Ignited competition: Impact of bioactive extracellular compounds on organelle functions and photosynthetic systems in harmful algal blooms
Huige Guo and Xiaochen Wang co-first authors.
Abstract
Prevalent interactions among marine phytoplankton triggered by long-range climatic stressors are well-known environmental disturbers of community structure. Dynamic response of phytoplankton physiology is likely to come from interspecies interactions rather than direct climatic effect on single species. However, studies on enigmatic interactions among interspecies, which are induced by bioactive extracellular compounds (BECs), especially between related harmful algae sharing similar shellfish toxins, are scarce. Here, we investigated how BECs provoke the interactions between two notorious algae, Alexandrium minutum and Gymnodinium catenatum, which have similar paralytic shellfish toxin (PST) profiles. Using techniques including electron microscopy and transcriptome analysis, marked disruptions in G. catenatum intracellular microenvironment were observed under BECs pressure, encompassing thylakoid membrane deformations, pyrenoid matrix shrinkage and starch sheaths disappearance. In addition, the upregulation of gene clusters responsible for photosystem-I Lhca1/4 and Rubisco were determined, leading to weaken photon captures and CO2 assimilation. The redistribution of lipids and proteins occurred at the subcellular level based on in situ focal plane array FTIR imaging approved the damages. Our findings illuminated an intense but underestimated interspecies interaction triggered by BECs, which is responsible for dysregulating photosynthesis and organelle function in inferior algae and may potentially account for fitness alteration in phytoplankton community.
1 INTRODUCTION
Marine phytoplankton coMADSntribute nearly half of the total primary production on earth, despite comprising only 1% of the photosynthetic biomass (Falkowski, 2012; Field et al., 1998). The disturbance of marine phytoplankton community structure triggered by long-range stressors related to global climate change, such as temperature and essential nutrients, which signify alteration of dominant populations, has been documented extensively (Burson et al., 2018; Cabrerizo et al., 2020; Garmendia et al., 2013; Gruber, 2011; Hu et al., 2011; Liu et al., 2021; Van Gennip et al., 2017). The escalation of harmful algal blooms (HABs) refers to the increasing fitness of harmful algae during the prevalent long-range interactions and increases negative effects on marine ecosystems and human health (Ralston & Moore, 2020; Thangaraj et al., 2022). Climate may shape the distribution of single harmful species directly, but the underlining mechanism of the species blooming may be mediated by interspecies interactions (Ockendon et al., 2014). In addition, several studies have reported that harmful algal species have adopted another strategy for hindering the physiological activities of their competitors; that is, releasing bioactive extracellular compounds (BECs) (Long et al., 2021a; Tillmann & John, 2002).
However, little is known regarding the enigmatic interactions among lower-trophic level phytoplankton in immediate environment, which are induced by BECs (Gong et al., 2014). Moreover, the BECs-induced interactions among related harmful algae that generate similar shellfish toxins remain unknown. According to the IOC-ICES-PICES Harmful Algae Event Database (HAEDAT) (Hallegraeff et al., 2021), over the past two decades, bloom events caused by Alexandrium spp. (312 events) have nearly doubled when compared with those attributed to Gymnodinium spp. (161 events) in relevant waters. Researchers speculate that Alexandrium spp. employ overlooked stressors to inhibit their competitors (Long et al., 2021a; Ma et al., 2011). This study focused on the interspecies interaction between two notorious algae, Alexandrium minutum and Gymnodinium catenatum, reportedly co-occur in various regions (such as Galician coast and Xiamen Bay) (Band-Schmidt et al., 2005; Bravo et al., 2010; Hernández-Sandoval et al., 2022; Liu et al., 2022). The two algal species share similar paralytic shellfish toxins (PSTs) profiles, including STX, GTX1/4, GTX2/3 and dcGTX2,3 (Supporting Information S1: Tables S1 and S2) (Lewis et al., 2018; Liu et al., 2020; Liu et al., 2022; Long et al., 2021b; Yang et al., 2010), to facilitate the alleviation of the influences solely attributed to PSTs.
The role of PST-independent BECs of A. minutum in algal physiology requires further research. We examined the interspecies interaction based on the cell morphology, suborganelle structure and biomacromolecules signatures, by performing numerous laboratory experiments and a comprehensive analysis. Transmission electron microscopy (TEM) and transcriptome analysis were employed to explore the possible changes in subcellular organelles and gene expression after incubation. Particularly, in situ focal plane array (FPA)-based Fourier transform infrared (FTIR) imaging, which can rapidly collect thousands of IR spectra for an entire cell, was employed, and high spatial resolution images were generated to elucidate biomacromolecular characteristics at the subcellular level (Baker et al., 2014). The technique has also been used to monitor cancer progression and plant pathology (Chatchawal et al., 2020; Girometta et al., 2020; Wrobel et al., 2017).
