Water wisteria genome reveals environmental adaptation and heterophylly regulation in amphibious plants
Abstract
Heterophylly is a phenomenon whereby an individual plant dramatically changes leaf shape in response to the surroundings. Hygrophila difformis (Acanthaceae; water wisteria), has recently emerged as a model plant to study heterophylly because of its striking leaf shape variation in response to various environmental factors. When submerged, H. difformis often develops complex leaves, but on land it develops simple leaves. Leaf complexity is also influenced by other factors, such as light density, humidity, and temperature. Here, we sequenced and assembled the H. difformis chromosome-level genome (scaffold N50: 60.43 Mb, genome size: 871.92 Mb), which revealed 36 099 predicted protein-coding genes distributed over 15 pseudochromosomes. H. difformis diverged from its relatives during the Oligocene climate-change period and expanded gene families related to its amphibious habit. Genes related to environmental stimuli, leaf development, and other pathways were differentially expressed in submerged and terrestrial conditions, possibly modulating morphological and physiological acclimation to changing environments. We also found that auxin plays a role in H. difformis heterophylly. Finally, we discovered candidate genes that respond to different environmental conditions and elucidated the role of LATE MERISTEM IDENTITY 1 (LMI1) in heterophylly. We established H. difformis as a model for studying interconnections between environmental adaptation and morphogenesis.
1 INTRODUCTION
Amphibious plants can grow both in water and on land, switching their morphology and physiology according to various environmental cues (Agarie et al., 1997). While genomic data are available for several aquatic plants (Abramson et al., 2022; Lan et al., 2017; Lu et al., 2021; Shi et al., 2022; Xue et al., 2020), amphibious plants remain largely unexplored at the genomic level. Climate change poses new challenges for plant growth and survival, and one strategy they use to cope with these challenges is phenotypic plasticity (Alpert & Simms, 2002), the ability to change phenotypes in response to environmental cues. Phenotypic plasticity connects plant evolution, ecology and molecular biology, and understanding it will be instrumental in designing resilient crops (Arnold et al., 2019; Cornelis & Hazak, 2022; Nicotra et al., 2010; Stotz et al., 2021). A striking example of phenotypic plasticity is heterophylly, the ability of a plant to alter its leaf shape depending on the surrounding environment (van Veen & Sasidharan, 2021). Heterophylly is a plant adaptation, and is common in amphibious plants (Kim et al., 2018; Koga et al., 2021; Li et al., 2017; Nakayama et al., 2014). Many heterophyllous plants can adjust their leaf morphology to various environmental factors such as submergence (Han et al., 2021), light (Momokawa et al., 2011; Nakayama et al., 2014), temperature (Nakayama & Kimura, 2015), and CO2 (Titus & Gary Sullivan, 2001), which might enable prediction of plant responses to novel environments caused by climate change. Whereas studies on model plants such as Arabidopsis thaliana have greatly contributed to identifying genes and processes regulating plant phenotypes, a suitable phenotypic plasticity model system is needed to understand the relationships between environmental factors and plant phenotypes. Interestingly, heterophyllous plants across diverse taxa often show similar leaf variations in response to the amphibious environment, making them potential plant models for convergent evolution and environmental adaptation.
Recently, in a few amphibious species, several mechanisms regulating heterophylly have been suggested. For example, ethylene and abscisic acid (ABA) control adaxial–abaxial leaf polarity and induce heterophylly in Ranunculus trichophyllus (Ranunculaceae) (Kim et al., 2018). Similarly, cell elongation and division contribute to leaf differentiation in Callitriche palustris (Plantaginaceae) (Koga et al., 2021). In Rorippa aquatica (Brassicaceae), KNOTTED1-LIKE HOMEOBOX (KNOX1) modulates the levels of endogenous phytohormone gibberellic acid (GA), influencing temperature-dependent heterophylly (Nakayama et al., 2014). However, despite transcriptomic data availability for several heterophyllous plants (Han et al., 2021; He et al., 2018; Horiguchi et al., 2023; Ikematsu et al., 2023; Koga et al., 2021; van Veen et al., 2013), the molecular mechanisms underlying heterophylly are largely unknown.
Hygrophila difformis (Acanthaceae; water wisteria), is an amphibious plant that exhibits heterophylly by developing deeply lobed, complex leaves when submerged and simple leaves when on land (Figure 1a). It has recently shown promise as a new model for heterophylly studies, due to its beneficial traits such as easy gene transformation, fast propagation, and conspicuous leaf shape change in response to various environmental stimuli (Li et al., 2017, 2020). It could also be used to explicitly address interactions between environmental responses, morphological features, and variation in gene expression in an ecological context (Li et al., 2021). Although others have studied heterophylly molecular mechanisms in Rorippa, Ranunculus and Callitriche, H. difformis is the only plant to have established a transformation system that allows their study using transgenic methods. Previously, we confirmed that SHOOT MERISTEMLESS (STM) participates in H. difformis heterophylly regulation (Li et al., 2022), but key underlying genes and molecular mechanisms remain unclear.
Hygrophila comprises nearly 90 species, many of which are amphibious and display phenotypic plasticity (Figure S1), but H. difformis displays the most conspicuous heterophylly form. Hygrophila includes ornamental plants (Karataş et al., 2013) and medicinal herbs (Ingale et al., 2013), and species used as models for photosynthetic studies (Silver Botts et al., 1990; Horiguchi et al., 2021). Presently, however, there are no genomic resources available for this genus.
Here, we report H. difformis chromosome-scale genome, assembled by integrating PacBio, Illumina, and High-throughput Chromosome Conformation Capture (Hi-C) sequencing technologies. We performed genome and transcriptome analyses to reveal the molecular mechanisms underlying heterophylly in response to submerged and terrestrial environments. We also identified and validated LATE MERISTEM IDENTITY 1 (LMI1) as a candidate gene for leaf shape regulation. Our study provides the first genome and transcriptome for the genus Hygrophila, establishing H. difformis as a novel environmental model for investigating environmental impacts on plant phenotypes. This study offers a comprehensive framework and functional data for future genetic, genomic, and molecular studies of plant phenotypic plasticity. We believe this will attract more attention to plant phenotypic plasticity and environmental adaptation, and promote H. difformis as an emerging model for plant Eco/Evo/Devo studies.
2 MATERIALS AND METHODS
2.1 Plant materials and phenotypic analysis
Sequenced H. difformis individuals were collected from South China Botanical Garden, Chinese Academy of Sciences, Guangdong Province, China (N113.373781°, E23.18794°). For the terrestrial treatment, young plants were planted in soil for 2 weeks, and then subjected to different temperatures and humidity. For the submergence treatment, plants were moved into aquariums filled with tap-water, and then exposed to different light intensities. Plants were then grown under different experimental conditions for at least 1-month (A: terrestrial plants grown at 23°C, 60% RH, 60 μmol m−2 s−1 illumination; B: terrestrial plants grown at 18°C, 60% RH, 60 μmol m−2 s−1 illumination; C: submerged plants grown at 23°C, 60 μmol m−2 s−1 illumination, D: submerged plants grown at 23°C, 10 μmol m−2 s−1 illumination, E: terrestrial plants grown at 23°C, 30% RH, 60 μmol m−2 s−1 illumination). For morphological analysis, mature leaves were photographed with a Canon EOS80D camera, and all light microscopy observations were performed under a Sunny EX20 light microscope and photographed with a ToupCam TP605100A digital camera. Images were integrated using MvImage media software (ToupCam). Leaf complexity was estimated based on the dissection index (), and epidermal cell complexity was also quantified (). Stomatal and vein density were calculated as described by (Li et al., 2017). All calculations were performed using ImageJ 1.47v (http://rsb.info.nih.gov/ij/). Statistical differences were determined using Student's t-test or one-way analysis of variance, and data represent the results from at least 20 independent individuals. Chlorophyll content analysis was performed following our previous methods (Sun et al., 2023). Briefly, 0.1 g fresh samples were homogenised in 95% (vol/vol) ethanol and maintained at 4°C for 24 h in the dark. Their photosynthetic pigment contents were then determined by absorbance measurements at 663 and 645 nm, using a spectrophotometer (BioDrop uLite80-3006-51; Biochrom). Fv/Fm, reflecting photosynthetic performance, was measured on mature leaves using a plant efficiency analyser after dark-adapted treatment for 10 min (Hansatech Instruments).
