A viroid-derived small interfering RNA targets bHLH transcription factor MdPIF1 to regulate anthocyanin biosynthesis in Malus domestica
Zhenlu Zhang, Zhao-Yang Li, and Fu-Jun Zhang contributed equally to this work.
Abstract
Fruit colour is a critical determinant for the appearance quality and commercial value of apple fruits. Viroid-induced dapple symptom severely affects the fruit coloration, however, the underlying mechanism remains unknown. In this study, we identified an apple dimple fruit viroid (ADFVd)-derived small interfering RNA, named vsiR693, which targeted the mRNA coding for a bHLH transcription factor MdPIF1 (PHYTOCHROME-INTERACTING FACTOR 1) to regulate anthocyanin biosynthesis in apple. 5’ RLM-RACE and artificial microRNA transient expression system proved that vsiR693 directly targeted the mRNA of MdPIF1 for cleavage. MdPIF1 positively regulated anthocyanin biosynthesis in both apple calli and fruits, and it directly bound to G-box element in the promoter of MdPAL and MdF3H, two anthocyanin biosynthetic genes, to promote their transcription. Expression of vsiR693 negatively regulated anthocyanin biosynthesis in both apple calli and fruits. Furthermore, co-expression of vsiR693 and MdPIF1 suppressed MdPIF1-promoted anthocyanin biosynthesis in apple fruits. Infiltration of ADFVd infectious clone suppressed coloration surrounding the injection sites in apple fruits, while a mutated version of ADFVd, in which the vsiR693 producing region was mutated, failed to repress fruit coloration around the injection sites. These data provide evidence that a viroid-derived small interfering RNA targets host transcription factor to regulate anthocyanin biosynthesis in apple.
1 INTRODUCTION
Apple dimple fruit (ADF) disease is a viroid-induced disease that was first observed in apple cultivar ‘Starking Delicious’ in Italy and characterised with green depressed spots (around 3–4 mm in diameter) on the red skin of fruits (Di Serio et al., 1996, 2001). Some of these symptoms are quite similar to the dapple fruits induced by apple scar skin viroid (ASSVd) in certain red-skinned cultivars (Koganezawa, 1989). ADF, as well as the dapple symptom induced by ASSVd, severely affect the fruit appearance, which renders those fruits unmarketable and causes economic loss to apple growers.
The causal agent of ADF disease is apple dimple fruit viroid (ADFVd), belonging to the genus Apscaviroid, family Pospiviroidae (Di Serio et al., 1996, 2001; Owens et al., 2012). ADFVd is an independently replicating circular RNA of 306–307 nucleotides in length (Di Serio et al., 1996, 2001). It usually forms a quasi-rod-like conformation that containing the core nucleotides of the central conserved region (CCR), a typical feature for the genus Apscaviroid and its type member ASSVd (Di Serio et al., 1996, 2001; Hashimoto & Koganezawa, 1987). The viroid has been detected in apple from multiple countries (Navarro et al., 2022), including China (Ni et al., 2023; Ye et al., 2013) and Japan (He et al., 2010). The symptoms induced by ADFVd vary by different cultivars. For instance, it induces crater-shaped green spots in ‘Pink Lady’ and ‘Fuji’, while it is symptomless in ‘Golden Delicious’ (Di Serio et al., 2001). In addition to apple, pear (Pyrus pp, cultivar ‘Fieud 37’) (Di Serio et al., 2001), fig (Ficus carica L.) (Buzkan & Balsak, 2022; Chiumenti et al., 2014), and pomegranate (Punica granatum) (Ruiz-Garcia et al., 2022) have been reported to be natural hosts of ADFVd.
Viroids are naked, noncoding RNAs, they do not encode any protein but rely on host cellular resources and apparatus to achieve self-replicating and pathogenesis (Flores et al., 2011; Ortolá & Daròs, 2023). Then how do viroids incite severe diseases in so many plant species? A growing body of evidence confirms that RNA interference (RNAi)-mediated by viroid-derived small RNAs (vd-sRNAs) play a critical role in this process (Di Serio et al., 2023; Ramesh et al., 2020). The viroid's rod-like secondary structures are particular double stranded RNA structures that can be recognised by host Dicer-like proteins, and are digested into double stranded small interfering RNAs (siRNAs) (Di Serio et al., 2023). These siRNAs are then loaded into argonaute proteins to target their cognate RNAs for degradation under the favour of RNA induced silencing complex (Di Serio et al., 2023).
vd-sRNAs have been detected from multiple viroids through deep RNA sequencing (Hammann & Steger, 2012; Navarro et al., 2009), and their functions in viroid pathogenesis have been determined in different viroid-host combinations. For example, potato spindle tuber viroid (PSTVd) is an extensively investigated member in the family Pospiviroidae that induces tuber disease in potato and is infectious to many other plant species (Kochetov et al., 2021). A PSTVd-derived small RNA could target and mediate the silencing of callose synthase genes CalS11/12-like in tomato (Solanum lycopersicum) (Adkar-Purushothama et al., 2015). vd-sRNAs produced from viroid mutants that are incapable of targeting these two genes failed to incite typical symptoms (Adkar-Purushothama et al., 2015). In addition, peach latent mosaic viroid (PLMVd) replicates in the chloroplast and induces a severe albinism (peach calico) in its natural host peach (Prunus persica) (Rodio et al., 2006, 2007). Two small RNAs derived from PLMVd target and mediate the cleavage of mRNA of chloroplastic heat-shock protein 90 (cHSP90), which is involved in chloroplast biogenesis (Cao et al., 2003; Navarro et al., 2012). Thus, vd-sRNAs originating from viroid genomes may play a critical role in causing disease-associated symptoms by targeting and mediating the cleavage of host mRNAs.
Fruit colour directly affects the commercial value and marketability of apple, especially for those red-skinned cultivars, such as ‘Fuji’. Apple fruit coloration is mainly determined by anthocyanins, a class of water-soluble flavonoid pigments widely distributed in various plant tissues (Tanaka et al., 2008; Zhao et al., 2022). In higher plants, anthocyanins are produced through the phenylpropanoid pathway using phenolic precursor on the cytoplasmic face of endoplasmic reticulum (Tanaka et al., 2008; Zhao et al., 2022). A set of correlative enzyme-encoding genes are involved in this pathway: upstream biosynthetic gene phenylalanine ammonialyase (PAL); early biosynthetic genes chalcone synthase (CHS), chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H); and late biosynthetic genes dihydroflavonol 4-reductase (DFR) and UDP-glucose flavonoid glucosyl transferase (UFGT) (Tanaka et al., 2008; Zhao et al., 2022).
Anthocyanin biosynthesis is affected by both external factors (e.g. light and temperature) and internal phytohormones, transcription factors (TFs), and microRNAs (Gao et al., 2021; Liu et al., 2021a). The role of TFs in anthocyanin biosynthesis has drawn much attention. Currently, a huge body of evidence supports that a ternary protein complex consisting of myeloblastosis (MYB), basic helix-loop-helix (bHLH), and tryptophan-aspartic acid repeat (WDR) proteins, named MBW complex, plays a central role in this process (Gonzalez et al., 2008; Liu et al., 2021a; Zhao et al., 2022). Among these TFs, bHLH TFs function by directly binding to the promoter of anthocyanin structural genes or interact with other TFs to coregulate anthocyanin biosynthesis (Chen et al., 2021). As the second largest TF family in plants, multiple bHLH TFs have been determined to play critical roles in regulating anthocyanin biosynthesis, such as MdbHLH3 and MdbHLH33 (Wang et al., 2017; Xie et al., 2012).