In short, this study aimed to elucidate short-range interaction triggered by BECs among phytoplankton within a short timescale, particularly the mechanism of PST-independent BECs produced by A. minutum mediate G. catenatum cells (Figure 1). This rapid and intense response to the enigmatic interactions inspired us to further insight into phytoplankton physiology dynamics from the perspective of intracellular microenvironment.
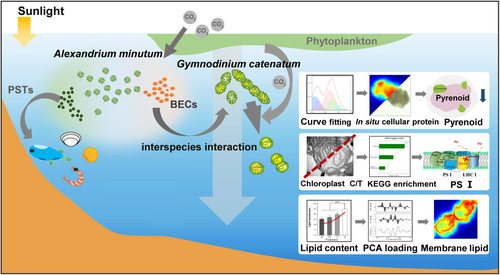
2 MATERIALS AND METHODS
2.1 Algal cultures and filtrate treatments
G. catenatum and A. minutum used in the present study are known to produce PSTs. Previous studies have reported that both strains exhibit a similar toxin profile, as shown in Supporting Information S1: Table S2. The algae were cultured in a modified f/2 medium supplemented with autoclaved seawater (Band-Schmidt et al., 2004; Guillard & Ryther, 1962). Cultures in all subsequent experiments were maintained in a climate-controlled incubator (Dengxun) at 20°C under a 12 L:12D regime with a light intensity of 100 μmol m−2s−1. To minimize the risk of spatial effects and cell deposition, the flasks were reassigned randomly and shaken manually twice daily. To determine the microalgae cytotoxicity, cell-free filtrate was prepared by filtering A. minutum supernatant in exponential phase (10 000 cells mL−1) through 0.2-μm PC filters (Millipore) and diluted to different concentrations (H: 10 000 cells mL−1, M: 1000 cells mL−1, L: 400 cells mL−1). The absence of residual cells in the filtrate was confirmed by light microscopy (Olympus). After measuring the concentration of nitrogen and phosphorus in the filtrate with an ultraviolet spectrophotometer (Thermo Scientific), an extra stock solution with nutrient levels similar to that of the f/2 medium was replenished to provide sufficient nutrients. The pH of the filtrate formulations was adjusted to 8.0 (±0.1) using NaOH before application to G. catenatum (Thermo Scientific). Salinity measurements of filtrate were performed with a hand-held refractometer (ATAGO) to ensure that the salinity of the filtrate is consistent with that of the f/2 medium.
G. catenatum pellets, harvested gently by centrifugation (300 g, 5 min) from cultures in the exponential phase, were resuspended in 300 mL of fresh f/2 medium and inoculated into diluted filtrates of different concentration, at a final density of 400 cells mL−1, comparable to previously reported densities of G. catenatum during blooming periods. Three treatments were used: including treatment L (A. minutum filtrate at 400 cells mL−1), treatment M (A. minutum filtrate at 1000 cells mL−1), and treatment H (A. minutum filtrate at 10 000 cells mL−1) and treatment R (fresh f/2 medium, control). Three replicate flasks were used for each treatment (Supporting Information S1: Figure S1).
2.2 Cell staining and optical observation
The cultured cells (1 mL) were removed aseptically from each flask daily and transferred into a Sedgewick Rafter counter chamber (Dengxun) for cell abundance determination. The cells were then fixed with Lugol's iodine solution and examined under an inverted microscope (Olympus). The growth rates (µ) were calculated according to the method of Guillard and Ryther (Guillard & Ryther, 1962). Two dyes were selected for cell morphology characterization. The cells were observed under a light microscope after staining with Giemsa (Sigma-Aldrich) for 30 min, as it stains the nucleus dark and cytoplasm light purple, we can focus on the morphology of the nucleus (Megha et al., 2022). In addition, the cells were visualized by fluorescence microscopy (at an excitation wavelength range of 515–565 nm) with red channel compensation (Image Pro) following staining with Rhodamine B (Aladdin) for live cell protein imaging (Wang et al., 2021). All the images were captured using a digital video camera connected to an optical microscope.
2.3 Image acquisition using scanning electron microscopy and transmission electron microscopy
Cells were fixed with 2.5% glutaraldehyde in the presence of A. minutum extracts and dehydrated in an ethanol gradient. After drying in supercritical CO2, the cells were coated with gold and observed under scanning electron microscopy (SEM) (Zeiss) at accelerating voltages of 10–20 kV. TEM was used for a more detailed ultrastructure analysis. Cells from 5-d incubation cultures were centrifuged (300 g, 5 min) (Hitachi) to remove the suspension and then fixed with 2.5% glutaraldehyde in sodium cacodylate buffer (0.1 M, pH 7.2) overnight at 4°C. After dehydration, a routine resin embedding protocol was used for 12 h, and ultrathin sections (70–90 nm) were obtained with a diamond knife (Leica) and visualized using TEM (Hitachi) at the organelle level.
