrates and/or in metabolic isotope fractionations. Accordingly, N nutrition had an influence on respiration, PEPC activity, sugar and organic acid content (Figure 4), the most visible effect being a decline in organic acids as the ammonium proportion increased. In bean, the sugar content changed little in leaves but increased significantly in roots under 100% NH4+, while both malate and citrate contents decreased. This is consistent with previous reports (reviewed in Andrews et al., 2013; Britto & Kronzucker, 2013). It is believed that the biosynthesis of sugars and their storage in the vacuole under high NH4+ supply represent a detoxification strategy (Bittsánszky et al., 2015) while lower contents in malate and citrate reflect the disappearance of their metabolic function as nitrate proportion declines in the nutrient solution. In fact, under nitrate nutrition, a proportionally higher flux of PEPC activity is generally observed, allowing the formation of negative charges, counterbalancing excess inorganic cations and ensuring pH homeostasis (Krapp et al., 2014), as opposed to what occurs under ammonium nutrition (Britto & Kronzucker, 2002, 2005; Gerendás et al., 1997). Surprisingly, there was no progressive decrease in PEPC activity as the ammonium proportion increased, under our conditions (except in bean leaves under high NH4+, Figure 4b), despite the change in organic acid content. This demonstrates that N nutrition impacted various pathways and the relative flux of PEPC activity −rather than its absolute flux value− declined compared to other metabolic fluxes. This effect was visible in fitted relative flux values in Figure 6, where one can see that PEPC activity was always low under our conditions, while other flux values (such as utilisation and remobilisation) increased as ammonium proportion increased.
Interestingly, δ13C of CO2 respired by roots was not as 13C-depleted as found previously (in practice, CO2 was more 13C-enriched than total root organic matter), for example in French bean (Bathellier et al., 2009). Therefore, under our experimental conditions, roots and leaves did not compensate for each other in terms of CO2 loss, so that δ13C of whole plant respired CO2 was higher than, rather than equal to, δ13C of whole plant organic matter (see Introduction). Such a situation has been encountered before in woody plants and occasionally in herbaceous species (reviewed in Ghashghaie & Badeck, 2014). Presumably, the isotope composition of root-respired CO2 is very sensitive to metabolic differences between species and growth conditions. In addition, the sampling of root-respired CO2 may involve root manipulation (e.g., washing, transfer in vials, etc.) and this could cause differences on average δ13C between methods and laboratories. Effects of different methods for the preparation of roots for respiration measurements and diverse approaches for quantification of isofluxes should be scrutinised with further research.
4.2 Is δ13C of metabolites related to δ13C of respired CO2?
Variations in δ13C of respired CO2 was not simply caused by a source effect (i.e. change in respiratory substrate) and did not directly reflect changes in δ13C of potential respiratory substrates (such as organic acids). In fact, we measured the carbon isotope composition of various metabolites (Figure 3) and found that (i) no specific metabolite had a response to N nutrition that was comparable to that of respired CO2; and (ii) no metabolite was always as 13C-enriched as respired CO2. In particular, we observed very limited changes in the δ13C of soluble sugars (except for sucrose in spinach leaves) across N conditions. Interestingly, differences in carbon isotope composition between glucose, fructose and sucrose in leaves and roots were in line with previous findings: sucrose was considerably 13C-enriched compared to glucose and fructose (Ghashghaie et al., 2001). We also note that maltose, which derives from starch degradation, was 13C-enriched, in accordance with the general 13C-enrichment in leaf starch compared to sucrose due to chloroplast enzymes isotope effects (Tcherkez et al., 2004). It is also unsurprising to observe some 13C-enrichment in root sucrose (compared to glucose and fructose) too, since there is no isotope fractionation during sucrose export (Maunoury-Danger et al., 2009).
Organic acids were significantly 13C-enriched relative to total soluble sugars, while the 13C-difference between them was more pronounced in leaves than in roots. Unsurprisingly, malate exhibited a strong 13C-enrichment, demonstrating the impact of the carbon input by PEPC activity. In fact, when dissolved CO2 equilibrates with bicarbonate, the equilibrium isotope effect associated with bicarbonate formation enriches in 13C by 9‰ (Marlier & O'Leary, 1984). Nevertheless, it should also be recognised that malate can be enriched as the result of isotope fractionations in malate degradation, leaving behind 13C-enriched malate molecules. For example, the malic enzyme is associated with an isotope fractionation (kinetic isotope effect) of 32‰ for CO2 liberation (C-4 atom of malate) (Edens et al., 1997). Parenthetically, it is remarkable that palmitate, which is synthesised from acetyl-CoA, shows limited changes in δ13C overall (Figure 3, dark green), suggesting that metabolic modifications impacting δ13C of respired CO2 were downstream of pyruvate dehydrogenation (i.e., acetyl-CoA production), that is, in organic acid pool turn-over and/or Krebs cycle metabolism.
The δ13C of amino acids showed a differential response in spinach and bean: while amino acids tended to be more 13C-enriched as the ammonium proportion increased in bean, they remained stable in spinach (Figure 3, bright green). It suggests that the metabolism of amino acids incorporated 13C-enriched material from carbon skeletons in bean, for example a greater proportion of 13C-enriched respiratory substrates. In this species, the demand for carbon skeletons to assimilate N was probably higher since the elemental % of N was higher (Supporting Information S1: Figure S5). Also, bean appeared to be slightly more selective against ammonium under intermediate ammonium nutrition (Figure 1) and therefore high ammonium conditions can be expected to have a more drastic effect on amino acids.