PHYTOCHROME-INTERACTING FACTORs (PIFs), a subset of the bHLH TF superfamily, are well known for their central roles in light-regulated plant growth and development (Cordeiro et al., 2022; Pham et al., 2018). In addition to light signalling, PIFs are also involved in regulating anthocyanin biosynthesis in plants. For example, PIF3 binds to the promoter of several anthocyanin biosynthetic genes and activates their expression to promote anthocyanin biosynthesis in Arabidopsis (Shin et al., 2007). Moreover, PIF4 and PIF5 are negative regulators of anthocyanin biosynthesis (Liu et al., 2015, 2021c; Qin et al., 2022). Specifically, PIF4 competitively interacts with an MYB TF, PRODUCTION OF ANTHOCYANIN PIGMENT 1 (PAP1), and directly represses its transcription, leading to disrupted function of MBW complex in regulating anthocyanin biosynthesis in Arabidopsis (Liu et al., 2021c; Qin et al., 2022). Moreover, a recent finding proves that MdPIF7 negatively regulates anthocyanin biosynthesis in apple calli (Liu et al., 2022).
ADFVd-induced green spots on the red skins severely affect the market value of apple fruits, however, the mechanism behind is unclear. We hypothesised that anthocyanin biosynthesis might be involved in this process. In the present study, we screened out a vd-sRNA, named vsiR693, which targeted the mRNA of a bHLH TF MdPIF1. We confirmed that vsiR693 could target and mediate the cleavage of mRNA of MdPIF1 via 5’-RLM RACE and artificial microRNA system. We further showed that MdPIF1 positively regulated anthocyanin biosynthesis by binding to the promoter of anthocyanin biosynthetic genes, MdF3H and MdPAL, to promote their transcription. Expression of vsiR693 negatively regulated anthocyanin biosynthesis in both apple calli and fruits. Moreover, infiltration of ADFVd infectious clone suppressed the coloration of apple peels surrounding the injection sites, while the mutated ADFVd failed to incite the similar phenotype.
2 MATERIALS AND METHODS
2.1 Plant materials
The apple fruits with dapple symptom together with the symptomless fruits from the same species were collected. Specifically, fruits from cultivar ‘Red Fuji’ (9-year-old) and ‘Hirosaki Fuji’ (14-year-old) were collected from Tai'an, Shandong Province; fruits from cultivar ‘Red Fuji’ (16-year-old) and ‘Southern Crisp’ (10-year-old) were collected from Linyi, Shandong Province; fruits from ‘Hirosaki Fuji’ (14-year-old) were collected from Yantai, Shandong Province; and fruits from cultivar ‘Fuji 2001’ (18-year-old) were collected from Qingyang, Gansu Province.
Apple calli were obtained from the apple cultivar ‘Orin’ (Malus domestica Borkh) and were cultivated on Murashige and Skoog (MS) medium supplemented with 1.5 mg/L 2,4-dichlorophenoxyacetic acid and 0.4 mg/L 6-benzylaminopurine at 24°C under dark conditions. The calli were subcultured every 2 weeks, and fresh calli (12-day-old) were used for transformation or various treatments.
2.2 Plasmid construction and genetic transformation
Coding sequence of MdPIF1 (MD10G1170600 or GenBank accession number NC_041798) or antisense fragment of MdPIF1 (1-320 bp of the coding region, GenBank accession number XM_008384439) were inserted into a pRI-101-AN vector. VsiR693 corresponding DNA sequence (5’-CGTCGTCGACGAAGGCT-3’) was constructed into an artificial microRNA backbone of miR319a from Arabidopsis (Liang et al., 2012), the whole cassette was inserted into a binary vector pCAMBIA1300. The resultants were agrotransformed into apple calli following the method described by (Zhang et al., 2021). Briefly, fresh apple calli were treated with Agrobacterium containing different constructs with gentle shaking for 15 min. The calli were then kept in the dark for 1 day, followed by selection for positive transformants on medium supplemented with corresponding antibiotics.
2.3 Determination of anthocyanin and chlorophyll contents
Anthocyanin was extracted using a methanol-HCl method as described before (Lee & Wicker, 1991). Briefly, apple fruit peel or calli were ground and incubated in methanol:HCl (100:1, V:V) solution overnight in the dark. After centrifugation, the upper supernatant was subjected to spectrophotometric quantification at 530, 620, and 650 nm using a spectrophotometer (UV-2550, Shimadzu, Japan). The relative content of anthocyanin was determined by the formula: optical density (OD) = (Abs530-Abs620)−0.1 (Abs650-Abs620).
For chlorophyll content, apple peels were obtained and pulverised. Then 80% acetone (V/V) was used to extract chlorophyll in the dark for 48 h. After centrifugation, the supernatant was subjected to an ultraviolet spectrophotometer (UV-2550, Shimadzu, Japan) to detect the absorbance at 663 and 645 nm. The total chlorophyll content (CT) is then calculated using the following equation: CT = 20.29 Abs645 + 8.05 Abs665.
2.4 Quantitative real-time PCR (qRT-PCR) analysis
Samples were first ground with liquid nitrogen and total RNAs were extracted using kit (Tiangen RNAprep pure, Hangzhou, China) per the manufacture's instruction. The first-strand cDNA was synthesised using a kit (PrimeScriptTM RT Reagent Kit, TaKaRa, Dalian, China). qRT-PCR reaction programme was performed in StepOnePlusTM Real-Time PCR Instrument (Applied Biosystems, Singapore). Primers used for qRT-PCR were list in Table S1. 18 S rRNA served as housekeeping gene.
2.5 Detection of ADFVd and ASSVd
Apple peels were collected and ground with liquid nitrogen. Total RNAs were extracted with hot phenol method (Zhang et al., 2018). The first-strand cDNA was synthesised as described above. Detection primers for ASSVd (ASSVd-F1/-R1) and ADFVd (ADFVd-F1/-R1) were designed according to the sequence of ASSVd (accession number: NC_001340) and ADFVd (accession number: EF088662) (Table S2). PCR products were separated using 1% agarose gel electrophoresis. The target bands were recovered and sent to company for sequencing.
2.6 5’ RLM-RACE assay
The 5’ RLM-RACE assay was performed based on the previous report by Adkar-Purushothama et al. (2015). Briefly, apple peel of dapple apple fruits (the same samples for small RNA sequencing) were collected and used for total RNA extraction. Then an RNA adaptor ologonucleotide was ligated to the total RNA using T4 RNA ligase (ThermoFisher Scientific) incubating at 37°C for 4 h. cDNA was synthesised using random-primed reverse transcription reaction. A nested PCR was performed to obtain the 5′ end of target fragment using primers listed in Table S3. The nested PCR product was separated by 1.5% agarose gel electrophoresis, and the expected band was collected and purified. The purified products were then cloned into pEASY-Blunt cloning vector (TransGen Biotech) and sent to company for sequencing.