2.4 In situ FPA-FTIR imaging
The G. catenatum cells were fixed with 4% formaldehyde, and the harvested residues were transferred into Eppendorf tubes after 30 min. The culture medium was removed by repeated centrifugal washing in step-gradient seawater (70%–50%–30%–0%, vol/vol) and excess water was removed from the upper layer. The pellets were then seeded onto the surface of BaF2 windows by pipetting. Before spectrum collection, the BaF2 window (Yunxiang) with specimens attached were placed in a dryer (Minbo) for at least 40 min. In situ FPA-FTIR chemical imaging was performed using a FT-IR imaging microscope system (Bruker) equipped with a 64 × 64 FPA detector and a 36× IR objective, with a final pixel size of 1.1 μm. In addition, to improve the signal-to-noise ratio (S/N), an external water-cooled high-power glowbar source (Bruker) was coupled to the system. Spectra of each sample over the 1000–3100 cm−1 range were acquired with the following parameters: transmission mode, and 128 coadded scans at 4 cm−1 spectral resolution. Since individual cells were tiny, adjacent pixel binning was not selected for clearer images (i.e., 64 × 64 pixel), and background spectra were obtained from a nearby cell-free region.
Representative spectra of each cell were calculated by averaging 40 evenly selected spectra in OPUS 8.2 (Bruker). Several R packages designed for data preprocessing were used. For the Savitzky–Golay smoothing, a 9-point window and second-order polynomial were selected for the best results. After cutting off the CO2 peaks that shifted in the 2200–2400 cm−1 range, in situ false colour images of the integrated area of the protein-specific range (1500–1700 cm−1) and lipid-specific range (2800–3100 cm−1) were performed using CytoSpec v 2.00.06 (CytoSpec) with a straight baseline drawn between the integral limits. The biomacromolecules corresponding to the characteristic peaks of interest in this study are shown in Supporting Information S1: Table S1.
2.5 RNA sequencing and whole transcriptome analysis
Total RNA was extracted from G. catenatum cells at two-time points (2 and 5 d) after subjection to two treatments (L and H) and a control (R), with each treatment having three replicates. The concentration and quality of the extracted RNA were determined using NanoDrop 2000 spectrophotometer (Thermo Scientific) and Agilent 2100 bioanalyzer (Agilent Technologies). The Illumina TruSeqTM RNA Sample Prep Kit (Illumina) was used to generate mRNA libraries, with 15 PCR cycles and then sequencing was performed on the Illumina Novaseq. 6000 platform (Illumina). To assess differential gene expression among the treatments, DESeq. 2 was used to construct PCA clusters. Additionally, SMRT sequencing of DNA from the isolated chloroplasts fraction was performed, as described by Del Cortona et al. (2017).
2.6 Statistics analysis
One-way ANOVA was used to compare the means of four treatment groups, and a p-value < 0.05 was considered significant. To determine the intrinsic proximities and distances within the data set and to cluster treatments according to spectra similarity, two unsupervised analyses, including hierarchical cluster analyses (HCA) using Ward's algorithm, and principal component analyses (PCA), were performed.
3 RESULTS
3.1 Growth and morphological alternations were observed in interspecies interactions induced by BECs
G. catenatum cells were cultured in four different concentrations of A. minutum cell-free filtrates, at concentrations of 0 (control), 400, 1000 and 10000 cells mL−1 (concentration of BECs in the filtrates were replaced by the cell concentrations in the medium before filtration). Thereafter, the four protocols in diverse concentrations of filtrates were referred to as treatment R (control), L, M, and H from low to high.
Even filtrates from low A. minutum cell concentrations reduced G. catenatum densities significantly, as evidenced by a decrease in biomass accumulation after 24 h (Figure 2a). This underscores the efficiency and significance of BECs in inhibiting potential competitors. The exudates had a similar inhibitory effect on G. catenatum at lower cell concentrations (treatment L and M), with an inhibition range of 30%–50%. The maximal inhibition of cell growth was achieved in treatment H in the initial 5 days, with the highest inhibition ratio of 59% (Figure 2a). After 4 d of growth, cell biomass in treatment R tended to be stable, and more than 29% of the cells formed chains of 4–8 cells, while an upward trend in the proportion of individual cells was observed in three treatments (Figure 2b). Over the 10 days of exposure to A. minutum filtrate, virtually all G. catenatum cells in treatments transformed from their typical subspherical or biconical shape to irregular shapes as shown in Figure 2c. The indistinct cingulum shown in the ventral view was a major feature of the swollen cells. Within 24 h, the naked cell wall began to shed from the surface of the cells as the BECs retracted. Similar changes were also observed in G. catenatum after exposure to free fatty acids, which were considered as effective metabolites in allelopathy (Encinas-Yánez et al., 2024). At the subcellular scale, organelle membranes collapsed into the cytoplasm on elevation of concentration of BECs, leaving no apparent compartmentation (Figure 3a).
