3-ketoacyl-CoA synthase 19 contributes to the biosynthesis of seed lipids and cuticular wax in Arabidopsis and abiotic stress tolerance
Na Luo and Yulu Wang are contributed equally to this study.
Abstract
Very-long-chain fatty acids (VLCFAs) are essential precursors for plant membrane lipids, cuticular waxes, suberin, and storage oils. Integral to the fatty acid elongase (FAE) complex, 3-ketoacyl-CoA synthases (KCSs) function as crucial enzymes in the VLCFA pathway, determining the chain length of VLCFA. This study explores the in-planta role of the KCS19 gene. KCS19 is predominantly expressed in leaves and stem epidermis, sepals, styles, early silique walls, beaks, pedicels, and mature embryos. Localized in the endoplasmic reticulum, KCS19 interacts with other FAE proteins. kcs19 knockout mutants displayed reduced total wax and wax crystals, particularly alkanes, while KCS19 overexpression increased these components and wax crystals. Moreover, the cuticle permeability was higher for the kcs19 mutants compared to the wild type, rendering them more susceptible to drought and salt stress, whereas KCS19 overexpression enhanced drought and salt tolerance. Disrupting KCS19 increased C18 species and decreased C20 and longer species in seed fatty acids, indicating its role in elongating C18 to C20 VLCFAs, potentially up to C24 for seed storage lipids. Collectively, KCS19-mediated VLCFA synthesis is required for cuticular wax biosynthesis and seed storage lipids, impacting plant responses to abiotic stress.
1 INTRODUCTION
Very-long-chain fatty acids (VLCFAs), characterized by fatty acid chains containing 20 or more carbon atoms, play a pivotal role as essential building blocks for biologically significant lipids. These lipids, including cuticular waxes, aliphatic suberin, phospholipids, sphingolipids, and triacylglycerols (TAGs) stored in plant seeds, are indispensable for various physiological processes such as membrane trafficking, cell division and differentiation, prevention of water loss, and energy storage (Haslam & Kunst, 2013; Kunst et al., 1992; Nobusawa et al., 2013). VLCFA biosynthesis initiates with the elongation of shorter saturated and monounsaturated C16 and C18 fatty acids produced within plastids. The elongation process occurs within the endoplasmic reticulum (ER), orchestrated by fatty acid elongase (FAE) complexes. The process involves condensation of the C2 carbon unit to acyl-CoA, followed by reduction, dehydration, and reduction reactions catalyzed by specific enzymes: 3-ketoacyl coenzyme A synthase (KCS), 3-ketoacyl coenzyme A reductase (KCR), 3-hydroxyacyl-CoA dehydratase (HCD/PAS2), and trans-2-enoyl-CoA reductase (ECR/CER10) (Supporting Information S1: Figure 1) (Haslam & Kunst, 2013). Iterative execution of this reaction cycle yields VLCFAs spanning a range of chain lengths from C20 up to C38 and beyond.
Cuticular waxes, comprising a complex mixture of VLCFAs and their derivatives such as aldehydes, alkanes, primary alcohols, secondary alcohols, ketones, and wax esters (Kunst & Samuels, 2003; Post-Beittenmiller, 1996) undergo specific conversions within epidermal cells. These conversions lead to the production of primary alcohols and wax esters through an acyl reduction pathway or the generation of aldehydes, alkanes, secondary alcohols, and ketones through a decarbonylation pathway (Supporting Information S1: Figure 1) (Kunst & Samuels, 2003; Samuels et al., 2008). Moreover, a distinct polyketide synthase pathway responsible for the synthesis of beta-diketone cuticular waxes has been identified (Sun et al., 2023). The complex cuticular wax mixtures are transported to the plant's outer surface through ATP-binding cassette transporters on the plasma membrane (Bird, 2008; Mcfarlane et al., 2010; Pighin et al., 2004) and glycosylphosphatidylinositol-anchored lipid transfer proteins (Kim et al., 2012). Cuticular waxes not only play crucial roles in reducing non-stomatal water loss, repelling pathogens and environmental particles, and protecting plants from harmful ultraviolet (UV) radiation (Barthlott & Neinhuis, 1997; Reicosky & Hanover, 1978; Riederer & Schreiber, 2001), but also underscore the multifaceted significance of VLCFAs in plant biology.
Apart from their role in cuticular waxes, VLCFAs serve as versatile precursors in various plant structures, including aliphatic suberin, membrane phospholipids, and seed storage lipids. Aliphatic suberin, acting as a barrier that restricts water and nutrient movement, provides protection against environmental stresses (Franke et al., 2012; Pollard et al., 2008). The suberin biosynthesis process involves the oxidation of the ω-carbon of VLCFAs, leading to the production of α, ω-dicarboxylic acids (Compagnon et al., 2009; HöFER et al., 2008; Molina et al., 2009). These intermediates can be further modified into glycerol-3-phosphate or ferulic acid (Molina et al., 2009; Yang et al., 2010). Additionally, VLCFAs are integrated into membrane phospholipids (Devaiah et al., 2006), sphingolipids (Markham et al., 2013), and seed storage lipids (Millar & Kunst, 1997), contributing to the unique properties of these lipids.