2.7 Transient dual luciferase assay
The promoter of MdPAL (−1395 ~ −1 bp) or MdF3H (−1741 ~ −1 bp) were cloned into pGreenII 0800-LUC vector to drive the expression firefly luciferase (LUC), resulting in the construct MdPALpro-LUC and MdF3Hpro-LUC, respectively. The coding sequence of MdPIF1 was inserted into pGreenII-62-SK controlled by Cauliflower mosaic virus (CaMV) 35 S promoter to obtain the construct 62-SK-MdPIF1. The constructs were transformed into Agrobacterium strain GV1301. Agrobacterium containing different combinations were then infiltrated into leaves of N. benthamiana. Three days post infiltration, the leaves were sprayed with the substrate of luciferase and were kept in the dark for 5 min. The bioluminescent signals were then captured under an in vivo imaging system (IVIS, Lumina II, Caliper Lifescience, the USA). The relative LUC/REN was detected using the Dual Luciferase Reporter Gene Assay Kit (Yeasen Biotech Co., Ltd, Shanghai, China).
2.8 Artificial microRNA transient expression
The assay was modified from Adkar-Purushothama et al. (2015). Briefly, a 35 S promoter was inserted into pGreenII 0800-LUC (35 S::LUC) to drive the expression of LUC. The vsiR693 targeted sequence in MdPIF1 (35 S::LUC-MdPIF1) or mutated MdPIF1 fragment (35 S::LUC-mMdPIF1) was inserted into the the 3’-untranslated region (3’-UTR) of LUC. Additionally, two in tandem vsiR693 corresponding DNA sequences or mutated ones were similarly inserted to construct 35 S::LUC-2×TS and 35 S::LUC-2×mTS. The vd-sRNA vsiR693 expression construct (35 S::amR-vsiR693) was obtained as described above (Liang et al., 2012). The primers used in these constructions were listed in Table S4. All the above constructs were transformed into Agrobacterium strain GV3101. Different combinations were co-agroinfiltrated into N. benthamiana leaves. Then the bioluminescence signals were observed and the enzymatic activity was tested as described in the ‘Transient dual luciferase assay’.
2.9 Transient expression assay in apple fruits
Transient expression assay in apple fruits were performed based on the method described by Peretz et al. (2007) and Ratcliff et al. (2001). Generally, coding sequence of MdPIF1 or the vsiR693 expression cassette was inserted into pIR vector to obtain pIR-MdPIF1 or pIR-amiR-vsiR693, respectively. The plasmid DNA (approximately 200 ng) of pIR, pIR-MdPIF1, or pIR-amiR-vsiR693 was infiltrated together with helper plasmid IL-60-BS into the skin of fresh-bagged apple fruit (cultivar ‘Starking Delicious’) using a needleless syringe. The injected apples were kept in the dark at 24°C for 48 h, and were then kept in a growth chamber with the following conditions: continuous white light (200 μM m−2 s−1), 19°C. The apples were used for photograghy and subsequent experiments 7–10 d later.
For decreasing expression, an MdPIF1 fragment (1–320 bp of the CDS) was inserted in the tobacco rattle virus (TRV) vector (TRV2-MdPIF1). TRV2, TRV2-MdPIF1, and the helper vector TRV1 were transformed into A. tumefaciens strain GV3101, respectively. Agrobacterium containing TRV2 or TRV2-MdPIF1 were separately mixed with TRV1 and infiltrated into apple fruits similarly as above. Infiltrated apple fruits were treated similarly to that in overexpressing assay. Primers used in this assay are listed in Table S2.
2.10 Yeast-one-hybrid assay
Coding sequence of MdPIF1 was inserted into pGAD424 vector (pGAD424-MdPIF1), while promoter fragment of MdPAL (−1395 ~ −1 bp), MdF3H (−1741 ~ −1 bp), MdANS (−1610 ~ −1 bp), and MdDFR (−1540 ~ −1 bp) were inserted into pHIS2 vector, respectively. pGAD424-MdPIF1 was co-transformed with different constructs into yeast competent cells of strain Y187 (WEIDI, Shanghai, China), and the yeast cells were plated on synthetic defined (SD) medium lacking leucine (Leu) and tryptophan (Trp). Empty pGAD424 vector served as negative control. 3-amino-1,2,4-triazole (3-AT) was supplied in the selection medium SD-Leu/-Trp/-histidine (His) to eliminate the effect of leaky expression of HIS (Ouwerkerk & Meijer, 2001). Primers used in this assay are listed in Table S2.
2.11 ChIP-qPCR
Transgenic apple calli of 35 S::MdPIF1-eGFP or 35 S::eGFP were used in the ChIP assay, which was conducted as described before (Qin et al., 2012). A ChIP kit (EpiQuikTM Plant ChIP Kit, Epigentek, the USA) was used to conduct the immunoprecipitation using anti-GFP antibody (Abmart, Shanghai, China), elution and reverse cross-linking of chromatin per the manufacture's protocol. Enriched DNA fragment was used as template for the qPCR assay to analyse the amount of immunoprecipitated chromatin. Then 1 μl of immunoprecipitated samples were used for qPCR analysis. The immunoprecipitated chromatin in each sample were considered as one biological replicate, and each ChIP assay was repeated three times. Three regions of the MdF3H promoter and nine regions of the MdPAL promoter were analysed. Primers used in the assay are listed in Table S5.
2.12 Electrophoretic mobility shift assays (EMSA)
The EMSA was performed generally based on the method described by Xie et al. (2012). The fragment that encoding the C-terminal DNA binding domain (315–533 amino acid) of MdPIF1 was inserted into pGEX-4T-1 vector. The recombinant protein GST-MdPIF1-C was obtained through prokaryotic induction system and purified using a PurKineTM GST-Tag Protein Purification Kit (Abbkine, Wuhan, China) per the manufacture's protocol. Single stranded oligonucleotides of the G-box with flanking sequences from promoter of MdPAL and MdF3H were labelled with biotin using an EMSA Probe Biotin Labelling Kit (Beyotime, Shanghai, China), and the double stranded probed were obtained by annealing the labelled single-stranded oligonucleotides. Non-labelled same oligonucleotides served as competitors. Then, biotin-labelled probes and purified recombinant proteins were incubated in incubation buffer (Beyotime, Shanghai, China) at 24°C for 30 min.
After a pre-run in the 0.5% TBE buffer for 0.5 h at 100 V, the reaction mixtures were then subjected to 6% acrylamide gels containing 0.5% TBE and 3.6% glycerol for gel electrophoresis for another 1.5 h under the same condition as in pre-run. The DNA-protein mix were then transferred to positive charged nylon membranes, and the signals were detected using the chemiluminescent detection kit (Beyotime, Shanghai, China) per the manufacture's protocol.
2.13 GUS activity assay
Promoter sequence of MdPAL (−1395 ~ −1 bp) or MdF3H (−1741 ~ −1 bp) were inserted into the vector p1300-GN containing the GUS (β-glucuronidase) reporter. MdPALpro-GUS and MdF3Hpro-GUS were transiently co-transformed with 35 S::MdPIF1 construct into apple calli via agrobacterium method. Apple calli were then kept in the dark at room temperature for 5 d and collected for GUS staining and activity analysis. For GUS activity determination, a fluorometric 4-methylumbelliferyl-β-D-glucironide method was applied.