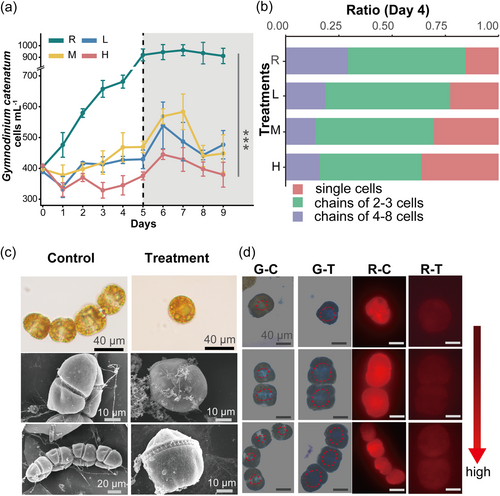
After staining with Rhodamine B, the red fluorescence of cytoplasmic proteins of treatment cells faded remarkably when compared to the control (Figure 2d). In addition, nuclei reacted blue with Giemsa in cells and were scattered and irregular in all treatments (Figure 2d). Extracellular attachments were visible on the surface of treated cells, as shown in the scanning electron microscope (SEM) images (Figure 2c). Overall, the findings of chain and cell morphologies may reflect the possible mechanism of the toxic effect of BECs produced by A. minutum.
3.2 Organelle distortion and subcompartment disorder: main signals for G. catenatum response
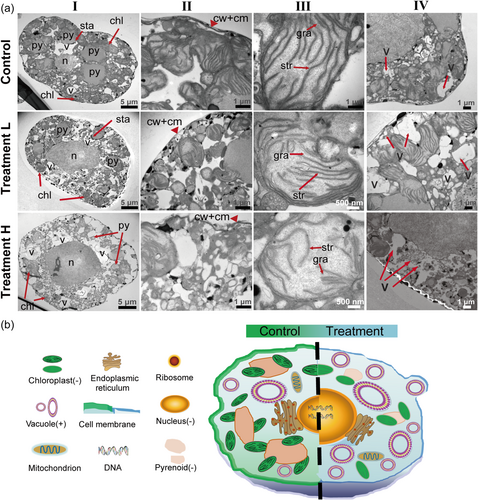
In the TEM analysis, significant changes were observed in the organelles and subcompartments of G. catenatum cells after treatment with BECs produced by A. minutum after 5 days. Compared to the control cells, subcellular structure of cells in treatment L and H showed light and significant damages, respectively. The cells lost their subspherical shape (Figure 3a [I]), and their thinner cell membrane was locally deformed (Figure 3a [II]), resulting in an enlarged nucleus. The deformation appeared to account for the modification of plasmalemma by BECs, which is consistent with the earlier results that Alexandrium spp. may have lytic compounds, which may disrupt cell membranes (Hernandez-Garcia & Martinez-Jeronimo, 2023; Long et al., 2021a). Besides, accumulation of vacuoles around the plasma membrane was observed (Figure 3a [IV]), suggesting that G. catenatum may adapt to BECs by mediating lipid distribution and contents, as well as regulation of the proportions of the components of the bilayer. The evidence provided a compelling reason to investigate the disrupting role of BECs (Figure 3b).
Furthermore, pyrenoids, the organelles responsible for carbon uptake (Wang & Jonikas, 2020), decreased in size and even disappeared in some cells, and the remaining ones loosely associated with chloroplasts, relative to their counterparts in control cells (Figure 3a [I]). Average diameters of pyrenoids decreased by almost 50% when compared with control cells (3.2 vs. 5.3 μm).
TEM data suggest that the pyrenoids in G. catenatum cells treated with BECs produced by A. minutum were attached to the chloroplasts loosely, followed by their localization spreading out from the cell centre. In addition, starch sheaths that prevent CO2 diffusion from the pyrenoids were shed from the periphery of the pyrenoids. Furthermore, inspection of subcompartments in the chloroplasts revealed additional characteristics. Unlike the control, chloroplast envelopes of treated cells were swollen, the grana lamellar structure was degraded and occupied a smaller area and thylakoids were distributed with any stacking pattern. The modifications of organelles, including decompartmental pyrenoid and disorganized grana thylakoids (Figure 3a [III]) affected mean photosynthetic production of G. catenatum, which is consistent with the decrease of red fluorescence in Chatoceros neogracile (Lelong et al., 2011). The results may suggest that the photosynthetic apparatus in G. catenatum was damaged by the BECs produced by A. minutum.
3.3 Redistribution of cellular lipids and proteins
After 5 days incubation under BECs, changes in biochemical properties of the G. catenatum under the effect of BECs produced by A. minutum were evaluated by FPA-FTIR. Different from traditional techniques, using FPA-FTIR, we can observe the substances composition in individual cells with high resolution on an in situ basis, so as to depict the dynamics of the macromolecular pool in G. catenatum cell after BECs incubation. The vibrational values were assigned and compared to the different types of biomacromolecules reported in previous studies (Meng et al., 2014). After scattering correction of each spectrum of each individual cell, average spectra were used to perform comparisons between the control group and treatments (Supporting Information S1: Figure S2), significant differences can be observed in HCA results (Figure 4a). Notably, unsupervised clustering accurately classified nearly all spectra from multiple replicates across the full range (1000–3050 cm−1). All treatments clustered in one-branch, while the control was segregated, facilitating better probing of the differentiated pathway between the control and treatments through examination of the main biomacromolecule pools (Baker et al., 2014).