The initial step in the elongation of fatty acids, catalyzed by KCS, is a key determinant of VLCFA production. In Arabidopsis, a diverse family of 21 KCS members is categorized into eight subclasses: α, β, γ, δ, ɛ, ζ, η, and θ (Joubès et al., 2008). Specific roles and substrate preferences characterize individual KCS members, influenced by carbon chain length and unsaturation. For instance, FAE1/KCS18, a seed-specific condensing enzyme, elongates fatty acids up to C22 to produce seed storage lipids (Blacklock & Jaworski, 2006; James et al., 1995; Trenkamp et al., 2004). KCS6/CER6/CUT1 and KCS5/CER60 contribute to the elongation of fatty acyl-CoAs longer than C28, crucial for cuticular waxes in the epidermis and pollen coat lipids (Fiebig et al., 2000; Hooker et al., 2002; Millar et al., 1999). KCS20 and KCS2/DAISY function redundantly in the two-carbon elongation to C22 VLCFAs for cuticular wax and root suberin biosynthesis (Lee et al., 2009). KCS9 extends C22 to C24 fatty acids for cuticular waxes, aliphatic suberins, and membrane lipids (Kim et al., 2013). KCS16 is associated with C36 and C38 VLCFAs for wax production in leaf trichomes (Hegebarth et al., 2017), as well as C22 and C24 VLCFAs essential for lateral root development (LV et al., 2021). KCS4 is implicated in VLCFA elongation during root and pollen tube growth (Kim et al., 2021), while KCS17 synthesizes C24 VLCFAs crucial for suberin in seed coats (Kim et al., 2023). It's crucial to emphasize that while these examples provide valuable insights into KCS functionality, they do not present a comprehensive list of known functions within the KCS family. Addressing the challenges associated with characterizing enzymes within the KCS family, such as KCS 19, as will be discussed later, requires further exploration.
A previous report from epidermis/stem gene expression ratios suggested a potential role in cuticular wax biosynthesis for KCS19 (Suh et al., 2005). However, attempts to ectopically express Arabidopsis KCS19 in yeast and Nicotiana benthamiana leaves failed to significantly affect VLCFA accumulation (Batsale et al., 2023; Blacklock & Jaworski, 2006). Consequently, uncertainty remains regarding the association of the KCS19 gene with VLCFAs and cuticular wax biosynthesis. This study addresses these uncertainties by isolating Arabidopsis kcs19 mutants and analyzing cuticular waxes and seed fatty acids across various lines (wild type, kcs19 mutants, KCS19 complementation lines, and overexpression lines), and exploring the impact of KCS19 on plant tolerance to drought and salt stress. This research aims to provide a comprehensive understanding of KCS19's involvement in cuticular wax and seed fatty acid biosynthesis, shedding light on its potential role in stress tolerance mechanisms.
2 MATERIALS AND METHODS
2.1 Plant materials and growth conditions
Arabidopsis kcs19-1 (SALK_131405) and kcs19-2 (SALK_030654) T-DNA insertion lines were obtained from the Arabidopsis Biological Resource Centre. Selection of homozygous lines was based on kanamycin antibiotic resistance and further confirmed by PCR genotyping. All Arabidopsis plants, including the wild-type (Col-0 ecotype), mutants, and transgenic lines, were cultivated in 1/2 MS (Murashige and Skoog) media or in soil under controlled growth conditions with a 16-h light/8-h darkness photoperiod, maintaining a light intensity of 12 000 lux at 22°C. To examine the effects of hormones and stress conditions on gene expression, 4-week-old plants were subjected to liquid cultures supplemented with 200 µM Salicylic acid (SA), Methyl Jasmonate, 1-Aminocyclopropane-1-carboxylic acid, and 20 µM Abscisic acid (ABA), or 150 mM NaCl, for 6 or 12 h. Drought treatments involved transferring plants to filter papers and air-dried for 6 h and 12 h under a light intensity of 12 000 lux, while cold treatments were performed by exposing the plants to 4°C for the same durations under the same light intensity.
2.2 Generation of KCS19 overexpression lines and kcs19 complementation lines
To generate the KCS19 overexpressing plants and kcs19 complementation plants, the KCS19 coding sequence was amplified with the primers FKCS19/RKCS19 (Supporting Information S1: Table 1) and inserted into the pC1301 vector containing the CaMV 35 S promoter. Following sequence validation, these constructs were introduced into Agrobacterium tumefaciens GV3101 and subsequently transformed into both Arabidopsis wild-type and mutant plants via the floral dip method. Transgenic seedlings were screened on 1/2 MS medium supplemented with 100 mg mL–1 hygromycin and verified through PCR analysis. Homozygous seedlings were selected for further analysis, specifically for cuticular wax or fatty acid analysis.
2.3 Gene expression analysis
Total RNA was extracted from various plant organs and 5-week-old plant lines using Trizol UP (Monad) following the manufacturer's instructions. Subsequently, cDNA was prepared through reverse transcription, as outlined in the TaKaRa manual. To assess KCS19 expression in leaves and seeds of the mutant lines, PCR was conducted using 1 μg of cDNA as a template, amplifying KCS19 and Actin2. The PCR conditions included an initial denaturation at 95°C for 3 min, 30 cycles of denaturation at 95°C for 15 s, annealing at 58°C for 15 s, elongation at 72°C for 1.5 min, and a final extension at 72°C for 5 min. The samples underwent analysis via 1% agarose gel electrophoresis.
For quantitative RNA transcript analysis in response to hormonal and abiotic stress treatments, across transgenic lines and various Arabidopsis organs, real-time reverse transcription polymerase chain reaction (RT-PCR) was performed using the BioRad CFX Connect Real-Time System with SYBR® Premix Ex Taq™ II (TaKaRa) according to the manufacturer's guidelines. Reference gene EF1a was used to normalize RNA quantity differences. The real-time PCR protocol included a single cycle at 95°C for 30 s, followed by 45 cycles at 95°C for 10 s and 58°C for 30 s. Relative gene expression levels were determined utilizing the method. All experimental samples were assayed in triplicates. Gene-specific primers for RT-PCR are provided in Supporting Information S1: Table 1.
2.4 Histochemical β-glucuronidase (GUS) analysis
The KCS19 promoter (1431 bp) was amplified from Arabidopsis genomic DNA utilizing primers FKCS19p/RKCS19p (Supporting Information S1: Table 1). The resultant PCR product was integrated into the pBI121 vector carrying the β-glucuronidase (GUS) gene and confirmed by PCR. After transformation into GV3101, the construct was introduced into Arabidopsis through the floral dip method. Selection of homozygous lines was based on Kanamycin resistance, and transgenic seedlings, along with various organs and tissues at different developmental stages, were gathered for GUS activity analysis, following established procedures (Vitha et al., 1995). For visual examination, dehydrated samples were observed and photographed using a stereomicroscope (OLYMPUS). To visualize stem and leaf cross-sections, dehydrated GUS samples were embedded in paraffin (Biosharp) and sectioned using a microtome (Leica RM2245) at 2−8 μm thicknesses. The sliced tissues were examined and photographed with a light microscope (Nikon).