2.14 Statistical analysis
All experiments were conducted in triplicate. Error bars show the standard deviation of three biological replicates. Significant differences were determined using ANOVA single factor test (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
3 RESULTS
3.1 Apple dimple fruit disease affects anthocyanin accumulation in apple peel
Green depressed spots on the red skin of fruits is one of the typical symptoms of ADF disease. We hypothesised that anthocyanin accumulation might be involved in this symptom development. Diseased apple fruits with the typical symptom were then collected from apple trees of cultivar ‘Southern Crisp’, and non-diseased fruits from the same cultivar served as control (Figure 1a). ADFVd was firstly confirmed to be present in the diseased fruits, but not in the non-diseased fruits (Figure S1a). We further determined that the diseased apple peel contained much less anthocyanin than that of the non-diseased fruits (Figure 1b, c). However, no significant difference of the chlorophyll content was found between them (Figure 1d). Moreover, the expression levels of multiple anthocyanin biosynthetic genes were significantly reduced in diseased apple peel compared to the non-diseased fruits (Figure 1e). In addition, the expression of several critical transcription factors involved in anthocyanin synthesis was also affected in diseased apple fruits, especially for MdMYB1 (Figure S2a). These data indicated that anthocyanin biosynthesis is closely associated with the symptom development of ADF disease.
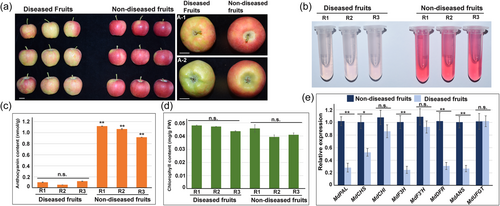
In addition, our results also showed that the single-fruit weight of diseased fruits was much lower than non-diseased fruits (Figure S2b). However, the fruit shape index and the hardness had no significant difference between them (Figure S2c,d). Moreover, the soluble solid contents in the diseased fruits were much lower than the non-diseased (Figure S2e).
3.2 Identification of ADFVd-derived small RNA vsiR693 and its predicted target MdPIF1
Because ADFVd shares high similarity with ASSVd and induces similar symptom to some extent, both of them were included in the test for their presence in the diseased fruits. The result showed that ADFVd was present in all samples collected from different places (Figure 2a). Interestingly, ASSVd was also detected in most of the diseased fruits (Figure 2a).
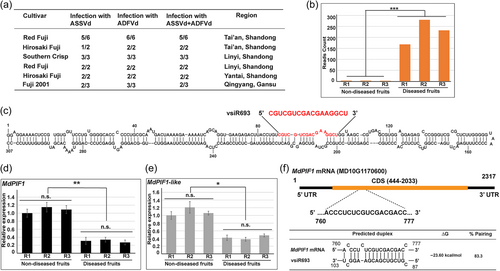
We next performed small RNA sequencing and target prediction to identify the potential vd-siRNA-target combinations that might be involved in anthocyanin biosynthesis. It is worth to mention that the apple peel collected from ‘Southern Crisp’ that containing both ASSVd and ADFVd were used for small RNA sequencing (Figure S1a and 1b). We first screened out those small RNAs with low reads count (less than 100 on average), and 65 small RNAs were identified (Figure S3a and Table S6). Among them, siRNAs in length of 20-22 nucleotides (nt) account more than 60% (Figure S3b), and siRNAs in length of 17 nt account 13.8% (Figure S3b). By analysing the predicted targets of those siRNAs, we finally focused on ADFVd-derived siRNA vsiR693 that targeted the mRNA of MdPIF1s.
Sequencing data showed that the reads count of vsiR693 were 168, 232, and 280 in three replicates of diseased fruits, while that were 1, 3, and 3 in the non-diseased (Figure 2b). Sequence alignment revealed that vsiR693, with a length of 17 nt, was originated from the 87 to 103 nt of ADFVd (Figure 2c). Furthermore, two MdPIF1 homologs, MdPIF1 (MD10G1170600) and MdPIF1-like (GenBank accession number, XM_008384439), were predicted to be targeted by vsiR693. We further found that their expressions were significantly lower in diseased apple fruits than that in non-diseased fruits (Figure 2d,e). Because MdPIF1-like lacks the bHLH domain that is responsible for DNA binding capacity (Figure S4), we thus focused on MdPIF1 for further investigation. The predicted binding sites of vdiR693 for MdPIF1 located to the 760–777 nucleotides of the mRNA of MdPIF1 (Figure 2f, upper section). The minimal energy for the predicted duplex formation was −23.60 kcal/mol (Figure 2f, bottom section).
3.3 VsiR693 mediates the cleavage of MdPIF1 target sequence
To confirm the direct cleavage of MdPIF1 by visR693, 5’ RNA ligase-mediated rapid amplification of cDNA ends (5’ RLM-RACE) assay, an efficient method to detect the direct cleavage of a target by a small RNA (Thomson et al., 2011), were performed. A PCR product of 312 bp in length was obtained from the nested PCR using the template prepared from diseased apple peel (Figure 3a). The nested PCR products had the same 5’ termini, between positions 10 and 11 from the 5’ termini of the vsiR693 (Figure 3b). However, no PCR product was obtained from the RNAs extracted from the apple peels of non-diseased fruits (Figure 3a), indicating a specific cleavage of MdPIF1 mediated by ADFVd-derived vsiR693.
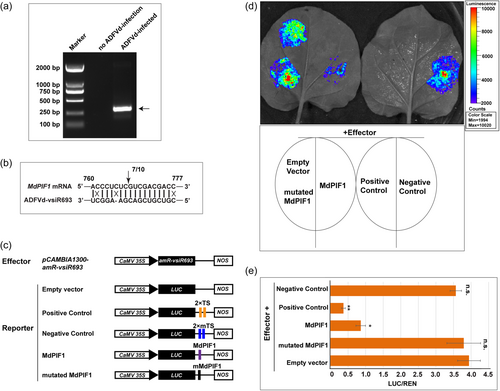
Then, an artificial microRNA (amiRNA) system was used to further demonstrate vsiR693-meidated targeting of MdPIF1 mRNA. The vsiR693 expression cassette was constructed and inserted into pCAMBIA1300, resulting in the construct 35 S::amR-vsiR693 (Figure S5a). The reporter system was modified from pGreenII-0800-LUC, in which a CaMV 35 S promoter was inserted to drive the expression of LUC, resulting in the construct 35 S::LUC (Figure S5b). Through an in vivo imaging system, we observed a strong bioluminescence signal where 35 S::LUC were agroinfiltrated, but not for pGreenII-0800-LUC (Figure S5c). The relative LUC/REN ratio of 35 S::LUC was significantly higher than that of pGreenII-0800-LUC (Figure S5d). These data suggested that the 35 S promoter was successfully inserted into the vector.
The predicted targeting sequence of MdPIF1 or a mutated version was inserted into the 3’-UTR of LUC gene in the 35 S::LUC to obtain 35 S::LUC-MdPIF1 or 35 S::LUC-mMdPIF1, respectively (Figure 3c). If the vsiR693 amiRNA binds the target, the bioluminescence signal will decrease because binding blocks the translational process and/or stability of the target mRNA (Adkar-Purushothama et al., 2015). Two tandem sequences (2×TS) that perfectly complementary to the vsiR693 or two tandem mutated sequences (2×mTS) were inserted into the 3’-UTR of LUC to serve as positive and negative control (Figure 3c). As shown in Figure 3d, bioluminescence signal was extremely lower in positive control. When 35 S::amR-vsiR693 was co-expressed with 35 S::LUC-MdPIF1, the bioluminescence signal was much lower than that of the empty vector (35 S::LUC), however, the signals were comparable to empty vector when co-expressed with 35 S::LUC-mMdPIF1 (Figure 3d). The relative LUC/REN ratio also confirmed that these results (Figure 3e). Given that the absorbance at 465 nm (the peak absorbance for REN luminescence) was similar among different combinations (Figure S5e), therefore, the variation of the LUC/REN ratio was due to the different enzymatic activity of firefly luciferase caused by the visR693-mediated targeting of MdPIF1 (Figure 3e). qRT-PCR assay also showed that the LUC expression levels were reduced in 35S-LUC-MdPIF1, but not in 35S-LUC-mMdPIF1 (Figure S5f). Collectively, these data validated the cleavage of MdPIF1 mRNA mediated by vsiR693.