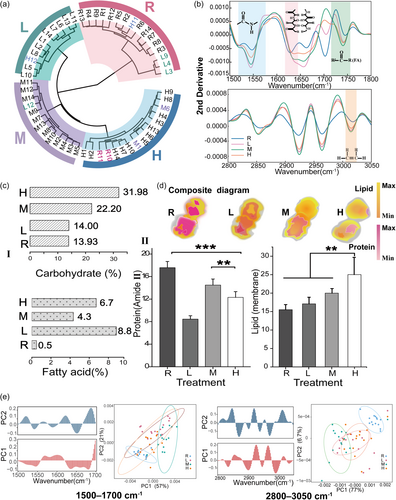
Notably, differences were observed in the bands at 3013 cm−1, which are assigned to =CH groups of polyunsaturated lipids (Figure 4b) (Fung et al., 1996). The bands were less intensive in cells when exposed to higher concentrations of treatments of BECs produced by A. minutum (treatment M and H), resulting in a reduction in unsaturated acyl chains of lipids. The increase in membrane lipid saturation of G. catenatum may be an attempt to increase fitness. An inverse relationship has been previously observed between unsaturated hydrocarbons and lipid peroxidation (Fung et al., 1996), and it is generally agreed that lipid peroxidation inhibits tumour cell growth (Girometta et al., 2020; Morisaki et al., 1982; Simsek Ozek et al., 2010). In photosynthetic organisms, several research with respect to the active restructuring of membranes maintaining adequate membrane fluidity refer to marine phytoplankton (Sepulveda & Cantarero, 2022; Vigneault et al., 2000). In more detail, bands arising at ∼2923 and ∼2853 cm−1 belong to the asymmetric and symmetric CH2 group stretching motions (Clède et al., 2014; Thangaraj et al., 2022), and yielded greater absorbance values in G. catenatum cells exposed to BECs filtrates (Figure 4b). In addition, the values of full width at half-maximum for the asymmetric CH2 band increased distinctly(Garip et al., 2007). Lower membrane fluidity occurred if the bandwidth of the asymmetric CH2 band was lower than normal (Simsek Ozek et al., 2010). We also calculated the area range of the spectra from 2800 to 3025 cm−1 using curve fitting (Supporting Information S1: Figure S3), which is specific to total membrane lipid content. In the filtrates from low, medium and high-density A. minutum cell cultures, 12%–42% more lipids accumulated when compared with those in the control (p < 0.05) (Figure 4c). Similar changes in lipid content have been observed in Thalassiosira pseudonana (Mao et al., 2021).
A characteristic decrease in the ratio of symmetric/asymmetric vibrations of CH2 was observed in all treatments, particularly in the culture with the highest number of cells (treatment H); conversely, the methylene/methyl ratios (CH2/CH3) were relatively high post filtrate addition (Supporting Information S1: Table S4). Previous studies have reported that low-branched fatty acid contents may be associated with thick cell membrane (Mao et al., 2021; Melin et al., 2001); thus, in our results, the high value of CH2/CH3 provided evidence of the thickened membrane after exposure. Even more apparent was the slight band downshift (~4 cm−1) of the ester carbonyl peak at approx. 1740 cm−1 in the spectrum of treated cells (p < 0.05) (Liu et al., 2022).
The first principal components (PC1) primarily accounted for the majority of differences (in the 2800–3050 cm−1 range) between the control and three treatment groups (Figure 4e). The lipid region from the control cells was grouped in the positive direction of the graph, whereas the cells treated with BECs had mostly negative PC1 values. At 2800–3050 cm−1, PC1 (77%) highlighted predominant positive loadings with a band centre of 2925 and 2963 cm−1. Evaluation of the PC1 and PC2 loading plot indicated the influence of a peak at 3013 cm−1, which was characteristic of vibrating cis-alkene group, and revealed a decrease in unsaturation level of lipids (see scree information in Supporting Information S1: Figure S4) (Kunyaboon et al., 2021). Considering some of the most remarkable changes at 3013 cm−1 were detected in the cells exposed to filtrates, FTIR mosaic imaging has been used to visualize the spatial distribution of lipids in individual cells in situ (Figure 5). An increase in lipid content was observed in incubated cells following exposure to BECs. The lipid-bilayer phase is thicker due to the higher packing of acyl chains than of nontreated cells. As the cell concentrations of A. minimum increased, in treatment H, there was a higher degree of lipid expression throughout the G. catenatum membrane. The apparent disorder of the first defensive barrier observed in the images was consistent with the TEM depiction (lost their membrane integrity) and staining consequences (more permeable) (Figures 3a and 2d). Changes in membrane fluidity and thickness suggest that unprevailed competitor fitness in short-range interactions may modulate through lipid dynamics.