2.5 Subcellular localization
The KCS19 coding sequence was amplified using FKCS19s/RKCS19s primers (Supporting Information S1: Table 1) and inserted into the GFP-containing vector pAN580. The resulting construct was transiently transformed into Arabidopsis mesophyll protoplasts by PEG-mediated transformation (Yoo et al., 2007). Concurrently, the ER-specific marker ER-mKate was co-transformed into Arabidopsis protoplasts with the recombinant 35 S: GFP-KCS19 construct. After an overnight incubation at 28°C in the dark, the expression was examined using a confocal microscope (Nikon C2-ER). Fluorescent signals were observed utilizing a mKATE filter (excitation: 561 nm, emission: 580 nm) and a GFP filter (excitation: 488 nm, emission: 510 nm). Chlorophyll (Chl) Autofluorescence was visualized with excitation at 640 nm and emission collected at 675 nm.
2.6 Bimolecular fluorescence complementation assay (BiFC)
The coding sequences of KCS19 were cloned into pCAMBIA1300S-YN and pCAMBIA1300S-YC vector using the ClonEXPress II One Step Cloning Kit. Simultaneously, the coding sequences of other FAE complex proteins (KCS1, KCR1, PAS1, PAS2 and CER10) were cloned into pCAMBIA1300S-YC vector. The resulting constructs were transformed into Agrobacterium tumefaciens strain GV3101, which were then used to infiltrate the leaves of 4-week-old Nicotiana benthamiana plants. Following a 48-h infiltration period, yellow fluorescent protein (YFP) fluorescence was observed using a confocal laser scanning microscope (TCS sp5II, Leica) with a YFP filter (514 nm excitation, 527 nm emission).
2.7 Luciferase complementation assay (LCA)
The coding sequences of KCS19 and other FAE complex proteins (KCS1, KCR1, PAS1, PAS2 and CER10) were amplified and subcloned into pCAMBIA1300-nLUC and pCAMBIA1300-cLUC vector, respectively. Following infiltration of N. benthamiana leaves with different combinations of the aforementioned vectors for 48 h, 1 mM D-luciferin was sprayed on the abaxial surfaces of the leaves and kept in the dark for 7 min. Luminescence images were captured using a luminescence imaging system (Newton7.0, Vilber). Combinations containing empty vectors serving as negative controls.
2.8 Cuticular wax analysis
The cuticular wax analysis was conducted according to the established procedure outlined by (Millar et al., 1999), with minor modifications. Briefly, the cuticular waxes were extracted twice by immersing stems or leaves into 5 mL of chloroform (CHCl3) at room temperature for 30 s. Ten micro gram tetracosane was added to the chloroform extract as an internal standard. The extracts were dried using a moderate nitrogen flux, then incubated with 20 µL of N, N-bistrimethylsilyltrifluoroacetamide and 20 µL of pyridine for 45 min at 70°C. After drying under a nitrogen blower, 100 μL of chloroform was added. Samples were subsequently analyzed using gas chromatography (GC) systems equipped with flame ionization detector (FID) and mass spectrometric detector (MS) for both qualitative and quantitative assessment. The wax amounts were quantified as micrograms of wax per milligram of dry weight (µg mg−1), with three biological replicates conducted, each comprising five plants per replicate.
2.9 Scanning electron microscopy (SEM) analysis
To observe epicuticular wax crystals, the upper and second internode sections of stems from 6-week-old Arabidopsis were dried, affixed onto standard aluminum stubs, and gold-coated using a sputter coater (Quorum SC-500). These prepared sections were subsequently examined using a Hitachi S3000-N scanning electron microscope.
2.10 TB staining, water loss and Chl leaching assays
To stain the leaf with toluidine blue (TB), 3-week-old Arabidopsis seedlings were immersed in TB solution for 30 s, and then observed using a stereomicroscope. For the water loss assay, rosette leaves of 4-week-old Arabidopsis plants were excised and kept in complete darkness for 3 h. After an hour of soaking in water in complete darkness, the leaves were dried and their weight was measured at specified time intervals using a microbalance. The water loss percentage was calculated as described by (Kosma et al., 2009). In measuring Chl, rosette leaves of 4-week-old plants were weighed, then incubated on ice for 30 min before being placed in 80% ethanol at 25°C. The Chl contents were determined by assessing the absorbance at 647 and 664 nm using a spectrophotometer.
2.11 Stress tolerance assays
To investigate the impact of stress on seed germination, surface-sterilized seeds were placed on 1/2 MS medium with varying NaCl concentrations (0, 50, and 100 mM) or mannitol concentrations (0 and 150 mM). Plates were cold-treated at 4°C in darkness for 2 days and then transferred to a growth chamber at 22°C under long-day conditions. Radicle emergence was documented at specific intervals. For assessing stress effects on root growth, 3-day-old seedlings were moved to fresh media supplemented with 50 mM or 100 mM NaCl or 150 mM mannitol for vertical cultivation. The primary root length was measured after 8 days.
To evaluate drought tolerance in Arabidopsis plants, those grown in soil for 4 weeks were deprived of water for 14 days. Leaf relative water content (RWC) from surviving leaves was quantified. Following the water deprivation, plants were re-watered for 2 days, and survival was determined, with plants showing > 50% green tissue considered as surviving. Each experiment was performed three times, with 10 plants per replication for each genotype. To assess salt tolerance, plants were grown in soil and watered every 2 days with 80 mL of a 250 mM NaCl solution. The phenotypes of the plants were observed and photographed on designated days.