2.2 Genome size and ploidy estimation
Short reads from the MGIseq. 2000 platform were filtered and used for genome size estimation by SOAPnuke (Chen et al., 2018). Initially, adaptors were removed from the sequencing reads. Read pairs were then excluded if any single end had an average quality <20, and subsequently, read ends were trimmed if the average quality was <20 in a 5 bp sliding window. Finally, read pairs with any end shorter than 75 bp were removed. These quality-filtered reads were used for genome size estimation. We generated the 17-mer occurrence distribution of sequencing reads from short libraries, and genome size was estimated by 17-K-mer analysis based on the K-mer method (Manekar & Sathe, 2018). The sequenced H. difformis chromosome number was determined by a cytological method (Li et al., 2017). Briefly, root tips were fixed in an acetic-alcohol mixture (1:3) overnight, then cells were dissociated by 1 M hydrochloric acid at 60°C for 1 h. After thorough washing with ultrapure water, they were stained with Modified Carbol fuchsin solution and squashed for chromosome number count. Genome size was estimated by flow cytometry as previously reported (Wang et al., 2014). We collected young H. difformis leaves and immediately conducted flow cytometry (Dolezel & Bartos, 2005) to determine genome size using Sorghum bicolor (BTX623, genome size = 0.71 Gb) and Zea mays (Mo17, genome size = 2.18 Gb) as external standards.
2.3 De novo sequencing and genome assembly
Fresh leaves from the same H. difformis individual were utilised to isolate high molecular weight genomic DNA for genome sequencing. Extracted DNA quality and quantity were examined using a NanoDrop spectrophotometer (NanoDrop Technologies). Genomic DNA was isolated using a Qiagen DNeasy Plant Mini Kit (Qiagen). We also collected a wide range of tissue samples (stems, shoots, roots, flowers, and leaves) from the same plant for RNA sequencing (RNA-seq) to support genome assembly.
Two long-insert continuous long-read libraries (40 kb) were prepared following PacBio Sequel platform (Pacific Biosciences, Frasergen). A SMRT (Single Molecule Real-Time) library was constructed using a SMRTbell Express Template Prep kit 2.0 (Pacific Biosciences). Briefly, DNA was carried into the first enzymatic reaction to remove single-stranded overhangs followed by treatment with repair enzymes. Subsequently, the double-stranded fragment ends were polished and tailed with T-overhang SMRTbell adapters and the SMRTbell library was purified using AMPure PB beads. Library size distribution and concentration were assessed using a FEMTO Pulse automated pulsed-field capillary electrophoresis instrument (Agilent Technologies) and a Qubit 3.0 Fluorometer (Life Technologies). Following library characterisation, BluePippin (Sage Science) was used to remove SMRTbells ≤25 kb. After size selection, the library was purified with AMPure PB beads. Library size and quantity were assessed using FEMTO Pulse and Qubit dsDNA HS reagents Assay kits. Sequencing primer and Sequel II DNA Polymerase were annealed and bound, respectively, to the final SMRTbell library. SMRT sequencing was performed using a single SMRT Cell on the Sequel II System with a Sequel II Sequencing Kit (Frasergen Bioinformatics Co., Ltd.). The sequenced genome draft assembly was assembled using MECAT2 (v20190226) with the default parameters (Xiao et al., 2017). A SMRT link toolkit was then used to correct errors after genome initial assembly.
To anchor hybrid scaffolds onto chromosomes, genomic DNA was also used to construct a Hi-C library. Briefly, samples were cross-linked under vacuum infiltration and subsequently lysed. Endogenous nuclease was inactivated with 0.3% SDS, then chromatin DNA was digested by MboI (NEB), marked with biotin-14-dCTP (Invitrogen), and then ligated by T4 DNA ligase (NEB). After reversing cross-links, the ligated DNA was extracted with a QIAamp DNA Mini Kit (Qiagen) according to manufacturers’ instructions. Purified DNA was sheared to 300- to 500-bp fragments which were further blunt-end repaired, A-tailed and adaptor added, followed by purification through biotin-streptavidin–mediated pull-down and PCR amplification. Finally, the Hi-C libraries were quantified and sequenced on the Illumina Nova-seq, or MGI-seq platform (BGI). We obtained sequencing data using a BGI MGISEQ-2000 platform on 150 PE mode. For anchored contigs, 381 400 686 clean read pairs were generated from the Hi-C library and mapped to the polished H. difformis genome using BWA (bwa-0.7.16) (H. Li & Durbin, 2010). Lachesis (Burton et al., 2013) was applied to orient the clustered contigs, and the Juicer and Juicebox tool (v1.8.8) with default parameters (Durand et al., 2016) was used to correct the assembly error in the Hi-C assembled genome. Paired reads mapped to different contigs were used for the Hi-C-associated scaffolding. Self-ligated, non-ligated, and other invalid reads were filtered out. We applied 3D-DNA to orient the clustered contigs and performed Juicer to filter the sequences and cluster them. Juicebox with default parameters was applied to manually adjust chromosome construction. We also used purge haplotigs to remove heterozygosity. Finally, we anchored the scaffolds on 15 chromosomes. Additionally, we used the BUSCO pipeline to assess the completeness and accuracy of our assembled H. difformis genome as previously outlined (Simão et al., 2015).
2.4 Protein-coding genes prediction and annotation
We predicted H. difformis protein-coding genes using three methods: ab initio, homology-based, and transcriptomic prediction. AUGUSTUS (v3.3.1) (Stanke et al., 2006) and GlimmerHMM (v3.0.4) (Majoros et al., 2004) were used to perform ab initio gene prediction. We used EXONERATE (v2.2.0) (Slater & Birney, 2005) for the homology-based prediction. First, protein sequences were aligned to the genome assembly and predicted coding genes using EXONERATE with the default parameters. For transcriptomic prediction, we used RNA-seq analysis. First, we assembled clean RNA-Seq reads into transcripts using trinity (Haas et al., 2013), and gene structures were formed using PASA (Trapnell et al., 2010). Finally, MAKER (v3.00) (Cantarel et al., 2008) was used to integrate the prediction results. Gene functions were inferred according to the best match of alignments to the non-redundant database (NR) of the National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/), TrEMBL (Boeckmann et al., 2003), SWISS-PROT (Boeckmann et al., 2003), and InterPro (Mitchell et al., 2015). Motifs and protein domains were annotated using PfamScan (Mistry et al., 2007) and InterProScan (http://www.ebi.ac.uk/InterProScan). We also performed BUSCO analysis (dataset: embryophyta_odb10 models) using the predicted protein to assess our H. difformis genome annotation completeness.