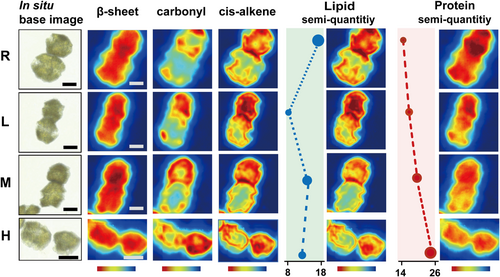
Bands associated with protein in the 1500–1700 cm−1 region, including Amide I and Amide II, were assigned to C = O stretching, C–N stretching, and N–H in-plane bending, respectively (Wood et al., 1998; Yano et al., 2000). In comparison to the control, the most significant increments in the peaks at ∼1656 and ∼1635 cm−1, which corresponded to α-helix and β-sheet, the shifting was generally assigned to disordered protein secondary structure. The α-helix band underwent a shift toward a high wavenumber at 6 cm−1 after treatment, β-sheet displayed a left shift 10 cm−1 apart (Figure 4b). Previous publications have shown that such shifts are due to changes in the intensities of hydrogen bonds between polypeptide groups, which lead to deformation of the protein's secondary structure and protein folding damage (Subirade et al., 1994). An increase in the ratio of 1637/1657 cm−1 also supported this view (Supporting Information S1: Table S4) (Allain et al., 1999). Combined with stoichiometry, PC1 versus PC2 score plot (1500–1700 cm−1) revealed that control cells had negative PC1 and negative PC2 values (Figure 4e). Treated cells yielded values around zero for PC1, whereas PC2 values were positive. The loading plot for PC1 (77.6%) indicates the key differences in wavenumbers that are responsible for grouping the samples along PC1, more specifically the protein pool feature that differentiated the cell according to the peak at 1650 cm−1 (see scree information in Supporting Information S1: Figure S4).
Based on the TEM data, the acutely different localization patterns of large amount of pyrenoids, support the view that they may have lost part of their functions. Exposure to BECs resulted in significant reduction in total protein (p < 0.01) (Figure 4d). The carbohydrate-to-protein and fatty acid-to-protein ratio were calculated based on the band area of C–O stretching and bending band and ester carbonyl band relative to the total area of the Amide II (Figure 4c [I], [II]). Significant increase in both ratios were observed in all treatments. Recent reports on the lipid/protein ratios may help to improve our understanding of cell compartments, such as the distribution of Golgi apparatus and endoplasmic reticulum (Clède et al., 2014; Meng et al., 2014).
3.4 Upregulated genes associated with photosynthetic apparatuses increase fitness of disadvantaged members
Interestingly, at all concentrations, G. catenatum incubated in 400 and 10 000 cells mL−1 filtrate had striking results on Days 2 and 5 (Figure 2a). The discriminative characteristics were explored by assuming a time dynamic, including short-term instability and long-term variation. The control and four treatments were named R, L2, L5, H2 and H5, respectively, for clarity. Concentration was the primary effect among four treatments as evidenced by hierarchical clustering (K-means) (Figure 6c).

The number of detailed differential expressed genes (DEGs, >2-fold change; p < 0.05) between all treatments and control are shown in Figure 6e. The expression levels of 708 genes were upregulated and 56 were downregulated in the L2, while 2493 genes were upregulated and 119 genes were downregulated in L5. In both H2 and H5, more than 5000 genes were upregulated. The upregulated genes are likely to be crucial to the dynamic response between related dinoflagellates (Collén et al., 2007). Furthermore, DEG profiles at different concentrations followed the distinct short-term and long-term patterns. A total of 6050 genes were shared among the R, L2 and L5 (Figure 6d), which were less diverged than between the R and H treatments (2070). L2 had 1270 unique genes shared with L5, and H2 had 229 genes shared with H5. Seven representative expression clusters from DEGs in response to different durations were identified using the STEM program, including three significant clusters (Figure 6f).
Following a KEGG enrichment analysis, gradually downregulated clusters of pathways related to carbohydrate metabolism (Figure 6g), such as glycolysis/gluconeogenesis, tricarboxylic acid (TCA) cycle and starch/sucrose metabolism, were observed in H2 and H5. However, majority of the photosynthesis pathways, including antenna proteins, oxidative phosphorylation and carbon fixation, exhibited upregulated changes, possibly in an attempt to offset the metabolism stress response to the toxic filtrate. Similarly, the clusters of regulated patterns were uncovered at low concentrations (L2 and L5). Additionally, in an effort to improve the annotation of the G. catenatum chloroplast transcriptome, the single-molecule real-time (SMRT) method allowed us to perform long-read sequencing with higher accuracy (Del Cortona et al., 2017).