2.12 Seed fatty acid analysis
The seed total lipids were extracted from 0.1 g ground seeds, and then were converted into fatty acid methyl esters (FAMEs) for quantification using GC with flame ionization detection according to the methods described by Karki and Bates (2018). FAMEs were produced in 1 mL 5% sulfuric acid in methanol at 85°C for 1.5 h together with 40 or 20 μg 17:0 TAG in 0.2 mL toluene as an internal standard. Then, FAMEs were extracted by adding 0.2–0.5 mL hexane and 1.5 mL 0.88% potassium chloride. GC of FAMES was conducted using an Agilent 7890 A GC fitted with a DB-23 capillary column (100 m length, 0.25 mm inner diameter, 0.2 µm thickness; J & W, Folsom, CA, USA). After an initial hold for 2 min at 125°C, the temperature was ramped at a rate of 5°C/min until reaching 230°C, and then held for 8 min. The fatty acids were identified by comparison of retention times and mass spectra with those of standards (fatty acids with chain length ranging from C4 to C22). Fatty acids were identified by comparing retention times and mass spectra with established standards. The content of each fatty acid was expressed as a percentage of the total fatty acids. The experiments were conducted with three biological replicates.
2.13 Seed size measurements
Approximately 50 mature dried seeds of each genotype were photographed using a SMZ-T2 microscope, and the seed length and seed width were determined using IMAGEJ software. Data are presented as the average of three independent experiments, with three biological replicates in each experiment. Student's t-test was used to determine the significance of differences in seed length and seed width between the mutants, transgenic and wild-type plants.
2.14 Phylogenetic analysis
Multiple alignment of amino acid sequences was prepared using ClustalX 2.1, and the phylogenetic tree was constructed using the neighbor-joining (N-J) method by MEGA 7.0. Bootstrap analysis was performed with 1000 replicates.
3 RESULTS
3.1 Expression patterns of KCS19 in Arabidopsis
To investigate the expression profile of the KCS19 gene, we conducted quantitative RT-PCR using total RNA extracted from various Arabidopsis organs. As shown in Figure 1a, KCS19 exhibited higher expression levels in reproductive organs, including flowers, siliques, and seeds, followed by leaves and stems, while displaying minimal expression in roots. This expression pattern is consistent with data obtained from microarray analyses (Suh et al., 2005). For a more detailed examination of KCS19's spatial and temporal expression, we generated transgenic Arabidopsis lines expressing the GUS reporter gene under the control of the KCS19 promoter. Our findings unveiled KCS19 expression across diverse developmental stages and tissue types, encompassing young seedlings, rosette leaves, cauline leaves, inflorescence stems, sepals, the upper portion of the styles, early developing silique walls, silique beaks and pedicels, and mature embryos, but not in seed coats (Figure 1b). Notably, higher KCS19 expression was observed in leaf and stem epidermal cells compared to adjacent internal tissues (Figure 1b,iii,vi), suggesting a potential association with wax-related functions. Furthermore, our investigation revealed the regulatory responsiveness of KCS19 to environmental stresses. The expression of KCS19 was significantly induced by SA, ABA, drought, and salt stresses (Supporting Information S1: Figure 2).
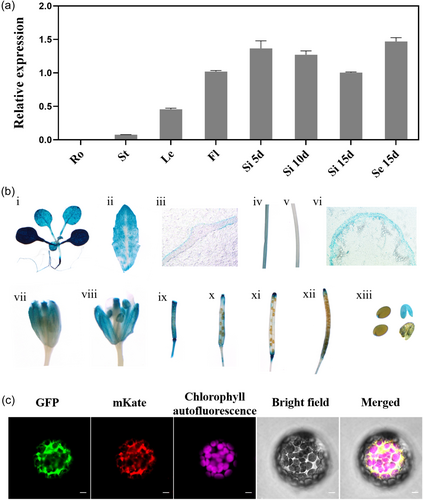
To examine the subcellular localization of KCS19, we constructed a GFP-KCS19 fusion protein vector and introduced it into Arabidopsis protoplasts for transient expression. As shown in Figure 1c, the fusion protein containing the target gene exhibited green fluorescence signals within the ER, and it overlapped with the signal from the ER-localized mKate protein (an ER-localized marker), indicating that KCS19 protein was localized in the ER.
3.2 Isolation of kcs19 T-DNA insertion lines and generation of transgenic lines
To investigate the function of KCS19 in Arabidopsis, mutants with T-DNA insertions—kcs19-1 (SALK_131405) and kcs19-2 (SALK_030654) —positioned in the upstream and downstream regions of the KCS19 coding sequence (Figure 2a), were identified via genomic DNA PCR analysis (Figure 2b). Semi-quantitative RT-PCR and qRT-PCR showed that the expression of the KCS19 transcripts in mutants was much lower when compared to the wild-type (Figure 2c,d). Under normal growth conditions, both kcs19-1 and kcs19-2 mutants did not exhibit any discernible differences in seed germination, root growth, overall plant development, and growth when compared to wild-type plants (Figure 2e; Supporting Information S1: Figures 3–5). Due to the abundant expression of KCS19 in mature seeds/embryo (Figure 1a,b), we examined the phenotype of wild-type, kcs19-1, and kcs19-2 seeds. As shown in Figures 2f,g, both mutant lines exhibited identical seed morphology to the wild-type, showing no observable alterations in seed size or weight.
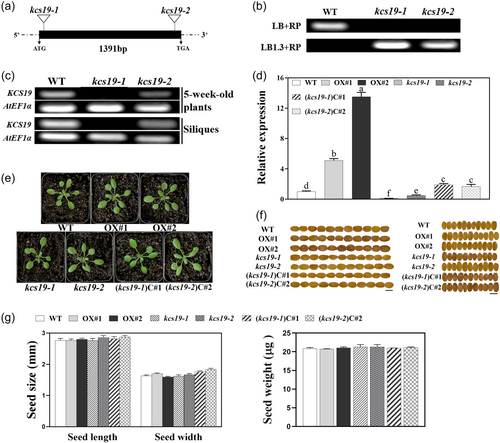
To further investigate the functional implications of KCS19 and its potential for manipulating cuticular wax and seed fatty acids in plants, we generated KCS19 overexpression transgenic lines, labeled as OX#1 and OX#2, along with complementation lines, referred to as (kcs19-1) C#1and (kcs19-2) C#2. The overexpression lines showed significantly elevated KCS19 transcript levels compared to the WT, and even the complementation lines demonstrated notably higher KCS19 transcript levels relative to the WT (Figure 2d). Additionally, both KCS19 overexpression and complementation lines displayed WT-like plant and seed morphology (Figure 2e−g; Supporting Information S1: Figure 5).