4.3 Metabolic origin of δ13C of respired CO2
Since potential respiratory substrates were not sufficiently enriched to account for the observed 13C-enrichment in respired CO2, it was necessary to address metabolic determinants of CO2 generation by respiration. The multivariate analysis conducted with all data points and samples suggested that CO2 could be predicted statistically via a multivariate component comprising δ13C of organic acids but also other variables including PEPC activity and δ13C in other substrates (Figure 5c). Our results show the important role of the δ13C in organic acids already found by Ghiasi et al. (2021) across NH4+:NO3- ratios in tobacco leaves. Nevertheless, it should be noted that δ13C of organic acids is always lower than that of respired CO2. As stated above, it indicates that there is either (a) a fractionation against 12C during decarboxylations, or (b) utilisation of a 13C-enriched pool that was not reflected in our compound-specific analysis (Figure 3). Hypothesis (a) is very unlikely since enzymes responsible for CO2 production are associated with a normal kinetic isotope effect, i.e., against 13C (Tcherkez et al., 2011). To our knowledge, there is only one exception, namely NADP-dependent isocitrate dehydrogenase, which has a very small kinetic isotope effect (Grissom & Cleland, 1988) and may exhibit an inverse thermodynamic isotope effect at equilibrium (Lin et al., 2008). Hypothesis (b) is much more plausible, since the consumption of organic acids by catabolism, N assimilation, malic enzyme, conversion to C5 branched acids, etc. likely fractionates against 13C, leaving behind a 13C-enriched pool (Rayleigh effect). In addition, this effect is believed to be responsible for the general 13C-enrichment in -COOH groups of organic acids (see, e.g., Hobbie & Werner, 2004; Schmidt, 2003), along with the natural 13C-enrichment in C-atom positions C-3 and C-4 of glucose, that are at the origin of C-atoms (COOH group) decarboxylated by pyruvate dehydrogenase (Gilbert et al., 2012; Rossmann et al., 1991).
We explored the credibility of hypothesis (b) with a model that accounts for the co-occurrence of a metabolically active pool and a pool of stored organic acids (described by Equations 4 and 5 in Material and methods). In fact, the model could generate relative δ13C of respired CO2 (Δδ13CR) in broad agreement with observed values (R² = 0.73; Figure 6b). Interestingly, such an agreement was observed regardless of species and organs, suggesting that the metabolic mechanism explaining the δ13C of respired CO2 was sufficiently generic. We recognise that there is still substantial variance (27%), due to probable species and organ effects as well as the contribution of metabolic pathways not accounted for, like OPPP. Modelling confirmed (1) the critical influence of the turn-over of the metabolically active pool, fed by remobilisation and used by respiration and other uses (with flux value that were relatively high); and (2) the fact that non-stationarity is probably important. In practice, it means that during plant development or during a diel cycle, the metabolically active organic acid pool is very dynamic and thus when respired CO2 is sampled, this pool is probably not in the steady state. Such a non-stationarity contributes to the observed 13C-enrichment because organic acids are consumed by fractionating reactions, thereby exaggerating the 13C-enrichment in acids left behind. Our results further suggest that this effect depends on N conditions, simply because utilisation, remobilisation and variation in pool size are impacted by the NH4+:NO3- balance (Figure 6c).
4.4 Perspectives
Taken as a whole, our results show that δ13C of respired CO2 is not simply linked to a unique metabolic source but rather, is a combination of flux values and δ13C of different components and this could be made apparent via both multivariate and modelling approaches. We nevertheless recognise that the drivers of respired CO2 found here may not apply to other situations, such as prolonged darkness (where the shift from sugar consumption to lipid degradation is involved) or the transition from light to dark in leaves (where light-enhanced dark respiration relates to malate degradation by the malic enzyme). Still, in all cases, emphasis should be given to non-stationarity of metabolic pools and substrates used by respiration. That is, respiratory substrates are very dynamic and their modification or their turn-over contributes to the observed 13C-signature of respired CO2. Of course, we also recognise that our modelling exercise was very simplified, since it did not account for all metabolic fluxes and furthermore, disregarded intramolecular isotope compositions. As stated above, there are important δ13C differences between C-atom positions, including those in organic acids. Unfortunately, there is presently no method implementable routinely to analyse δ13C of organic acids. Typically, using quantitative 13C-NMR would require sample preparation to convert COOH groups to their reduced forms (-CH2OH), break molecular symmetry (e.g., in the case of citrate) and block configuration changes and equilibria. To our knowledge, no such method has been published yet. This technical challenge will be addressed in a subsequent study so as to gain further insights into the isotope signature of CO2 generated by plant respiration.
ACKNOWLEDGEMENTS
Yang Xia wishes to thank the China Scholarship Council (CSC) for her PhD scholarship funding. The authors are grateful to Laboratoire d'Ecologie, Systématique et Evolution, ESE (University of Paris-Sud, Orsay, France) for the financial support of the experiments; Laboratory of ECOSYS (INRA, Grignon) for training on and assistance in isotope analyses of respired CO2; Annika Ackermann and Institute of Agricultural Sciences (ETH Zürich) for isotope analyses of organic material; Steffen Ruehlow and Max Planck Institute (Jena, Germany) for training on and assistance in LC-C-IRMS analyses of sugars and organic acids; Marlène Lamothe-Sibold for training on EA-IRMS; and the gardeners of ESE for helping in plant culture/care. Guillaume Tcherkez thanks the financial support by the Région Pays de la Loire and Angers Loire Métropole via the grant Connect Talent Isoseed.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data are shown in main text or supplementary material.