3.4 MdPIF1 positively regulates anthocyanin biosynthesis and fruit coloration in apple
To determine the function of MdPIF1 in anthocyanin biosynthesis, we first obtained the transgenic apple calli overexpressing or suppressing MdPIF1 expression, which were confirmed at both DNA and transcriptional levels (Figure S6). Transgenic lines MdPIF1-OE1, -OE2, MdPIF1-Anti-1, and -Anti-2 were selected for further investigation because of their relative higher and lower expression of MdPIF1, respectively (Figure S6c). The results showed that transgenic calli overexpressing MdPIF1 displayed a redder colour than WT, and anthocyanin contents were higher in transgenic calli than WT (Figure 4a); while the coloration of transgenic lines MdPIF1-Anti-1 and −2 were much lighter than that of WT, and they also accumulated less anthocyanins compared to WT (Figure 4a). Moreover, overexpressing MdPIF1 promoted expression of anthocyanin biosynthetic genes, including MdPAL, MdCHS, MdF3H, MdDFR, MdANS, and MdUFGT; while expression of these structural genes were significantly downregulated in MdPIF1-Anti transgenic lines (Figure 4b). These data suggested that MdPIF1 positively regulated anthocyanin biosynthesis in apple calli.
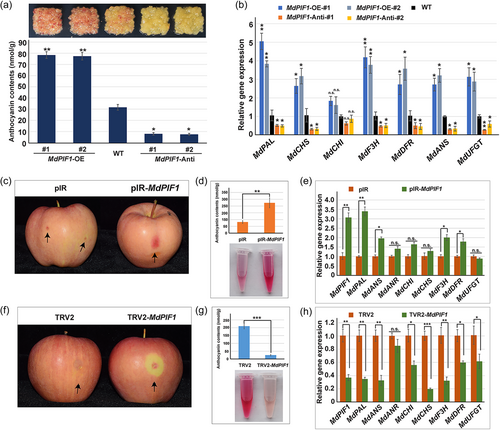
To verify the function of MdPIF1 in apple fruit coloration, the expression of MdPIF1 was overexpressed or suppressed through a viral vector-based transient transformation method. qRT-PCR analysis confirmed that MdPIF1 expression was indeed affected in the corresponding treatment (Figure 4e,h). Overexpressing MdPIF1 promoted red coloration in skin around the infiltration sites (compared with empty vector of pIR) (Figure 4c), while suppressing MdPIF1 expression inhibited the red coloration (compared with the empty vector of TRV2) (Figure 4f). Anthocyanin contents in skins around the infiltration sites also confirmed these results (Figure 4d,g). Moreover, expression levels of multiple anthocyanin biosynthetic genes showed a positive correlation with the expression of MdPIF1, including MdPAL, MdANS, MdF3H, and MdDFR (Figure 4e,h), implying MdPIF1 might directly or indirectly regulate the expression of these genes. In addition, MdPIF1 had no significant effect on MdHY5 transcription (Supplementary Fig. S7a,b), which encodes a b-ZIP transcription factor that play key roles in anthocyanin biosynthesis (An et al., 2017). While the expression of MdMYB1 and MdMYB11, which encode another two key regulators of anthocyanin synthesis, were downregulated upon MdPIF1 suppression, but were not significantly affected when MdPIF1 was overexpressed (Figure S7a,b). Collectively, these data prove that MdPIF1 is a positive regulator of anthocyanin biosynthesis in both apple calli and fruits.
3.5 MdPIF1 specifically binds to the promoter of MdPAL and MdF3H
Based on the above results, four anthocyanin biosynthetic genes (MdPAL, MdANS, MdF3H, and MdDFR) were significantly modulated in both MdPIF1 overexpression and suppression treatments. Thus, MdPIF1 may function as TF to interact with the promoter of the four genes, and a yeast-one-hybrid assay was then performed to determine the potential target(s) of MdPIF1. In the presence of promoter of MdPAL (−1395 ~ −1 bp) or MdF3H (−1741 ~ −1 bp), the yeast cells grew on SD-L/-W/-H medium supplemented with 3-AT at concentrations of 100 and 125 mM, respectively (Figure 5a). While the yeast cells harbouring the promoter of MdANS (−1610 ~ −1 bp) or MdDFR (−1540 ~ −1 bp) did not grow in the selective medium with 3-AT (Figure 5a). This indicated that the promoter of MdPAL and MdF3H might be the potential targets of MdPIF1 (Figure 5a).
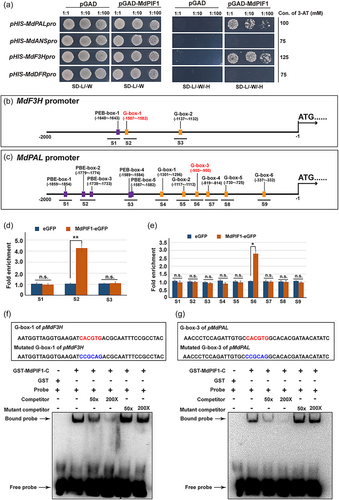
It has been reported that AtPIF1, the ortholog of MdPIF1 in Arabidopsis, preferentially bind to PBE-box (CACATG) and G-box (CACGTG) of target promoters (Pfeiffer et al., 2014). We thus analysed the promoter sequence of the two genes, and found that MdF3H promoter had one PBE-box and two G-box (Figure 5b), while MdPAL promoter contained five PBE-box and six G-box (Figure 5c). To determine the specific motif(s) responsible for MdPIF1 recognition and binding, a ChIP-PCR assay was performed. Chromatin samples prepared from transgenic apple calli of 35 S::MdPIF1-eGFP or 35 S::eGFP were immunoprecipitated using anti-GFP antibody, the enriched DNA fragments were tested using specific primers designed to amplify different promoter regions of MdPAL and MdF3H. The results showed that the fragments containing G-box-1 (5’-CACGTG-3’) of MdF3H promoter (region S2) (Figure 5d) and G-box-3 (5’-CACGTG-3’) of MdPAL promoter (region S6) (Figure 5e) were significantly enriched in 35 S::MdPIF1-eGFP transgenic apple calli compared to 35 S::eGFP, indicating the two motifs might be the responsible binding sites of MdPIF1.
To verify whether MdPIF1 binds to the two motifs, an EMSA assay was performed. The GST fused C-terminal (315-533 aa) of MdPIF1 (GST-MdPIF1-C) was incubated with the biotin labelled DNA fragments of the G-box-3 (flanked by 17 bp on both side) from MdF3H promoter (Figure 5f, the top boxed region) or the G-box-1 (flanked by 17 bp on both side) from MdPAL promoter (Figure 5g, the top boxed region). The results showed that specific DNA-GST-MdPIF1-C complexes were detected when the two G-box-containing sequences were used as probes, while no signal was detected in the absence of GST-MdPIF1-C (Figure 5f,g). The formation of these complexes was reduced upon the addition of increasing amount (50×, 200×) of unlabelled competitor probes (Figure 5f,g). However, this competition was not observed when mutated version of unlabelled G-box-containing fragments (mutant competitor) were used in the assay (Figure 5f,g). All these data confirmed that MdPIF1 could bind to the promoter of MdPAL and MdF3H via binding the G-box-1 and G-box-3 motif, respectively.