3.3 KCS19 participates in the biosynthesis of VLCFAs in seeds
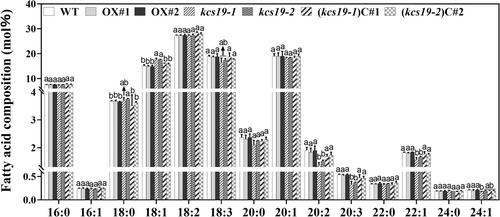
Given the notably high expression of KCS19 in developing seeds, we investigated the seed fatty acid content to understand the potential impact of KCS19 on seed fatty acid composition. Quantitative analysis unveiled noteworthy changes in the fatty acid content. In the kcs19-1 and kcs19-2 mutants, notable increases were observed in the levels of C18:1 compared to the wild-type. In contrast, the levels of C20 species (C20:0, C20:1, C20:2, and C20:3) experienced a reduction, with a particularly significant decrease in the levels of C20:2 and C20:3 (Figure 3). Remarkably, reintroducing KCS19 expression into these mutants effectively restored the levels of C18:1, C20:2, and C20:3 in seeds to those of the wild type. Furthermore, a significant reduction in the accumulation of C22:1 fatty acid was observed in kcs19 mutants, and this reduction was successfully restored to wild-type levels in KCS19 complementation lines (Figure 3). In contrast, KCS19 overexpression lines exhibited seed fatty acid composition resembling those of the wild type (Figure 3).
3.4 KCS19 was required for the biosynthesis of cuticular wax in stems and leaves
To address the molecular function of KCS19 in wax biosynthesis, we analyzed the compositions of cuticular wax covering the surfaces of stems and leaves in both kcs19 mutants and transgenic lines. As shown in Figure 4a, the total wax amounts in the stems of kcs19-1 and kcs19-2 were significantly decreased. This notable change in total wax amounts primarily resulted from a substantial reduction in alkanes and acids (Figure 4a). For instance, the total alkanes in the stems of kcs19-1 and kcs19-2 were reduced to 73.0% and 59.4% of wild type, respectively, with a notable decline observed in C29 alkane (Figure 4b). Additionally, within the stem wax compounds, a significant reduction was observed in the levels of C24 fatty acid, C28 aldehyde, and C24 primary alcohol in both kcs19-1 and kcs19-2 mutants compared to the wild type (Figure 4b), alongside a significant reduction of C26 acid in the kcs19-2 mutant, while no significant differences were noted in other wax compounds.
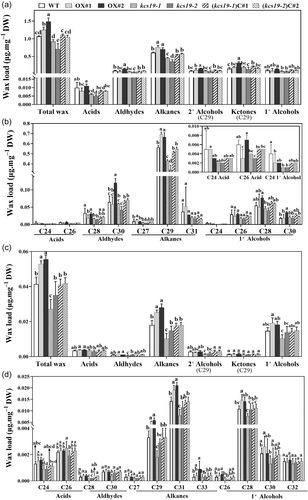
In contrast, the stems of KCS19 overexpression lines displayed substantial increases in both total wax loads and the contents of alkanes, particularly C29 alkane, presenting a distinct contrast to the wild type (Figure 4a,b). An elevated abundance of C29 homologues, including C29 secondary alcohol, and C29 ketone, was significantly evident in OX#2 and displayed an increasing trend in OX#1 relative to the wild type. Furthermore, significant increases in the amount C30 aldehyde, and C30 primary alcohol were observed in OX#2 (Figure 4b). Additionally, the wax phenotypes observed in kcs19 mutants were successfully rescued by the reconstitution of KCS19 expression in the kcs19 background, reflected in the recovery of total wax loads, alkanes, and acids to wild-type levels in the complementation lines.
Similar to inflorescence stems, rosette leaves of kcs19-1 and kcs19-2 mutants also exhibited decreased levels of alkanes and total wax loads (Figure 4c). As the predominant component, the total alkane levels in kcs19-1 and kcs19-2 were reduced to 56.4% and 70.2% of wild type, respectively, with significant decreases observed in C29 and C31 alkanes (Figure 4d). Additionally, C29 secondary alcohol exhibited significantly decreased levels in both mutants compared to WT. Conversely, in the leaves of KCS19 overexpression lines, substantial increases were observed in both total wax loads and the contents of alkanes, particularly C29 and C31 alkane, demonstrating a distinct contrast to the wild type (Figure 4c,d).
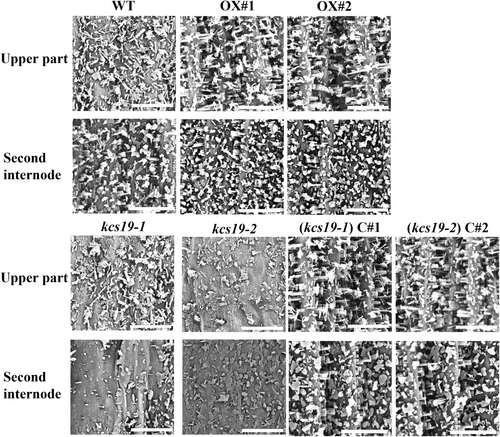
To ascertain the impact of these observed wax alterations on epicuticular wax crystal formation, stem surface of WT, kcs19 mutants, complementation and overexpression lines were observed using SEM. These observations revealed a substantial reduction in epicuticular wax crystals on the upper stem and secondary internode of kcs19-1 and kcs19-2 mutants compared to the wild type. In contrast, there was a notable increase in epicuticular wax crystal presence in the complementation and overexpression lines (Figure 5). This highlights the role of KCS19 in the biosynthesis of cuticular wax.