2.5 Repetitive sequences annotation
The homology-based and de novo prediction methods were combined to identify repeats in the genome. For homology-based analysis, we identified the known TEs within the H. difformis genome using RepeatMasker (open-4.0.9) (Tarailo-Graovac & Chen, 2009) with the Repbase TE library (Jurka et al., 2005). RepeatProteinMask searches were also conducted using the TE protein database as a query library. For de novo prediction, we constructed a H. difformis genome de novo repeat library using RepeatModeler (http://www.repeatmasker.org/RepeatModeler/), which can automatically execute two core de novo repeat-finding programmes RECON (v1.08) (Bao & Eddy, 2002) and RepeatScout (v1.0.5) (Price et al., 2005) to comprehensively conduct, refine and classify consensus models of putative interspersed repeats for the H. difformis genome. Furthermore, we performed a de novo search for long terminal repeat (LTR) retrotransposons against the H. difformis genome sequences using LTR_FINDER (v1.0.7) (Z. Xu & Wang, 2007). Finally, we merged the library files of the two methods and used Repeatmaker to identify the repeat contents.
2.6 Gene family identification and phylogenetic analysis
We selected 14 species to construct putative gene families (Oryza sativa (Poaceae), Boea hygrometrica (Gesneriaceae), Mimulus guttatus (Phrymaceae), Olea europaea (Oleaceae), Sesamum indicum (Pedaliaceae), Andrographis paniculata (Acanthaceae), Genlisea aurea (Lentibulariaceae), Actinidia chinensis (Actinidiaceae), Arabidopsis thaliana (Brassicaceae), Cucumis sativus (Cucurbitaceae), Glycine max (Leguminosae), Ipomoea nil (Convolvulaceae), Vitis vinifera (Vitaceae) and Solanum lycopersicum (Solanaceae), and downloaded their genome data from the NCBI database (https://www.ncbi.nlm.nih.gov).
To cluster protein-coding genes into families, protein sequences from the longest transcript of each gene from H. difformis and other species were extracted and aligned to each other using the BLAST programme with a maximal e-value of 1e−5. The genome sequences and corresponding protein sequences were downloaded from NCBI (https://www.ncbi.nlm.nih.gov/). Genes with identity <30% or coverage <50% were filtered out to exclude putative fragmented genes. The OrthoMCL (v14-137) (Li et al., 2003) method was used to cluster genes from these different species into gene families. To reveal phylogenetic relationships among H. difformis and other closely related species, protein sequences from 196 single-copy orthologous genes were used for phylogenetic tree reconstruction. The protein sequences of the single-copy orthologous genes were aligned with the MUSCLE (v3.8.31) programme (Edgar, 2004), and corresponding coding sequence alignments were generated and concatenated with the guidance of protein alignment. RAxML (v8.2.11) (Stamatakis, 2014) and TimeTree (Hedges et al., 2006) were used to construct the phylogenetic tree using the maximum likelihood method.
2.7 Gene family expansion and contraction analysis
Based on the identified gene families and constructed phylogenetic tree with predicted species divergence times, we used CAFÉ to analyse gene family expansion and contraction (Mendes et al., 2020). A random birth or death model in CAFÉ was used to study gene gain or loss in gene families across the phylogenetic tree, and an adjusted p-value (Q-value) was calculated for H. difformis genes to test for significant expansion or contraction (Fisher test, Q < 0.01). These expansion and contraction genes were mapped to GO pathways for functional enrichment analysis, which was conducted using the enrichment methods. This method implemented hypergeometric test algorithms and the Q-value false discovery rate (FDR) was calculated to adjust the p-value using the R package (https://github.com/StoreyLab/qvalue).
2.8 Collinearity and WGD analysis
BLASTp (e-value <1e−5) was used to detect orthologous genes in H. difformis, Strobilanthes cusia and A. paniculata, to assess synteny level between species. Then, syntenic paralogous blocks were identified with MCScanX (Wang et al., 2012) integrated in TBtools software with default parameters, and visualised by TBtools (Chen & Chen, Zhang, et al., 2020). BLASTp (e-value <1e−5) was also used to detect orthologous pairwise, and MCScanX analysis was then performed for Ks evaluation of V. vinifera, Avicennia marina, H. difformis, S. cusia and A. paniculata. Genome data and annotation of V. vinifera and A. paniculata were downloaded from the NCBI database (https://www.ncbi.nlm.nih.gov). Genome data and annotation of S. cusia were downloaded from its database (http://indigoid-plant.iflora.cn) (Xu et al., 2020). Genome data and annotation of A. marina were downloaded from its database (https://datadryad.org/stash/dataset/doi:10.5061/dryad.3j9kd51f5) (Friis et al., 2021). These analyses were performed using a haploid genome. We extracted all paralogous and orthologous gene pairs from syntenic blocks in those species to further estimate the Ks distribution and infer WGD dates by assuming a molecular clock (Schmutz et al., 2010).
2.9 RNA sequencing, gene expression analysis, and co-expression analyses
To extract total RNA, shoots were harvested at 15:00 pm, from plants grown under each treatment for at least 1 month. Each sample was biologically replicated three times. RNA extraction, messenger RNA (mRNA) purification, library preparation and sequencing for RNA-seq were conducted by Wuhan Frasergen Bioinformatics Co., Ltd. (http://www.frasergen.com/; Wuhan, China). Total RNA was extracted and purified using TRNzol reagent (Invitrogen) according to the manufacturer's instructions. RNA purity was checked using the Nanodrop 2000 system, and RNA integrity was assessed using the Agilent 2100 system (Agilent Technologies). After sequence library generation through mRNA enrichment and reverse transcription PCR, the preparations were sequenced on a BGI PE150 platform and generated 150 bp paired-end reads. We used SOAPnuke (Chen et al., 2017) to filter the raw reads, and HISAT2 (Kim et al., 2015) to align filtered clean reads to the H. difformis genome. Read counts of these samples were calculated, and differential gene expression analysis was conducted using the R package DESeq. 2 (Love et al., 2014) (FDR < 0.05 and expression-level log2 [fold-change] >1). For differentially expressed genes (DEGs) identification and naming, putative orthologs were identified by tBLASTn and BLASTp (e-value <1e−5) analysis to published Arabidopsis databases (https://www.arabidopsis.org/). To identify relationships between DEGs, co-expression networks were constructed using the WGCNA package (Langfelder & Horvath, 2008) integrated in TBtools software with default parameters, and DEGs were filtered according to their gene expression profiles (discarded genes that FPKM < 0.9). Cytoscape (Shannon et al., 2003) was used to visualise the network of genes related to phytohormones' responses. Expression heatmaps were also generated using TBtools software (Chen & Chen, Zhang, et al., 2020).
2.10 Phytohormones and NPA treatments quantification
To measure phytohormone levels, plants were grown in growth chambers at 23°C under a white light flux density of 60 µmol m−2 s−1 for 1 month. Shoots (including leaf primordia) were excised from the plants and immediately frozen in liquid nitrogen. Extraction and quantification of endogenous hormones (IAA, trans-Zeatin, and ABA) were performed as described previously (Yoshimoto et al., 2009). Experiments were conducted in triplicate from three independent plants. For naphthylphthalamic acid (NPA) treatments, plants grown in submerged conditions were shifted to an aquarium that contained a 10 µM NPA solution. After 1 month of treatment, emerging leaves were harvested for morphological analysis.
2.11 Reactive oxygen species content (ROS) detection
Terrestrial and submerged mature leaves (at same stage, P6) were harvested and stained with DAB and NBT solution for H2O2 and O2− analysis, respectively, as previously described (Chen et al., 2021). Leaves were harvested from 20 independent plants and immediately stained for H2O2 (1% 3,3-diaminobenzidine, DAB), and O2− (0.1% nitro blue tetrazolium, NBT) distribution overnight. Leaves were then rinsed in an ethanol series and stored for subsequent analysis.