The overexpression of genes encoding light harvesting complex I (LHC I) proteins, especially Lhca1 and Lhca4, was observed in both treatments L and H. The expression of Lhca1 was upregulated approximately two-fold at Day 5 when compared with that at Day 2 upon low concentration incubation (treatment L), while an inverse ratio was noted at treatment H (Supporting Information S1: Figure S5). This is also the case for Lhca4, which may be because Lhca1 and Lhca4 were arranged in the form of a heterodimer. Considering four antenna complexes combined in pairs as Lhca1–4 and Lhca 2–3 dimers, the absorption cross-section of the PS I core could be raised by 60% (Croce & van Amerongen, 2020). The upregulated Lhca1 and Lhca4 genes may have promoted the fitness of G. catenatum cells by accelerating antenna protein production for light harvesting, which minimized the BECs stress on the cells. TEM (Figure 3a) revealed the thylakoids swelled gradually and became undistinguishable, and the grana stacks were loosely connected by the stroma lamella, which adversely affected the efficiency of light harvesting and excitation energy transfer. In situ images (Figure 5) also revealed the weight ratio between protein and lipids does not follow the primary value (1:1) (Barber & Gounaris, 1986).
Moreover, the level expression of Rubisco, which is responsible for efficient assimilation of CO2 into the pyrenoids, was increased significantly in all four treatments (Supporting Information S1: Figure S5). This suggests that the addition reaction of CO2, which is catalyzed by Rubisco, was partly inhibited. TEM observations provided critical clues for evaluating functional damage to chloroplasts and pyrenoids at the suborganelle scale. In G. catenatum, multiple-stalked pyrenoids were supported by at least two chloroplast projections. However, after incubation, the thylakoids that penetrated pyrenoids deformed, which impairs the conversion of carbonic anhydrase (CA) in the lumen. In addition, the matrix, which was the main subcompartment in pyrenoids for Rubisco distribution, shrank. Under low-CO2 conditions and decreased Rubisco enzyme coexistence, the generation of 3-phosphoglyceric acid (3-PGA) in the matrix was considerably inhibited (Figure 6a,b). The inhibition of the C3 or C4 pathway was similar in this case (Treves et al., 2022). Moreover, the exposure of A. minutum resulted in the disappearance of starch sheaths surrounding the pyrenoids (Figure 3a), thereby promoting CO2 leakage across the weakened barrier (Toyokawa et al., 2020).
4 DISCUSSION
This study unveiled interactions among marine phytoplankton in immediate environment, which influences algal physiology induced by BECs. This stressor can trigger acute stress responses to competitors in phycosphere within a short timescale. The underestimated but ignited interspecies interaction may potentially affect the community structure of marine primary producers.
4.1 BECs can weaken PS I photon capture and deform thylakoids of algal chloroplast
G. catenatum was exposed to BECs conditions caused by another key harmful alga, A. minutum, and a significant inhibition effect on the growth rhythm of G. catenatum was observed. The study focused on the interspecies interaction in the phycosphere, in which pyrenoid and chloroplast subcompartment damages were observed in G. catenatum as stress responses, particularly involved in starch sheath loosing, spheroidal matrix disorder and grana thylakoids swelling (Figure 3a). The redistribution of lipids and proteins occurred at a subcellular level as revealed by in situ FPA-FTIR imaging and dynamic regulations of transcriptome approved the damages (Figure 5). The results revealed that PST-independent BECs produced by A. minutum disturbed the inferiors' physiological dynamics by weakening functions of photosystem-I light-harvesting complex I and CO2 concentration organelles (pyrenoids and thylakoids) in G. catenatum. Changes in membrane lipid composition and proportion under specific stresses have been reported to be an important mechanism of stress adaption, although these adaptations may be at the expense of partial photosynthetic functions. Suaeda glauca (Bunge) can enhance salt tolerance by increasing the content of phosphatidylglycerol, but the accumulation of Na+ in cytoplasm leads to the change of glycolipids (Sui & Han, 2014), which constitute the basic skeleton of the thylakoid membrane. The light reaction site is located on the thylakoid membrane, abnormal glycolipids composition interferes with the formation of thylakoid stacks and thus reduces the light reaction rate. In Triticum aestivum L. cells, when under high temperature, a large amount of monogalactosyldiacylglycerol gathered in the thylakoids and phase transition occurs (Djanaguiraman et al., 2020; Higashi et al., 2015), further destroys its normal function of thylakoids. In our study, we hypothesized that the regulations in the structure and the membrane lipid composition of deformed thylakoids in G. catenatum may be a positive response of their photosynthetic system to BECs injury.