3.5 KCS19 influences the cuticle permeability and drought and salt tolerance of A. thaliana
Given the established link between cuticular wax and cuticle permeability in prior studies (Seufert et al., 2022), we evaluated cuticle permeability by employing TB staining, Chl-leaching, and water-loss rate analyses in both mutant and transgenic lines, aiming to evaluate the influence of altered KCS19 expression on cuticle permeability. The kcs19-1 and kcs19-2 mutants exhibited a distinct staining pattern characteristic of impaired cuticles (Figure 6a). Rosette leaves of kcs19-1 and kcs19-2, but not the cotyledons, displayed noticeable staining. By contrast, the complementation lines showed patterns of leaf cuticle permeability to TB that closely resembled those of the wild type, whereas overexpression lines did not exhibit any changes in leaf cuticle permeability to TB (Figure 6a). Furthermore, when compared to wild-type plants, Chl extraction was notably swifter from kcs19-1 and kcs19-2 mutants and slower from the overexpression lines OX#1 and OX#2 (Figure 6b). The water loss assay revealed that the kcs19 mutant lines displayed higher rates of water loss, while the overexpression lines exhibited lower rates compared to the wild-type plants (Figure 6c).
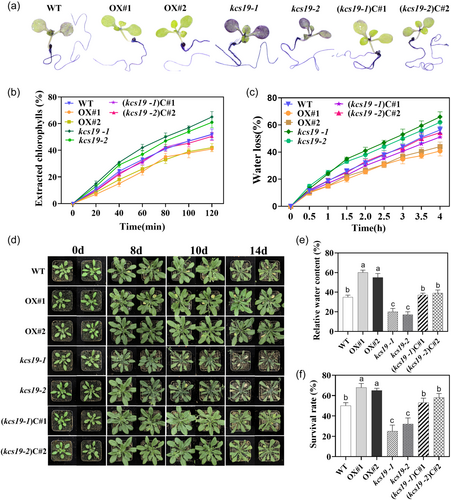
To ascertain the impact of cuticle permeability on plant drought tolerance, we subjected 4-week-old plants to a 14-day water deprivation period and subsequently recorded their phenotypes, leaf RWC and survival rate. After 14 days, wild-type plants displayed wilting and curled leaf edges, whereas KCS19 overexpression lines retained their healthy green appearance. In contrast, the kcs19 mutants exhibited severe wilting and pronounced leaf curling, while KCS19 complementation lines exhibited performance similar to the wild type (Figure 6d). These observations aligned with the RWC measurements from the leaves of the kcs19 mutant lines and KCS19 overexpression lines, which exhibited significantly lower and higher RWC values compared to wild-type plants, respectively. The RWC of KCS19 complementation lines closely mirrored that of the wild type (Figure 6e). Further substantiating the findings, the survival rate of kcs19 mutant lines was notably lower compared to the wild type, complementation, and overexpression lines, as shown in Figure 6f. These results underscore the pivotal role of KCS19 in modulating cuticle permeability and its consequential impact on plant drought tolerance. Meanwhile, simulated drought stress with mannitol was applied to assess its impact on seed germination and root growth. The findings revealed no significant differences in root length and germination rate between the two kcs19 mutants and the wild-type under both normal and stress conditions (Supporting Information S1: Figure 3).
Furthermore, Arabidopsis plants were subjected to salt stress to investigate the impact of KCS19 on plant salt tolerance. Following exposure to salt stress, the KCS19 overexpression lines exhibited significantly more green leaves, while the kcs19 mutants exhibited signs of leaf chlorosis and leaf whitening at the vegetative stage (Supporting Information S1: Figure 6A). At the reproductive stage, the development of flowers and siliques in kcs19 mutants was severely affected (Supporting Information S1: Figure 6B). Conversely, The KCS19 overexpression lines exhibited healthy growth of silique compared to the WT. The KCS19 complementation lines closely resembled wild-type plants (Supporting Information S1: Figure 6). These findings emphasize the role of KCS19 in Arabidopsis plants' response to salt stress. Additionally, we sowed wild-type and mutant seeds on 1/2 MS solid culture medium with varying NaCl concentration and recorded root length and germination rates. As shown in Supporting Information S1: Figure 4, there were no significant differences in root length and germination rate between the wild type and kcs19 mutants. This suggests that, under the tested circumstances, KCS19 deficiency did not significantly affect seed germination and root growth.
3.6 Protein–protein interaction between KCS19 and other FAE complex proteins
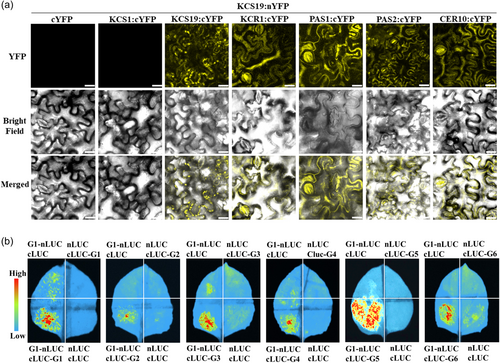
As previously reported (Haslam and Kunst, 2013), VLCFAs are synthesized by ER-associated FAE complexes, which comprise core enzymes KCS, KCR, PAS2/HCD, and ECR/CER10 (Supporting Information S1: Figure 1). Additionally, the immunophilin PASTICCINO1 (PAS1) serves as a scaffold to associate with KCR, PAS2/HCD, and ECR/CER10 in the ER (Roudier et al., 2010). Given the role of KCS19 in wax production and lipid biosynthesis, we investigated its potential interactions with FAE core complex subunits using BiFC and luciferase complementation assays. The BiFC assay revealed homo-interactions of KCS19 (Figure 7a). Furthermore, KCS19 formed hetero-interactions with KCR1, PAS1, PAS2, and CER10 proteins in the BiFC analysis (Figure 7a). The Split-Luciferase assay confirmed both homo-interactions of KCS19 and hetero-interactions between KCS19 and FAE core complex proteins (KCR1, PAS1, PAS2, and CER10) (Figure 7b), indicating that KCS19 may coordinate with other FAE complex proteins to regulate the synthesis of VLCFAs. Considering that KCS1 is involved in VLCFA and wax synthesis in Arabidopsis vegetative tissues, exhibiting broad substrate specificity for saturated and monounsaturated acyl-CoAs (Blacklock & Jaworski, 2006; Todd et al., 1999), we further investigated the potential interaction between KCS19 and KCS1. This investigation was based on the tendency of KCS isoforms to interact with each other, as reported by Kim et al. (2022) for KCS2, KCS6, and KCS9. However, our findings showed that KCS19 did not physically interact with KCS1 (Figure 7), suggesting that KCS19 protein acts independently of KCS1 and does not modulate the steric structure of KCS1.