2.12 Gene functional studies and expression analysis
To amplify the HdLMI1A, HdLMI1B, and HdLMI1C complementary DNA (cDNA) sequences, total RNA was extracted from plant shoots (including leaf primordia) grown for a month and then used to synthesise cDNA as described previously (Li et al., 2020). Amplified fragments of expected length were purified and cloned into a pEASY-T1 cloning vector (TransGen) for sequencing. HdLMI1A, HdLMI1B, and HdLMI1C functions were investigated using the VIGS method as previously described (Zhang et al., 2020). Specific fragments (171 bp for HdLMI1A, 268 bp for HdLMI1B, and 252 bp for HdLMI1C) were amplified, purified, and independently introduced into the pTRV2 vector (http://www.bt-lab.cn/) using a Trelief SoSoo Cloning Kit (Tsingke Biological Technology) to generate the constructs TRV2-HdLMI1A, TRV2-HdLMI1B, and TRV2-HdLMI1C. Recombinant vectors and empty vectors were then introduced into Agrobacterium tumefaciens strain LBA4404 by the liquid nitrogen freeze-thaw method. Empty vectors were introduced as mock controls. At least 20 wild-type individuals were used for each treatment. After two rounds of infiltration (7 and 14 days), plants were grown for 3 additional days in terrestrial conditions and then shifted to aquaria. After 20 days of growth in submerged conditions, representative leaves from 10 individuals showing phenotypic changes were photo-documented for analysis. VIGS treatments silencing efficiency was determined by reverse transcription quantitative PCR as previously described (Li et al., 2022). The cDNAs from individual developing leaves of the VIGS-treated plants were used as templates. Primers used in this study are provided in Table S1. Significant differences in relative expression levels between mock and transgenic lines at p < 0.01 were evaluated using Dunnett's test with the two-sided function in IBM SPSS version 24.
2.13 Carbonic anhydrase (CA) identification
CA protein sequences in Arabidopsis were downloaded from the TAIR database (https://www.arabidopsis.org/) and used to perform local BLASTp to the H. difformis protein database. Subsequently, HMM profiles of the Carb_anhydrase (PF00194), Pro_CA (PF00484), and Hexapep (PF00132) domains were obtained from the Pfam database (http://pfam.xfam.org/) and HMMER (http://hmmer.org/) to identify CA proteins. Putative CA protein sequences were further examined using CDD programmes (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) and the Simple Modular Architecture Research Tool (SMART) (http://smart.embl-heidelberg.de). All obtained CAs were then renamed based on gene IDs and their similarity to Arabidopsis CAs.
2.14 Data availability
The genome dataset is available on the NCBI BioProject GenBank with the accession number PRJNA872356. Genome sequencing data (BGI seq reads, PacBio Sequel reads, and Hi-C interaction reads) and environmental treatment RNA-Seq reads are available in the NCBI Sequence Read Archive under accessions PRJNA855313 and PRJNA859677. H. difformis annotations were deposited in Figshare (10.6084/m9.figshare.25441387). Sequence data from this article can be found in the GenBank data library under the following accession numbers: HdLMI1A (OR296694), HdLMI1B (OR296695) and HdLMI1C (OR296696).
3 RESULTS
3.1 Genome sequencing, assembly, and annotation
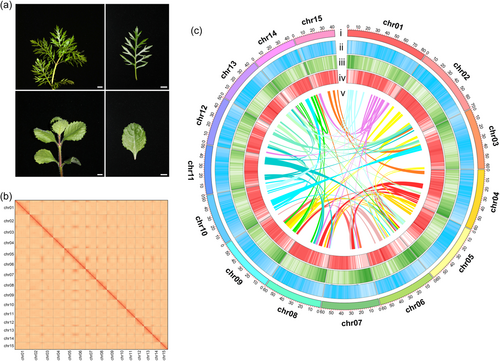
We previously reported that H. difformis reproduces vegetatively (Li et al., 2017), may be infertile, and likely a triploid. Here, we generated 142.27 Gb sequencing data (genome coverage: 150×), with 52 199 178 926 k-mers and a k-mer depth of 54 (Table S2 and Figure S2), which estimated a genome size of 913.38 Mb. K-mer analysis confirmed that H. difformis is triploid (Figure S2), as its two peaks have triple values (K-mer depth = 18 and 54) (Ranallo-Benavidez et al., 2020). We also performed karyotype analysis and cytogenetic studies, which showed 45 chromosomes present (2n = 3X = 45) (Figure S3). We further evaluated genome size by flow cytometry, and it was 0.89 Gb (Figure S4), slightly smaller than the k-mer estimated genome size (Table S2). The H. difformis genome was sequenced, separated, and assembled to the haploid genome using the PacBio Sequel platform (Zhou et al., 2021). The initial genome assembly consisted of 2752 contigs covering 914.25 Mb. Using the Hi-C seq platform (Burton et al., 2013; Durand et al., 2016), we obtained 15 pseudochromosome sequences covering 871.92 Mb (95.37%), with a Scaffold N50 of 60.43 Mb (Table S3-5). BUSCO (Benchmarking Universal Single-Copy Orthologs) (Simão et al., 2015) analysis suggested that 95.50% of total genes were recovered (Table S6). Based on the Hi-C contig contact matrix heatmap and genome features (Figure 1b,c), we verified that clustering, ordering, and orientation of the contigs were successful.
We identified 36 099 protein-coding gene models (Table S7) according to homology-based and de novo predictions and 90.62% (32 714) could be mapped with functional annotations using public databases (Table S8). We also performed BUSCO analysis using the predicted protein sequences to assess our annotation completeness, which indicated that 94.40% of the total genes were recovered (Table S6). These results suggest successful assembly and annotation of major genic regions in the H. difformis genome.
Repetitive DNA sequences are a major component of eukaryotic genomes, reflecting their organisation and evolution (Mehrotra & Goyal, 2014). Using a combination of homology-based searches and de novo prediction, we identified repetitive sequences accounting for 74.92% of the genome (Table S9), which is higher than other related terrestrial species within Acanthaceae, including Andrographis paniculata (53.3%) (Sun et al., 2019b), Strobilanthes cusia (74.4%) (Xu et al., 2020), Avicennia marina (48.8%) (Ma et al., 2022), and the related aquatic species Utricularia gibba (42.18%) (Lan et al., 2017) within the Lamiales. Among repeat sequences in the H. difformis genome, LTRs were dominant (62.39%) (Table S9), which is also higher than U. gibba (12.44%), A. marina (24.92%), A. paniculata (38.54%), and S. cusia (51.32%). These results suggest that the H. difformis genome is relatively more repetitive than the genomes of other reported related species.
3.2 Comparative genomics and genome evolution
To investigate H. difformis evolution, we compared its genome to 14 representative species of diverse evolutionary status, including one monocot, Oryza sativa (Poaceae), and 13 eudicots. We identified a total of 14 656 ortholog groups, of which 1362 were unique to H. difformis (Table S10). We identified 196 single-copy orthologous gene sets among the 15 genomes (Table S11) and subsequently constructed a phylogenetic tree based on the molecular clock (Kumar et al., 2017). The phylogenetic placement of these species is consistent with references therein (Kordyum & Mosyakin, 2020). Among the genomes of other Lamiales, we estimated the divergence times of around 69.8 Mya (million years ago) between O. europaea and the other six species, 57.8 Mya between B. hygrometrica and the remaining five species, and 30.6 Mya between H. difformis and A. paniculata (Figure 2a). H. difformis differentiation time was approximately 25.4–37.9 Mya, coinciding with the evolution and diversification of East Asian Flora (EAF) during the Oligocene (33.9 to 23.0 Mya) (Ma et al., 2019; O'Brien et al., 2020).