3.6 MdPIF1 promotes the expression of MdPAL and MdF3H
We next determined whether MdPIF1 modulated the expression of the two targets using a transient dual luciferase assay. Co-expression of 35 S::MdPIF1 (62-SK-MdPIF1) with MdF3Hpro-LUC or MdPALpro-LUC produced higher luminescence signals (Figure 6a,c, combination 3) than the combination 4, in which 35 S::MdPIF1 was absent (Figure 6a,c). Moreover, the relative ratio of LUC/REN indicated that the combination 3 was significant higher than that of the combination 4 (Figure 6b,d), suggesting MdPIF1 promoted the LUC transcription driven by the promoter of MdF3H or MdPAL.
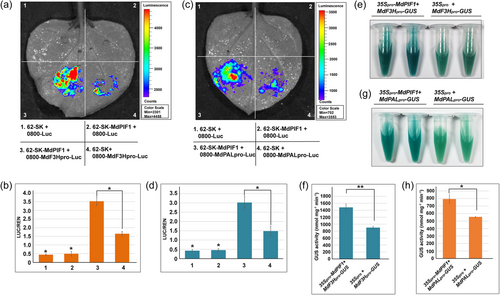
To further confirm MdPIF1 activating the expression of the two targets, a GUS reporter assay was performed. MdF3Hpro-GUS or MdPALpro-GUS was first transiently transformed into apple calli together with 35Spro-MdPIF1, while 35Spro served as control. GUS histochemical assay showed that co-transformation of 35Spro-MdPIF1 with MdF3Hpro-GUS or MdPALpro-GUS displayed a darker staining than that of control (35Spro with MdF3Hpro-GUS or MdPALpro-GUS) (Figure 6e,g). Moreover, apple calli transiently transformed with 35Spro-MdPIF1 plus MdF3Hpro-GUS or MdPALpro-GUS exhibited much higher GUS activity than that of control (Figure 6f,h), suggesting MdPIF1 promoted GUS transcription driven by the MdF3H or MdPAL promoter. Collectively, these data indicated that MdPIF1 promoted the expression of MdF3H and MdPAL.
3.7 VsiR693 negatively regulates anthocyanin biosynthesis in apple
Given that MdPIF1 was a target of vsiR693, and MdPIF1 positively regulated the anthocyanin biosynthesis in apple, we hypothesised that vsiR693 might also play a role in regulating anthocyanin biosynthesis. To verify our hypothesis, 35 S::amR-vsiR693 was introduced into apple calli through agrobacterium-mediated transformation. By verification at DNA level and transcriptional level (the downregulated MdPIF1 expression), three transgenic lines were obtained and used for subsequent experiments (Figure S8a,b). In addition to MdPIF1, six other PIF members have also been identified in our previous reports (Zheng et al., 2020), we next verified whether these MdPIFs were affected by vsiR693 using qRT-PCR. The results showed that their transcription levels in vsiR693 expression apple calli were not significantly affected compared to those in wt (Figure S8b), suggesting visR693 specifically recognised and mediated the degradation of mRNA of MdPIF1. Under continuous light and low temperature (19°C), transgenic apple calli accumulated less anthocyanin than WT, as indicated by a lighter red colour than WT (Figure 7a). Moreover, the expression of MdPAL and MdF3H, as well as other anthocyanin biosynthetic genes, decreased in the transgenic apple calli (Figure 7b), indicating vsiR693 negatively regulated anthocyanin biosynthesis in apple calli.
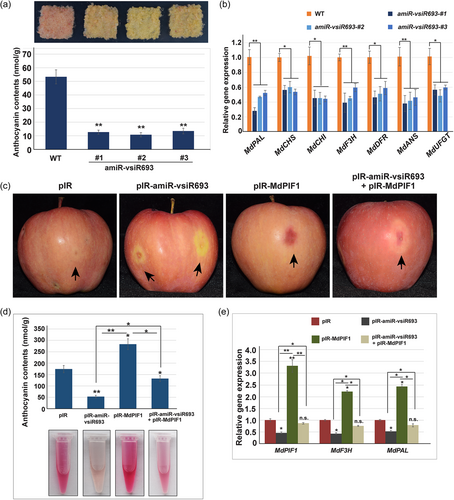
We next determined the role of MdPIF1 in vsiR693-regulated anthocyanin biosynthesis using apple fruits. Transient expression of vsiR693 (pIR-amiR-vsiR693) significantly inhibited coloration around the infiltration sites (Figure 7c). Anthocyanin contents and expression levels of MdPIF1, MdPAL, and MdF3H in pIR-amiR-vsiR693 infiltration apple fruits were significantly lower than fruits with empty vector (pIR) infiltrated (Figure 7d,e), confirming the negative role of vsiR693 in anthocyanin biosynthesis in fruits. On the contrary, overexpressing MdPIF1 (pIR-MdPIF1) promoted coloration around the infiltration sites as well as anthocyanin content and related biosynthetic gene expression (Figure 7c–e), which are in consistence with the results in Figure 4a–c. However, when co-expressing pIR-amiR-vsiR693 and pIR-MdPIF1, the coloration of apple peels surrounding the infiltration sites were lighter than that of pIR-MdPIF1 but redder than that of pIR-amiR-vsiR693 (Figure 7c). The anthocyanin contents of apple peel adjacent to the co-infiltration sites, as well as the expression of MdPAL and MdF3H, were in consistence with the phenotype (Figure 7d,e). These data indicated that vsiR693 negatively regulated anthocyanin biosynthesis in an MdPIF1-dependent manner.
3.8 Infection with ADFVd affects the coloration in apple fruits
We next intended to verify whether infection with ADFVd affected the fruit coloration in apple. A dimeric viroid infectious clone of ADFVd (EF088662, GenBank accession number) was synthesised and constructed by assembling the viroid dimeric sequence into pCAMBIA1300. When inoculating the N. benthamiana leaves with the infectious clone, no symptom was observed in either inoculated or upper leaves compared with the mock (empty vector pCAMBIA1300 was agroinfiltrated into leaves of N. benthamiana). Then RNA samples from the upper leaves were prepared and the corresponding cDNAs were synthesised using random primer. RT-PCR assay was performed using the primer pairs to obtain the full length of ADFVd, and results showed that specific bands with similar size of positive control (using plasmid harbouring ADFVd full length as template) were amplified (Figure S9a). Sequencing analysis of PCR products showed that the amplicons were identical to ADFVd sequence, suggesting ADFVd infectious clone was successfully constructed. Then, the ADFVd infectious clone was agroinfiltrated into apple peel, and the apples were subsequently placed in a growth chamber that facilitating anthocyanin accumulation, while empty vector (pCAMBIA1300) served as mock. The results showed that infiltration with ADFVd infectious clone suppressed the coloration of apple peels surrounding the injection sites, while that was not observed in the mock (Figure 8a). We also determined that ADFVd infection decreased anthocyanin accumulation in apple peel adjacent to the injection sites (Figure 8b). Moreover, the expression of MdPIF1, MdPAL, and MdF3H, as well as other anthocyanin biosynthetic genes, were also significantly decreased upon ADFVd infection (Figures 8c–e and S9b–f), suggesting viroid repressed coloration of apple peel.