4 DISCUSSION
4.1 KCS19 functions in VLCFA synthesis for cuticular waxes and stress resistance in Arabidopsis
In Arabidopsis, the extensive family of 21 KCS members is classified into eight subclasses: α, β, γ, δ, ɛ, ζ, η and θ (Supporting Information S1: Figure 7) (Joubès et al., 2008). The challenge in accurately determining the biochemical activity and in planta function of KCSs stems from functional redundancy, which arises from overlapping substrate specificities and expression patterns. Known contributors to wax production in Arabidopsis include KCS1 (Todd et al., 1999), KCS2/DAISY (Lee et al., 2009), KCS3 (Huang, Yang, Zheng, Chen, et al., 2023), KCS4(Kim et al., 2021), KCS5 (Huang et al., 2022), KCS6 (Fiebig et al., 2000; Millar et al., 1999), KCS9 (Kim et al., 2013), KCS12 (Huang, Yang, Zheng, Lü, et al., 2023), KCS16 (Hegebarth et al., 2017), and KCS20 (Lee et al., 2009). KCS19, along with KCS3 and KCS12, belongs to the θ subclass within the KCS family (Supporting Information S1: Figure 7). Recent research on the θ subclass suggests that KCS3 and its paralogue KCS12 act as negative regulators of wax synthesis, but there is no conclusive evidence to determine KCS19's association with wax production (Huang, Yang, Zheng, Chen, et al., 2023). Cuticular wax biosynthesis is believed to occur exclusively within epidermal cells (Kunst & Samuels, 2003). Our findings, using histochemical GUS analysis, confirmed heightened expression of KCS19 in the leaf and stem epidermis compared to internal tissues, suggesting a direct association with wax-related functions (Figure 1b). The observed localization of the KCS19 protein within the ER aligns with the established knowledge that the FAE complex operates within the ER membrane (Figure 1c) (Joubès et al., 2008; Kunst & Samuels, 2003).
Similar to KCS9 (Kim et al., 2022) and KCS17 (Kim et al., 2023), KCS19 formed homo- and hetero-interactions with KCR1, HCD/PAS2, and ECR1/CER10 (Figure 7), suggesting that it might be efficient for the synthesis of VLCFAs with different carbon chain lengths. In addition, KCS19 also physically interacted with PAS1 (Figure 7), a molecular scaffold protein for FAE (Roudier et al., 2010), further indicating its elongase activity. However, our findings showed that KCS19 does not interact with KCS1 (Figure 7), which is involved in VLCFA and wax synthesis in Arabidopsis vegetative tissues and exhibits broad substrate specificity for saturated and monounsaturated acyl-CoAs (Blacklock & Jaworski, 2006; Todd et al., 1999). This lack of interaction suggests that KCS19 functions independently of KCS1 in VLCFA synthesis in vegetative tissues. However, given the presence of numerous other KCS family members, KCS19 may still interact with other KCS isoforms. Further studies are needed to identify potential interactions between KCS19 and other KCS proteins to fully understand its role and functional interactions within the FAE complex.
Disruption of KCS19 in Arabidopsis resulted in a significant decrease in the total wax content in both stems and rosette leaves, primarily affecting alkanes—the key constituents of Arabidopsis cuticular wax (Figure 4). In contrast, KCS19 overexpression led to a notable increase in alkane and total wax loads, reinforcing its role in Arabidopsis wax biosynthesis. These results demonstrate that KCS19 is involved in cuticular wax biosynthesis in Arabidopsis, functioning similarly to other KCS family members such as KCS2, KCS3, KCS6 and KCS11 (Huang, Yang, Zheng, Chen, et al., 2023; Yang et al., 2021). Furthermore, the loss of KCS19 expression led to a decrease in wax components, including C24 primary alcohol, C28 aldehyde, and C29 alkane in stems, as well as C24 acid, C29 and C31 alkanes in leaves compared to the wild type. This finding strongly suggests the involvement of KCS19 in elongating VLCFAs with C24 and beyond for wax production. However, the loss of KCS19 expression does not lead to complete absence of any individual wax component. Other KCS isoforms could partially compensate for the absence of KCS19 in the mutants, thereby supporting the redundancy observed in elongase KCS activities involved in wax synthesis (Batsale et al., 2023). Previous reports indicated that the complete loss of KCS1 expression in Arabidopsis had relatively minor effects on the total wax load and major wax components, such as C29 alkanes and C29 ketones in stems (Todd et al., 1999). However, our findings revealed the alterations in KCS19 expression had a significant impact on the total wax load and particularly on alkanes, notably C29 alkane. Additionally, a notable increase in C29 secondary alcohol and C29 ketone levels was observed in the stems of the overexpressed line OX#2, with a similar trend observed in OX#1 (Figure 4), suggesting that KCS19 might predominantly influence the decarbonylation pathway. Notably, as indicated by the lack of interaction between KCS19 and KCS1 (Figure 7), these results imply functional distinctions between KCS19 and KCS1 in Arabidopsis, underscoring their potentially independent roles in wax synthesis.