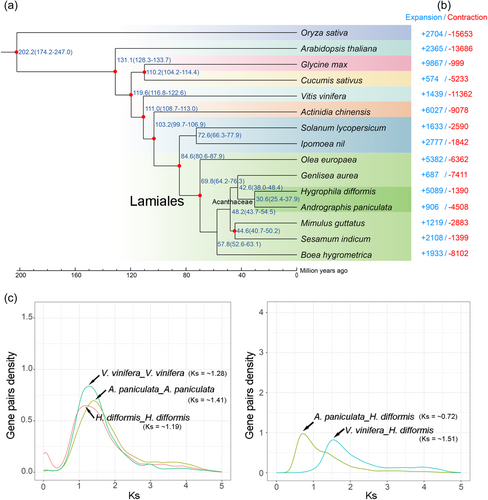
Examining gene expansion and contraction in the phylogenetic analysis revealed that H. difformis had greater gene expansion than its related species (Figure 2b). A total of 5089 ortholog groups expanded in the H. difformis lineage, while 1390 ortholog groups contracted. GO pathway enrichment analysis showed that significantly expanded (Q < 0.01) genes were enriched in traits related to environmental adaptation, such as “tropism” (GO:0009606), “response to freezing” (GO: 0050826), and “response to blue light” (GO:0009637), and also in energy metabolisms and physiological processes, such as “carbohydrate metabolic process” (GO:0005975), “oxidation-reduction process” (GO:0055114) and “regulation of photosynthesis” (GO:0010109). We also detected expanded genes in “hormone metabolic process” (GO:0042445) and “regulation of hormone levels” (GO:0010817), which may regulate H. difformis hormone levels and homeostasis (Table S12). Significantly contracted (Q < 0.01) genes were enriched in “carbon-oxygen lyase activity” (GO:0016838), “terpene synthase activity” (GO:0010333), and some other pathways (Table S13).
Whole genome duplication (WGD) events are major evolutionary forces associated with macroevolutionary changes and evolutionary innovations that happen relatively frequently in plants (Ren et al., 2022). Many Lamiales genera underwent additional WGD events after the gamma event (Godden et al., 2019). To study H. difformis evolution within Acanthaceae, we characterised the synonymous substitution divergence (Ks) of each collinear gene pair within a genome or between genomes for H. difformis and other reported species (Figure 2c; Figure S5). The paralogs Ks distribution indicated that H. difformis, grape (Vitis vinifera, the Acanthaceae outgroup) and A. paniculata had only one peak at Ks = ~1.19, ~1.28 and ~1.41, respectively (Figure 2c). Notably, H. difformis likely experienced a recent WGD event that led to a triploid karyotype, which was not captured in this analysis. The H. difformis–A. paniculata and H. difformis–V. vinifera orthologous distribution peaks also corresponded to Ks values of ~0.72 and ~1.53, respectively (Figure 2c). These results showed that the H. difformis genome may have only experienced ancient whole-genome triplication (γ), which is similar to its closely-related Acanthaceae species A. paniculata and S. cusia (Sun et al., 2019a; Xu et al., 2020). We revealed that the V. vinifera Ks distribution peak was lower than the H. difformis-V. vinifera Ks peak, indicating that the γ event occurred after their differentiation (Figure 2c). We also analysed the Ks distribution of recently reported Acanthaceae species A. marina and S. cusia (Figure S5). Interestingly, the Ks H. difformis–S. cusia and H. difformis–A. paniculata distribution peaks were lower than for H. difformis, S. cusia and A. paniculata (Figure 2c; Figure S5), indicating that these species underwent a common γ event. Additionally, A. marina (2n = 64) experienced a special recent WGD (Ks = ~0.49) (Figure S5) (Ma et al., 2022).
3.3 H. difformis morphological, physiological, and molecular adaptation to the terrestrial or submerged environment
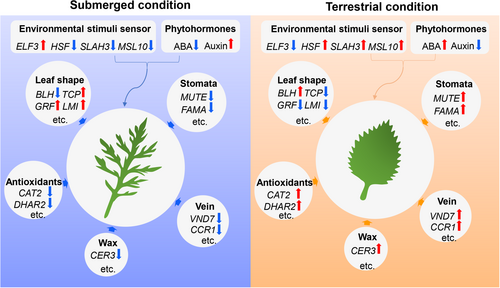
Heterophyllous plants growing in submerged conditions often develop filiform, dissected or linear leaves that lack a cuticle and stomata, while terrestrial leaves are thicker, with differentiated mesophylls, and are cutinised with stomata (Koga et al., 2021; Li et al., 2021). We found that H. difformis leaf area, dissection index (DI), and epidermal cell complexity significantly increased in submerged conditions (Student's t-test, p < 0.01), while stomatal density, vein density, and chlorophyll content significantly increased under terrestrial conditions (Student's t-test, p < 0.01) (Figure 3a; Figure S6), which is similar to other heterophyllous plants (Kim et al., 2018; Koga et al., 2021).
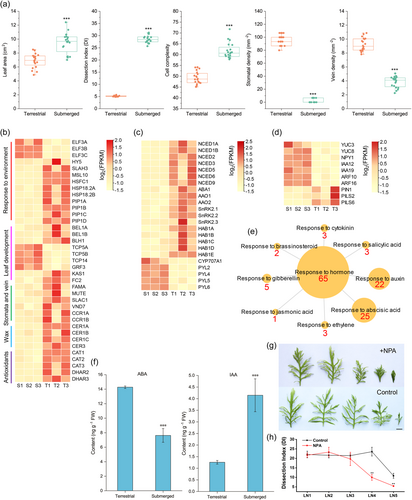
To elucidate the molecular mechanisms underlying H. difformis phenotypic plasticity, we performed RNA-seq analysis on both submerged and terrestrial shoots using annotations based on our genomic data (Tables S14 and S15). A total of 6226 DEGs (FDR < 0.05 and expression-level log2 [fold-change] >1) were identified, with 4100 upregulated genes and 2126 downregulated genes in terrestrial shoots. We then analysed DEGs involved in environmental sensing or response (Figure 3b). Expression of the thermosensor gene EARLY FLOWERING 3 (ELF3) (Jung et al., 2020), a negative regulator of thermomorphogenesis, was downregulated in terrestrial conditions; while heat shock factor and heat shock proteins were upregulated (Figure 3b). The proton sensor gene SLAC1 HOMOLOGUE 3 (SLAH3) (Lehmann et al., 2021), the mechanical stimuli sensor gene MSCS-LIKE 10 (MSL10) (Tran et al., 2021), and the PLASMA MEMBRANE INTRINSIC PROTEIN (PIP) aquaporin genes (Ding et al., 2022; Yaneff et al., 2015) were also upregulated in terrestrial conditions (Table S15).
Morphological changes in leaf shape are the most obvious heterophyllous plant characteristics. The antagonistic interaction of KNOX1 and BELL1-LIKE HOMEOBOX (BLH) genes modulate leaf development, with KNOX1 overexpression enhancing leaf lobes (Hareven et al., 1996) and BLH overexpression reducing leaf serration (Kimura et al., 2008; Kumar et al., 2007). We observed increased expression of BLH genes in terrestrial shoots, which may underlie the leaf simplification observed. TfTCP13 (from Torenia fournieri) overexpression in A. thaliana leads to narrower leaves (Zhang et al., 2021), and we detected increased expression of TCP5, one of the paralogs of TCP13 in H. difformis submerged shoots. TCP14 expression was reduced in H. difformis terrestrial leaves, mimicking the broad, short leaf phenotype induced by the dominant negative construct TCP14::SRDX in A. thaliana (Kieffer et al., 2011). Plant size directly correlates with water use efficiency in Arabidopsis (de Ollas et al., 2023). GROWTH-REGULATING FACTORs (GRFs) control A. thaliana leaf size (Debernardi et al., 2014) and GRF3 was elevated in H. difformis submerged shoots, which may account for increased leaf size (Figure 3a,b). Genes related to chloroplast development and biosynthesis were also elevated in terrestrial shoots (Figure 3b; Figure S6 and Table S15).