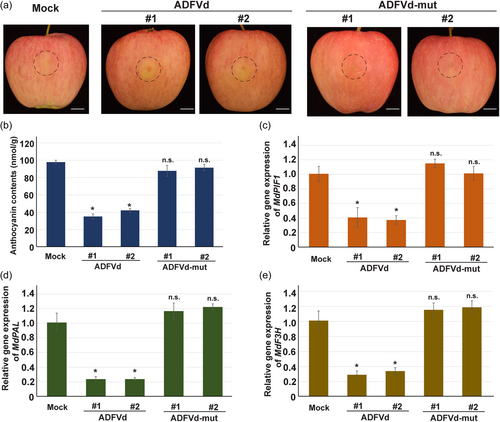
To test whether vsiR693 play a role in ADFVd-affected fruit coloration, we generated a mutant ADFVd infectious clone, in which site mutation was introduced into the vsiR693 producing region (Figure S10a). The secondary structure of mutated ADFVd was similar with the original one, with minor changes in the mutated region (Figures S10a, 2c). The predicted mutated vsiR693 had three G to C mutation at its 5’ end seed region. It is worthnoting that the site mediating the cleavge of MdPF1 mRNA was also mutated (Figure S10b). The infectious clone of mutated ADFVd was constructed and agroinfiltrated into N. benthamiana, and the RT-PCR testing showed expected bands at similar size with the positive control (using plasmid harbouring ADFVd-mut full length as template) (Figure S10c), suggesting the introduced mutation did not affect the viroid replication capacity. The mutated version was then used in apple fruit coloration assay. After infiltration with the mutated version, the coloration surrounding the injection sites were not affected, showing similar phenotype with that of mock (Figure 8a). In accordance with the phenotype, anthocyanin accumulation (Figure 8b) and expression of MdPIF1, MdPAL, or MdF3H and other anthocyanin biosynthetic genes (Figures 8c–e, S9b–f) all had no significant difference with that of mock. This indicated that the mutated region, where vsiR693 was produced, might play a key role in ADFVd-regulated fruit coloration in apple.
4 DISCUSSION
We report here that an ADFVd-derived small RNA, vsiR693, negatively regulates anthocyanin biosynthesis by targeting and mediating the cleavage of mRNA of MdPIF1, whose product promotes anthocyanin biosynthesis by directly binding to the promoter of anthocyanin biosynthetic genes (MdPAL and MdF3H) to promote their transcription (Figure 9). Anthocyanin biosynthesis is regulated by many external factors, such as light, temperature, drought, and even wounding (An et al., 2019; Gao et al., 2021), however, the viroid-affected synthesis of anthocyanin has been rarely reported. Therefore, our work not only uncovers a molecular pathway which is explored by ADFVd to regulate anthocyanin biosynthesis, but also complements and expands the external factors that are involved in regulating anthocyanin biosynthesis.
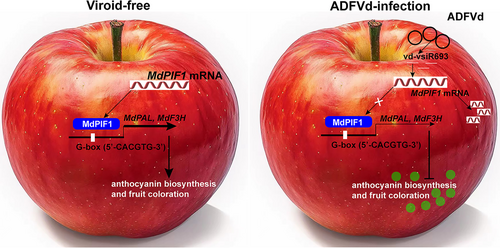
4.1 Viroid-derived siRNAs play a critical role in viroid pathogenicity
ASSVd and ADFVd belong to the same genus and they share a 63.5% sequence similarity, and ADFVd contains the core nucleotide sequence of the central and terminal conserved region of ASSVd (Di Serio et al., 1996). More importantly, they incite similar symptom in the field, suggesting the two viroids may trigger a common pathogenic alteration (Di Serio et al., 2001). Thus, the apple fruits with dimple symptom that are infected with both ASSVd and ADFVd were subjected for RNA sequencing to seek the potential symptom-associated sRNAs derived from both. In this study, we identified an ADFVd-derived small RNA vsiR693, which is predicted to target and mediate the cleavage the mRNA of MdPIF1. 5’ RLM-RACE and transient expression of artificial microRNA system were used to confirm the cleavage of the mRNA of MdPIF1 mediated by vsiR693 (Figure 3). Moreover, 5’ RLM-RACE assay revealed that the cleavage site occurs between positions 10 and 11 on mRNA:sRNA (mRNA of MdPIF1 and vsiRNA693) duplex starting from the 5’ termini of vsiR693 (Figure 3b). In fact, viroid derived small RNA-mediated cleavage between 10 and 11 of the mRNA:sRNA duplex starting from the 5’ end of the sRNA have been previously reported in multiple cases, such as sRNAs derived from PTSVd or chrysanthemum chlorotic mottle viroid (CChMVd) target and mediate the cleavage of mRNA of TCP (teosinte branched1/Cycloidea/Proliferating cell factor) or choroplastic TKT (transketolase), respectively, to incite corresponding symptoms (Bao et al., 2019; Serra et al., 2023).
Vd-sRNAs are derived from genomes of viroids, and viroid mutants that producing mutated sRNAs or even failing to produce specific sRNAs might affect corresponding pathogenesis. For example, PSTVd mutants failing to produce sRNA that are incapable of targeting the mRNAs of callose synthase fail to incite typical symptom in tomato (Adkar-Purushothama et al., 2015). In another report, sRNA produced from a point mutating version of CChMVd forms a more stable hybrid duplex with its target mRNA, leading to earlier and more severe symptom in chrysanthemum (Chrysanthemum morifolium Ramat) (Serra et al., 2023). In this study, we observed that infiltrating apple fruits with ADFVd infectious clone repressed fruit coloration around the injection sites (Figure 8). However, infiltration with a mutated version, in which the region producing vsiR693 was point mutated (Figure 8), failed to suppressed the fruit coloration, suggesting the vd-sRNAs play a critical role in this process.
Vd-sRNAs production entirely rely on host mechanisms, thus, the majority of vd-sRNAs are 21-24 nucleotides in length (Dadami et al., 2013). For viroids belonging to the family Avsunviroidae, which usually replicating and accumulating in the chloroplast, predominantly producing vd-sRNAs of 21- and 22-nt in length; while viroids belonging to the family Pospiviroidae, which usually replicating and accumulating in the nucleus, predominantly producing vd-sRNAs of 21-, 22-, and 24-nt in length (Adkar-Purushothama et al., 2018; Chiumenti et al., 2014; Di Serio et al., 2023; Jiang et al., 2019). However, it does not mean that small RNAs of shorter (<20 nt) or longer (>25 nt) in length are dysfunctional. A recent study reported that the abundance of small RNAs with length of >26 nt, which are produced in DICER-independent manner and are preferentially associated with ARGONAUTE1, increased upon exposure to nitrogen or sulfure deprivation in alga (Chlamydomonas reinhardtii), indicating their possible role in nutritional stress tolerance (Li et al., 2023).