The role of KCS19 in cuticular wax biosynthesis was substantiated by the reduction in epicuticular wax crystal observed on the stems of kcs19 mutants compared to the wild type, while a distinct increase in epicuticular wax crystal in the overexpression lines (Figure 5). Furthermore, it was noted that epicuticular wax crystals in the complementation lines showed an increase compared to the wild type, although there are no significant differences in cuticular wax load between the wild type and complementation lines. The elevated expression of KCS19 in complementation lines corresponds with the observed increase in epicuticular wax crystals. It's crucial to acknowledge that cuticular wax consists of both intracuticular and epicuticular waxes, with wax crystals protruding from the epicuticular layer into the atmosphere (Buschhaus & Jetter, 2011). The rise in epicuticular wax crystals in the complementation lines strongly suggests a shift in epicuticular wax amounts, although the exact amounts were not quantified.
Significant alterations in KCS19 transcript levels under drought and salt stresses suggest a pivotal role for KCS19 in the plant's response to abiotic stress (Supporting Information S1: Figure 2). KCS19 disruption resulted in reduced alkanes and total wax loads, compromising resistance to water loss and making the Arabidopsis plant more susceptible to drought stress. Conversely, KCS19 overexpression increased the levels of alkanes and total wax, enhancing the plant's ability to withstand drought conditions (Figures 4 and 6). Furthermore, kcs19 mutants exhibited sensitivity to salt stress, whereas the overexpression lines showed tolerance to salt stress (Supporting Information S1: Figure 6). The water barrier properties of the plant cuticle primarily rely on the presence of cuticular waxes (Buschhaus and Jetter, 2011). Alkanes, as major wax compounds in Arabidopsis, play a specific role in cuticle properties and resistance to water stress (Bourdenx et al., 2011; Wang et al., 2020, 2021). The accumulation of cuticular waxes and reduction in residual transpiration are crucial for enhancing tolerance to salinity stress, potentially optimizing water use efficiency during challenging conditions such as drought or salinity (Hasanuzzaman et al., 2017).
Furthermore, the expression of KCS19 was significantly induced by SA, prompting speculation regarding its potential connection with plant responses to pathogens (Lowe-Power et al., 2016; Zhong et al., 2021). The synthesis of specific wax components induced by exogenous SA may directly or indirectly affect the production of defense-related compounds (Yuan et al., 2020), thereby enhancing the plant's resistance to pathogen attack. Further research is needed to elucidate the specific role of KCS19 in plant-pathogen interactions and its contribution to overall plant defense strategies.
4.2 Role of KCS19 in VLCFA synthesis for seed storage lipids
Arabidopsis seeds generally hold neutral lipid pools containing C16 to C22 fatty acids, commonly with zero to three double bonds (Zhou et al., 2014). The expression or inhibition of different KCS genes in seeds can alter the length or types of VLCFAs that accumulate (Millar & Kunst, 1997; Taylor et al., 2009). KCS19 exhibits high expression levels in seeds (Figure 1), indicating its involvement in seed fatty acid metabolism. Compared with the wild type, kcs19 mutants displayed significant reductions in C20:2, C20:3, and C22:1VLCFAs, with a trend of reduction in C24:1, but significant increases in C18:1 VLCFA, with a trend of increase in C18:0 (Figure 3). Conversely, the kcs19 complementation lines (kcs19-1)C#1 and (kcs19-2)C#2 showed seed VLCFA composition similar to the wild type, confirming that the mutant phenotype stemmed from the misexpression of KCS19.
The fatty acid chain length ratio (CLR), representing the ratio between the total amount of C20 to C24 fatty acids and the total amount of C16 to C18 fatty acids, serves as a main indicator of VLCFA proportion (Jasinski et al., 2012; O'Neill et al., 2003). Our data demonstrated a decrease in the CLR value in kcs19 mutant seeds compared to the wild type, indicating the involvement of KCS19 activity in the elongation of VLCFAs in seeds. Furthermore, the ratio of (20:1 + 22:1 + 24:1)/18:1 proportion was utilized to gauge the efficiency of substrate conversion to VLCFAs in Arabidopsis seeds (MA et al., 2021). The kcs19 mutant seeds exhibited a reduction in the ratio of (20:1 + 22:1 + 24:1)/18:1, with the proportions of 20:1, 22:1, and 24:1 being 18.47%–18.50%, 1.59%–1.71%, and 0.18%–0.20% in mutant lines compared to 18.98%, 1.82%, and 0.21% in the wild type, respectively. Taken together, our results demonstrate that the kcs19 mutants exhibit a deficiency in chain lengths C20 and greater while being enriched in chain length C18. This suggests that KCS19 is involved in elongating C18 to C20 VLCFAs, possibly up to C24. It is worth noting that an increase in 18:1 fatty acid level in kcs19 mutants does not affect the levels of 18:2 and 18:3 fatty acids, indicating that the increase in substrate abundance (18:1) caused by KCS19 disruption might not be sufficient for substrate availability for biosynthesis of polyunsaturated fatty acids (18:2 and 18:3).
Remarkably, when transgenic plants overexpressing KCS19 were studied, no observed alteration were noted in the fatty acid composition of the seeds or any abnormal morphological changes. Previous reports indicated that FAE1/KCS18 serves as the rate-limiting enzyme for VLCFA biosynthesis in Arabidopsis seeds (Millar & Kunst, 1997). Overexpression of FAE1/KCS18 leads to significant accumulation of monounsaturated 20:1, as well as other saturated and polyunsaturated VLCFAs in seeds (Ma et al., 2021; Millar & Kunst, 1997). Altered plant morphology and changed floral phenotype were observed in FAE1/KCS18 overexpression lines (Millar et al., 1998). Collectively, these results suggest that KCS19 may not serve as the rate-limiting enzyme for VLCFA biosynthesis in seeds, unlike FAE1/KCS18, despite both participating in the elongation of C18 to C20 fatty acids in seeds.
ACKNOWLEDGEMENTS
This research was funded by National Natural Science Foundation of China (32171938).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