MUTE, FAMA, and SLOW ANION CHANNEL-ASSOCIATED 1 (SLAC1), which regulate stomatal development and movement, were upregulated in terrestrial shoots, possibly facilitating stomatal formation in terrestrial leaves (Figure 3b; Table S15). Although EPIDERMAL PATTERNING FACTOR-LIKE9 (EPFL9/STOMAGEN) is a positive stomatal development regulator (Hunt et al., 2010; Zhao et al., 2022), it is often absent in aquatic plants, including Z. marina, S. polyrhiza, Nymphaea colorata, and Wolffia australiana (Park et al., 2021). However, we identified its putative homologous gene (Hdi026079) in H. difformis, but its expression was not detected in either terrestrial or submerged shoots, suggesting it might be a pseudogene or it acquired a different function in H. difformis.
Genes related to vascular development, lignin biosynthesis, and wax biosynthesis were upregulated in terrestrial shoots (Figure 3b), which may enhance terrestrial acclimation. In terrestrial conditions, both UV and drought increase stress-associated symptoms such as MDA accumulation and ROS (Jansen et al., 2022). We also observed increased expression of genes responsive to oxidative stress in the terrestrial environment, which is in agreement with our finding that ROS accumulates in H. difformis terrestrial leaves (Figure 3b; Figure S7 and Table S15).
3.4 Phytohormones role in terrestrial and submerged acclimation
The role of phytohormones in the environmental control of morphogenesis has been reviewed recently (Li et al., 2019; Nakayama et al., 2017; Song et al., 2023). We detected numerous DEGs involved in the “response to hormone”, especially related to the “response to auxin” and “response to abscisic acid” (Figure 3e). Previously, we showed that exogenous ABA can induce H. difformis leaf simplification (Li et al., 2017). Here, we observed increased expression of genes related to ABA biosynthesis (e.g., NINE-CIS-EPOXYCAROTENOID DIOXYGENASE [NCED], ABA DEFICIENT1 [ABA1], and ALDEHYDE OXIDASE [AAO]) and ABA signalling-related genes in terrestrial shoots, consistent with their increased ABA contents (Figure 3c and 3f; Table S15). CYP707A, which encodes an ABA-degradation enzyme, was reduced in terrestrial conditions, as were the PYRABACTIN RESISTANCE 1-LIKE (PYL) genes (Figure 3c; Table S15).
We also observed increased expression of auxin biosynthesis gene YUCCA (YUC) and YUC-related NAKED PINS IN YUC MUTANTS 1 (NPY1) in submerged shoots, along with increased auxin contents (Figure 3d and 3f). INDOLE-3-ACETIC ACID (IAA)-INDUCIBLE and AUXIN RESPONSE FACTOR 10 (ARF10) were also elevated in submerged conditions. Interestingly, the auxin efflux carriers PIN-FORMED (PIN) and PIN-LIKES (PILS) were downregulated under submerged conditions, indicating auxin transport may affect underwater morphogenesis (Figure 3d; Table S15).
To further examine the role of auxin transport in H. difformis, we treated submerged plants with the polar auxin transport inhibitor naphthylphthalamic acid (NPA), which caused an obvious reduction of leaf complexity in emerging leaves, and eventually the formation of oval leaves (Figure 3g,h; Figure S8). During heterophylly, the roles of ABA and ethylene in Ludwigia arcuata (Kuwabara et al., 2003), H. difformis (Li et al., 2017) and R. trichophyllus (Kim et al., 2018), GA in R. aquatica (Nakayama et al., 2014), and cytokinin (CK) in H. difformis (Li et al., 2020) have been elucidated. Here, we demonstrate for the first time that auxin metabolism and transport are also implicated in H. difformis heterophylly regulation.
Previously, we found that CK content is related to leaf complexity and environmental factors, including temperature and humidity (Li et al., 2020). Therefore, we further detected t-Zeatin content in terrestrial and submerged shoots, and found that it was significantly higher in terrestrial, than submerged shoots (Figure S9a). Moreover, ISOPENTENYLTRANSFERASE 7 (IPT7), which encodes cytokinin synthase involved in cytokinin biosynthesis was upregulated in terrestrial shoots (Figure S9b). CYTOKININ OXIDASE 5/6/7 (CKX5/6/7), which catalyse cytokinin degradation and RESPONSE REGULATOR 7 (RR7), which mediates cytokinin signalling, were also upregulated in terrestrial shoots (Table S15). These results indicate that CK metabolism and signalling are active in terrestrial conditions.
3.5 DEGs expression patterns under diverse environmental factors
Expression patterns of candidate genes in the heterophyllous plant C. palustris were revealed under phytohormone treatments (Koga et al., 2021). However, expression patterns of candidate genes in H. difformis under different environmental factors remain unclear. To address this, H. difformis plants were grown in submerged/terrestrial, normal/low light density, normal/low temperature, and high/normal humidity conditions, and sampled for RNA-seq (Figure 4a; Table S14). We also measured morphological and physiological characteristics between these groups (Figure S10), to determine how they were affected by environmental factors. A total of 6226 DEGs were identified under submerged and terrestrial conditions; 9213 under different light densities; 2851 under different temperatures; and 4065 under different humidity levels (Figure 4b,c; Tables S15–18). A total of 180 genes were identified as co-upregulated DEGs under terrestrial, low light density, low temperature, and low humidity conditions, while 119 were identified as co-downregulated DEGs (Table S19-20). NCED3, ETHYLENE-INSENSITIVE 3 (EIN3), CYTOKININ OXIDASE 5 (CKX5), and GA INSENSITIVE DWARF1A (GID1A) genes, which are related to phytohormone pathways, were co-upregulated DEGs under terrestrial, low light density, low temperature, and low humidity conditions (Figure 4d). Genes related to antioxidants were also upregulated, while NPY1 was downregulated under terrestrial, low light density, low temperature, and low humidity conditions (Figure 4e). Wu et al. (2022) showed that expression and functional divergence of SUGARS WILL EVENTUALLY BE EXPORTED TRANSPORTERS (SWEET) genes contribute to the specific developmental strategy of Euryale ferox leaves. Here, we observed that three SWEET putative orthologs were upregulated under terrestrial, low light density, low temperature, and low humidity conditions, implying their potential roles in heterophylly. We also identified 19 upregulated TFs and eight downregulated TFs (Figure 4f) from these common DEGs, which may regulate H. difformis acclimation to different environmental conditions.
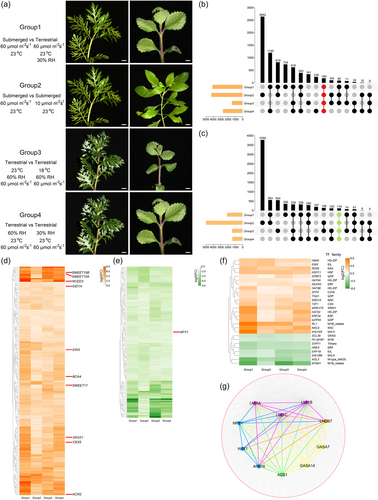
To further pinpoint key heterophylly genes, co-expression analysis was conducted on a total of 15 295 DEGs (all DEGs that were changed in at least one group) and genes from 15 samples were filtered and clustered into 10 modules using weighted gene co-expression network analysis (WGCNA) (Figure S11). We focused on the yellow module which showed high gene expression levels in the high leaf complexity groups (Figure S11, S12, Table S21). We identified three leaf development-related genes, putative orthologs of LATE MERISTEM IDENTITY1 (LMI1A, LMI1B, and LMI1C), which were positively co-expressed with three auxin-related genes (NPY1, AUX1, and ARF16), two GA-STIMULATED IN ARABIDOPSIS (GASA7 and GASA14) genes, the ethylene synthesis gene ACC SYNTHASE1 (ACS1), and the photosynthesis-related gene LIGHT-HARVESTING COMPLEX B7 (LHCB7) (Figure 4g). It is known that heterophylly includes morphological, anatomical and physiological adaptation (Horiguchi et al., 2019; Li et al., 2021). Candidate genes we identified regulating leaf formation, along with phytohormone pathways and physiological adaptation to environmental changes, might influence H. difformis heterophylly.