PS I with Lhca1 and Lhca4 antenna proteins is essential for photosynthesis (Su et al., 2019). During the short-range interaction, BECs can weaken PS I photon capture and deform thylakoids, indicating that the synthesis capacity of Lhca1–Lhca4 dimer is insufficient to meet the high demand for newly synthesized antenna proteins (Figure 6a). Previous studies have focused on the redistribution of mobile LHC II antenna proteins or regulation of the LHC II-related genes under stress such as heat, light and persistent organic pollutants (Iwai et al., 2008; Minagawa & Takahashi, 2004); however, our findings suggest that BECs are more likely to attack LHC I, which affects the normal functioning of PS I, that is essential for cyclic electron flow (CEF) and linear electron flow (LEF) (Girometta et al., 2020; Lunde et al., 2000). In the thylakoid membrane, inhibition of CEF and LEF does not generate a sufficient proton gradient to match the ATP:NADPH ratio of 3:2 to drive Calvin cycle, especially for C3 plants (Joliot & Joliot, 2006). In addition, massive transformation of grana stacks and loosening of uniform stromal thylakoids interfere with light reaction. Such changes indicate that G. catenatum is sensitive to BECs produced by A. minutum. Together with the conformational changes and redistribution of proteins as revealed by in situ FPA-FTIR images, we depict the major molecular mechanisms that link the phenotypic changes to the pronounced degradation of the G. catenatum photosystem. The functional inhibition of the photosystem is explained by the overexpression of genes coding for antenna proteins, which forces the regulators in LHC I to tap into in vivo stress cues to trigger and boost the productivity of photosynthesis to resist the inhibition caused by related harmful algae (Figure 6g).
4.2 Upregulated rubisco acts as a defense response involving structural destruction of pyrenoids
Upregulated Rubisco acts as a defense response involving structural destruction of pyrenoids, increasing fitness through CO2 uptake and assimilation under the effect of BECs. Adaptive strategies on Rubisco regulation may have a strong correlation with pyrenoid disorganization. Some mutants that fail to form intact pyrenoids highlight the importance of the pyrenoid in the CO2 concentration mechanism (CCM) (Toyokawa et al., 2020; Zalutskaya et al., 2019). After the induction of BECs, the cross-sectional area shrunk by more than half. Shrunk matrix and missing starch sheaths, along with the loss of integrity of tubules formed by thylakoid membrane, indicate that all processes in which CO2 occurs in the pyrenoid were hindered, presenting a defense mechanism involving structural destruction of pyrenoids. Dynamic biomacromolecular changes of cellular lipids and proteins are also well signatured by in situ FTIR imaging (Figure 5), and the results correspond to the shrinkage of pyrenoids. The change in pyrenoid is similar to the result observed by Iswarya and Zhang (Iswarya et al., 2015; Zhang et al., 2020). A few studies have revealed a correlation between the induction of CCM and fatty acid desaturation in Chlamydomonas reinhardtii, the increase in saturation of membrane lipid of G. catenatum could be a potential adaption mechanism for degraded CCM to modulate membrane fluidity (Pronina et al., 1998). This may represent an energetic trade-off in some phytoplankton: to quickly respond to negative stress, they could divert energy away from photosynthesis towards stress protection such as the remodelling of fatty acids (Britton et al., 2020). According to the results, G. catenatum may have a remarkable protective mechanism for increasing carbon assimilation efficiency.
4.3 Interspecies interactions among harmful algae affect phytoplankton physiology directly
Competition between A. minutum and G. catenatum could serve as a new pattern for determining interactions among marine neighbouring phytoplankton, which provides insights into community homoeostasis. Several studies have shown the adaptive acclimation of the highly destructive Alexandrium genus to climate change (Garrido et al., 2023; Mardones et al., 2017; Thangaraj et al., 2022). Differ from concentrating on BECs toxic effects of Alexandrium spp. on remote species (Su et al., 2019; Wang et al., 2017; Wang et al., 2024), we demonstrate that A. minutum can substantially inhibit related harmful algae, such as G. catenatum by influencing their light harvesting efficiency and CO2 addition effectiveness without cell contact, after alleviation of the interference from PSTs. Chain and cell damage mechanism will enhance our understanding to make projections of this overlooked stressor on marine primary ecosystem will kill not only just higher trophic level species but also inhibit the physiology of related phytoplankton (Hallegraeff et al., 2023).
This study briefly investigated an underestimated short-range interaction among marine harmful algae, which ultimately influences algal physiology through BECs within a short timescale, though we could not fully define the core components of BECs in A. minutum. This is an attempt to determine the potential competitive relationships among phytoplankton and the cellular physiology that influences these relationships in their natural environment. Significantly, these key physiological attributes may not be unique to a specific genus but are widespread across many harmful algal taxa. Competition between individuals is part of the structural dynamics of communities, and inquiry into enigmatic interspecies interaction can guide understanding of the ecology of harmful phytoplankton communities under a pressing climate scenario. This pattern of ignite interaction between harmful algae may also be helpful when considering the prediction and management of harmful algal blooms.
ACKNOWLEDGEMENTS
This work was financially supported by the National Key Research and Development Program of China (2019YFE0124700), the Scientific Research Foundation of Third Institute of Oceanography, Ministry of Natural Resources (2019002) and Bundesministerium für Bildung und Forschung (01LP2007A). The authors thank Dr. Peter Lasch from Cytospec for providing access to their software. Huige Guo and Xiaochen Wang have made the equal work.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the Supporting Information of this article.