Vd-sRNAs with a shorter (<20 nt) or longer length (>25 nt) are also detectable in viroid-infected plant tissues, such as in hop stunt viroid (HSVd)-infected cucumber leaves (Martinez et al., 2010), PLMVd-infected peach leaves (Bolduc et al., 2010), and PSTVd-infected tomato (Tsushima et al., 2015). This indicates that shorter vd-sRNAs that accounting for a small proportion of the total sRNA library might also play critical roles in viroid pathogenesis. In this study, our sequencing data revealed that vd-sRNAs of 20- to 22-nt account for the majority of the sRNA library (Figure S3). However, by analysing the predicted targets, we identified a shorter vd-sRNA vsiR693, which is 17 nt in length, and demonstrated its role in regulating anthocyanin biosynthesis in apple. In fact, vsiR693 account for 13.8% of the selected vd-sRNAs, and their reads count were 168, 232, and 280 in the three replicates of diseased apple fruits, while that were 1, 3, and 3 in the control (Figure 2b). Thus, vd-sRNAs in shorter length may also be important for viroid pathogenesis. It should be mentioned that the CCR region of ADFVd, where visR693 was originated, is conserved across the genus Apscaviroid (Di Serio et al., 1996, 2001). Therefore, it is of high possibility that, in addition to vsiR693, other small RNAs derived from this region of ADFVd, as well as from ASSVd, might contribute to the symptom development and pathogenesis in apple fruits.
4.2 PIFs are key regulators of anthocyanin biosynthesis in multiple plant species
In addition to the critical roles in light-regulated signalling pathway (Balcerowicz, 2020; Sanchez et al., 2020), PIFs are also involved in regulating anthocyanin biosynthesis, including PIF3 (Kim et al., 2003), PIF4 and PIF5 (Liu et al., 2015; Qin et al., 2022) in Arabidopsis, MdPIF7 in apple (Liu et al., 2022), and PyPIF5 and PpPIF8 in pear (Liu et al., 2021b; Ma et al., 2022). For instance, MdPIF7 negatively regulates anthocyanin biosynthesis in apple. Specifically, MdbZIP44 and MdERF38 interact with and promote the transcriptional activity of MdMYB1 on anthocyanin biosynthetic genes (An et al., 2020). However, MdPIF7 could interact with MdbZIP44 and MdERF38 to disturb the interactions between MdbZIP44-MdMYB1 and MdERF38-MdMYB1, resulting decreased anthocyanin biosynthesis in apple (Liu et al., 2022). In pear (Pyrus L.), PyPIF5 also negatively regulates anthoycanin biosynthesis through a module composed of PymiR156a-PySPL9 (SQUAMOSA promoter binding protein-like 9)-MYB114/PyMYB10 (Liu et al., 2021b). Although the aforementioned MdPIF7 and PyPIF5, together with Arabidopsis PIF4 and PIF5, are negative regulators in anthocyanin biosynthesis, certain PIF members may also act as positive regulators in anthocyanin biosynthesis. In addition to the positive role of PIF3 in Arabidopsis anthocyanin synthesis (Kim et al., 2003), PpPIF8 is also reported to positively regulate anthocyanin biosynthesis by promoting the expression of anthocyanin biosynthetic genes in both pear fruits and calli (Ma et al., 2022). In this study, we determined that MdPIF1 was a positive regulator in anthocyanin biosynthesis in apple. Our data proved that overexpressing MdPIF1 promoted anthocyanin biosynthesis in both apple calli and fruits (Figure 4). Moreover, MdPIF1 could directly bind the promoter of two anthocyanin biosynthetic genes, MdPAL and MdF3H, to promote their expression, leading to increased anthocyanin biosynthesis (Figures 5 and 6). Therefore, various PIF members function differently in different plant species, and even in the same species.
In addition to structural genes, PIFs may also modulate the expression of anthocyanin regulatory TFs. For example, PIF4 could interact with the promoter and repress the transcription of PAP1, which encoding an R2R3-MYB TF that regulating anthocyanin synthesis in Arabidopsis (Liu et al., 2021c). Moreover, MdPIF7 interacts with and compromises the transcriptional activation of MdBBX23 (B-box 23) on MdHY5 (Long Hypocotyl 5), both of which are positive regulators in anthocyanin biosynthesis, leading to inhibited anthocyanin biosynthesis (An et al., 2017; Liu et al., 2022). We also tested the effect of MdPIF1 on transcription of MdHY5, MdMYB1, and MdMYB11, three key TFs in regulating anthocyanin synthesis in apple (Figure S7a,b) (An et al., 2015, 2017; Li et al., 2012). qRT-PCR analysis showed that MdPIF1 had no effect on the expression of MdHY5 (Figure S7a, b), which is in consistence with previous results that PIF3 and HY5 do not regulate each other at the transcriptional level in Arabidopsis (Shin et al., 2007). Downregulating MdPIF1 significantly inhibited the expression of MdMYB1 and MdMYB11, but overexpression of MdPIF1 had no effect on their transcription (Figure S7a,b), suggesting MdPIF1 might indirectly regulate expression of these two TFs.
It has been well-investigated that PIFs accumulate in the dark and will be degraded in a phytochrome-dependent manner upon exposure to light (Pham et al., 2018; Xu et al., 2015). However, light is an indispensable factor for anthocyanin biosynthesis and accumulation (Li et al., 2012). Therefore, when testing the effect of PIFs on the accumulation of anthocyanin, light condition must be carefully considered. When determining the effect of PIF3 on anthocyanin biosynthesis, the Arabidopsis seedlings were treated with far-red light, which will not induce the degradation of PIF3 (Shin et al., 2007). In this study, the infiltrated apples were kept in the dark for 2 days, allowing MdPIF1 staying in the nucleus to execute its transcriptional function (Figures 4 and 7). The samples were then exposed to light condition to facilitate anthocyanin biosynthesis and accumulation. Our data showed that overexpressing MdPIF1 promoted anthocyanin synthesis and accumulation compared to that in WT, while repressing MdPIF1 expression decreased the synthesis and accumulation of anthocyanin in both apple calli and fruits (Figure 4).
In summary, our work identifies a viroid-derived small RNA that mediating the cleavage of mRNA coding for a bHLH transcription factor MdPIF1, which could directly bind to the promoter of anthocyanin biosynthetic genes MdPAL and MdF3H to promote their expression, resulting in decreased synthesis of anthocyanin in apple fruits. Our data determine a regulatory pathway explored by ADFVd to modulate anthocyanin biosynthesis, which may complement and extend the external factors that manipulating anthocyanin biosynthesis.
4.3 Accession number of genes used in the manuscript
The GenBank accession number of genes and viroid sequences used in this study are listed as following. MdPIF1 (NC_041798), MdPIF1-like (XM_008384439), MdPIF2 (NC_041800), MdPIF3 (NC_041792), MdPIF4 (NC_041805), MdPIF5 (NC_041797), MdPIF7 (NC_041802), MdPIF8 (NC_041795), MdPAL (NC_041789), MdF3H (NC_041790), MdCHS (NC_041792), MdDFR (NC_041803), MdCHI (NC_041789), MdANS (NC_041794), MdANR (NC_041798), MdUFGT (NC_041789), MdMYB1 (NC_041797), MdMYB11 (NC_041797), MdHY5 (NC_041796), MdbHLH3 (NC_041799), ASSVd (NC_001340), ADFVd (EF088662).
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (grant number 31901988) and Shandong Province Key R&D Projects (2022TZXD008). We thank Prof. Yanxiu Wang (Gansu Agricultural University), Prof. Laiqing Song (Yantai Academy of Agricultural Science) and Prof. Lingling Zhao (Yantai Academy of Agricultural Science) for helping to collect the apple fruit samples. We thank Prof. Xiaofeng Wang (Virginia Tech) and Prof. Ying Wang (Mississippi State University) for discussing the experiments. We thank Prof. Xiaofeng Wang (Virginia Tech) for critical reading of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the supplementary material of this article. The data supporting the findings of this study are available within the manuscript and its Supporting Information.