3.6 HdLMI1 genes regulate H. difformis leaf shape in submerged conditions
LMI1 and its putative orthologs have been studied in several terrestrial species and shown to have conserved roles in leaf shape diversity (Andres et al., 2017; Vlad et al., 2014). Here, we identified three putative paralogous LMI1 orthologs in H. difformis, and examined their expression in different samples (Figure 5a). These genes were highly expressed in humid and aquatic samples (A1-A3, and C1-C3), which had complex leaves (Figure S11). Conversely, their expression was downregulated in simple-leafed groups (Figure 5a).
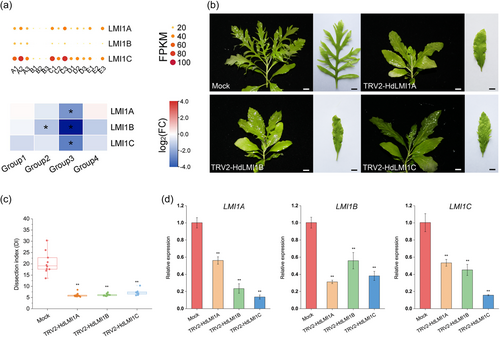
To further examine HdLMI1s roles in regulating H. difformis leaf development, we knocked down these genes using virus-induced gene silencing (VIGS) (Zhang et al., 2020) with the Tobacco rattle virus (TRV) constructs TRV2-HdLMI1A, TRV2-HdLMI1B and TRV2-HdLMI1C. Compared to mock plants, TRV2-HdLMI1A, TRV2-HdLMI1B, and TRV2-HdLMI1C transgenic plants grown in submerged conditions were similar, developing crinkled, complete, or shallowly serrated leaf margins (Figure 5b). Mock plants had significantly higher leaf complexity than TRV2-HdLMI1A, TRV2-HdLMI1B, and TRV2-HdLMI1C transgenic plants (Dunnett's test, p < 0.01) (Figure 5c). In addition to their morphological features, these plants showed a significantly reduced expression of all three HdLMI1s compared to mock (Dunnett's test, p < 0.01) (Figure 5d). LMI1 orthologs in Medicago truncatula cooperatively regulate leaf margin development (Wang et al., 2021). These results confirmed our hypothesis that HdLMI1s are important heterophylly regulators in H. difformis.
4 DISCUSSION
Our knowledge of genetic regulatory networks that contribute to plant phenotype variations has been advanced by the use of the latest technologies, but phenotypic plasticity mechanisms still remain elusive mostly due to the scarcity of suitable models (Hoffmann & Sgro, 2011; van Veen & Sasidharan, 2021). Our published H. difformis genome and transcriptome provide resources for further studies to expand adaptations knowledge to enable this plant to thrive in a highly fluctuating environment. The H. difformis genome also provides an opportunity to investigate convergent evolution among heterophyllous species.
Many aquatic species became polyploid during speciation (Albert & Renner, 2020; Lu et al., 2021), which was verified as a factor in plants expanding their ranges or invading new environments as polyploid progenies often possess novel traits (Cheng et al., 2021). Polyploidy and hybridisation in aquatic or amphibious plants are also associated with complex evolutionary histories (Les & Philbrick, 1993; Prančl et al., 2014). For example, water caltrop (Trapa natans) (Lu et al., 2021) and C. palustris are tetraploid (Koga et al., 2021), while Cabomba caroliniana has polyploid (3×, 6×, 8×) and aneuploid variation between individuals (McCracken et al., 2013). The number of chromosomes in Hygrophila species varies, such as in H. spinosa (2n = 32), H. augustifolia (2n = 44) and H. balsamica (2n = 34) (Govindarajan & Subramanian, 1983). We previously reported that H. difformis always reproduces through vegetative propagation (Li et al., 2017) and does not seem to produce viable seeds. Our genome survey and cytogenetic studies revealed 45 chromosomes, and further analyses suggest that the sequenced H. difformis is triploid (2n = 3x = 45). The Lamiaceae exhibit widespread but asymmetrical gene duplication patterns and ancient polyploidy (Godden et al., 2019). For example, Paulownia tomentosa (Paulowniaceae) had 3 inferred WGDs, while there were 7–18 WGDs in Nepetoideae (Godden et al., 2019). A. paniculata (2n = 48) and S. cusia (2n = 32) only experienced one γ event (Sun et al., 2019a; Xu et al., 2020), however, A. marina (2n = 64) had two WGDs, the most recent attributed to a novel specific adaptation to global warming that occurred during the Palaeocene–Eocene maximum (Ma et al., 2022). Our analysis confirmed the recent WGD in A. marina, but did not detect the common γ event, which could be due to data or methodological differences. We also observed that H. difformis possessed the same γ event as A. paniculata and S. cusia, and lacked any additional WGD during its evolutionary history (Figure 2e). Polyploidy is widely regarded as a common speciation strategy that has pronounced implications for plant evolution and ecology (Van De Peer et al., 2017). H. difformis appears to be a natural triploid. There are only four Acanthaceae species with genome information reported so far (Ma et al., 2022), and thus more data about other Hygrophila species is essential for evolutionary studies. Future studies comparing subgenomes by haplotype-resolved genome assembly would also be helpful in this regard.
Our comparative genome data showed that H. difformis differentiation time was about 30.6 Mya (Figure 2a), coinciding with the evolution and diversification of EAF during the Oligocene (33.9 to 23.0 Mya). Notably, atmospheric CO2 decline and global cooling culminated in the Early Oligocene Aridification event at 31 Mya (Wasiljeff et al., 2022), which is close to the H. difformis differentiation time. We found significantly expanded (Q < 0.01) genes of H. difformis were enriched in “response to freezing”, “regulation of photosynthesis”, and “carbohydrate metabolic process”, which may reflect the environmental response, physiological plasticity and metabolic adaptations to the changing environments (Table S12). Additionally, the formation of the Asian monsoon system in the Oligocene influenced plant evolution and diversification (Ma et al., 2019). Rainfall seasonality increased progressively, achieving modern monsoon-like wet summers and dry winters, by the early Oligocene (W. Y. Chen & Su, 2020). Therefore, plant species must have adapted to rainfall seasonality and drought. For example, habitat diversification (terrestrial and aquatic/marshland) within Pogostemon (Lamiaceae) is related to adaptations to seasonally changing climate triggered by the Asian monsoon in southern and southeast Asia (Yao et al., 2016). It is known that monsoon systems are involved in seasonal rain and fluctuating water levels, and water level change was regarded as the heterophylly driver in many amphibious species (Nakayama et al., 2014; Wanke, 2011). Hygrophila species are widely spread in East Asia, South America, and Southern Africa, and many are amphibious plants (Figure S1). However, due to the lack of sufficient Hygrophila genome and fossil data, it would be premature to conclude that the H. difformis amphibious habitat and phenotypic plasticity resulted from Oligocene climate change.
Cardamine hirsuta leaflet development depends on REDUCED COMPLEXITY (RCO), a LMI1 homolog that represses leaf flank growth and promotes leaflet formation (Vlad et al., 2014). RCO originated from gene duplication in the Brassicaceae family and was lost in A. thaliana, leading to leaf simplification (Vlad et al., 2014). However, LMI1 has diverse functions in different species, for instance, it regulates simple serrated leaf formation in A. thaliana. The lmi1 mutation resulted in decreased leaf serration but induced leaflet formation in A. thaliana (Saddic et al., 2006). In M. truncatula, MtLMI1a and MtLMI1b are expressed in leaf margin, and they redundantly promote of leaf serrations development, as revealed by the smooth leaf margin in their double mutants (Wang et al., 2021). In rapeseed (Brassica napus), BnA10.LMI1 positively regulates the development of leaf lobes (Hu et al., 2018). In cotton (Gossypium hirsutum), the downregulation of GhLMI1-D1b leads to strongly reduced leaf lobing (Andres et al., 2017). To assess LMI1 function in H. difformis heterophylly, we silenced HdLMI1 genes by VIGS and observed reduced leaf complexity in transgenic plants (Figure 5). However, the three HdLMI1 genes may have cross-suppressed together by the TRV constructs due to their sequence similarities, which points to functional redundancies among the HdLMI1 genes. In M. truncatula, two LMI1 paralogous are separately expressed in leaf margin tooth and sinus, and cooperatively regulate leaf serrations (Wang et al., 2021). Thus, it is necessary to distinguish the effects of silencing each HdLMI1 gene via CRISPR/Cas9-mediated genetic modifications. Moreover, heterophylly may involve epigenetic regulation such as non-coding RNAs, DNA methylation, and nucleosome assembly (Li et al., 2021; Nakayama et al., 2014), and more advanced analyses such as target mimicry, single-cell sequencing, and CRISPR/Cas9 genetic modification are warranted in future studies.
Auxin is a key phytohormone that orchestrates development and thus participates in plant evolution (Finet & Jaillais, 2012; Moon & Hake, 2011). Auxin transport is essential for leaf primordia initiation and leaf margin formation (Shani et al., 2006). In Arabidopsis, auxin transport disruption leads to the absence of leaf serration, whereas PIN1 localisation determines the local auxin maxima at the tips of emerging serrations (Hay et al., 2006). In compound-leaved species auxin transport perturbation causes a dramatic simplification of leaf morphology (DeMason & Chawla, 2004; Wang et al., 2005). Although auxin accumulation has been detected in some waterlogged plants (Qi et al., 2019), its role in heterophylly-induction of amphibious plants remains elusive. Previous studies measured various C. palustris phytohormones under submerged/terrestrial conditions (Koga et al., 2021), but they focused on the importance of ABA and GA, and did not analyse the detailed role of auxin. Here, we show that auxin levels significantly increased under submergence, and the genes related to auxin metabolism, transport and response (e.g., YUC8, NPY1, PIN1) are differentially expressed (Figures 3 and 4). Moreover, we demonstrate that auxin transport inhibition abolishes heterophylly in H. difformis. The interplay between auxin maxima and KNOX1 genes underlies serrations evolution in A. thaliana and leaflets in C. hirsuta (Canales et al., 2010). The LMI1-like and KNOX1 genes also jointly control leaf development in both cotton and C. hirsuta (Chang et al., 2019; Wang et al., 2022). It was revealed that M. truncatula LMI1 regulates leaf margin development by activating auxin transporter genes (Wang et al., 2021). Thus, putative orthologs of LMI1, KNOX1, and the auxin transport genes may cooperate in H. difformis heterophylly regulation.
We also detected DEGs related to phytohormones ABA and CK metabolism under terrestrial and submerged conditions. Genes related to ABA biosynthesis and ABA signalling-related genes were upregulated in terrestrial shoots, while CYP707A, which encodes an ABA-degradation enzyme, was reduced in terrestrial conditions, as were the PYL genes. PYLs code ABA receptors, therefore, PYLs downregulation may contribute to terrestrial morphogenesis by modulating the sensitivity to ABA or inducing differential responses downstream of the ABA signalling pathway. Additionally, t-Zeatin content is higher in terrestrial shoots, whereas both IPT7 and CKX5/6/7, which mediate cytokinin biosynthesis and degradation, were also upregulated in terrestrial shoots (Figure S9). These results indicate that ABA biosynthesis and CK metabolism are active in terrestrial conditions.
Because many aquatic plants perform gas exchange by diffusion through the epidermis, stomatal development is always inhibited or abolished. Zostera marina, the submerged sea grass, even lost a set of key regulatory genes driving stomatal differentiation (Olsen et al., 2016). Studies on R. trichophyllus and C. palustris detected reduced expression of SPCH, MUTE, FAMA, and other core stomatal regulators, together with stomatal reduction in their submerged leaves (Kim et al., 2018; Koga et al., 2021). In R. aquatica, ethylene downregulates RaSPCH and RaMUTE expression, a mechanism sufficient for the inhibition of stomatal differentiation under submergence (Ikematsu et al., 2023). We also identified MUTE, FAMA, and SLAC1, which regulate stomatal development and movement, were upregulated in H. difformis terrestrial shoots (Figure 3), indicating that submergence-inhibited stomatal formation may be conserved among heterophyllous species.
CA, an enzyme that can catalyse interconversion between CO2 and HCO3−, plays important roles in HCO3− uptake under submergence (Han et al., 2021). Horiguchi et al. (2023) identified a CA group upregulated in submerged H. difformis leaves, including two α-CAs and one β-CA, and claimed that HCO3– use for photosynthesis is driven by the cooperation between CAs and HCO3– transporters. To make a comparison, we further identified CAs in H. difformis based on our genome data, and analysed their expression under terrestrial and submerged conditions (Table S22). Two α-CAs were significantly downregulated in submerged shoots, while four β-CAs and two α-CAs were significantly upregulated in terrestrial shoots. The results from these two studies are inconsistent, possibly because Horiguchi et al. (2023) performed RNA-seq on fully expanded terrestrial and submerged leaves, while our RNA-seq was performed on terrestrial and submerged shoots. Another possible reason is that plant growth conditions may have been different (e.g., different temperature, light density or other environmental factors).
In the present era of rapid climate change, flooding stress (submergence or waterlogging) is a major challenge to many terrestrial plants, especially crops (Bailey-Serres et al., 2012). Plants have evolved two main strategies to cope with the lethal effects of submergence (Akman et al., 2012; Bailey-Serres & Voesenek, 2008; Müller et al., 2021). The “escape” strategy involves shoot elongation to reach the surface and restore air contact, while the “wait” strategy involves growth reduction and energy conservation to survive until the floods recede. Recent studies have mainly focused on the crop “escape” strategy, especially in deep-water rice (Kuroha et al., 2018; Mittal et al., 2022; Nagai et al., 2020). Interestingly, the aquatic plant N. nucifera also adopts the “escape” strategy during early submergence (Deng et al., 2022). Heterophyllous plants appear to have evolved a third strategy, “variation”, to adapt to constant changes in submergence and dehydration (Figure 6). Understanding heterophylly mechanisms could provide novel avenues to engineer resilient crops and develop agricultural practices to mitigate the effects of climate change.
ACKNOWLEDGEMENTS
We thank professor Shilin Chen of the Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences for kindly providing the genome annotation of A. paniculata and Dr. Markus Stetter, University of Cologne for critical reading of the manuscript. We thank Li Xie and Huan Wang of Wuhan Frasergen Bioinformatics Co., Ltd. for kindly providing the after service of our genome project. This study was supported by the International Partnership Programme of the Chinese Academy of Sciences (152342KYSB20200021), the National Natural Science Foundation of China (32101254), and the National Key Research and Development Programme of China (2017YFE0128800). This work was also supported by the China Scholarship Council.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




